Abstract
Isorhapontigenin (ISO) is a new derivative of stilbene compound that was isolated from the Chinese herb Gnetum Cleistostachyum, and has been used for treatment of bladder cancers for centuries. In our current studies, we have explored the potential inhibitory effect and molecular mechanisms underlying ISO anti-cancer effects on anchorage-independent growth of human bladder cancer cell lines. We found that ISO showed a significant inhibitory effect on human bladder cancer cell growth and was accompanied with related cell cycle G0/G1 arrest as well as downregulation of Cyclin D1 expression at the transcriptional level in UMUC3 and RT112 cells. Further studies identified that ISO down-regulated Cyclin D1 gene transcription via inhibition of SP1 transactivation. Moreover, ectopic expression of GFP-Cyclin D1 rendered UMUC3 cells resistant to induction of cell cycle G0/G1 arrest and inhibition of cancer cell anchorage-independent growth by ISO treatment. Together, our studies demonstrate that ISO is an active compound that mediates for Gnetum Cleistostachyum’s induction of cell cycle G0/G1 arrest and inhibition of cancer cell anchorage-independent growth through down-regulating SP1/Cyclin D1 axis in bladder cancer cells. Our studies provide a novel insight into understanding the anti-cancer activity of the Chinese herb Gnetum Cleistostachyum and its isolate ISO.
Keywords: Bladder cancer, Isorhapontigenin, cell cycle progression, Cyclins, SP1, Signal transduction pathway
Introduction
Bladder cancer (BC) is one of the most common cancers in the Western world and the fifth most common cancer in United States (1). According to the American Cancer Society, 73,510 new cases of BC are expected to be diagnosed and 14,880 patients will die from this disease in the U.S. in 2012. Because high-grade invasive bladder cancers can progress to life threatening metastases and are responsible for almost 100% of death from this disease (2, 3), identifying a natural compound that specifically inhibits BC invasion and metastasis is of tremendous importance for potentially reducing mortality as a result of this disease. Previous studies have addressed the clinical relevance of Cyclin D1 alteration in bladder cancer development (4, 5). A significant proportion of bladder cancer cases showed that overexpression of the Cyclin D1 gene and increased Cyclin D1 expression were associated with poor prognosis and decreased postoperative patient survival (4, 6). Aberrant Cyclin D1 expression has been observed early in carcinogenesis as well (7). Cyclin D1 is a key cell cycle regulatory protein, playing a critical role in the G1-to-S transition of the cell cycle progression through binding to cyclin dependent kinase 4 (CDK4) to phosphorylate (8) and inactivate the retinoblastoma protein (pRb) (9), heterozygous deletion of which occurs in ~50% of human muscle-invasive BC. Thus, identifying a new anti-cancer drug targeting and downregulating Cyclin D1 expression and function are one of the first priorities in the field of anti-cancer research.
Due to the multi-faced biological activities of natural oligostibenes, in the past two decades, more and more attention has been focused on the anti-cancer activities of this kind of compound (10, 11). Isorhapontigenin (ISO) is a new derivative of stilbene compound that was isolated from the Chinese herb Gnetum Cleistostachyum, which has been used for treatment of bladder cancers for centuries (12). To determine the anti-cancer activity and mechanisms of this Chinese herb, in this study, the potential anti-cancer activity, inhibition of Cyclin D1 expression as well as molecular events implicated in these activities were elucidated in human bladder cancer cells.
Materials and Methods
Plasmids, Antibodies, and Reagents
The GFP-tagged Cyclin D1 expression construct was described in our previous publication (13). The Cyclin D1 promoter driven luciferase reporter (Cyclin D1 Luc) came from Dr. Anil Rustgi (Gastroenterology Division, University of Pennsylvania, Philadelphia, PA) (14). Human Cyclin D1 -163 and -163 mSP1 (point mutation at -130 of SP1 binding site) promoter-driven leuciferase reporter was gift from Dr. Richard G. Pestell (Kimmel Cancer Center, Thomas Jefferson University, PA) (15). The transcription factor Specific protein 1 (SP1) luciferase reporter, containing three consensus SPl binding sites, was kindly provided by Dr. Farnham Peggy J (McArdle Laboratory for Cancer Research, University of Wisconsin, Madison) (16). The antibodies against p53, P-ATFII, were purchased from Cell Signaling Technology (Boston, MA). The antibodies against CDK4, CDK6, FOS (C-FOS), Cyclin A, Cyclin B1, Cyclin D1, Cyclin E, p21 and Specific Protein 1 (SP1) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The antibodies against C-Jun, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), nuclear factor kappa B (NF-κB) p65, P-C-Jun Ser 63, P-C-Jun Ser 73, and P-NF-κB p65 were obtained from Cell Signaling Technology (Boston, MA). The antibody against heat shock factor-1 (HSF-1) was obtained from Stressgene Inc. (San Diego, CA). The antibody against p27 was obtained from Abcam Inc. (Cambridge, MA). ISO with purity over 99% was obtained from Dr. Qi Hou, Materia Medica of Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China. ISO was dissolved in Dimethyl sulfoxide (DMSO) to make a stock concentration at 10 mM and the same concentration (0.1%, v/v) of DMSO was used as a negative control in all experiments.
Cell Culture and Transfection
Human bladder cancer cell line RT4, RT112 and UMUC3 were provided by Dr. Xue-Ru Wu (Departments of Urology and Pathology, New York University School of Medicine, New York, NY) (17). Normal mouse epidermal cell line Cl41 cells was provided by Dr. Zigang Dong (Hormel Institute, University of Minnesota) (18–20) and was cultured with Eagle’s MEM with 5% FBS, 2 mM L-glutamine, and 25 μg/ml gentamicin. All cell lines were subjected to DNA tests and authenticated before utilization for researches. UMUC3 cells were maintained at 37 °C in a 5% CO2 incubator in DMEM supplemented with 10% fetal bovine serum (FBS), and RT112 cells were cultured with RPMI 1640 supplemented with 10% FBS. Stable co-transfections were performed with specific cDNA constructs and/or pSuper vector using PolyJet™ DNA In Vitro Tranfection Reagent (SignaGen Laboratories, Gaithersburg, MD) according to the manufacturer’s instructions and our previous studies (21).
Cell Cycle Analysis
UMUC3 cells were cultured in each well of six-well plates to 70%–80% confluence with normal culture medium. The cell culture medium was replaced with 0.1% FBS DMEM with 2 mmol/L L-glutamine and 25 μg gentamicin and cultured for 24 hours. The cells were then exposed to ISO (5μM) for the indicated time. The ISO-treated and control cells were harvested and fixed in 75% ethanol overnight. The cells were then suspended in staining buffer (containing 0.1% Triton X-100, 0.2 mg/ml RNase A, and 50 μg/ml propidium iodide (PI)) at 4 °C for 1 hour and then DNA content was determined by flow cytometry utilizing a Epics XL flow cytometer (Beckman Coulter Inc., San Diego, CA) and EXPO32 software as previously described in reference(13).
Anchorage-independent growth Assay
The potential ISO inhibitory effect of anchorage-independent growth (soft agar assay) on human bladder cancer cells was determined in UMUC3 cell line (21). In brief, 1×104 UMUC3 cells were exposed to various concentrations of ISO in 10% fetal bovine serum (FBS) basal medium Eagle (BME) containing 0.33% soft agar, was seeded over bottom layer of 0.5% agar in 10% FBS BME/in each well of 6-well plates. The cultures were maintained at 37°C in 5% CO2 incubator for 21 days and the cell colonies with over 32 cells were scored, as described in our previous studies (21, 22). Colonies were observed and counted under microscope. The results were presented as mean±SD of colony number per 10,000 seeded cells in soft agar from 3 independent experiment wells.
Animal experiment and ISO Pharmacokinetics analysis in vivo
Thirty (30) Wistar male mice, weighing 20~25g, were purchased from Experimental Animal Center of the Chinese Academy of Military Medical Sciences and kept under controlled conditions with a 12-h light cycle with accessing water ad libitum overnight. Mice were then administered with ISO (150 mg/kg) via gastric gavage. Three mice were sacrificed and blood samples were taken at each time points of 0.033h, 0.083h, 0.17h, 0.25h, 0.5h, 0.75h, 1h, 1.5h, 2h and 4h after ISO was given. The serum was collected from each mouse by centrifuging of blood sample at 4000rpm for 30 min and stored at −20°C for further analyses. To determine pharmacokinetics of ISO in serum of mice, a 50 μL aliquot of each serum sample was transferred to 1.5 mL polypropylene tubes, and 300 μL methanol (LC grade) was added to each sample with vortex for 5 min. After centrifugation for 10 min at 10000 rpm, the supernatant was filtered through 0.45 μm filter membrane and then applied to the LC/MS/MS. The LC/MS/MS system that was used consisted of an Applied Biosystems Sciex QTrap 5500 mass spectrometer (Thornhill, Ontario, Canada) coupled to a Shimadzu UPLC system (Shimadzu, Columbia, MD). ISO and IS naringenin were separated on a Shimpack C18 ODS column (150 mm × 2.3 mm id, 3μm particle size) with a gradient elution of the mobile phase system consisting of 0.1% acetic acid solution (A) and methanol (B). The elution program was performed with flow rate at 0.2 mL/min under column temperature at 30°c. The mass spectrometer was performed using electrospray ionization (ESI) with an ionspray voltage of −4500V and 550°c. The negative ion multiple-reaction-monitoring (MRM) mode analysis was performed using nitrogen as the collision gas. Precursor/product ion pairs for ISO and naringenin were m/z 257.0/241.1 and m/z 271.1/151.1. Data acquisition and processing were performed using Sciex Analyst 1.5.1 software package (SCIEX).
Western Blotting Assay
After the cells were exposed to the indicated concentration of ISO or for the indicated time with 5 μM ISO, cells were extracted in a cell lysis buffer (10 mM Tris-HCl (pH 7.4), 1% SDS, and 1 mM Na3VO4) and total protein was quantified with a DC protein assay kit (Bio-Rad). The membranes were probed with the indicated primary antibodies and the AP-conjugated second antibody. Signals were detected by the ECF Western-blotting system, as described previously described (23).
RT-PCR
Total RNA was extracted with TRIzol reagent (Invitrogen Corp., Carlsbad, CA) after ISO treatment and the cDNAs were synthesized with the Thermo-Script RT-PCR system (Invitrogen Corp., Carlsbad, CA). The mRNA amount present in the cells was measured by semiquantitative RT-PCR. The primers were 5′-AGAAGGCTGGGGCTCATTTG -3′ and 5′-AGGGGCCATCCACAGTCTTC -3′ for human gapdh, and 5′-GAGGTCTGCGAGGAACA GAAGTG-3′ and 5′-GAGGGCGGATTGGAAATGAACTTC-3′ for human cyclin d1. The PCR products were separated on 2% agarose gels and stained with ethidium bromide, and the results were imagined with Ahpha Innotech SP Image system (Alpha Innotech Corporation, San Leandro, CA), as previously described (13, 21).
Luciferase assay
UMUC3 cells with stable transfection of the Cyclin D1 promoter–driven luciferase reporter or SP1 luciferase reporter were seeded into 96-well plates (1×104 per well) and subjected to the ISO treatments when cell density reached 80–90% confluence. The cells were extracted with lysis buffer [25 mmol/L Tris-phosphate (pH 7.8), 2 mmol/L EDTA, 1% Triton X-100, and 10% glycerol], and the luciferase activity was determined by the microplate luminometer (Microplate Luminometer LB 96V, Berthold GmbH & Co.KG,Germany) using the luciferase assay system (Promega Corp., Madison, WI) as described in our previous studies (24).
Nuclear Extract Preparation
UMUC3 cells were seeded into 10-cm culture dishes and treated either with DMSO or 5 μM ISO for 12 hours. The nuclear proteins were extracted according to the protocol of Nuclear/Cytosol Fractionation Kit (BioVison Technologies, Mountain View, CA). Preparation of nuclear extracts was assessed as previously described in reference (25) and nuclear extracts were stored at −80°C until they were used.
ChIP (Chromatin Immunoprecipitation) Assay
The ChIP assay was performed with EZ- ChIP kit (Millipore Technologies, Billerica, MA) according to the manufacturer’s instructions. Briefly, UMUC3 cells were untreated or treated with ISO (5 μM) for 12 hours. Then genomic DNA and the proteins were isolated in the same manner as in our previous publication (26). To specifically amplify the region containing the putative responsive elements on the human Cyclin D1 promoter, PCR was performed with the following pair of primers as follows: 5′-TTCTCTGCCCGGCTTTGATCTC-3′ (From −92 to −73) and 5′-CTCTCTGCTACTGCG CCAACA- 3′ (From +7 to +27) (15). The PCR products were separated on 2% agarose gels and stained with ethidium bromide, and the images were scanned with a UV light.
Bioinformatic Analysis
Cyclin D1 promoter region was analyzed for potential transcription factor binding sites using TFANSFAC® Transcription Factor Binding Sites Software (Version 7.0).
Statistical Methods
Student’s t test was utilized to determine the significance of differences between different groups. The differences were considered to be significant at P< 0.05.
Results
ISO inhibited cell proliferation and anchorage-independent growth, and induced G0/G1 growth arrest in human BC UMUC3 cell line
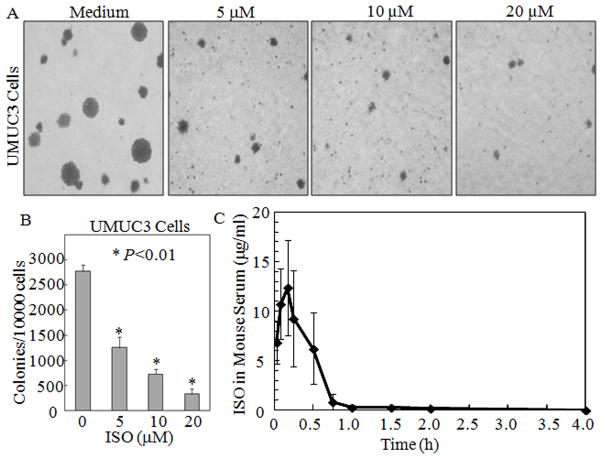
The chemical structure of ISO is a chemical compound 4-methoxyresveratrol with a molecular weight of 258 as described in our published study (Fig. 1A) (21). To evaluate the potential inhibition of ISO in human bladder cancer, we first examined the effects of ISO on cell viability in non-cancerous Cl41 cells, non-invasive human bladder tumor cell line RT4 and high invasive human bladder cancer cell line UMUC3. As shown in Fig. 1B, UMUC3 and RT4 cells with ISO treatment at concentration of 5–60 μM for 48 h resulted in significant reduction of cell viability in a concentration-dependent manner in ATPase activity assays. The IC50 of the UMUC3 and RT4 cell lines was 22.4±3.3 μM (n=3) and 38.6±2.9 μM (n=3) respectively, whereas there was no obvious reduction of cell viability in normal Cl41 cells. The cell morphology showed that ISO at 20 μM induced UMUC3 cells undergoing markedly morphologic changes such as shrinkage, rounding, detachment and membrane blebbing (Fig. 1C), which is consistent with our most recent findings that ISO induced apoptosis in UMUC3 and other invasive BC cells at 20μM (21). More importantly, ISO at concentration of 5 μM did show inhibition of cell proliferation (Figs. 1B and 1C) without induction of observable apoptosis in UMUC3 cells (Fig. 1D). This notion was further verified with the results obtained from cell cycle and apoptotic analyses by flow cytometry. Exposure of subconfluent UMUC3 cells to 5 μM ISO led to significant induction of G0/G1 growth arrest at both 12h (47.58 vs 57.98%) and 24h (47.58 vs 62.62%) (Fig. 1D and 1E) respectively, whereas it did not induce any increases of apoptotic cells (Fig. 1D). These results suggested that the inhibition of high invasive BC MUMC3 cell proliferation by low concentration (5 μM) of ISO was associated with its induction of cell G0/G1 growth arrest. To determine whether a low concentration of ISO was able to inhibit anchorage-independent growth of BC cells, UMUC3 was exposed to ISO in soft agar. As shown in Fig. 2A and 2B, ISO also markedly inhibited anchorage-independent growth in a concentration-dependent manner at concentration as low as 5 μM (P<0.01), indicating that ISO induction of cell G0/G1 growth arrest might be associated with its anti-cancer activity in high invasive human bladder cancers.
Figure 1. ISO induced cell cycle G0/G1 arrest of human bladder cancer.
(A) The chemical structure of ISO. (B) Incubation with the ISO caused concentration-dependent growth effects on UMUC3, RT4, and Cl41 cells in vitro, as observed in ATPase assays. Results are presented from three independent experiments in the presence of varying concentrations of ISO for 48 hrs. (C) These morphology changes were observed in UMUC3 cells exposed to different concentrations of ISO for 24 hours. (D) & (E) Flow-cytometry analysis of cell cycle alteration in UMUC3 cells upon ISO treatment. UMUC3 cells were treated with 5 μM ISO as indicated time. The result represents one of three independent experiments.
Figure 2. ISO inhibited cell proliferation in human bladder cancer cells.
(A) Representative images of colonies of UMUC3 cells in soft agar assay without or with various concentrations of ISO. (B) Quantification of results of colony formation of UMUC3 cells in soft agar assay obtained from three independent experiments. Colonies were visualized and counted under a microscope with size over 32 cells of each colony. (C) ISO concentration vs. time curve in serum of mice (n=3).
To determine whether ISO concentrations (5–20 μM) used in current in vitro studies are reachable animal models in vivo, thirty (30) Wistar male mice were administered via gastric gavage with ISO (150 mg/kg). Blood samples from each group (n=3) were taken at each time points of 0.033h, 0.083h, 0.17h, 0.25h, 0.5h, 0.75h, 1h, 1.5h, 2h and 4h after ISO was given. The serum was collected for determination of ISO concentration in serum of mice using LC/MS/MS system. The mean of ISO concentration versus time profiles was shown in Table 1 and the corresponding curve is shown in Fig. 2C following oral administration of 150 mg/kg of ISO. The pharmacokinetic parameters of ISO were obtained by DAS 3.0 computer software analysis using non-compartmental model and summarized in Table 2. Maximum observed concentration (Cmax) at 12.35μg/ml (47.9 μM) in mouse serum rapidly reached at 0.17 h (10 min). The elimination half-time of ISO was 1.7 h and the MRT was 0.7 h in vivo. The results demonstrated that ISO oral administration could result in a rapid absorption in mice, and 5–20 μM of ISO concentrations applied in current in vitro studies are reachable in vivo mice.
Table 1.
Serum ISO concentration vs. time in mouse serum after administration of 150 mg/kg body weight (n=3)
| Time (h) | Caverage ± SD (μg/mL) |
|---|---|
| 0.033 | 6.80±2.10 |
| 0.083 | 10.74±3.52 |
| 0.170 | 12.35±4.79 |
| 0.250 | 9.23±4.84 |
| 0.500 | 6.22±3.60 |
| 0.750 | 0.83±0.80 |
| 1.000 | 0.29±0.24 |
| 1.500 | 0.28±0.14 |
| 2.000 | 0.17±0.12 |
| 4.000 | 0.03±0.01 |
Table 2.
Non-compartmental pharmacokinetic parameters of ISO
| Parameter | Unit | Value ±SD |
|---|---|---|
| AUC(0-t)a | μg/mL *h | 6.09±2.81 |
| AUC(0-∞)a | μg/mL *h | 6.10±2.80 |
| MRT(0-t)b | h | 0.70±0.20 |
| t1/2zc | h | 1.72±0.27 |
| Tmaxd | h | 0.14±0.05 |
| CLz/Fe | L/h/kg | 27.7±10.3 |
| Cmaxef | μg/mL | 12.7±6.5 |
AUC (0-t) and AUC (0-∞): area under the curve from the time of dosing to the last measurable concentration or the time of the last observation.
MRT(0-t): mean residence time.
t1/2z: terminal half-life.
Tmax: time of maximum observed concentration.
CLz/F: apparent clearance.
Cmax: maximum observed concentration.
ISO treatment downregulated Cyclin D1 protein expression in human BC cells
The results above showed that ISO pretreatment led to a G0/G1 phase growth arrest. To elucidate the molecular mechanisms underlying this biological effect of ISO, we determined the alteration in Cyclin D1 expression upon ISO treatment. Treatment of UMUC3 with different concentrations of ISO for 24 hours resulted in a concentration-dependent reduction of Cyclin D1 protein expression compared to the DMSO-treated cells (Fig. 3A and 3E), whereas it did not show observable inhibition of other cycle regulators, including Cyclin A, Cyclin E, Cyclin B1, CDK4, CDK6, P53, P27, and P21 (Fig. 3A). Since ISO at 5 μM showed the induction of cell cycle arrest without any apoptotic effect, it was used for the time course investigation and in the following experiment. Similarly, the ISO showed a markedly inhibition of Cyclin D1 expression in a high grade RT112 cell line (Fig. 3B), a slight inhibition in a low grade human RT4 cell line (Fig. 3C), and marginal induction of Cyclin D1 in a normal Cl41 cell line (Fig. 3D). The significant reduction of Cyclin D1 expression by ISO could be observed as early as 6 h upon ISO treatment in both UMUC3 cells (Fig. 4A and 4B) and RT112 cells (Fig. 4C). Consistently, expression of Cyclin A, Cyclin E, CDK4, CDK6, P53, P27, and P21 were not affected under the same experimental conditions and Cyclin B1 expression was slightly reduced at 24 h of treatment by ISO in UMUC3 cells (Fig. 4A). These results suggest that ISO downregulates Cyclin D1 expression, and that might be associated with its induction of G0/G1 growth arrest in human BC cells.
Figure 3. ISO downregulated Cyclin D1 protein expression.
(A–D) Protein expressions, as indicated in UMUC3, RT112, RT4, and Cl41 cells, were determined with Western blotting after cells were treated with indicated concentrations of ISO for 24h. (E) Quantitative analysis of Cyclin D1 expression, relative to GADPH (Ratio of Cyclin D1/GAPDH) in ISO-treated UMUC3 cells using the scanning software. “*” indicates a significant difference from medium control (P<0.01, n=3).
Figure 4. ISO downregulated Cyclin D1 protein expression in UMUC3 and RT112 cells.

Protein expression as indicated in UMUC3 (A) and RT112 (C) cells, were determined with Western blotting after cells were treated with 5 μM of ISO for the indicated time periods. GAPDH was used as the protein loading control. (B) Quantitative analysis of Cyclin D1 expression relative to GADPH (Ratio of Cyclin D1/GAPDH) in 5μM ISO-treated UMUC3 cells using the scanning software. “*” and “**” indicate a significant difference from medium control (n=3).
Ectopic expression of GFP-CyclinD1 in UMUC3 cells rendered the transfectant resistant to G0/G1 growth arrest induction and anchorage-independent growth inhibition by ISO
To evaluate the contribution of Cyclin D1 downregulation by ISO to cell cycle and anchorage-independent growth regulation, we stably transfected GFP-Cyclin D1 expression construct into UMUC3 cells and the stable transfectant UMUC3 (GFP-Cyclin D1) was established, as indicated in Fig. 5A. UMUC3 (GFP-Cyclin D1) and its vector control transfectant UMUC3 (GFP) were exposed to ISO for determination of ectopic expression of GFP-Cyclin D1 on regulation of cell cycle and anchorage-independent growth. As shown in Fig. 5A, ISO treatment only downregulated endogenous Cyclin D1 protein expression, and not exogenous GFP-Cyclin D1 expression. Consistent with ISO effects on endogenous Cyclin D1 and exogenous GFP-Cyclin D1 protein expression, ISO-induced a G0/G1 growth arrest in UMUC3(GFP) cells (62.74% vs. 74.60%) was impaired by ectopic expression of GFP- Cyclin D1 in UMUC3(GFP-Cyclin D1) cells (51.01% vs. 54.61%) (Fig. 5B). More importantly, ISO inhibition of anchorage-independent growth in UMUC3 (GFP) cells was reversed by ectopic expression of GFP-Cyclin D1 in UMUC3 cells (Fig. 5C). These results demonstrate that downregulating of Cyclin D1 expression mediates ISO induction of G0/G1 growth arrest and inhibition of anchorage-independent growth of UMUC3 cells.
Figure 5. Ectopic expression of GFP-Cyclin D1 was not inhibited by ISO and was able to reverse ISO-mediated induction G0/G1 growth arrest and inhibition of anchorage-independent growth in UMUC3 cells.
UMUC3 stable transfectants, as indicated, were treated with ISO for 24 h. The cells were extracted for Western Blotting (A) or subjected to cell cycle analysis using Flow-cytometry assay (B). (C) UMUC3 stable transfectants as indicated were subjected to anchorage-independent growth in soft agar in the absence or presence of ISO.
ISO downregulated Cyclin D1 expression at transcriptional level
Our above results that ISO treatment only downregulated endogenous Cyclin D1 protein expression but not exogenous GFP-Cyclin D1 expression, excluded the possibility of ISO inhibiting Cyclin D1 expression at regulation of protein stability. To further elucidate the underlying mechanisms of ISO-induced down-regulation of Cyclin D1 protein expression, we examined the effect of ISO on cyclin d1 mRNA expression. As shown in Figs. 6A & 6B, UMUC3 cells treatment with ISO resulted in a marked reduction of cyclin d1 mRNA in concentration- and time-dependent manners, which was consistent with the results obtained at protein levels. These results indicate that ISO treatment attenuates Cyclin D1 expression at either the transcription level or mRNA stability level. To test whether transcription was involved in Cyclin D1 downregulation by ISO, the Cyclin D1 promoter-driven luciferase reporter was stably transfected into UMUC3 cells. The results showed that treatment of UMUC3 cells with ISO led to a marked inhibition of Cyclin D1 promoter transcriptional activity in a time-dependent manner (Fig. 6C). These results indicated that ISO mainly regulated the Cyclin D1 protein expression at the transcriptional level.
Figure 6. ISO downregulated Cyclin D1 transcription and SP1 protein expression.
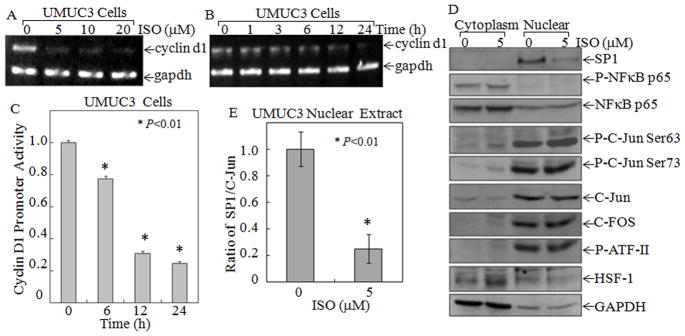
(A & B) Total RNA isolated from the UMUC3 cells treated with the indicated concentration of ISO for 24 h (A) or 5 μM ISO for indicated time periods (B) were subjected to RT-PCR for determination of Cyclin D1 expression level. The GAPDH was used as a loading control. (C) UMUC3 stably transfected with Cyclin D1 promoter-driven luciferase reporter was treated with ISO (5 μM) for indicated times to determine the inhibitory effect of ISO on Cyclin D1 promoter transcriptional activity. “*” indicated P<0.01. (D) Cytoplasmic and nuclear extracts that were isolated from the UMUC3 cells treated with either 0.1% DMSO or 5 μM ISO for 12 h and were subjected to Western Blotting, with the specific antibodies as indicated. GADPH was used as protein loading control. (E) Quantitative analysis of SP1 in nuclear relative to C-Jun expression (Ratio of SP1/C-Jun) in 5μM ISO-treated UMUC3 cells using the scanning software. “*” indicates a significant difference from medium control (n=3).
ISO downregulated transcription factor SP1 expression
To identify the related nuclear transcription factors responsible for the down-regulation of Cyclin D1 by ISO, we used the TFANSFAC® Transcription Factor Binding Sites Software (Version 7.0) to bio-informatic analysis of the Cyclin D1 promoter region. The results revealed that promoter region of the human Cyclin D1 gene contained multiple putative DNA-binding sites of transcription factors, including Activator protein 1 (AP-1), Heat shock factor protein 1 (HSF-1), Activating transcription factor 2 (ATFII), NF-κB, and SP1. We further determined protein expression and nuclear translocation of those transcription factor components upon ISO treatment. The results showed that ISO (5 μM) treatment only downregulated SP1 protein expression (Fig. 6D & E), whereas it did not show any observable inhibition of other transcription factor expression, activation, or nuclear translocation, including C-FOS, P-C-JUN(ser 73), P-C-JUN(ser63), C-JUN, HSF-1, P-ATFII, P-NF-κB p65, or NF-κB p65 (Fig. 6D), thus suggesting that SP1 was a major transcription factor that might be targeted by ISO for downregulation of Cyclin D1 transcription. To determine the effect of ISO on SP1-depedent transcriptional activity, SP1-luciferase reporter was transfected into UMUC3 cells to establish the stable transfectant. ISO treatment led to a dramatically inhibition of SP1-dependent transcriptional activity in a time-dependent manner (Fig. 7B). These results indicated that ISO not only inhibited SP1 protein expression and its nuclear translocation, it also inhibited its dependent transcriptional activity.
Figure 7. SP1 was a major target of ISO for its inhibition of Cyclin D1 transcription.
(A) Schematic representation of the transcription factor SP1 binding sites in the human Cyclin D1 promoter region. (B) The inhibition of SP1-dependent transactivation by ISO. SP1-luciferase reporter plasmid that contains three SP1 consensus binding sites was stably co-transfected with PRL-TK-Luciferase expression vector and pSuper gene into UMUC3 cells. Cells were exposed to ISO (5μM) for indicated time periods. Luciferase activity was determined by Dual-Luciferase Reporter Assay System, and the results are presented as relative SP1 activity of means±SE from three independent experiments. * P<0.01. (C) WT Cyclin D1 luciferase reporter, -163 Cyclin D1 and its mutant SP1 −163 Cyclin D1 were cotransfected with pSuper plasmid into UMUC3 cells respectively, and the stable transfectants were exposed to ISO (5μM) for determination of Cyclin D1 promoter activity. (D) ChIP assay was used to determine ISO effect on SP1 binding activity to the Cyclin D1 promoter region, as described in “Materials and Methods”. (E) The proposed model for ISO regulation of Cyclin D1 expression and G0/G1 cell growth arrest.
The transcription factor SP1 binding sites in Cyclin D1 promoter region was represented in schematic diagram in Fig. 7A. Previous studies reported that deletion of the promoter sequentially from −163 to −22 dramatically reduced Cyclin D1 promoter activity (15). To identify the promoter regions that were necessary for ISO down-regulating Cyclin D1 expression, and to understand the mechanisms that regulate this expression, the wild-type −163 Cyclin D1(WT- Cyclin D1-Luc) and mutated −163 SP1 Cyclin D1 (SP1mut-Cyclin D1-Luc) luciferase reporters were co-transfected with pSuper plasmid into UMUC3 cells respectively, and the stable transfectants UMUC3/WT- Cyclin D1-Luc and UMUC3/SP1mut-Cyclin D1-Luc, were established. As shown in Fig. 7C, ISO treatment inhibited Cyclin D1 transcription in UMUC3/WT Cyclin D1-Luc transfectant, while this treatment did not shown a significant inhibition of Cyclin D1 transcription in UMUC3/SP1mut-Cyclin D1-Luc transfectant, suggesting that ISO’s inhibition of Cyclin D1 transcription was specifically targeting SP1.
ISO impaired SP1 binding to its binding site in Cyclin D1 promoter
To test whether down-regulation of the SP1 level by ISO was associated with its specific binding to Cyclin D1 promoter in vivo, we performed ChIP assays followed by PCR with primers, specifically targeting SP1 binding region from −92 to +27 in the human Cyclin D1 promoter in UMUC3 cells (15). As shown in Fig. 7D, SP1 showed its binding to Cyclin D1 promoter region between −92~+27, and this binding was impaired in the cells treated with ISO (5 μM). Taken together, the above results showed that ISO inhibited Cyclin D1 promoter transcription activity in WT Cyclin D1 reporter, but not in SP1 mutant reporter (Fig. 7C). We anticipated that down-regulation of Cyclin D1 transcription induced by ISO was mediated by its targeting and inhibiting SP1 expression, transactivation and specific binding to SP1 binding sites of Cyclin D1 promoter region as summarized in Fig. 7E.
Discussion
ISO is isolated from the Gnetum Cleistostachyum, and belongs to a group of naturally occurring polyhydroxy stilbenes (27). Several studies have indicated that ISO exhibits an inhibitory effect on oxidized low-density lipoprotein (oxLDL)-induced proliferation and mitogenesis of bovine aortic smooth muscle cells (BASMCs) (28). ISO also inhibits cardiac hypertrophy by anti-oxidative activity and attenuating oxidative stress-mediated signaling pathways (29). ISO has been used for treatment of bladder cancers for centuries. There are reports of side effects from super-high dose (6000 mg/kg/day) application of the Chinese herb Gnetum Cleistostachyum in clinical patients (30), which include dry mouth and dizziness, followed by blurred vision, dry nasopharynx, and stomach pain. Our most recently published results also indicate that ISO at concentration over 20 μM show apoptosis in human bladder cancer cells via downregulation of XIAP expression, while at concentration at lower than 20 μM, such as the concentration used in the current studies, do not show cytotoxic effect on human bladder cancer cell lines (21). Moreover, the pharmacokinetics of ISO in mice indicated that the maximum observed concentration (Cmax) could reach to 47.9 μM in mouse serum, suggesting that 5–20 μM of ISO concentrations applied in current in vitro studies are relevant to in vivo, and further providing crucial information in future ISO application in either animal studies or clinical trials.
We find that ISO at concentration within 20~60 μM exhibits a significant inhibitory effect on anchorage-independent growth, a marked apoptotic induction, as well as downregulation of X-linked inhibitor of apoptosis protein (XIAP) in human bladder cancer cells, whereas overexpression of exogenous HA-XIAP reverses the apoptotic effects and colony formation inhibition by ISO at concentration of 20~60 μM (21). In the current studies, we explored the potential inhibitory effect of ISO at non-apoptotic low concentration on anchorage-independent growth, cell cycle alteration, and the molecular mechanisms underlying these biological effects in high grade bladder cancer cell line, UMUC3 and RT112 cells. We found that ISO not only inhibited anchorage-independent cell growth of cancer cell lines, it also induced cell cycle G0/G1 arrest in a non-cell death concentration of 5 μM in high grade bladder cancer cell line, UMUC3 and RT112 cells, whereas it only showed a slight inhibition of Cyclin D1 expression in low grade human bladder tumor RT4 cells. Moreover, we observed that ISO had no inhibitory effect on cell proliferation and Cyclin D1 expression in non-cancerous Cl41 cells, suggesting that ISO might have a strong inhibitory effect on invasive cancers, rather than low grade and non-cancerous cells. Further studies indicated that the ISO anti-cancer activity was mediated by its downregulation of Cyclin D1 expression via direct inhibition of SP1 transactivation and binding activity to Cyclin D1 promoter region.
Growing evidence had indicated that cell cycle alterations occur in responses of cells to various carcinogens (31, 32). Cyclin D1 is one of the key regulators in the control of cell cycle progression from G0-G1 to S phase, and inducible Cyclin D1 forms a complex with CDK4/6, which phosphorylates the retinoblastoma tumor suppressor protein (33), sequestrates pRb growth inhibitory effects on E2F and enables E2F transcription factors to transcriptional regulate genes required for entry into the DNA synthetic phase (S) of the cell division cycle (34). Cyclin D1 overexpression prevails over that of Cyclin D2 and D3 (35), and overexpression of Cyclin D1 is one of the cancer features and is responsible for inducing excessive cellular proliferation in many human cancers, including bladder cancer (4), breast (36), cervix (37), colon (38), prostate (39), and skin cancer (40). Thus, Cyclin D1 is one of the most frequently altered cell cycle regulating protein in cancers and therefore, is a potential therapeutic target (41, 42). For example, Meng H. et al demonstrated that Cyclin D1-associated protein, PACSIN 2, regulates cell spreading and migration, both of which are dependent on Cyclin D1 expression (43). Li Z. et al demonstrated Cyclin D1-deficient mouse embryo fibroblasts (MEFs) exhibited increased adhesion and decreased motility compared with wild-type MEFs (44). Molecular approaches for targeting Cyclin D1 expression include Cyclin D1α isoform (full-length Cyclin D1) with a small molecule CDK4/6 inhibitor PD0332991 (45), small interfering RNA (siRNA) (42, 46), genomic deletion of Cyclin D1 gene (43), and modulation of Glycogen synthase kinase 3β (GSK3β) activity (47). However, the major limitations of these genomic therapies are their poor stability, poor membrane permeability, and inadequate stable transfection efficiency (48). While there are no chemical inhibitors targeting Cyclin D1 so far, identifying and exploring a natural compound that specifically downregulates Cyclin D1 expression is of tremendous importance for cancer therapy and the reduction of mortality as a result of cancers. In current studies, we identified that ISO at a concentration as low as 5 μM was able to downregulate Cyclin D1 expression at the transcriptional level. At this level, ISO exhibited its induction of G0/G1 cell cycle arrest and inhibition of anchorage-independent growth of human high grade bladder cancer cells, without affecting cell viability or the other cell cycle regulators, including Cyclin A, Cyclin B, Cyclin E, CDK4, and CDK6. These findings demonstrated ISO as a novel mechanism-based cancer therapeutic agent against human bladder cancer, and provide a basis for possible clinical trials exploring the usefulness of ISO as a preventive and therapeutic agent against bladder and other cancers with abnormal expression of Cyclin D1 in patients.
Cyclin D1 levels could be regulated at transcriptional and post-transcriptional levels (49). The signaling pathways that have been reported to regulate Cyclin D1 expression include NF-κB (50), SP1 (15, 51), ras/mitogen activated protein kinases (MAPK)(52), phosphatidylinositol 3-kinase (PI-3K)/Akt (53, 54), and GSK3β/β-catenin (55). Cyclin D1 promoter was first reported almost 20 years ago (56, 57), and many transcription factors have been identified to directly bind to, or otherwise regulate, the Cyclin D1 promoter (36, 58). SP1 was an important transcription factor involved in the regulation of many gene expression and cellular functions, including Cyclin D1 (5, 15, 51, 59). Here, we demonstrate that the ISO-mediated transcriptional down-regulation of the Cyclin D1 gene was achieved by inhibition of transcription factor SP1. Our results indicated that ISO treatment down-regulated Cyclin D1 expression, accompanied by its inhibition of transcription factor SP1 expression, transactivation, and binding activity to the Cyclin D1 promoter region. Our studies further show that that putative SP1 binding sites were between −92 and +27 bp with the 5′-untranslated region, which is consistent with the previous find regarding SP1-mediated regulation of Cyclin D1 expression (15). Based on our results obtained from ChIP assay, SP1 was found to be a major participant transcription factor binding to the GC-box site of the Cyclin D1 promoter and down-regulating Cyclin D1 transcription upon ISO treatment.
In summary, our studies demonstrate that ISO is an active compound that is responsible for Gnetum Cleistostachyum inhibition of bladder cancer cell anchorage-independent growth. This anti-cancer activity of ISO is mediated by its down-regulation of Cyclin D1 expression, and in turn, its induction of cell cycle G0/G1 arrest via specific targeting of transcription factor SP1 in bladder cancer cells. Our studies provided a novel insight into understanding the anti-cancer activity of the Chinese herb Gnetum Cleistostachyum isolate, ISO, as proposed in Fig. 7E. Although in vivo animal verification and extensive in vitro studies will be required for further translational application of ISO in the management of clinical patients, particularly gene models with highly expressed Cyclin D1, the understanding of the molecular mechanisms responsible for ISO action would provide valuable information for the design of more effective strategies for utilization of ISO in therapy and prevention of high grade bladder cancers, in order to substantially impact the field of bladder cancer therapy.
Acknowledgments
We thank Dr. Peggy J Farnham from McArdle Laboratory for Cancer Research, University of Wisconsin, for the gift of transcription factor Spl luciferase reporter; Dr. Anil Rustgi from Gastroenterology Division, University of Pennsylvania, Philadelphia, PA, for the cyclin D1 promoter driven luciferase reporter; and Dr. Richard G. Pestell from the Department of Cancer Biology and Medical Oncology, Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA, for generous gift of human Cyclin D1 -163 and -163 mSP1 promoter-driven leuciferase reporter.
Grant Support
This work was partially supported by grants from NIH/NCI CA112557 (to C. Huang) and CA177665 (to C. Huang), NIH/NIEHS ES000260 (to C. Huang), and NSFC 81229002 (to C. Huang).
Footnotes
Conflict of interest: The authors declare that they have no actual or potential competing financial interests.
References
- 1.Holick CN, Giovannucci EL, Stampfer MJ, Michaud DS. A prospective study of fish, marine fatty acids, and bladder cancer risk among men and women (United States) Cancer Causes Control. 2006;17:1163–73. doi: 10.1007/s10552-006-0059-x. [DOI] [PubMed] [Google Scholar]
- 2.Gerullis H, Ecke TH, Janusch B, Arndt C, Heidari M, Oniani J, et al. Long-term response in advanced bladder cancer involving the use of temsirolimus and vinflunine after platin resistance. Anticancer Drugs. 2011;22:940–3. doi: 10.1097/CAD.0b013e328347a86a. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–49. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 4.Shariat SF, Ashfaq R, Sagalowsky AI, Lotan Y. Correlation of cyclin D1 and E1 expression with bladder cancer presence, invasion, progression, and metastasis. Hum Pathol. 2006;37:1568–76. doi: 10.1016/j.humpath.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Ikoma T, Ozawa S, Suzuki K, Kondo T, Maehata Y, Lee MC, et al. Calcium-calmodulin signaling induced by epithelial cell differentiation upregulates BRAK/CXCL14 expression via the binding of SP1 to the BRAK promoter region. Biochem Biophys Res Commun. 2012;420:217–22. doi: 10.1016/j.bbrc.2012.01.157. [DOI] [PubMed] [Google Scholar]
- 6.Yuan L, Gu X, Shao J, Wang M, Wang M, Zhu Q, et al. Cyclin D1 G870A polymorphism is associated with risk and clinicopathologic characteristics of bladder cancer. DNA Cell Biol. 2010;29:611–7. doi: 10.1089/dna.2010.1018. [DOI] [PubMed] [Google Scholar]
- 7.Lin DI, Lessie MD, Gladden AB, Bassing CH, Wagner KU, Diehl JA. Disruption of cyclin D1 nuclear export and proteolysis accelerates mammary carcinogenesis. Oncogene. 2008;27:1231–42. doi: 10.1038/sj.onc.1210738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciznadija D, Liu Y, Pyonteck SM, Holland EC, Koff A. Cyclin D1 and cdk4 mediate development of neurologically destructive oligodendroglioma. Cancer Res. 2011;71:6174–83. doi: 10.1158/0008-5472.CAN-11-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tashiro E, Maruki H, Minato Y, Doki Y, Weinstein IB, Imoto M. Overexpression of cyclin D1 contributes to malignancy by up-regulation of fibroblast growth factor receptor 1 via the pRB/E2F pathway. Cancer Res. 2003;63:424–31. [PubMed] [Google Scholar]
- 10.Yamada M, Hayashi K, Ikeda S, Tsutsui K, Ito T, Iinuma M, et al. Inhibitory activity of plant stilbene oligomers against DNA topoisomerase II. Biol Pharm Bull. 2006;29:1504–7. doi: 10.1248/bpb.29.1504. [DOI] [PubMed] [Google Scholar]
- 11.Hagiwara K, Kosaka N, Yoshioka Y, Takahashi RU, Takeshita F, Ochiya T. Stilbene derivatives promote Ago2-dependent tumour-suppressive microRNA activity. Sci Rep. 2012;2:314. doi: 10.1038/srep00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang KS, Wang YH, Li RL, Lin M. Stilbene dimers from the lianas of Gnetum hainanense. Phytochemistry. 2000;54:875–81. doi: 10.1016/s0031-9422(00)00151-5. [DOI] [PubMed] [Google Scholar]
- 13.Ouyang W, Ma Q, Li J, Zhang D, Liu ZG, Rustgi AK, et al. Cyclin D1 induction through IkappaB kinase beta/nuclear factor-kappaB pathway is responsible for arsenite-induced increased cell cycle G1-S phase transition in human keratinocytes. Cancer Res. 2005;65:9287–93. doi: 10.1158/0008-5472.CAN-05-0469. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins TD, Mueller A, Odze R, Shahsafaei A, Zukerberg LR, Kent R, et al. Cyclin D1 overexpression combined with N-nitrosomethylbenzylamine increases dysplasia and cellular proliferation in murine esophageal squamous epithelium. Oncogene. 1999;18:59–66. doi: 10.1038/sj.onc.1202296. [DOI] [PubMed] [Google Scholar]
- 15.Marampon F, Casimiro MC, Fu MPM, Popov VM, Lindsay J, Zani BM, et al. Nerve Growth factor regulation of cyclin D1 in PC12 cells through a p21RAS extracellular signal-regulated kinase pathway requires cooperative interactions between Sp1 and nuclear factor-kappaB. Mol Biol Cell. 2008;19:2566–78. doi: 10.1091/mbc.E06-12-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slansky JE, Li Y, Kaelin WG, Farnham PJ. A protein synthesis-dependent increase in E2F1 mRNA correlates with growth regulation of the dihydrofolate reductase promoter. Mol Cell Biol. 1993;13:1610–8. doi: 10.1128/mcb.13.3.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang HY, Shariat SF, Sun TT, Lepor H, Shapiro E, Hsieh JT, et al. Persistent uroplakin expression in advanced urothelial carcinomas: implications in urothelial tumor progression and clinical outcome. Hum Pathol. 2007;38:1703–13. doi: 10.1016/j.humpath.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C, Ma WY, Ryan CA, ZD Proteinase inhibitors I and II from potatoes specifically block UV-induced activator protein-1 activation through a pathway that is independent of extracellular signal-regulated kinases, c-Jun N-terminal kinases, and P38 kinase. Proc Natl Acad Sci U S A. 1997;94:11957–62. doi: 10.1073/pnas.94.22.11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C, Ma WY, Young MR, Colburn N, ZD Shortage of mitogen-activated protein kinase is responsible for resistance to AP-1 transactivation and transformation in mouse JB6 cells. Proc Natl Acad Sci U S A. 1998;95:156–61. doi: 10.1073/pnas.95.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C, Ma WY, Dong Z. Requirement for phosphatidylinositol 3-kinase in epidermal growth factor-induced AP-1 transactivation and transformation in JB6 P+ cells. Mol Cell Biol. 1996;16:6427–35. doi: 10.1128/mcb.16.11.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang Y, Yu Y, Hou Q, Zhen X, Zhang M, Zhang D, et al. The Chinese herb isolate isorhapontigenin induces apoptosis in human cancer cells by downregulating overexpression of antiapoptotic protein XIAP. J Biol Chem. 2012;287:35234–43. doi: 10.1074/jbc.M112.389494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang D, Li J, Costa M, Gao J, Huang C. JNK1 mediates degradation HIF-1alpha by a VHL-independent mechanism that involves the chaperones Hsp90/Hsp70. Cancer Res. 2010;70:813–23. doi: 10.1158/0008-5472.CAN-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang D, Li J, Zhang M, Gao G, Zuo Z, Yu Y, et al. The Requirement of c-Jun N-terminal kinase2 in Regulation of hypoxia inducing factor-1&[alpha] mRNA Stability. J Biol Chem. 2012;287:34361–71. doi: 10.1074/jbc.M112.365882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Chen H, Tang MS, Shi X, Amin S, Desai D, et al. PI-3K and Akt are mediators of AP-1 induction by 5-MCDE in mouse epidermal Cl41 cells. J Cell Biol. 2004;165:77–86. doi: 10.1083/jcb.200401004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song L, Li J, Zhang D, Liu ZG, Ye J, Zhan Q, et al. IKKbeta programs to turn on the GADD45alpha-MKK4-JNK apoptotic cascade specifically via p50 NF-kappaB in arsenite response. J Cell Biol. 2006;175:607–17. doi: 10.1083/jcb.200602149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song L, Gao M, Dong W, Hu M, Li J, Shi X, et al. p85alpha mediates p53 K370 acetylation by p300 and regulates its promoter-specific transactivity in the cellular UVB response. Oncogene. 2011;30:1360–71. doi: 10.1038/onc.2010.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang KS, Zhou S, Lin M, Wang YH. An isorhapontigenin tetramer and a novel stilbene dimer from Gnetum hainanense. Planta Med. 2002;68:916–20. doi: 10.1055/s-2002-34951. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, GL Isorhapontigenin and resveratrol suppress oxLDL-induced proliferation and activation of ERK1/2 mitogen-activated protein kinases of bovine aortic smooth muscle cells. Biochem Pharmacol. 2004;67:777–85. doi: 10.1016/j.bcp.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Li HL, Wang AB, Huang Y, Liu DP, Wei C, Williams GM, et al. Isorhapontigenin, a new resveratrol analog, attenuates cardiac hypertrophy via blocking signaling transduction pathways. Free Radical Biology and Medicine. 2005;38:243–57. doi: 10.1016/j.freeradbiomed.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 30.China Pharmaceutical University, editor. Chinese medicine Cihai _Volume 2. China Medical Science and Technology Press; 1997. pp. 2365–66. [Google Scholar]
- 31.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–7. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 32.Lee CC, Yamamoto S, Wanibuchi H, Wada S, Sugimura K, Kishimoto T, et al. Cyclin D1 overexpression in rat two-stage bladder carcinogenesis and its relationship with oncogenes, tumor suppressor genes, and cell proliferation. Cancer Res. 1997;57:4765–76. [PubMed] [Google Scholar]
- 33.Keenan SM, Lents NH, Baldassare JJ. Expression of cyclin E renders cyclin D-CDK4 dispensable for inactivation of the retinoblastoma tumor suppressor protein, activation of E2F, and G1-S phase progression. J Biol Chem. 2004;279:5387–96. doi: 10.1074/jbc.M310383200. [DOI] [PubMed] [Google Scholar]
- 34.Singh RP, Agarwal C, Agarwal R. Inositol hexaphosphate inhibits growth, and induces G1 arrest and apoptotic death of prostate carcinoma DU145 cells: modulation of CDKI-CDK-cyclin and pRb-related protein-E2F complexes. Carcinogenesis. 2003;24:555–63. doi: 10.1093/carcin/24.3.555. [DOI] [PubMed] [Google Scholar]
- 35.Kim JK, Diehl JA. Nuclear cyclin D1: an oncogenic driver in human cancer. J Cell Physiol. 2009;220:292–6. doi: 10.1002/jcp.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajabi H, Ahmad R, Jin C, Kosugi M, Alam M, Joshi MD, et al. MUC1-C oncoprotein induces TCF7L2 transcription factor activation and promotes cyclin D1 expression in human breast cancer cells. J Biol Chem. 2012;287:10703–13. doi: 10.1074/jbc.M111.323311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satinder K, Chander SR, Pushpinder K, Indu G, Veena J. Cyclin D1 (G870A) polymorphism and risk of cervix cancer: a case control study in north Indian population. Mol Cell Biochem. 2008;315:151–7. doi: 10.1007/s11010-008-9799-0. [DOI] [PubMed] [Google Scholar]
- 38.Ogino S, Nosho K, Irahara N, Kure S, Shima K, Baba Y, et al. A cohort study of cyclin D1 expression and prognosis in 602 colon cancer cases. Clin Cancer Res. 2009;15:4431–8. doi: 10.1158/1078-0432.CCR-08-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleischmann A, Rocha C, Saxer-Sekulic N, Zlobec I, Sauter G, Thalmann GN. High-level cytoplasmic cyclin D1 expression in lymph node metastases from prostate cancer independently predicts early biochemical failure and death in surgically treated patients. Histopathology. 2011;58:781–9. doi: 10.1111/j.1365-2559.2011.03800.x. [DOI] [PubMed] [Google Scholar]
- 40.Burnworth B, Popp S, Stark HJ, Steinkraus V, Bröcker EB, Hartschuh W, et al. Gain of 11q/cyclin D1 overexpression is an essential early step in skin cancer development and causes abnormal tissue organization and differentiation. Oncogene. 2006;25:4399–412. doi: 10.1038/sj.onc.1209474. [DOI] [PubMed] [Google Scholar]
- 41.Kristt D, Turner I, Koren R, Ramadan E, Gal R. Overexpression of cyclin D1 mRNA in colorectal carcinomas and relationship to clinicopathological features: an in situ hybridization analysis. Pathol Oncol Res. 2000;6:65–70. doi: 10.1007/BF03032661. [DOI] [PubMed] [Google Scholar]
- 42.Lehn S, Tobin NP, Berglund P, Nilsson K, Sims AH, Jirström K, et al. Down-regulation of the oncogene cyclin D1 increases migratory capacity in breast cancer and is linked to unfavorable prognostic features. Am J Pathol. 2010;177:2886–97. doi: 10.2353/ajpath.2010.100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meng H, Tian L, Zhou J, Li Z, Jiao X, Li WW, et al. PACSIN 2 represses cellular migration through direct association with cyclin D1 but not its alternate splice form cyclin D1b. Cell Cycle. 2011;10:73–81. doi: 10.4161/cc.10.1.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Z, Wang C, Jiao X, Lu Y, Fu M, Quong AA, et al. Cyclin D1 regulates cellular migration through the inhibition of thrombospondin 1 and ROCK signaling. Mol Cell Biol. 2006;26:4240–56. doi: 10.1128/MCB.02124-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marzec M, Kasprzycka M, Lai R, Gladden AB, Wlodarski P, Tomczak E, et al. Mantle cell lymphoma cells express predominantly cyclin D1a isoform and are highly sensitive to selective inhibition of CDK4 kinase activity. Blood. 2006;108:1744–50. doi: 10.1182/blood-2006-04-016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao Y, Wang J, Lu J, Liu Y, Wang Y, Gao Y, et al. Down-regulation of cyclin D1 by small interfering RNA inhibits cell growth and induces apoptosis of laryngeal squamous cell carcinoma. Am J Otolaryngol. 2011;32:541–6. doi: 10.1016/j.amjoto.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Yang K, Guo Y, Stacey WC, Harwalkar J, Fretthold J, Hitomi M, et al. Glycogen synthase kinase 3 has a limited role in cell cycle regulation of cyclin D1 levels. BMC Cell Biol. 2006;30:33. doi: 10.1186/1471-2121-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nie J, Liu L, Zheng W, Chen L, Wu X, Xu Y, et al. microRNA-365, down-regulated in colon cancer, inhibits cell cycle progression and promotes apoptosis of colon cancer cells by probably targeting Cyclin D1 and Bcl-2. Carcinogenesis. 2012;33:220–5. doi: 10.1093/carcin/bgr245. [DOI] [PubMed] [Google Scholar]
- 49.Musgrove EA. Cyclins: roles in mitogenic signaling and oncogenic transformation. Growth Factors. 2006;24:13–9. doi: 10.1080/08977190500361812. [DOI] [PubMed] [Google Scholar]
- 50.Klein EA, Yang C, Kazanietz MG, Assoian RK. NFkappaB-independent signaling to the cyclin D1 gene by Rac. Cell Cycle. 2007;6:1115–21. doi: 10.4161/cc.6.9.4147. [DOI] [PubMed] [Google Scholar]
- 51.Bartusel T, Schubert S, Klempnauer KH. Regulation of the cyclin D1 and cyclin A1 promoters by B-Myb is mediated by Sp1 binding sites. Gene. 2005;351:171–80. doi: 10.1016/j.gene.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y, Hock JM, Sullivan C, Fang G, Cox AJ, Davis KT, et al. Activation of the p38 MAPK/Akt/ERK1/2 signal pathways is required for the protein stabilization of CDC6 and cyclin D1 in low-dose arsenite-induced cell proliferation. J Cell Biochem. 2010;111:1546–55. doi: 10.1002/jcb.22886. [DOI] [PubMed] [Google Scholar]
- 53.Ouyang W, Li J, Ma Q, Huang C. Essential roles of PI-3K/Akt/IKKbeta/NFkappaB pathway in cyclin D1 induction by arsenite in JB6 Cl41 cells. Carcinogenesis. 2006;27:864–73. doi: 10.1093/carcin/bgi321. [DOI] [PubMed] [Google Scholar]
- 54.Ouyang W, Luo W, Zhang D, Jian J, Ma Q, Li J, et al. PI-3K/Akt pathway-dependent cyclin D1 expression is responsible for arsenite-induced human keratinocyte mmn. Environ Health Perspect. 2008;116:1–6. doi: 10.1289/ehp.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D’Amico M, Hulit J, Amanatullah DF, Zafonte BT, Albanese C, Bouzahzah B, et al. The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3beta and cAMP-responsive element-binding protein-dependent pathways. J Biol Chem. 2000;275:32649–57. doi: 10.1074/jbc.M000643200. [DOI] [PubMed] [Google Scholar]
- 56.Motokura T, Arnold A. PRAD1/cyclin D1 proto-oncogene: genomic organization, 5′ DNA sequence, and sequence of a tumor-specific rearrangement breakpoint. Genes Chromosomes Cancer. 1993;7:89–95. doi: 10.1002/gcc.2870070205. [DOI] [PubMed] [Google Scholar]
- 57.Herber B, Truss M, Beato M, Muller R. Inducible regulatory elements in the human cyclin D1 promoter. Oncogene. 1994;9:2105–7. [PubMed] [Google Scholar]
- 58.Vartanian R, Masri J, Martin J, Cloninger C, Holmes B, Artinian N, et al. AP-1 regulates cyclin D1 and c-MYC transcription in an AKT-dependent manner in response to mTOR inhibition: role of AIP4/Itch-mediated JUNB degradation. Mol Cancer Res. 2011;9:115–30. doi: 10.1158/1541-7786.MCR-10-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seznec J, Silkenstedt B, Naumann U. Therapeutic effects of the Sp1 inhibitor mithramycin A in glioblastoma. J Neurooncol. 2011;101:365–77. doi: 10.1007/s11060-010-0266-x. [DOI] [PubMed] [Google Scholar]