Abstract
The blood-brain barrier (BBB) is an important interface between the peripheral and central nervous systems. It protects the brain against the infiltration of harmful substances and regulates the permeation of beneficial endogenous substances from the blood into the extracellular fluid of the brain. It can also present a major obstacle in the development of drugs that are targeted for the central nervous system. Several methods have been developed to investigate the transport and metabolism of drugs, peptides, and endogenous compounds at the BBB. In vivo methods include intravenous injection, brain perfusion, positron emission tomography, and microdialysis sampling. Researchers have also developed in vitro cell-culture models that can be employed to investigate transport and metabolism at the BBB without the complication of systemic involvement. All these methods require sensitive and selective analytical methods to monitor the transport and metabolism of the compounds of interest at the BBB.
Keywords: microdialysis, brain microvessel endothelial cell culture, microfluidics, peptides, drug analysis
1. INTRODUCTION
The blood-brain barrier (BBB) is a unique biological interface that maintains brain homeostasis by preventing and regulating the permeation of endogenous substances, ions, and xenobiotics (toxins, pollutants, and drugs, for example) into the extracellular space of the brain (1–3). Although beneficial for neurobiological purposes, this interface is also the major obstacle in the development of drugs for treatment of central nervous system (CNS) disorders and brain cancers (4–6). To better understand and evaluate the role of the BBB in drug delivery as well as the chemical communication between the CNS and the peripheral nervous system (PNS), a number of biological and analytical methods have been developed. This article reviews the different in vivo and in vitro approaches for studying the BBB and the analytical methods that are used to measure the transport, metabolism, and release of compounds at the blood-brain interface.
1.1. The Blood-Brain Barrier
The major component of the BBB is the brain microvessel endothelial cell (BMVEC). Specialized proteins, such as claudin, occludin, and cadherins, hold the endothelial cells together to produce tight junctions (TJs), areas where adjacent endothelial cells are physically held together, making passive transcellular transport of small hydrophilic molecules extremely difficult. The “tightness” of these junctions can be evaluated by measuring the transendothelial electrical resistance (TEER). The cells that make up the BBB exhibit TEER values that are almost three orders of magnitude higher than those in peripheral capillaries. In fact, the resistance across these endothelial cells is so great that even the movement of small hydrated ions, such as Na+ and Cl−, is significantly restricted. The surface of BMVECs is also strongly anionic and creates an electrostatic barrier for the transport of negatively charged compounds. In addition to the physical and electrostatic barriers to transport, these cells also create a metabolic barrier. There are a number of intracellular and extracellular enzymes, including peptidases, nucleotidases, monoamine oxidase, and cytochrome P450, that convert substrates into less permeable or less toxic compounds. Additionally, the BBB is an immunological barrier that prevents bacteria and viruses from entering the brain.
The endothelial cells that make up the BBB are part of the larger neurovascular unit (NVU) that also contains pericytes, astrocytes, microglia, and neurons (Figure 1) (1, 3). All the cells in the NVU play a role in the maintenance of the BBB as well as in blood-brain signaling in both directions (7). The endothelial cells are surrounded by a basement membrane that is shared with pericytes, generally undifferentiated cells that differentiate into support cells in the brain vasculature (e.g., vascular smooth muscle cells). Known functions of the pericytes include helping to build and maintain the basement membrane at the BBB. Astrocytes are connected to the endothelial cells via their end feet and provide growth factors and nutrients to both endothelial cells and local neurons (2, 3). These cells, therefore, play an important role in blood-to-brain communication. Microglia are also a part of the NVU and are involved in the immune response (8). Normally, these cells are in a resting state, but they quickly become activated if there is a disturbance in the homeostasis of the brain such as during ischemia, infection, or an influx of albumin from the blood.
Figure 1.
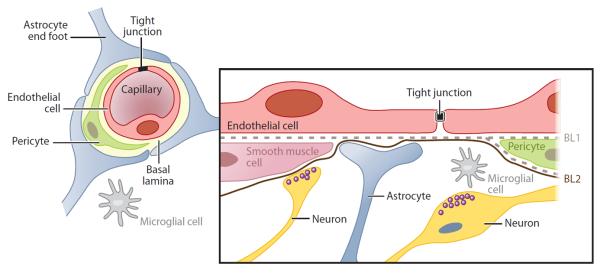
The cells that make up the blood-brain barrier and the neurovascular unit. Reprinted with permission from Reference 3.
As mentioned above, the primary role of the BBB is to maintain the homeostasis of the brain by inhibiting the uncontrolled influx of molecules from the blood into the brain. There are many natural substances that, if allowed into the brain, would severely disrupt neuronal activity or cause brain damage. For example, if glutamate, a neuroexcitatory amino acid that is also present at high concentrations in food, were allowed to pass freely into the brain, severe neurotoxicity would result. A complementary second role of the BBB is to supply nutrients to the brain in a regulated manner. This task is accomplished using specific transport systems for molecules needed to maintain the cells in the brain (9). Examples of such transport systems include transporters for glucose, insulin, amino acids, and neurotransmitter precursors. A third role of the BBB is to protect the brain against toxins present in the blood. Examples include poisons, pollutants, and drugs, as well as endogenous metabolites or proteins. Specialized transport proteins, expressed on the apical (blood) side of BBB endothelial cells, are responsible for the efflux of undesirable compounds, such as lipophilic toxins, that may otherwise cross the cell membrane.
The BBB is also an important participant in the brain's immune system, and it can direct inflammatory cells to act quickly in response to changes in the neurovascular space. For example, if albumin crosses the BBB (owing to head trauma or stroke), an inflammatory reaction in the brain, microglia cellular activation, and programmed cell death may result (10). Lastly, the BBB serves as an important chemical messaging system between the CNS and the PNS. Substances released in the periphery can be transported to the brain and generate a neuronal response. Likewise, substances that are released from the brain into the periphery can generate a physiological response in a remote tissue. In particular, cytokines and neuropeptides are important mediators of such signaling. This transport/messaging system can be involved in a variety of disorders, including depression, drug addiction, Alzheimer's, and Parkinson's (6).
1.2. Blood-Brain Barrier Transport Mechanisms
As mentioned above, the endothelial cells that make up the BBB are distinct from the cells that make up the blood vessels in peripheral systems. These endothelial cells have TJs that allow very few small hydrophilic molecules, such as ethanol or mannitol, to pass into the brain. The membrane is also highly negatively charged, so anionic compounds are generally excluded. Owing to these restrictions, most molecules are transported across the BBB by one of the following mechanisms (Figure 2) (11).
Transcellular passive diffusion: Small lipophilic compounds can passively diffuse across the endothelial membrane. In general, the more lipophilic a molecule is, the greater its ability to permeate. Examples of compounds that are transported by this mechanism include acetaminophen and fluoxetine.
Carrier-mediated transport: Various transporters are used to bring essential polar molecules into the brain. For example, there are specific amino acid, nucleoside, peptide, vitamin, and glucose transporters. Neurotransmitters such as dopamine and serotonin do not cross the BBB; however, their precursors, levodopa and tryptophan, are transported through this mechanism.
Receptor-mediated endocytosis: This is a common method of transport for large peptides and proteins and is facilitated by binding to a receptor on the membrane surfaces followed by endocytosis. Examples of molecules transported by this approach include insulin, transferrin, cytokines, and other large peptides.
Adsorptive-mediated endocytosis: Due to the highly anionic character of the BBB, cationic molecules adsorb nonspecifically to the membrane and undergo endocytosis. This mode of transport has a lower affinity and a higher capacity than receptor-mediated endocytosis. Highly positively charged molecules such as histones, cationized albumin, and arginine-containing peptides are examples of molecules transported by this approach.
TJ modulation: Typically, the TJs between the BMVECs restrict the passage of even very hydrophilic compounds from crossing the BBB via paracellular diffusion; however, if the TJs are disrupted, nonspecific passage into the brain of molecules that would normally be excluded can occur. Changes in the resistance of the TJs are usually due to a disease or administration of a drug that disrupts the proteins that make up the TJs. Experimentally, this can be accomplished through the administration of hyperosmolar solutions such as 25% mannitol. The high ionic strength causes the endothelial cells to shrink, opening the TJs. Clinically, this is employed for the treatment of brain tumors. Additionally, leukocytes and other immune cells can modify TJs or cross the BBB via transcellular mechanisms. Changes in the TJs can also occur during ischemia or brain trauma.
Figure 2.
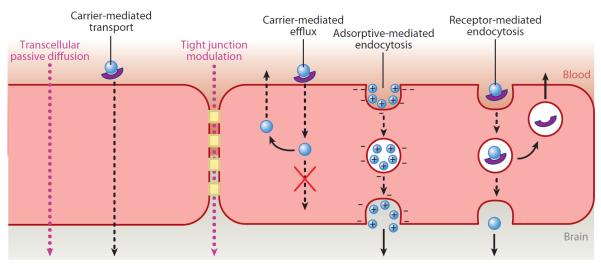
Mechanisms of transport across the blood-brain barrier.
Along with these transport mechanisms of substances from the blood to the brain, efflux mechanisms also shuttle substances out of the endothelial cells and back into the blood before they have a chance to enter the brain. These carrier-mediated efflux mechanisms pose a significant challenge to pharmaceutical scientists attempting to deliver drugs into the brain. Examples of carrier-mediated efflux systems that are present at the BBB include the multidrug-resistance proteins such as P-glycoprotein (P-gp).
1.3. Importance of the Blood-Brain Barrier
Understanding the BBB and the NVU is important for several reasons. If the integrity of the BBB is compromised due to diseases such as AIDS, undesired substances could leak into the brain, generating an immune or inflammatory response (12). Conversely, if drugs are unable to pass through the BBB, they will be ineffective for the treatment of neurological and psychiatric disorders, including depression, schizophrenia, Alzheimer's, and Parkinson's. Likewise, anticancer drugs must be able to enter the brain to treat brain tumors. In addition, the BBB plays an important role as a chemical messaging system between the CNS and the PNS. Peptides and other substances can be released in the brain or periphery, traverse the BBB, be transformed by a separate set of enzymes, and trigger a neurological or physical response at a remote location.
2. IN VIVO METHODS FOR STUDYING TRANSPORT OF SUBSTANCES ACROSS THE BLOOD-BRAIN BARRIER
In response to the great amount of interest in the BBB as a means to understand and treat neurological diseases, several methods have been developed to investigate BBB transport. These range from in silico and cell-culture models to the use of live animals and positron emission tomography (PET) (13–15). Here, we discuss the different approaches for studying BBB transport and metabolism as well as the analytical methods that are employed to determine the kinetics of the transport processes.
2.1. Intravenous Injection Methods
The most common approach for monitoring the permeation of a substance across the BBB is the intravenous injection method (16). In this case, the compound is administered to the animal intravenously, and the analyte concentration is determined at different times in the brain, plasma, and cerebrospinal fluid. With this approach, all the physiological and metabolic systems remain intact, thereby providing the most realistic assessment of what actually gets into the brain. However, a major disadvantage of the intravenous injection method is that a separate animal must be used for each data point. Because animal-to-animal variability is also an issue, large numbers of animals must be employed for brain pharmacokinetic studies to obtain statistically relevant data. It is also difficult to determine the specific action of hormones, ions, nutrients, and proteins on the delivery of a drug or other substances to the brain with this approach, and there is no straightforward way to study efflux mechanisms.
2.2. Brain Perfusion Techniques
For a more direct indication of the permeation of a substance across the BBB, the brain perfusion technique was developed by Smith & Allen (17) and Takasato et al. (18). In this technique, the compound of interest is dissolved in artificial blood, plasma, or saline and directly infused into the heart or a major vessel that leads directly to the brain by use of a perfusion pump. At a predetermined time, the perfusion is stopped, the animal is decapitated, and the amount of substance in the brain is determined. For ease of detection, radiolabeled compounds are frequently employed in these studies. This approach has an advantage over the intravenous injection method described above in that the compound does not undergo first-pass metabolism prior to entering the brain. In addition, the composition of the perfusate, the concentration of the substance under investigation, and the duration of the perfusion experiment can be carefully controlled. Inhibitors for metabolic enzymes and/or efflux transporters can be introduced into the perfusate to clarify their role in the transport and metabolism of the substance under investigation. By controlling the concentration of albumin in the perfusate, the effect of protein binding on BBB transport can also be elucidated. These experiments are most easily performed using radiolabeled substances, although liquid or gas chromatography/mass spectrometry (LC-MS or GC-MS, respectively) can also be employed.
The brain perfusion method also has several disadvantages. It is very animal and labor intensive because a new animal must be used for each experiment. As with the previous method, if kinetic studies are being performed, a large number of animals may be needed to obtain statistically relevant data. Nonspecific adsorption of a drug to the brain tissue can also lead to erroneous conclusions concerning the activity of the drug because only the free concentration of the drug is physiologically active. It is also impossible to make any correlation between drug concentrations in the brain and animal behavior with this technique.
2.3. Tomographic Methods
Recently, PET and single-photon emission-computed tomography (SPECT) have been employed to study brain uptake kinetics, cerebral blood flow, BBB integrity, and efflux mechanisms (19–22). In these experiments, a small amount of compound labeled with a positron-emitting radionuclide is injected intravenously and allowed to distribute to the different tissues in the body. The emitted γ radiation is then measured as a function of tissue depth by the instrument. Computer software is employed to create a three-dimensional image of the distribution of the substance in the brain and other tissues. PET provides higher-resolution images than SPECT does. Both techniques are noninvasive and can be used on both human and animal subjects. A major advantage of these methods is that it is possible to obtain excellent spatial resolution regarding drug distribution in a noninvasive manner. Figure 3 shows the use of PET to investigate the BBB permeation and tissue distribution of a series of 18F-labeled fluoropyridinyl compounds in rats (22). The authors of this study determined the kinetics of BBB permeation by measuring the radioactivity in the cerebral areas of the PET images and plotting the percentage of the original injected dose in that region over time.
Figure 3.
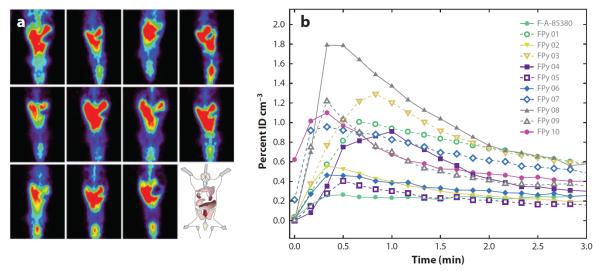
(a) Whole-body positron emission tomography (PET) acquired over two minutes after an intravenous injection of 18F-labeled fluoropyridinyl (FPy) derivatives. (b) In vivo cerebral pharmacokinetics determined using the PET images. Reprinted with permission from Reference 22.
Some disadvantages of tomography are that it requires expensive instrumentation and that radiolabeled analogs of the substance under study must be synthesized (13). PET uses 11C- or 18F-labeled compounds, whereas SPECT employs 123I. The short half-life of 11C-labeled compounds (20 min) means that they must be synthesized on site prior to administration. Compounds labeled with radioactive fluorine or iodine have longer half-lives, but they can exhibit different transport properties that can confound the results. Another major drawback of this method is that, because only radioactivity is measured, it does not distinguish between transport of the drug and its metabolites. It is also impossible to determine the free versus bound fraction in vivo (14).
2.4. Microdialysis Sampling
Microdialysis sampling was developed in the 1970s (23–27) as a minimally invasive method for monitoring neurotransmitters in the brain. It has been extensively used to investigate the transport and metabolism of drugs and other substances at the BBB. Compared with the aforementioned techniques, microdialysis sampling offers several key advantages. In microdialysis sampling, tissue concentrations are determined by direct sampling of the chemical makeup of the interstitial fluid. Therefore, along with drug concentrations, other analytes of importance, such as neurotransmitters and metabolic markers, can be measured at the same time. In addition, because long-term sampling can be accomplished on a single animal, it can serve as its own control, leading to a significant reduction in the number of animals needed to obtain statistically significant results in tissue-distribution studies.
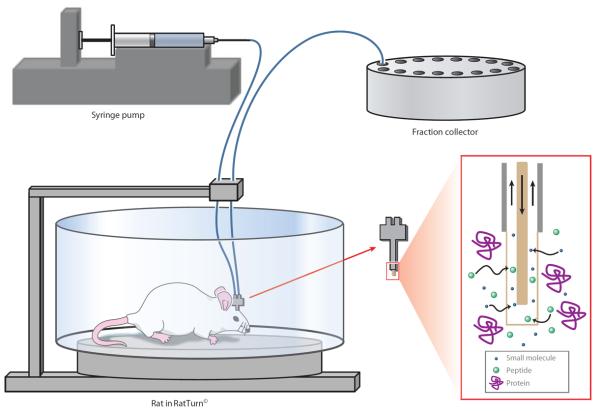
To perform microdialysis sampling, a probe consisting of a short length of hollow-fiber dialysis membrane connected to inlet and outlet tubing is implanted in the brain or other tissue. A solution that is similar in composition and ionic strength to the extracellular fluid (ECF) of the tissue of interest is then pumped slowly through the probe. Small molecules in the extracellular space that are not present in the perfusate diffuse across the membrane based on their concentration gradients and are transported by the syringe pump to a fraction collector or online analysis system. Figure 4 shows a typical microdialysis sampling setup.
Figure 4.
Microdialysis sampling system. The probe is implanted in the brain of the rat. Small molecules and peptides of interest from the extracellular fluid of the brain diffuse across the probe membrane and are transported in the perfusate to a fraction collector or online analytical system.
For studies involving the BBB, a stainless-steel concentric cannula probe is most commonly used for sampling the brain ECF, whereas a flexible probe can be used to monitor the blood levels (28). The concentric cannula probe is very rigid, whereas the flexible probe is composed of side-by-side fused silica capillaries or flexible tubing and can bend when the animal moves, minimizing any damage to blood vessels. An alternative method of blood sampling is the Culex automated blood sampler that is used to sample blood using a push-pull method; it then replaces the lost fluid with isotonic saline. The Culex blood-sampling method has been employed in conjunction with brain microdialysis sampling to investigate the transport of nicotine across the BBB (29). Due to the small size and relatively noninvasive nature of the microdialysis sampling probes, multiple probes may be used in a single animal. Therefore, it is also possible to measure blood, brain, and tissue concentrations of drugs or endogenous substances simultaneously.
2.4.1. Benefits of microdialysis sampling for blood-brain barrier studies
A major advantage of microdialysis sampling over the in vivo methods discussed above is that there is no net fluid loss from the tissue as a result of the sampling process. Thus, long-term studies are possible with minimal disruption of the physiological system. A unique advantage of this method for BBB studies is the possibility of continuously monitoring the concentrations of a substance in both the brain and blood of an awake and freely moving animal, which makes it feasible to correlate brain concentrations of a substance with behavior. Also, because of the low-molecular-weight cutoff of the dialysis membranes, samples obtained via microdialysis sampling are protein free, meaning that only the physiologically active free-drug concentration is measured by this technique. Enzymes are also excluded, preventing metabolic degradation of the sample contents.
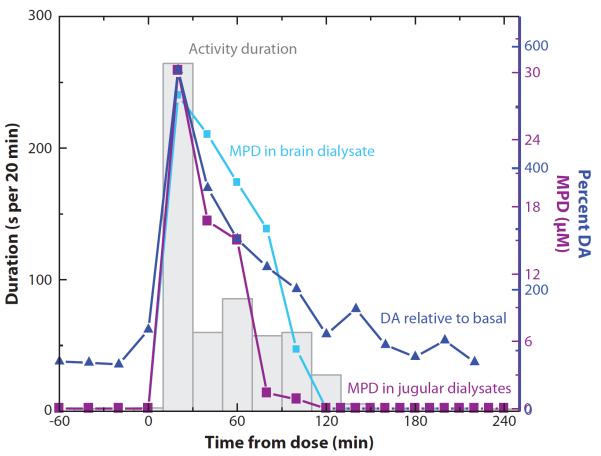
Another important advantage of microdialysis sampling for CNS-active drugs is the ability to simultaneously monitor drug concentrations and neurotransmitter release in the brain and correlate them to both blood levels and behavior. Figure 5 shows a nice example of the use of microdialysis sampling for a BBB study. A rat was given a single intravenous injection of methylphenidate (Ritalin) (30). Brain and blood sampling were accomplished using a concentric cannula probe and a flexible probe, respectively. Dialysates were collected off-line, and the concentrations of methylphenidate and dopamine in both the brain and blood were determined using LC with electrochemical detection. In this manner, it was possible to measure the transport of Ritalin across the BBB as well as monitor its effect on catecholamine release. Lastly, through the use of a RatTurn©, the extracellular concentration of these substances could be directly correlated with the overall activity level of the rat.
Figure 5.
In vivo monitoring of the transport of MPD into the brain correlated with dopamine (DA) release and behavior. Abbreviation: MPD, methylphenidate. Reprinted with permission from Reference 30.
In addition to looking at transport across the BBB, microdialysis sampling can also be employed for investigations involving site-specific transport and metabolism of drugs. An excellent example of such sampling is a report by de Lange et al. (31, 32), who examined differences in the distributions of an intravenous injection of methotrexate in tumor versus healthy brain tissue. Microdialysis sampling has also been used to investigate the metabolism of drugs and neuropeptides in specific areas of the brain (33–35). For substance P, the results obtained for metabolism in the neurovascular space were then compared with those obtained using cell-culture models of the BBB.
2.4.2. Drawbacks of microdialysis sampling for blood-brain barrier studies
A major concern with microdialysis sampling for BBB transport studies is the effect of probe implantation on the brain and the integrity of the BBB (26, 36). In particular, if the studies are to be performed over a long period of time, tissue damage associated with probe implantation and the potential for an immune response must be taken into consideration because fibrosis or gliosis occurs several days after probe implantation (37–39). A study by Jaquins-Gerstl & Michael (40) used immune labeling of tissue slices for glial fibrillary acidic protein to investigate glial activation by implantation of the microdialysis sampling probe. They found a 200% increase in glia immunoreactive cells 24 h after probe implantation (40). Jaquins-Gerstl et al. (41) also recently reported that inclusion of dexamethasone in the perfusate can reduce this glial response.
The integrity of the BBB following probe implantation has also been a controversial issue in brain microdialysis sampling (26, 42). Studies conducted using autoradiography with 14C-α-aminoisobutyric acid (which does not cross the BBB under normal conditions) as well as transport characteristics of hydrophilic and moderately lipophilic drugs (following intravenous injection) post surgery indicate that the BBB integrity is maintained overall (42, 43). However, other studies have shown a significant effect of probe implantation on BBB permeability by using 51Cr-EDTA transport (44). More recently, Gerhardt's group (45) showed that, although there was tissue damage and glial activation following probe implantation for both ceramic microelectrode arrays and microdialysis sampling, the BBB remained intact.
2.4.3. Quantitation and calibration issues
To quantitate the amount of an exogenous substance entering the brain from the blood, it is necessary to calibrate the microdialysis sampling probe (46). Calibration is usually accomplished by measuring the relative recovery, which is defined as the ratio of the concentration of the analyte inside the probe to that in the sampling solution. This number depends on a number of parameters, including the charge, size, and lipophilicity of the analyte, the microdialysis sampling flow rate, and the composition of the dialysis membrane. Although in vitro calibration can be accomplished by placing the probe in a stirred beaker containing the analyte of interest, it is rarely equivalent to the recovery in vivo (27, 46, 47).
The no-net-flux method is the “gold standard” for quantitation by microdialysis sampling (48). In this method, the animal is administered a constant intravenous dose of a drug until steady-state conditions are confirmed in the tissue of interest. After steady-state conditions have been reached in the brain, specified concentrations above and below the expected values for the compound of interest are perfused through the brain microdialysis probe. When the concentration in the brain is equal to that of the perfusate, the concentration in the dialysate does not change. This concentration of the substance of interest in the ECF of the brain can be determined by plotting the concentration of the dialysate minus the concentration of the perfusate (Cd–Cp) versus the concentration of the substance in the perfusate (Cp). The zero intercept is the concentration of the substance in the brain ECF (46, 49). This method provides the most accurate measurement of exogenous substances in the ECF of the brain but is a very time-consuming experiment.
The technique of retrodialysis has been evaluated to quantitate the free concentration of a substance in the brain ECF (27, 47, 50). This technique can be performed in one of two ways. The first uses the actual exogenous substance of interest as its own control and assumes that analyte delivery through the probe into the tissue is equivalent to the recovery. In this experiment, the substance under study is dissolved in the perfusate and delivered via the microdialysis probe to the brain region of interest. The delivery is then calculated as the ratio of the concentration of the substance coming out of the probe to the original perfusate concentration. Following the delivery experiment, the substance is washed out of the tissue by perfusion with artificial cerebrospinal fluid, and the drug is administered peripherally for the BBB experiment. The delivery is evaluated again at the end of the experiment. Experiments performed with caffeine and acetaminophen obtained ECF concentrations with the delivery method that were higher for caffeine but lower for acetaminophen in comparison to those obtained using the no-net-flux method (50). The higher values obtained for caffeine were believed to be due to saturable active transport across the BBB.
An alternative to the delivery experiment described above is retrodialysis using a calibrator (51, 52). This approach uses a compound with structural and physical properties similar to those of the analyte of interest as an internal standard. This calibrator is added to the perfusion medium, and the delivery of the substance through the probe is monitored continuously throughout the BBB experiment. Changes in the delivery of the calibrator during the course of the experiment correlate with changes in analyte recovery. One of the difficulties is finding a calibrator compound that has structural characteristics similar to those of the analyte of interest but is not physiologically active. Recently, deuterated analogs were shown to be useful calibrators when used with mass-spectrometric analysis (53).
Another calibration approach is to sample at a very low flow rate (100 nl min−1) in which the perfusate approaches complete equilibrium with the ECF, yielding close to quantitative recovery (within error) (54). The disadvantage of this approach is that only very small sample volumes (100 nl or less) are obtained per minute, so highly sensitive analytical methods that can analyze such small samples are necessary for good temporal resolution. Capillary and microchip electrophoresis are extremely useful methods for the analysis of such small sample volumes (55, 56). Another approach is to use the MetaQuant probe, which samples at a very low flow rate to obtain quantitative recoveries but then supplies makeup flow after sampling to provide an adequate sample for conventional analysis methods (57).
Despite some of the concerns regarding quantitation and calibration, microdialysis sampling is still extensively employed to evaluate the transport of drugs across the BBB (14, 26). Significant advantages of microdialysis sampling for these studies are that the animal can be used as its own control and several parameters (different concentrations of drug, administration of efflux inhibitor, etc.) can be investigated using the same animal. Doing so provides a clear advantage over the previously described methods, in which a different animal is needed for each time point and experimental variable. It is also impossible to determine the free (active) concentration of a drug in the brain of a freely moving animal by any other technique (58).
2.4.4. Analytical methods for microdialysis samples
LC is the most common method for analysis of microdialysis samples. To maintain good temporal resolution and high sensitivity, microbore and capillary columns are commonly employed. Detection methods include ultraviolet (UV), laser-induced fluorescence (LIF), and electrochemical (EC) detection, as well as MS (59). In an early BBB study, microbore LC-UV was used in conjunction with microdialysis sampling to determine the effects of anesthesia on the transport of tacrine across the BBB (60). More recently, microdialysis sampling, in conjunction with LC-EC, has been used to correlate brain concentrations of mercaptoproprionic acid, a chemical model for epilepsy, with electrical activity in the brain (61).
LC-MS has proven to be a popular method for the analysis of microdialysis samples due to its high specificity for the analytes of interest and potential for low limits of detection. Special considerations must be made to remove the high salt concentrations of the dialysate sample prior to analysis to minimize ion suppression (62). Microdialysis sampling with LC-MS analysis was used to investigate the metabolism of substance P in the neurovascular space and compare it with the metabolism of the peptide by bovine BMVECs (63). Using LC-MS, researchers can employ deuterated calibrators for more accurate quantitation of brain extracellular concentrations. Bengtsson et al. (53) used deuterated morphine as a calibrator to monitor the recovery of morphine and evaluate the performance of the probes.
The transport of L-3,4-dihydroxyphenylalanine (L-DOPA) into an “on-rat” collection system was investigated using LC-EC (64). Figure 6 shows a diagram of the experimental setup. An Alzet™ osmotic pump implanted in the back of the rat was used to provide the flow through the probe implanted in the brain. Because osmotic pumps do not require a power source, on-rat collection was made possible by attaching a small vial with a septum to the outlet of the microdialysis probe. The rat was then injected with L-DOPA, and the collection vial was removed from the rat's head at specific time intervals. The contents were subsequently analyzed for DOPAC (3,4-dihydroxyphenylacetic acid), HVA (homovanillic acid), and 5-HIAA (5-hydroxyindoleacetic acid) by LC-EC.
Figure 6.
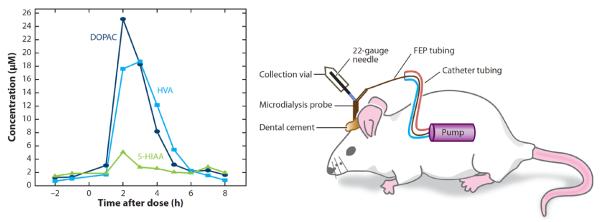
On-rat sampling system for monitoring drug transport and behavior. Abbreviations: DOPAC, 3,4-dihydroxyphenylacetic acid; FEP, fluorinated ethylene propylene; 5-HIAA, 5-hydroxyindoleacetic acid; HVA, homovanillic acid. Reprinted with permission from Reference 64.
Although most analyses of substances for BBB transport studies are developed using LC as the separation method, capillary electrophoresis (CE) is useful for the analysis of microdialysis samples owing to its low-sample-volume requirements. Thus, it is possible to employ very low flow rates to improve analyte recovery or, alternatively, to employ a single microdialysis sample of 1 to 10 μl to determine several different analytes. The ability to measure substances in very small volumes also improves temporal resolution, which can be particularly important if neurotransmitter release is being measured in conjunction with brain drug concentrations.
One of the first experiments using CE to analyze microdialysis samples involved the detection of kynurenine following peripheral administration of tryptophan (65). Samples were collected off-line and measured using CE-EC. A more recent example is the use of microdialysis sampling in conjunction with CE-LIF for the simultaneous determination of the concentrations of carbamathione in the brain following administration of disulfuram and the release of GABA (γ-aminobutyric acid) and glutamate (66, 67).
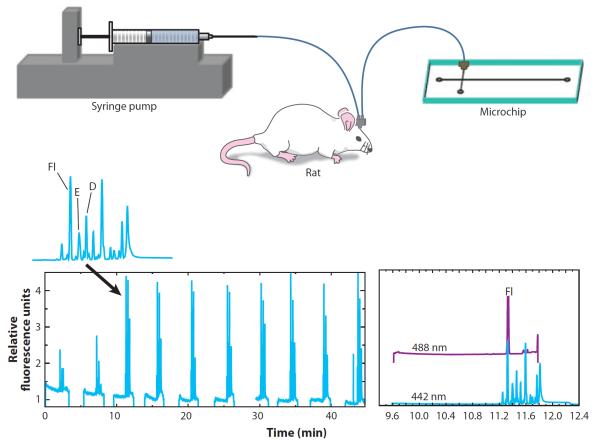
Microdialysis sampling coupled to microchip electrophoresis has recently been employed to simultaneously monitor neurotransmitter concentrations and BBB permeability (68). Rats were injected with a bolus dose of fluorescein that was used as an indicator of BBB integrity. The amino acids in the microdialysate were derivatized online using a microchip reactor. The detection of the excitatory amino acid neurotransmitters aspartate and glutamate, along with fluorescein that had entered the brain, is shown in Figure 7.
Figure 7.
Simultaneous monitoring of blood-brain barrier (BBB) integrity and excitatory amino acids in the brain via microdialysis sampling coupled to microchip electrophoresis. Fluorescein (Fl) was used as a marker of BBB integrity. Abbreviations: D, aspartic acid; E, glutamic acid. Reproduced from Reference 68.
Other approaches for the analysis of microdialysis samples include AMS (accelerator mass spectrometry) and electron spin resonance. AMS has been employed to investigate the transport of 14C-labeled polyphenols across the BBB as well as to monitor brain pharmacokinetics of morphine at very low concentrations (69, 70). Electron spin resonance has also been employed to detect the permeation of free-radical compounds. Representative examples of the different analytical methods in conjunction with microdialysis sampling are provided in Table 1 and in Reference 56.
Table 1.
Methods used for BBB microdialysis sample analysis
| Analytical method | Drug transported | Reference(s) |
|---|---|---|
| AMS | Morphine | 70 |
| Polyphenols | 69 | |
| CE-LIF | Carbamathione | 66,67 |
| Substance P | 33 | |
| Electron spin resonance | Levetiracetam | 108 |
| GC-MS | Aminopropylbutylphosphinic acid | 109 |
| LC-EC | Mercaptopropionic acid | 61 |
| Benserazide | 64 | |
| Nalbuphine | 110 | |
| Butorphanol | 110 | |
| Morphine | 110 | |
| L-DOPA | 111 | |
| LC-LIF | Bisphenol A | 112 |
| LC-MS | Rosiglitazone | 113 |
| Methotrexate | 114 | |
| LC-MS/MS | Morphine | 70 |
| Carbamathione | 115 | |
| Tetrahydroisoquinolines | 116 | |
| Cocaine | 117 | |
| LC-UV | Doxorubicin | 118 |
| Gastrodin | 119 | |
| Levamisole | 120 | |
| Thalidomide | 121 | |
| Cocaine | 117 | |
| LIF | Fluorescent nanospheres | 122 |
| Microchip electrophoresis–LIF | Fluorescein | 68 |
Abbreviations: AMS, accelerator mass spectrometry; BBB, blood-brain barrier; CE, capillary electrophoresis; EC, electrochemical detection; GC, gas chromatography; L-DOPA, L-3,4-dihydroxyphenylalanine; LC, liquid chromotography; LIF, laser-induced fluorescence; MS, mass spectrometry; UV, ultraviolet.
3. IN VITRO MODELS OF THE BLOOD-BRAIN BARRIER
Cell-culture systems have evolved as a first-pass screening tool for BBB permeability to drugs and other compounds. These systems can also be used to investigate the effects of the metabolic component of the BBB on transport. Using primary cultures isolated from the gray matter of bovine brains, Audus & Borchardt (71) developed one of the first cell-culture models of the BBB. These primary cultures express metabolic enzymes crucial for investigating peptide metabolism (72–74), and they exhibit many of the physical characteristics typical of the BBB, including decreased pinocytosis and minimal fenestration. Efflux transporters as well as specific uptake transport systems are expressed by these cells and have been extensively characterized, making them a useful method for investigating BBB permeability (75).
3.1. Primary Brain Microvessel Endothelial Cells
Bovine and porcine brain microvessel endothelial cells, known as BBMECs and PBMECs, respectively, are the most common primary cell models for BBB transport studies (75). For transport studies using the BBMEC culture system, cells are isolated from whole bovine brains, grown to confluency on polycarbonate membranes, and mounted in Side-by-Side™ diffusion chambers (Figure 8). This setup enables temperature maintenance via external circulating water baths as well as constant stirring, driven by an external console, to eliminate the effect of a stagnant layer at the monolayer surface. The Side-by-Side diffusion chambers provide access to both sides of the cell monolayer, allowing bidirectional permeation to be investigated by spiking either the apical or basolateral chambers with the compound of interest and monitoring transport in either direction. Once the compound is added to the donor side of the cell monolayer, aliquots are removed at discrete time points from the receiver chamber and stored for further analysis. To avoid changes in volume that could affect flux in the system, fluid is always replaced following the removal of each aliquot. Another advantage of this in vitro method is the ability to manipulate variables, including temperature and compound concentration, to evaluate their effects on permeability. For example, by performing experiments at 4°C, the effect(s) of reduced ATP activity on the BBB transport of a specific compound can be characterized (76), which enables the researcher to distinguish between passive and active transport processes. Passive transport does not require energy; however, active transport mechanisms, such as carrier- and receptor-mediated transport, require ATP. If a compound is actively transported across the BBB, the temperature dependence of transport can be used to determine the activation energy of the transport process. Receptor-mediated processes can be further elucidated by determining the effect of competing ligands for the same receptor on the transport process.
Figure 8.
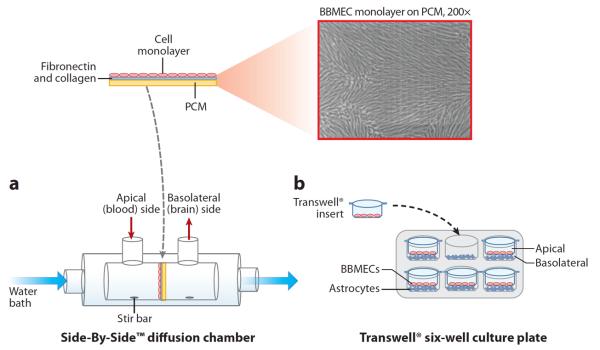
Systems used for monitoring in vitro transport across the blood-brain barrier. (a) In the Side-by-Side™ diffusion chamber, cells are grown on polycarbonate membranes (PCMs) that are subsequently mounted between two water-jacketed, thermally controlled chambers. (b) In the Transwell® system, a coculture setup is demonstrated in a six-well format in which brain endothelial cells are grown in the Transwell insert and astrocytes are grown in the bottom of the multiwall plates. Abbreviation: BBMEC, bovine brain microvessel endothelial cell.
In addition to the advantages that the primary cell line affords in these assays, the Side-by-Side diffusion chambers also provide advantages over Transwell® culture plates that have been used in Caco-2 studies. In the Transwell system, cells are grown on a polycarbonate insert tray that fits within a larger multiwelled culture plate. An issue that can arise when using this system with BBMECs, however, is the propensity for the cells not to reach confluency at the edges of the inserts, negatively impacting permeability data. Temperature control is also more difficult in these systems, and constant stirring presents a challenge. However, the Transwell system has some merit in studies involving the BBB, as has been demonstrated with coculture systems and immortalized cell lines (described below).
The BBMEC culture model also expresses the efflux transporter P-gp, which is a common obstacle to the delivery of anticancer agents for brain tumors. Therefore, in addition to transport studies aimed at determining permeation across the BBB, Rhodamine 123 uptake assays with BBMECs serve as a convenient in vitro assessment of a compound's affinity for P-gp. The fluores-cent dye Rhodamine 123 is known to be a substrate for P-gp, and accumulation of this dye within cultured BBMECs can be characterized via intracellular fluorescence determinations. Competitive assays in which BBMECs are incubated with both Rhodamine 123 and the compound of interest exhibit an increased accumulation of Rhodamine 123 intracellularly if the unlabeled drug of interest is also a P-gp substrate. In other words, a substrate for P-gp prevents the efflux of Rhodamine via P-gp; therefore, an increase in the fluorescence signal is observed due to a higher-than-normal accumulation of the dye within the cells, as can be seen with the anticancer agent Paclitaxol in Figure 9 (76, 77). This assay is useful for investigating structural modifications that may alter a drug's affinity for P-gp, thus providing insight into the modifications necessary to formulate CNS-active therapeutics that circumvent efflux mechanisms (76, 77).
Figure 9.
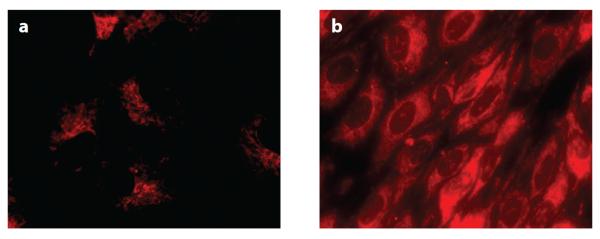
Accumulation of Rhodamine 123 in bovine brain microvessel endothelial cells following treatment with (a) 5 μM of Rhodamine 123 alone and (b) 5 μM of Rhodamine 123 with 10 μM of Paclitaxol, an anticancer drug and known P-gp substrate. Decreased Rhodamine 123 efflux is observed in the presence of Paclitaxol (76).
3.2. Immortalized Cell Lines
In recent years, work with in vitro models of the BBB has focused on the development of immortalized brain endothelial cell lines (78) to decrease the time necessary to reach confluency (7 versus 14 days) as well as lessen the workload on the laboratory scientist because the isolation procedures are time and labor intensive for primary cells. The immortalized cell lines form monolayers but not complete TJs, leading to a so-called leaky barrier. Therefore, they can be used to explore endothelial uptake of compounds, but they are not useful for transport studies. Given the leakiness of the immortalized cell lines, cells derived from other tissues have been evaluated as models for BBB transport. The Mardin-Darby canine kidney cell line transfected with the multidrug resistance gene (MDR1) is currently the best noncerebral epithelial model for BBB permeability studies (15, 79).
Additionally, the role of other cell types in the behavior of the brain endothelium is of interest and has driven the development of coculture systems. Specifically, the role of astrocytes in TJ formation has been investigated using cocultures with Transwell systems and endothelial cells grown in astrocyte-stimulated media (75, 80–82). Typically, the endothelial cells are grown on the Transwell insert tray, whereas the astrocytes are cultured on the bottom of the Transwell multiwell plate (Figure 8).
3.3. Methods for Measuring Transport and Metabolism with In Vitro Models
Once an in vitro method is chosen as a BBB model, researchers must have in place analytical techniques with sufficient sensitivity to monitor the transport of the compounds of interest across the cell monolayers. Low limits of detection are often required, especially for potent compounds. Therefore, several factors must be kept in mind when choosing an analytical technique to be used for the investigation of the transport or metabolism of drugs in cell-culture studies. These factors include the anticipated concentrations of the drug on both the donor and receiver sides of the transport assay, the ability to label the compounds of interest (fluorescently or radioactively) for subsequent detection, sample-volume requirements, and the compatibility of the cellular matrix with the desired technique.
3.3.1. Permeability markers
Once in vitro transport studies are completed using the BBMEC culture model, further analyses must be performed to quantitate the percent transport or apparent permeability coefficient of the compounds of interest. Essential to these determinations is the use of proper control compounds to assess cell monolayer integrity and, therefore, validate the experimentally determined transport properties of the drug or other compound. Typically, low-molecular-weight compounds that are membrane impermeant are added to the apical side of the diffusion chambers at the end of a study to assess the integrity of the endothelial cell TJs. Radiolabeled sucrose or fluorescein is most commonly employed. Samples are removed from the basolateral side of the membrane over the course of 1 h and are easily analyzed via scintillation counting or fluorescence spectrophotometry (often performed on basic benchtop plate readers). The ability of these compounds to permeate must fall within a defined range to accurately assess the transport of the compound of interest (83).
Another important consideration for in vitro transport studies is the maintenance of healthy cells during transport and metabolism studies. To achieve this, experiments must be carried out under appropriate conditions. Transport media must maintain isotonicity, TJ integrity, and physiological pH (7.4) as well as provide an energy source (typically glucose) for studies lasting longer than 1 h (71). Once the conditions are met to maintain healthy cells throughout the study, the sample requirements for the downstream analytical technique must also be taken into consideration.
3.3.2. Scintillation counting
One of the simplest methods to determine the transport of compounds across the BBB is the use of radiolabeled compounds and scintillation counting (84, 85). Scintillation counting is frequently employed in competitive studies that are performed to determine whether saturable, carrier-mediated processes are responsible for a compound's permeation. In these studies, a radiolabeled substance is added to the diffusion chamber, and its permeation is determined. Then, the effect of the unlabeled compound on the permeation of the radiolabeled compound is evaluated. Alterations in the permeation of the labeled compound indicate that the two species are competing for the same transport system. Once samples are diluted in scintillation fluid, additional sample preparation is unnecessary, adding to the simplicity of this technique. This method, however, can be costly due to the expense of producing labeled compounds and the required special training for handling radioactive materials.
3.3.3. Capillary electrophoresis
Because the cell studies employ relatively small (milliliter) volumes of sample on either side of the membrane in the Transwells, sample-volume requirements of the analytical method can become an issue for studies where multiple samples will be removed from the diffusion cell. CE provides considerable advantages in addressing this aspect of cell-based studies. In particular, fluorescence detection has been utilized with CE to analyze peptide transport samples. However, one drawback is the need for sample derivatization prior to analysis (86). Most labeling techniques require reaction with amine groups such as the peptide N terminus or lysine residues. Therefore, peptides with modified N termini or those lacking a lysine residue are not detected. Nonetheless, the small sample volumes necessary for CE analysis and the sensitivity of fluorescence detection still make it an attractive technique.
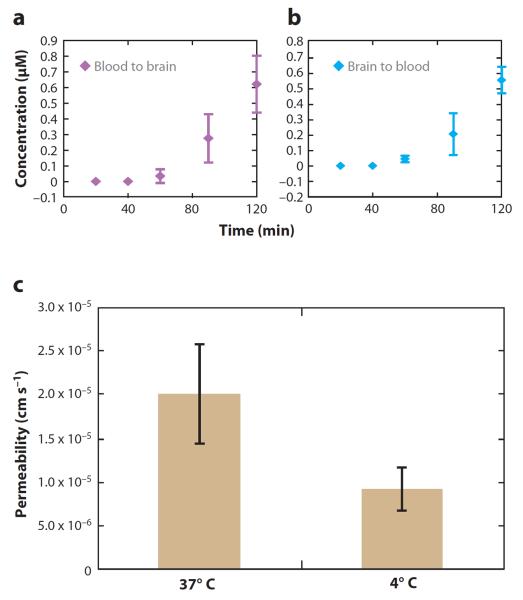
Utilizing CE-LIF Freed et al. (33, 86) investigated the metabolism and transport of substance P at the BBB following sample derivatization by NDA/CN−. Transport of this neuropeptide was characterized in both the apical-to-basolateral (blood-to-brain) and basolateral-to-apical (brain-to-blood) direction. Substance P was found to cross the BBB at similar percentages over the concentration range studied. Additionally, the temperature dependence of transport was determined by examining transport at 37, 25, and 4°C; transport of the peptide was completely abolished at 4°C.
3.3.4. Liquid chromatography/mass spectrometry
The matrices with high salt content that are essential for cellular function can present several downstream analytical challenges, especially when LC-MS is the desired technique. First, high-salt solutions are incompatible with electrospray ionization because significant damage to the ESI probe can occur. The first line of defense against such damage to expensive MS equipment is the use of a diverter valve to flush the salts present in the void volume to waste prior to introduction of the effluent into the MS. A second line of defense is to dilute samples in the mobile phase to decrease the salt concentration. Alternatively, liquid-liquid or solid-phase extraction can be employed prior to analysis (83).
Chappa et al. (63, 87) investigated substance P transport across a BBMEC monolayer, employing LC-MS/MS for peptide quantitation. One advantage of the LC-MS method over CE-LIF is the ability to investigate the effects of lower substance P concentrations on BBB transport owing to improved limits of detection. In this work, the transport of substance P was found to be a saturable process (87). Competitive studies with radiolabeled substance P provided further evidence for a carrier-mediated transport mechanism. The saturation kinetics in conjunction with the competition studies with radiolabeled peptides, as well as the temperature dependence of substance P transport, are all indicative of a carrier-mediated transport process and also suggest the involvement of a specific receptor, neurokinin-1. The presence of this receptor in the BBMEC culture model was confirmed via Western blot (87). The BBB can also act as a significant metabolic barrier for the delivery of peptides into the brain. The metabolism of substance P by enzymes present at the BBB was also investigated using the BBMEC model and LC-MS analysis (63). Figure 10 shows the products of substance P degradation by the BBB as a function of time.
Figure 10.
Metabolism of substance P (SP) by bovine brain microvessel endothelial cells. The cells were grown in multiwell culture plates and incubated with SP; then the aliquots were removed over time. The time course and appearance of each SP metabolite are shown. Reprinted with permission from Reference 63.
Another neuropeptide of interest, dynorphin (Dyn), is currently being investigated because of its involvement in pain and addiction through its interaction with κ-opioid receptors (89). Work with analogs of dynorphin, specifically Dyn A(1–11)NH2, utilized BBMECs and LC-MS/MS to evaluate the effects of structural changes to the peptide backbone on BBB permeability. All the dynorphin analogs exhibited a similar BBB permeability lag time, indicating a carrier-mediated transport mechanism was responsible for their transport (Figure 11) (90). However, the structural changes altered their ability to permeate the BBB. Peptide analogs that exhibit improved blood-to-brain transport have the potential to serve as improved therapeutics for cocaine addiction, whereas those that do not significantly enter the brain could be used to treat peripheral pain, selectively targeting κ receptors in other tissues.
Figure 11.
Transport of dynorphin A 1–11 amide by bovine brain microvessel endothelial cells. The lag time observed is indicative of a receptor-mediated process (90). (a) Blood to brain. (b) Brain to blood. (c) Effect of temperature on transport, indicating an active transport process.
BBMEC assays are also employed to determine the BBB permeation of small-molecule drugs. Desino et al. (91) investigated the BBB permeation of TCP-FA4, a derivative of the monoamine oxidase inhibitor, tranylcypromine, using the BBMEC culture model and subsequent analysis via LC-MS/MS. Compared with the parent drug, the derivative demonstrated improved BBB permeability as well as decreased monoamine oxidase inhibition. Temperature and inhibition studies suggest that passive diffusion is responsible for the compound's permeation. TCP-FA4 improved neuronal cell survival in the presence of the toxic protein β-amyloid, and it increased the level of BDNF, a protein involved in neuronal survival and differentiation, in HUVECs (human umbilical vein endothelial cells), suggesting its potential use as a neuroprotective agent.
3.3.5. Microfluidic applications
More recently, microfluidic devices have been used to investigate the transport and metabolism of substances by endothelial cells as well as the release of signaling compounds (92). These devices can be fabricated from biocompatible materials and can incorporate multiple functions, including cell culture, stimulation, and analysis (93–97), into a single device. An advantage of the microfluidic platform for studies of endothelial cell function is that the chip can be designed so that the size of the channels and flow of fluid over the cells mimic physiological conditions, and cells can be cultured directly into a microchip device (98). Because the overall channel volumes are extremely small (nanoliters), molecules secreted into the perfusing fluid are only slightly diluted and can be detected with better sensitivity than with bulk analysis methods.
Nitric oxide (NO) is a vasodilator that increases the permeability of the BBB (99). Cytokines and other inflammatory agents can stimulate inducible NO synthase and increase NO production of brain endothelial cells. Therefore, methods that are capable of monitoring NO production by endothelial cells would be very useful for understanding the role that inducible NO synthase plays in BBB permeation. Under physiological conditions, NO has a very short half-life. Therefore, the NO oxidation products nitrite (NO2−) and/or nitrate (NO3−) are normally used as an indirect measurement of NO production. However, the rapid analyses afforded by microfluidic devices allow NO to be measured directly.
The detection of NO release by bovine pulmonary artery endothelial cells (bPAECs) has been investigated using cells grown inside the channels of a polydimethylsiloxane (PDMS) microchip. A Nafion®-coated carbon electrode was employed to detect NO (100, 101). Release of NO from the bPAECs was accomplished by changing the perfusate from pure buffer to ATP-containing buffer. ATP binds the P2y purinergic receptors in the bPAECs and stimulates the release of NO. Due to the small dimensions of the channel and the close proximity of the electrode, it was possible to measure the NO release from the cells with good sensitivity and temporal resolution.
A benefit of microfluidics is the relative ease of fabricating devices that can combine multiple processes into a single substrate. Researchers have constructed devices that provide a representative model of the vasculature and that monitor the release of NO from erythrocytes (102, 103). Figure 12 shows a diagram of the current version of such a device. Flow-through channels were fabricated in a PDMS slab and then sealed to a second piece of PDMS containing sample wells with a polycarbonate membrane sandwiched in the middle. Cells were then seeded onto the membrane and allowed to grow to confluence. The device was built so that the TEER of the bPAEC layer could be measured to monitor the growth of the cells (103). Once the endothelial cells were grown to confluency, red blood cells were pumped through the fluidic channels in close proximity to the endothelial cells on the membrane. Release of ATP from the red blood cells stimulated the release of NO from the endothelial cells. DAF-FM was incubated with the endothelial cells to quantify the concentration of NO produced in the system. Unlike previous static models, this system provides a realistic in vitro representation of blood vessel circulation and the secretion of NO within the cells. Furthermore, these microchips were designed to allow many cells to be run in parallel and analyzed via high-throughput means. Multiple detection wells have been fabricated onto a single chip and analyzed using a standard 96-well-plate reader (102). This device could also have applications for BBB transport and metabolism studies.
Figure 12.
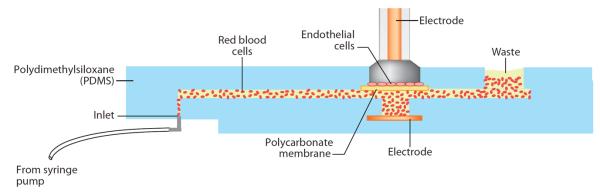
Microfluidic system for the investigation of nitric oxide release by endothelial cells in the presence of erythrocytes. Reproduced with permission from Reference 103.
Devices that can be used for both cell permeation and transport studies have been reported. In one study, investigators fabricated a microchip in which a top PDMS layer containing cell-culture chambers was sealed to a bottom glass fluidic network with a membrane sandwiched in the middle. Unlike the open-air well described above, the cells were immobilized in a closed channel. Pneumatic valves were incorporated into the design and used to trap HUVECs in predefined regions of the chip. Once the cells reached confluency, FITC (fluorescein isothiocyanate)-labeled albumin was perfused through the microfluidic channels and allowed to permeate the cell layer (104). After a designated period of time, a fluorescence measurement was made in the portion of the cell channel not in direct contact with the microfluidic channel. This was done to avoid detecting nonpermeated FITC flowing through the channel below. Introducing a compound known to increase permeation led to an increased amount of fluorescence in the reservoir, as expected (104).
Other cell types that are representative of those present in the NVU have also been investigated in the microfluidic format. One recent example is the development of a PDMS device to monitor the release of dopamine and norepinephrine from PC-12 cells. This device employed a pneumatic valve to isolate the cell perfusion channel from the electrophoresis channel. The valve could be rapidly actuated to allow a small plug of the cell perfusate sample to be introduced into the separation channel. Analytes were separated electrophoretically and then detected by fluorescence following postchannel derivatization (105, 106).
4. SUMMARY AND FUTURE DIRECTIONS
Methods are continuing to be developed to monitor the transport and metabolism of molecules at the BBB as well as to evaluate the integrity of the BBB. A number of new in vitro cell lines have been developed and evaluated for properties that mimic the BBB. The integration of these cell lines into a microfluidic format will facilitate the high-throughput screening of drugs and other substances for their ability to cross the BBB.
The ability to correlate behavior with concentrations of drugs or other substances in the brain will become increasingly important in drug development and in studies aiming to better understand neurological diseases. Microdialysis sampling enables the simultaneous monitoring of drug concentrations and neurotransmitter release in awake, freely moving animals. However, these animals are normally tethered to the syringe pump by tubing and a liquid swivel. Studies on freely roaming animals have been performed employing an osmotic pump and on-animal collection (64). However, sample analysis has thus far been performed only off-animal. In the future, the development of sampling and analysis methods that could be placed on-animal, allowing the animals to be freely roaming, would enable researchers to better investigate the effects of CNS-active drugs on behavior. Therefore, the next necessary advancement for neurochemical investigations is the development of portable on-animal separation-based sensors for drugs and neurotransmitters (107). Microfluidics, microelectronics, and telemetry are all tools that can be employed to make these sensors a reality. The advent of such devices will serve to elucidate neurochemical associations between drug administration, neurotransmitter release, and resulting behaviors.
ACKNOWLEDGMENTS
The authors acknowledge support received for this work from the National Institutes of Health (grants R01 NS042929 and R21 NS061202 and R01 DA023924). P.N. and T.H.L. gratefully acknowledge the American Heart Association for their respective predoctoral fellowships. C.D.K.S. acknowledges the support of a predoctoral fellowship from Pfizer. The authors also thank Nancy Harmony for her assistance in the preparation of this manuscript.
Glossary
- Xenobiotic
a nonendogenous compound present in an organism
- Central nervous system (CNS)
responsible for coordinating body movement and integrating information; consists of the brain and spinal cord
- Peripheral nervous system (PNS)
responsible for connecting the CNS to the limbs and organs; consists of nerves outside the brain or spinal cord
- Endothelial cells
cells that line the interior of blood vessels and are in direct contact with blood
- Brain microvessel endothelial cells (BMVECs)
endothelial cells in the brain and the main component of the BBB, different from capillary endothelial cells in the periphery due to the formation of tight junctions
- Neurovascular unit (NVU)
consists of cells found in the brain, including neurons, vascular cells (e.g., endothelial cells, pericytes), and glia (e.g., astrocytes, microglia)
- Pericytes
cells that help maintain the endothelial cells of the BBB by stabilizing them and by regulating capillary blood flow and BBB permeability
- Astrocytes
glial cells in the CNS responsible for providing nutrients to the endothelial cells in the BBB and repairing damage following trauma
- Microglia
glial cells involved in the immune response of the CNS
- Ischemia
restriction of blood flow to a tissue, resulting in a nutrient- and oxygen-deficient environment
- Apical
refers to the side of the cell-membrane surface facing the lumen (interior of a blood vessel)
- Cytokines
are a large family of proteins that serve as signaling molecules for intercellular communication
- Endocytosis
process of incorporating extracellular molecules into vesicles for transport into a cell
- P-glycoprotein (P-gp)
human glycoprotein expressed throughout the body, including on the apical side of brain endothelial cells; responsible for the efflux of compounds, preventing them from crossing the BBB
- Tomography
imaging technique that can visualize sections or planes in a three-dimensional sample
- Fibrosis
formation of fibrous connective tissue as part of a reparative response to trauma that causes scarring
- Gliosis
proliferation of astrocytes in response to trauma leading to scarring
- Accelerator mass spectrometry (AMS)
high-resolution MS technique that can separate rare isotopes from more abundant neighboring masses
- Pinocytosis
a form of endocytosis that nonspecifically transports molecules into the cell
- Fenestration
an opening through a cell membrane
- Basolateral
refers to the side of the cell-membrane surface facing away from the lumen and toward the interstitium
- Caco-2
cell line derived from human epithelial colorectal adenocarcinoma cells
Footnotes
DISCLOSURE STATEMENT The authors are not aware of any affiliations, memberships, funding, or financial holding that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Cardoso FL, Brites D, Brito MA. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res. Rev. 2010;64:328–63. doi: 10.1016/j.brainresrev.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Abbott NJ, Roennbaeck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 3.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Jain AJ, SK Drug targeting to brain: a review. Curr. Nanosci. 2011;7:21–36. [Google Scholar]
- 5.Jeffrey P, Summerfield S. Assessment of the blood-brain barrier in CNS drug discovery. Neurobiol. Dis. 2010;37:33–37. doi: 10.1016/j.nbd.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 6.Vangilder RL, Rosen CL, Barr TL, Huber JD. Targeting the neurovascular unit for treatment of neurological disorders. Pharmacol. Ther. 2011;130:239–47. doi: 10.1016/j.pharmthera.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lok J, Gupta P, Guo S, Kim WJ, Whalen MJ, et al. Cell-cell signaling in the neurovascular unit. Neurochem. Res. 2007;32:2032–45. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- 8.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 2009;27:119–45. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 9.Mangas-Sanjuan V, Gonzalez-Alvarez M, Gonzalez-Alverez I, Bermejo M. Drug penetration across the blood-brain barrier: an overview. Ther. Deliv. 2010;1:535–62. doi: 10.4155/tde.10.37. [DOI] [PubMed] [Google Scholar]
- 10.Nag S, Kapadia A, Stewart DJ. Review: molecular pathogenesis of blood-brain barrier breakdown in acute brain injury. Neuropathol. Appl. Neurobiol. 2011;37:3–23. doi: 10.1111/j.1365-2990.2010.01138.x. [DOI] [PubMed] [Google Scholar]
- 11.Abbott NJ, Dolman DEM, Patabendige AK. Assays to predict drug permeation across the blood-brain barrier, and distribution to brain. Curr. Drug Metab. 2008;9:901–10. doi: 10.2174/138920008786485182. [DOI] [PubMed] [Google Scholar]
- 12.Bentivoglio M, Mariotti R, Bertini G. Neuroinflammation and brain infections: historical context and current perspectives. Brain Res. Rev. 2011;66:152–73. doi: 10.1016/j.brainresrev.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Westerhout J, Danhof M, De Lange EC. Preclinical prediction of human brain target site concentrations: considerations in extrapolating to the clinical setting. J. Pharm. Sci. 2011;100:3577–93. doi: 10.1002/jps.22604. [DOI] [PubMed] [Google Scholar]
- 14.Hammarlund-Udenaes M, Bredberg U, Fridén M. Methodologies to assess brain drug delivery in lead optimization. Curr. Top. Med. Chem. 2009;9:148–62. doi: 10.2174/156802609787521607. [DOI] [PubMed] [Google Scholar]
- 15.Nicolazzo JA, Charman SA, Charman WN. Methods to assess drug permeability across the blood-brain barrier. J. Pharm. Pharmacol. 2006;58:281–93. doi: 10.1211/jpp.58.3.0001. [DOI] [PubMed] [Google Scholar]
- 16.Smith Q. A review of blood-brain barrier transport techniques. In: Nag S, editor. The Blood-Brain Barrier: Biology and Research Protocols. Humana; Totowa, NJ: 2003. pp. 193–207. [DOI] [PubMed] [Google Scholar]
- 17.Smith QR, Allan DD. In situ brain perfusion technique. In: Nag S, editor. The Blood-Brain Barrier: Biology and Research Protocols. Humana; Totowa, NJ: 2003. pp. 209–18. [Google Scholar]
- 18.Takasato Y, Rapoport SI, Smith QR. An in situ brain perfusion technique to study cerebrovascular transport in the rat. Am. J. Physiol. Heart Circ. Physiol. 1984;247:484–93. doi: 10.1152/ajpheart.1984.247.3.H484. [DOI] [PubMed] [Google Scholar]
- 19.Miller JM, Kumar D, Mann JJ, Parsey RV. Applications of positron emission tomography in neuropsychiatric pharmaceutical drug development. Curr. Radiopharm. 2008;1:12–16. [Google Scholar]
- 20.Pike VW. PET radiotracers: crossing the blood-brain barrier and surviving metabolism. Trends Pharmacol. Sci. 2009;30:431–40. doi: 10.1016/j.tips.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Syvanenl S, Hammarlund-Udenaes M. Using PET studies of P-gp function to elucidate mechanisms underlying the disposition of drugs. Curr. Top. Med. Chem. 2010;10:1799–809. doi: 10.2174/156802610792927997. [DOI] [PubMed] [Google Scholar]
- 22.Josserand V, Pelerin H, de Bruin B, Jego B, Kuhnast B, et al. Evaluation of drug penetration into the brain: a double study by in vivo imaging with positron emission tomography and using an in vitro model of the human blood-brain barrier. J. Pharmacol. Exp. Ther. 2006;316:79–86. doi: 10.1124/jpet.105.089102. [DOI] [PubMed] [Google Scholar]
- 23.Delgado JM, Defeudis FV, Roth RH, Ryugo DK, Mitruka BM. Dialytrode for long term intracerebral perfusion in awake monkeys. Arch. Int. Pharm. Ther. 1972;198:9–21. [PubMed] [Google Scholar]
- 24.Ungerstedt U. Introduction to intracerebral microdialysis. In: Robinson TE, Justice JB Jr., editors. Microdialysis in the Neurosciences. Elsevier; Amsterdam: 1991. pp. 3–22. [Google Scholar]
- 25.Westerink BHC, Cremers TIFH. Handbook of Microdialysis. Elsevier; Amsterdam: 2007. p. 697. [Google Scholar]
- 26.de Lange EC. Microdialysis as a method to study blood brain barrier transport mechanisms. In: Westerink BHC, Cremers TIFH, editors. Handbook of Microdialysis. Elsevier; Amsterdam: 2007. pp. 545–72. [Google Scholar]
- 27.Chaurasia CS, Müller M, Bashaw ED, Benfeldt E, Bolinder J, et al. AAPS-FDA Workshop white paper: microdialysis principles, application and regulatory perspectives. Pharm. Res. 2007;24:1014–25. doi: 10.1007/s11095-006-9206-z. [DOI] [PubMed] [Google Scholar]
- 28.Telting-Diaz M, Scott DO, Lunte CE. Intravenous microdialysis sampling in awake, freely moving rats. Anal. Chem. 1992;64:806–10. doi: 10.1021/ac00031a019. [DOI] [PubMed] [Google Scholar]
- 29.Woods JE, II, Cadle K, Solomon B, Gunaratna C, Duda C, Kissinger C. Simultaneous determination of nicotine and cotinine pharmacokinetics in the blood and brain using a combination of automated blood sampling and in vivo microdialysis in Sprague Dawley rats. Curr. Sep. Drug Dev. 2006;21:85–90. [Google Scholar]
- 30.Huff JK, Davies MI. Microdialysis monitoring of methylphenidate in blood and brain correlated with changes in dopamine and rat activity. J. Pharm. Biomed. Anal. 2002;29:767–77. doi: 10.1016/s0731-7085(02)00196-6. [DOI] [PubMed] [Google Scholar]
- 31.de Lange ECM, de Vries JD, Zurcher C, Danhof M, de Boer AG, Breimer DD. The use of intracerebral microdialysis for the determination of pharmacokinetic profiles of anticancer drugs in tumor-bearing rat brain. Pharm. Res. 1995;12:1924–31. doi: 10.1023/a:1016239822287. [DOI] [PubMed] [Google Scholar]
- 32.de Lange ECM, de Boer AG, Breimer DD. Microdialysis for pharmacokinetic analysis of drug transport to the brain. Adv. Drug Deliv. Rev. 1999;36:211–27. doi: 10.1016/s0169-409x(98)00089-1. [DOI] [PubMed] [Google Scholar]
- 33.Freed AL, Audus KL, Lunte SM. Investigation of the metabolism of substance P at the blood-brain barrier using capillary electrophoresis with laser-induced fluorescence detection. Electrophoresis. 2001;22:3778–84. doi: 10.1002/1522-2683(200109)22:17<3778::AID-ELPS3778>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 34.Kostel KL, Lunte SM. Evaluation of capillary electrophoresis with post-column derivatization and laser-induced fluorescence detection for the determination of substance P and its metabolites. J. Chromatogr. B. 1997;695:27–38. doi: 10.1016/s0378-4347(97)00173-4. [DOI] [PubMed] [Google Scholar]
- 35.Reed B, Bidlack JM, Chait BT, Kreek MJ. Extracellular biotransformation of beta-endorphin in rat striatum and cerebrospinal fluid. J. Neuroendocrinol. 2008;20:606–16. doi: 10.1111/j.1365-2826.2008.01705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Lange ECM, Danhof M, de Boer AG, Breimer DD. Methodological considerations of intracerebral microdialysis in pharmacokinetic studies on drug transport across the blood-brain barrier. Brain Res. Rev. 1997;25:27–49. doi: 10.1016/s0165-0173(97)00014-3. [DOI] [PubMed] [Google Scholar]
- 37.Brunner M, Derendorf H. Clinical microdialysis: current applications and potential use in drug development. Trends Anal. Chem. 2006;25:674–80. [Google Scholar]
- 38.Grabb MC, Sciotti VM, Gidday JM, Cohen SA, van Wylen DG. Neurochemical and morphological responses to acutely and chronically implanted brain microdialysis probes. J. Neurosci. Methods. 1998;82:25–34. doi: 10.1016/s0165-0270(98)00025-9. [DOI] [PubMed] [Google Scholar]
- 39.Benveniste H, Drejer J, Schousboe A, Diemer NH. Regional cerebral glucose phosphorylation and blood flow after insertion of a microdialysis fiber through the dorsal hippocampus in the rat. J. Neurochem. 1987;49:729–34. doi: 10.1111/j.1471-4159.1987.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 40.Jaquins-Gerstl A, Michael AC. Comparison of the brain penetration injury associated with microdialysis and voltammetry. J. Neurosci. Methods. 2009;183:127–35. doi: 10.1016/j.jneumeth.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaquins-Gerstl A, Shu Z, Zhang J, Liu Y, Weber SG, Michael AC. Effect of dexamethasone on gliosis, ischemia, and dopamine extraction during microdialysis sampling in brain tissue. Anal. Chem. 2011;83:7662–67. doi: 10.1021/ac200782h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Lange EC, Danhof M, de Boer AG, Breimer DD. Critical factors of intracerebral microdialysis as a technique to determine the pharmacokinetics of drugs in rat brain. Brain Res. 1994;666:1–8. doi: 10.1016/0006-8993(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 43.Choi YH, Fletcher PJ, Wong CCS, Andersen GH. Measurement of blood brain permeability in rats with α amino butyric acid during microdialysis: application to behavioral studies. Physiol. Behav. 1999;67:587–98. doi: 10.1016/s0031-9384(99)00110-9. [DOI] [PubMed] [Google Scholar]
- 44.Major O, Shdanova T, Duffek L, Nagy Z. Continuous monitoring of blood-brain barrier opening to Cr51-EDTA by microdialysis following probe injury. Acta Neurochir. Suppl. 1990;51:46–48. doi: 10.1007/978-3-7091-9115-6_16. [DOI] [PubMed] [Google Scholar]
- 45.Hascup ER, af Bjerkén S, Hascup KN, Pomerleau F, Huettl P, et al. Histological studies of the effects of chronic implantation of ceramic-based microelectrode arrays and microdialysis probes in rat prefrontal cortex. Brain Res. 2009;1291:12–20. doi: 10.1016/j.brainres.2009.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stenken JA. Methods and issues in microdialysis calibration. Anal. Chim. Acta. 1999;379:337–57. [Google Scholar]
- 47.Bungay PM, Morrison PF, Dedrick RL, Chefer VI, Zapata A. Chapter 2.2: Principles of quantitative microdialysis. In: Westerink BHC, Cremers TIFH, editors. Handbook of Behavioral Neuroscience. Elsevier; Amsterdam: 2006. pp. 131–67. [Google Scholar]
- 48.Lonnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am. J. Physiol. Endocrinol. Metab. 1987;253:228–31. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- 49.Kehr J. A survey on quantitative microdialysis: theoretical models and practical implications. J. Neurosci. Methods. 1993;48:251–61. doi: 10.1016/0165-0270(93)90096-a. [DOI] [PubMed] [Google Scholar]
- 50.Song Y, Lunte CE. Comparison of calibration by delivery versus no net flux for quantitative in vivo microdialysis sampling. Anal. Chim. Acta. 1999;379:251–62. [Google Scholar]
- 51.Wang Y, Wong SL, Sawchuk RJ. Microdialysis calibration using retrodialysis and zero-net flux: application to a study of the distribution of zidovudine to rabbit cerebrospinal fluid and thalamus. Pharm. Res. 1993;10:1411–19. doi: 10.1023/a:1018906821725. [DOI] [PubMed] [Google Scholar]
- 52.Dai H, Elmquist W. Drug transport studies using quantitative microdialysis. In: Nag S, editor. The Blood-Brain Barrier: Biology and Research Protocols. Humana; Totowa, NJ: 2003. pp. 249–64. [DOI] [PubMed] [Google Scholar]
- 53.Bengtsson J, Bostroem E, Hammarlund-Udenaes M. The use of a deuterated calibrator for in vivo recovery estimations in microdialysis studies. J. Pharm. Sci. 2008;97:3433–41. doi: 10.1002/jps.21217. [DOI] [PubMed] [Google Scholar]
- 54.Menacherry S, Hubert W, Justice JB., Jr. In vivo calibration of microdialysis probes for exogenous compounds. Anal. Chem. 1992;64:577–83. doi: 10.1021/ac00030a003. [DOI] [PubMed] [Google Scholar]
- 55.Kennedy RT, Watson CJ, Haskins WE, Powell DH, Strecker RE. In vivo neurochemical monitoring by microdialysis and capillary separations. Curr. Opin. Chem. Biol. 2002;6:659–65. doi: 10.1016/s1367-5931(02)00373-3. [DOI] [PubMed] [Google Scholar]
- 56.Nandi P, Lunte SM. Recent trends in microdialysis sampling integrated with conventional and microanalytical systems for monitoring biological events: a review. Anal. Chim. Acta. 2009;651:1–14. doi: 10.1016/j.aca.2009.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cremers TIFH, de Vries MG, Huinink KD, van Loon JP, Hart MVD, et al. Quantitative microdialysis using modified ultraslow microdialysis: direct rapid and reliable determination of free brain concentrations with the MetaQuant technique. J. Neurosci. Methods. 2009;178:249–54. doi: 10.1016/j.jneumeth.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 58.Hammarlund-Udenaes M. Active-site concentrations of chemicals: Are they a better predictor of effect than plasma/organ/tissue concentrations? Basic Clin. Pharmacol. Toxicol. 2010;106:215–20. doi: 10.1111/j.1742-7843.2009.00517.x. [DOI] [PubMed] [Google Scholar]
- 59.Nandi P, Lunte SM. Microdialysis sampling as a sample preparation method. In: Pawliszyn J, Lord HL, editors. Handbook of Sample Preparation. Wiley; Hoboken: 2010. pp. 103–24. [Google Scholar]
- 60.Telting-Diaz M, Lunte CE. Distribution of tacrine across the blood-brain barrier in awake, freely moving rats using in vivo microdialysis sampling. Pharm. Res. 1993;10:44–48. doi: 10.1023/a:1018964727833. [DOI] [PubMed] [Google Scholar]
- 61.Crick EW, Osorio I, Bhavaraju NC, Linz TH, Lunte CE. An investigation into the pharmacokinetics of 3-mercaptopropionic acid and development of a steady-state chemical seizure model using in vivo microdialysis and electrophysiological monitoring. Epilepsy Res. 2007;74:116–25. doi: 10.1016/j.eplepsyres.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiss DJ, Krisko RM, Lunte CE. Applications of microdialysis/mass spectrometry to drug discovery. In: Rossi DT, Sinz MW, editors. Mass Spectrometry in Drug Discovery. Marcel Dekker; New York: 2002. pp. 377–97. [Google Scholar]
- 63.Chappa AK, Cooper JD, Audus KL, Lunte SM. Investigation of the metabolism of substance P at the blood-brain barrier using LC-MS/MS. J. Pharm. Biomed. Anal. 2007;43:1409–15. doi: 10.1016/j.jpba.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cooper JD, Heppert KE, Davies MI, Lunte SM. Evaluation of an osmotic pump for microdialysis sampling in an awake and untethered rat. J. Neurosci. Methods. 2007;160:269–75. doi: 10.1016/j.jneumeth.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malone ML, Zuo H, Smyth MR, Lunte SM. Determination of tryptophan and kynurenine in brain microdialysis samples by capillary electrophoresis with electrochemical detection. J. Chromatogr. A. 1995;700:73–80. [Google Scholar]
- 66.Kaul S, Faiman MD, Lunte CE. Determination of GABA, glutamate and carbamathione in brain microdialysis samples by capillary electrophoresis with fluorescence detection. Electrophoresis. 2011;32:284–91. doi: 10.1002/elps.201000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaul S, Faiman MD, Lunte CE. Determination of GABA, glutamate, dopamine and carbamathione in brain microdialysis samples by micellar electrokinetic chromatography and laser-induced fluorescence (MEKC-LIF) Anal. Methods. 2011;3:1514–20. [Google Scholar]
- 68.Nandi P, Desai DP, Lunte SM. Development of a PDMS-based microchip electrophoresis device for continuous online in vivo monitoring of microdialysis samples. Electrophoresis. 2010;31:1414–22. doi: 10.1002/elps.200900612. [DOI] [PubMed] [Google Scholar]
- 69.Janle EM, Lila MA, Grannan M, Wood L, Higgins A, et al. Method for evaluating the potential of 14C-labeled plant polyphenols to cross the blood-brain barrier using accelerator mass spectrometry. Nucl. Instrum. Methods B. 2010;268:1313–16. doi: 10.1016/j.nimb.2009.10.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sadiq MW, Salehpour M, Forsgard N, Possnert G, Hammarlund-Udenaes M. Morphine brain pharmacokinetics at very low concentrations studied with accelerator mass spectrometry and liquid chromatography–tandem mass spectrometry. Drug Metab. Dispos. 2011;39:174–79. doi: 10.1124/dmd.110.036434. [DOI] [PubMed] [Google Scholar]
- 71.Audus KL, Borchardt RT. Characterization of an in vitro blood-brain barrier model system for studying drug transport and metabolism. Pharm. Res. 1986;3:81–87. doi: 10.1023/A:1016337202335. [DOI] [PubMed] [Google Scholar]
- 72.Spatz M, Mrsulja BB. Progress in cerebral microvascular studies related to the function of the blood-brain barrier. Adv. Cell. Neurobiol. 1982;3:311–37. [Google Scholar]
- 73.Thompson SE, Audus KL. Leucine-enkephalin metabolism in brain microvessel endothelial cells. Peptides. 1993;15:109–16. doi: 10.1016/0196-9781(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 74.Baranczyk-Kuzma A, Audus KL, Borchardt RT. Catecholamine-metabolizing enzymes of bovine brain microvessel endothelial cell monolayers. J. Neurochem. 1986;46:1956–60. doi: 10.1111/j.1471-4159.1986.tb08519.x. [DOI] [PubMed] [Google Scholar]
- 75.Gumbleton M, Audus KL. Progress and limitations in the use of in vitro cell cultures to serve as a permeability screen for the blood-brain barrier. J. Pharm. Sci. 2001;90:1681–98. doi: 10.1002/jps.1119. [DOI] [PubMed] [Google Scholar]
- 76.Desino KE. PhD thesis. Univ. Kans; 2008. Improving blood-brain barrier permeation of small molecules exhibiting chemotherapeutic and neuroprotective effects. [Google Scholar]
- 77.Turunen BJ, Ge H, Oyetunji J, Desino KE, Vasandani V, et al. Paclitaxel succinate analogs: anionic and amide introduction as a strategy to impart blood-brain barrier permeability. Bioorg. Med. Chem. Lett. 2008;18:5971–74. doi: 10.1016/j.bmcl.2008.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yazdanian M, Bormann BJ. Immortalized brain endothelial cells. U.S. Patent 6,093,553. 2000
- 79.Wang Q, Rager JD, Weinstein K, Kardos PS, Dobson GL, et al. Evaluation of the MDR-MDCK cell line as a permeability screen for the blood-brain barrier. Int. J. Pharm. 2005;288:349–59. doi: 10.1016/j.ijpharm.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 80.de Boer AG, Gaillard PJ. In vitro models of the blood-brain barrier: when to use which? Curr. Med. Chem. 2002;2:203–9. [Google Scholar]
- 81.Reinhardt CA, Gloor SM. Co-culture blood-brain barrier models and their use for pharmatoxicological screening. Toxicol. In Vitro. 1997;11:513–18. doi: 10.1016/s0887-2333(97)00039-8. [DOI] [PubMed] [Google Scholar]
- 82.Takakura T, Audus KL, Borchardt RT. Cultured brain microvessel endothelial cells as in vitro models of the blood-brain barrier. NIDA Res. Monogr. 1992;120:138–49. [PubMed] [Google Scholar]
- 83.Sloan CDK. PhD thesis. Univ. Kans; 2011. The development of analytical methods for investigations of dynorphin A 1–17 metabolism in the central nervous system and peripheral tissues and transport at the blood brain barrier. [Google Scholar]
- 84.Audus KL, Borchardt RT. Characteristics of the large neutral amino acid transport system of bovine brain microvessel endothelial cell monolayers. J. Neurochem. 1986;47:484–88. doi: 10.1111/j.1471-4159.1986.tb04527.x. [DOI] [PubMed] [Google Scholar]
- 85.Shah MV, Audus KL, Borchardt RT. The application of bovine brain microvessel endothelial-cell monolayers grown onto polycarbonate membranes in vitro to estimate the potential permeability of solutes through the blood-brain barrier. Pharm. Res. 1989;6:624–27. doi: 10.1023/a:1015913817221. [DOI] [PubMed] [Google Scholar]
- 86.Freed AL, Audus KL, Lunte SM. Investigation of substance P transport across the blood-brain barrier. Peptides. 2002;23:157–65. doi: 10.1016/s0196-9781(01)00592-7. [DOI] [PubMed] [Google Scholar]
- 87.Chappa AK, Audus KL, Lunte SM. Characteristics of substance P transport across the blood-brain barrier. Pharm. Res. 2006;23:1201–8. doi: 10.1007/s11095-006-0068-1. [DOI] [PubMed] [Google Scholar]
- 88.Cooper J. PhD thesis. Univ. Kans; 2003. Investigation of substance P metabolism and transport by liquid chromatography with tandem mass spectrometric detection. [Google Scholar]
- 89.Patkar KA, Murray TF, Aldrich JV. The effects of C-terminal modifications on the opioid activity of [N-benzylTyr1]dynorphin A-(1–11) analogues. J. Med. Chem. 2009;52:6814–21. doi: 10.1021/jm900715m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chappa AK. PhD thesis. Univ. Kans; 2007. Biopharmaceutical aspects of the development of peptides as CNS drug delivery vectors and therapeutic agents: studies with substance P and dynorphin analogs. [Google Scholar]
- 91.Desino KE, Pignatello R, Guccione S, Basile L, Ansar S, et al. TCP-FA4: a derivative of tranylcypromine showing improved blood-brain permeability. Biochem. Pharmacol. 2009;78:1412–17. doi: 10.1016/j.bcp.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 92.Spence DM. Microfluidic technology as a platform to investigate microcirculation. In: Landers JP, editor. Handbook of Capillary and Microchip Electrophoresis and Associated Microtechniques. CRC; Boca Raton: 2008. pp. 841–52. [Google Scholar]
- 93.Sanders GHW, Manz A. The incredibly shrinking laboratory reactions, separations and detections. J. Assoc. Lab. Autom. 2000;5:40–45. [Google Scholar]
- 94.Datta S, Ghosal S. Characterizing dispersion in microfluidic channels. Lab Chip. 2009;9:2537–50. doi: 10.1039/b822948c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roman GT, Kennedy RT. Fully integrated microfluidic separations systems for biochemical analysis. J. Chromatogr. A. 2007;1168:170–88. doi: 10.1016/j.chroma.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 96.Bennet D, Kim S. Implantable microdevice for peripheral nerve regeneration: materials and fabrications. J. Mater. Sci. 2011;46:4723–40. [Google Scholar]
- 97.Gauvin R, Khademhosseini A. Microscale technologies and modular approaches for tissue engineering: moving toward the fabrication of complex functional structures. Am. Chem. Soc. Nano. 2011;5:4258–64. doi: 10.1021/nn201826d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martin RS, Root PD, Spence DM. Microfluidic technologies as platforms for performing quantitative cellular analyses in an in vitro environment. Analyst. 2006;131:1197–206. doi: 10.1039/b611041j. [DOI] [PubMed] [Google Scholar]
- 99.Thiel VE, Audus KL. Nitric oxide and blood-brain barrier integrity. Antioxid. Redox Signal. 2001;3:273–78. doi: 10.1089/152308601300185223. [DOI] [PubMed] [Google Scholar]
- 100.Spence DM, Torrence NJ, Kovarik ML, Martin RS. Amperometric determination of nitric oxide derived from pulmonary artery endothelial cells immobilized in a microchip channel. Analyst. 2004;129:995–1000. doi: 10.1039/b410547h. [DOI] [PubMed] [Google Scholar]
- 101.Hulvey MK, Martin RS. A microchip-based endothelium mimic utilizing open reservoirs for cell immobilization and integrated carbon ink microelectrodes for detection. Anal. Bioanal. Chem. 2009;393:599–605. doi: 10.1007/s00216-008-2468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Halpin ST, Spence DM. Direct plate-reader measurement of nitric oxide released from hypoxic erythrocytes flowing through a microfluidic device. Anal. Chem. 2010;82:7492–97. doi: 10.1021/ac101130s. [DOI] [PubMed] [Google Scholar]
- 103.Vogel PA, Halpin ST, Martin RS, Spence DM. Microfluidic transendothelial electrical resistance measurement device that enables blood flow and postgrowth experiments. Anal. Chem. 2011;83:4296–301. doi: 10.1021/ac2004746. [DOI] [PubMed] [Google Scholar]
- 104.Shao J, Wu L, Wu J, Zheng Y, Zhao H, et al. A microfluidic chip for permeability assays of endothelial monolayer. Biomed. Microdevices. 2010;12:81–88. doi: 10.1007/s10544-009-9362-0. [DOI] [PubMed] [Google Scholar]
- 105.Li MW, Martin RS. Microchip-based integration of cell immobilization, electrophoresis, post-column derivatization, and fluorescence detection for monitoring the release of dopamine from PC 12 cells. Analyst. 2008;133:1358–66. doi: 10.1039/b807093h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Z, Cui Z. Application of microdialysis in tissue engineering monitoring. Progr. Nat. Sci. 2008;18:503–11. [Google Scholar]
- 107.Lunte SM, Nandi P, Regel A, Grigsby R, Hulvey MK, Scott D. The development of a miniaturized wireless microdialysis-microchip electrophoresis system for in vivo monitoring of drugs and neurotransmitters in awake and freely moving sheep. Presented at Int. Conf. Miniat. Syst. Chem. Life Sci., MicroTAS, 14th; Groningen, Neth. 2010. [Google Scholar]
- 108.Ueda Y, Doi T, Takaki M, Nagatomo K, Nakajima A, Willmore LJ. Levetiracetam enhances endogenous antioxidant in the hippocampus of rats: in vivo evaluation by brain microdialysis combined with ESR spectroscopy. Brain Res. 2009;1266:1–7. doi: 10.1016/j.brainres.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 109.Andren PE, Emmett MR, DaGue BB, Steulet A-F, Waldmeier P, Caprioli RM. Blood-brain barrier penetration of 3-aminopropyl-n-butylphosphinic acid (CGP 36742) in rat brain by microdialysis/mass spectrometry. J. Mass Spectrom. 1998;33:281–87. doi: 10.1002/(SICI)1096-9888(199803)33:3<281::AID-JMS631>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 110.Groenendaal D, Blom-Roosemalen MCM, Danhof M, de Lange ECM. High-performance liquid chromatography of nalbuphine, butorphanol and morphine in blood and brain microdialyzate samples: application to pharmacokinetic/pharmacodynamic studies in rats. J. Chromatogr. B. 2005;822:230–37. doi: 10.1016/j.jchromb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 111.Zhang J, Qu F-R, Nakatsuka A, Nomura T, Nagai M, Nomoto M. Pharmacokinetics of L-DOPA in plasma and extracellular fluid of striatum in common marmosets. Brain Res. 2003;993:54–58. doi: 10.1016/j.brainres.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 112.Sun Y, Nakashima MN, Takahashi M, Kuroda N, Nakashima K. Determination of bisphenol A in rat brain by microdialysis and column switching high-performance liquid chromatography with fluorescence detection. Biomed. Chromatogr. 2002;16:319–26. doi: 10.1002/bmc.161. [DOI] [PubMed] [Google Scholar]
- 113.Sheu WHH, Chuang H-C, Cheng S-M, Lee M-R, Chou C-C, Cheng F-C. Microdialysis combined blood sampling technique for the determination of rosiglitazone and glucose in brain and blood of gerbils subjected to cerebral ischemia. J. Pharm. Biomed. Anal. 2011;54:759–64. doi: 10.1016/j.jpba.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 114.Blakeley JO, Olson J, Grossman SA, He X, Weingart J, Supko JG. Effect of blood-brain barrier permeability in recurrent high-grade gliomas on the intratumoral pharmacokinetics of methotrexate: a microdialysis study. J. Neuro-Oncol. 2009;91:51–58. doi: 10.1007/s11060-008-9678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kaul S, Williams TD, Lunte CE, Faiman MD. LC-MS/MS determination of carbamathione in microdialysis samples from rat brain and plasma. J. Pharm. Biomed. Anal. 2010;51:186–91. doi: 10.1016/j.jpba.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Song Y, Xu J, Hamme A, Liu Y-M. Capillary liquid chromatography–tandem mass spectrometry of tetrahydroisoquinoline derived neurotoxins: a study on the blood-brain barrier of rat brain. J. Chromatogr. A. 2006;1103:229–34. doi: 10.1016/j.chroma.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 117.Chen KC. Evidence on extracellular dopamine level in rat striatum: implications for the validity of quantitative microdialysis. J. Neurochem. 2005;92:46–58. doi: 10.1111/j.1471-4159.2004.02848.x. [DOI] [PubMed] [Google Scholar]
- 118.Whitaker G, Lunte CE. Investigation of microdialysis sampling calibration approaches for lipophilic analytes: doxorubicin. J. Pharm. Biomed. Anal. 2010;53:490–96. doi: 10.1016/j.jpba.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin LC, Chen YF, Tsai TH. Analysis of brain distribution and biliary excretion of a nutrient supplement, gastrodin, in rat. Anal. Chim. Acta. 2007;590:173–79. doi: 10.1016/j.aca.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 120.Lin LC, Tsai TH. Pharmacokinetics and brain distribution of unbound levamisole in the anesthetized rats using microdialysis and microbore column liquid chromatography. Anal. Chim. Acta. 2006;569:145–50. [Google Scholar]
- 121.Huang YJ, Liao JF, Tsai TH. Concurrent determination of thalidomide in rat blood, brain and bile using multiple microdialysis coupled to liquid chromatography. Biomed. Chromatogr. 2005;19:488–93. doi: 10.1002/bmc.466. [DOI] [PubMed] [Google Scholar]
- 122.Yang CS, Chang CH, Tsai PJ, Chen WY, Tseng FG, Lo LW. Nanoparticle-based in vivo investigation on blood-brain barrier permeability following ischemia and reperfusion. Anal. Chem. 2004;76:4465–71. doi: 10.1021/ac035491v. [DOI] [PubMed] [Google Scholar]