Abstract
Developmental and Behavioral Pediatricians (DBP) diagnose and care for children with Fragile X syndrome (FXS). Their attitudes towards FMR1 newborn screening (NBS) and FMR1 carrier testing in childhood could highlight potential pitfalls with FMR1 NBS. We conducted a cross-sectional survey with an adjusted response rate of 61%. Among DBP, 74% supported universal FMR1 NBS, preferring to identify both full mutations and premutations. DBP also support FMR1 testing of asymptomatic siblings. Although DBP support testing for premutations at various points in lifespan, DBP are not familiar with the array of Fragile X associated disorders (FXAD). Targeted educational interventions are needed to ensure that all health care providers have the knowledge and competence to consent and to counsel families on FXAD.
Keywords: Fragile X, Newborn screening, Population screening, Professional attitudes
Introduction
Fragile X Syndrome (FXS) is the most common cause of inherited intellectual disability (ID) and part of an array of trinucleotide repeat disorders caused by mutations in the FMR1 gene. (Table 1) Multiple professional organizations including the American Academy of Pediatrics (AAP) recommend that DNA testing for FXS be part of the routine diagnostic algorithm for a child with unexplained developmental delays, ID or autism (Filipek et al., 2000; Moeschler et al., 2006; Myers et al., 2007; Sandler et al., 2001; Sandler et al., 2001; Shevell et al., 2003). Screening for FXS at other times in the lifespan has also been considered (Cronister, DiMaio, Mahoney, Donnenfeld, & Hallam, 2005; Saul et al., 2008; Song, Barton, Sleightholme, Yao, & Fry-Smith, 2003)and NIH-sponsored FMR1 newborn screening (NBS) programs are being piloted (Brice, 2008; Skinner et al., 2011).
Table 1.
Clinical Spectrum of Fragile X associated disorders
| FMR-1 genotype | Disorder | Symptoms | Onset of symptoms | Gender | Prevalence |
|---|---|---|---|---|---|
| Full mutation (>200 trinucleotide repeats) | Fragile X Syndrome1 |
|
Childhood | M>F Only 50% females with full mutation will have symptoms; 25% will have learning disabilities |
Males:1:3600–4000 Females: 1:4000–6000 |
| Premutation (55–200 trinucleotide repeats) | Fragile X Tremor Ataxia Syndrome (FXTAS)2 |
|
Adult | M>F | 30–40% among male premutation carriers 8–16% among female premutation carriers |
| Fragile X Premature Ovarian Insufficiency (FXPOI)3 | Early menopause | Adult | Only Females | 20–28% among female premutation carriers |
NBS for FMR1 identifies children at risk for FXS, but can also identify carriers who are at risk for disorders associated with having a FMR1 premutation, including adult-onset Fragile X Premature Ovarian Insufficiency (FXPOI) and Fragile X Tremor Ataxia Syndrome (FXTAS). While some studies have reported an increase in depression and anxiety among FMR1 carriers (Hunter et al., 2012; Tassone et al., 2012), population-based screening for FMR1 gene alleles revealed that while those with FMR1 premutations had higher rates of divorce than controls, they were no more likely than controls to report significant mental health issues (Seltzer et al., 2012). Because of adult-onset FMR1 premutation associated conditions, identifying FMR1 premutations in childhood is akin to the carrier identification in childhood of adult-onset conditions and is not recommended by many professional organizations (Clarke et al., 1994; Dalby, 1995; Hogben & Boddington, 2005; Nelson et al., 2001; Sherman, Pletcher, & Driscoll, 2005). Identification of premutation alleles in NBS may be justifiable if there were a childhood-onset condition that could benefit from timely intervention. Although there have been reports of mild behavioral, emotional and executive function impairments in children, predominantly boys, with FMR1 premutations (R. J. Hagerman et al., 2009; P. J. Hagerman & Hagerman, 2004), a detailed clinical characterization including population prevalence of the childhood premutation phenotype is unknown. Whether or not a FMR1 premutation phenotype with childhood-onset is confirmed as a distinct entity, proposed treatment for the reported impairments remain the same, regardless of etiology, and would include counseling, school accommodations and behavioral supports.
Even though geneticists and pediatricians report being familiar with FXS as a cause of ID, professional knowledge about the clinical nuances of FMR1 inheritance and awareness of the other FXAD, FXPOI and FXTAS, varies (Kemper & Bailey Jr., 2009). In a national survey of US pediatricians, Kemper et al demonstrated that only 50% knew that females could be affected and fewer than 1/3 knew that individuals with premutations could develop adult-onset health problems. Only 33% of pediatricians felt competent to counsel families about a FMR1 NBS program (Kemper & Bailey Jr., 2009). In contrast, Acharya et al surveyed physician geneticists and genetic counselors and found familiarity rates over 87% for FXAD, FXPOI and FXTAS (Acharya & Ross, 2009).
Developmental and behavioral pediatricians (DBP) are often asked to evaluate children with developmental delays and autism and thus are the ones who must obtain consent for FMR1 testing and counsel families on the results. If FMR1 NBS is implemented, DBP may be increasingly called upon to counsel families about the implications of having an FMR1 mutation. However, it is unknown how familiar DBP are with adult-onset FXAD. Additionally, examining their attitudes regarding the design of a FMR1 NBS program and current practice regarding FMR1 carrier testing in childhood could highlight potential future pitfalls with FMR1 NBS. Our objective was to describe the familiarity of DBP towards FXAD and their attitudes towards FMR1 NBS, and childhood carrier (premutation) testing.
Method
We conducted a cross-sectional survey of members of the 2010 edition of the web-based directory of the Society of Developmental and Behavioral Pediatrics (SDBP). We excluded individuals when they did not reside in the United States, or did not have an e-mail address listed in the professional directory. Each subject was contacted a maximum of 3 times by email or mail. The survey was initially piloted at the SDBP annual meeting held September 2010 in Boston. Feedback was used to refine phrasing and improve the face validity of the survey. Responses to the piloted survey were excluded from final analysis.
The primary survey measures were (1) respondents’ self-reported familiarity with the clinical syndromes associated with FMR1, specifically: ID, FXPOI, and FXTAS, (2) attitudes towards pre- and full mutation screening of newborns, and (3) FMR1 testing of an asymptomatic sibling of a child with FXS. Demographics were collected including whether respondents order FMR1 testing of symptomatic children with ID or autism.
DBP were asked about their familiarity with FMR1 full mutation causing ID in boys and girls (FXS), FMR1 premutation causing premature ovarian insufficiency (FXPOI) in young adult women and FMR1 premutation causing FXTAS in adults. DBP were asked to rate their familiarity with each clinical scenario on a 4 point Likert scale [1= not at all familiar; 2= minimally familiar; 3= moderately familiar and 4= very familiar].
After these items, a brief summary was provided (survey available from corresponding author) to ensure current knowledge of the state of prenatal testing and NBS for FMR1 mutations. Specifically, DBP were told that although American College of Obstetrics and Gynecology (ACOG) does not recommend universal prenatal screening, it does recommend FMR1 screening of pregnant women with a family history of ID. Regarding NBS, DBP were told that technology suitable for FMR1 NBS has been developed and pilot NBS programs are underway. Respondents were not provided with additional information about the clinical presentation or genetics of FXAD.
Following the descriptions, DBP were asked if they supported a mandatory FMR1 NBS program, one that required informed consent, or did not support universal NBS. DBP were also asked to rate how important it was to identify FMR1 full mutations and premutations in male and female newborns on a 4 point Likert scale [1= not important; 2= minimally important; 3= moderately important and 4= very important]. An additional option (5) was also offered: “It is important to NOT diagnose.” On a 4 point Likert scale, DBP were asked to rate how important the following reasons were to pursue FMR1 NBS: to give women reproductive choices about pregnancy, to help families prepare for the child’s disability, to ensure that affected newborns will have early access to available treatments and interventions, and to facilitate research.
For childhood testing, DBP were asked if they would agree to order FMR1 testing on a typically developing brother or sister of an 8 year-old boy with FXS at any age or only in adolescence with permission of parent and teenager. For these 2 questions, DBP were able to provide optional open-ended explanations for their choices.
Statistical analyses were conducted using SPSS for Windows Version 19 (SPSS Inc, Chicago, IL). First, descriptive statistics were generated for each survey measure. For statistical analysis, we excluded responses that were left blank or marked as “not sure”. For familiarity, “very familiar” and “moderately familiar” responses were combined and “minimally familiar” and “not familiar” responses were combined. For the universal NBS question, “yes” and “yes with consent” were combined. For questions with a 4 point Likert, “very important” and “moderately important” responses were combined and “minimally important” and “not important” responses were combined. Option 5 was combined with those who believed that FMR1 testing was minimally or not important. For sibling carrier testing, the responses “yes” and “yes only in adolescence” were combined. McNemar statistic was used to examine statistical differences in familiarity across conditions and in support for (1) full mutation and premutation identification in male and female newborns and for (2) asymptomatic testing of a typically developing male or female sibling. Bivariate analyses using the χ2 test was then used to examine differences in the primary survey measures by each of the demographic factors measured, and multivariate logistic regression analyses were performed to examine whether bivariate associations persisted after controlling for relevant covariates [gender, practice setting (academic, community-based academic affiliate, and non-academic community-based clinic) and years in practice]. The open-ended responses were coded independently by the authors KA and AS. Disagreements were reviewed and resolved with discussion. Approval from the University of Chicago Institutional Review Board for the project and for waived written consent was obtained before any of the clinicians were contacted.
Results
Of 484 eligible list-serv members, 294 (61%) returned partial or complete surveys. Demographics are listed in Table 2. Our respondents were mostly female, non-Hispanic (245/257, 96%), and Caucasian (232, 87%). Very few reported having personal experience with an individual with ID or FXTAS. The vast majority of DBP surveyed order FMR1 testing when they evaluate symptomatic children with ID (231/268, 86%) or autism (235/266, 88%). DBP who practiced at a referral center were more likely to order FMR1 testing to evaluate ID or autism (135/144, 94%) than DBP who practiced at a community based academic affiliate (38/47, 81%) or a non-academic community based setting (54/71, 76%) (p<0.01). No other demographic variable was significant.
Table 2.
Demographics
| Characteristic | % (N) |
|---|---|
| Gender (n=269) | |
| Male | 36% (96) |
| Female | 64% (173) |
| Years in Practice (n=239) | |
| >5 | 6% (14) |
| 6–15 | 26% (61) |
| 16–25 | 21% (51) |
| 26–35 | 32% (76) |
| > 35 | 15% (37) |
| Practice Setting (n=277) | |
| Academic referral center | 55% (152) |
| Academic Affiliate (Community-Based) | 17% (48) |
| Non-academic community-based | 27% (75) |
| Time in Direct Patient Care (n=212) | |
| 0–25% | 13% (27) |
| 26–50% | 17% (37) |
| 51–75% | 18% (39) |
| 76–100% | 51% (109) |
| Family History | |
| Intellectual Disability (n=272) | 13% (35) |
| FX Tremor/Ataxia (n=267) | 7% (19) |
DBP’s self-reported familiarity with the FXAD varied with condition. Almost all were at least moderately familiar with FMR1 full mutations as a cause of ID (97%, n=285/293). Respondents were significantly less familiar with the premutation-associated conditions of FXPOI (n=161/292, 55%; p<0.01) and FXTAS (181/292, 62%; p<0.01).
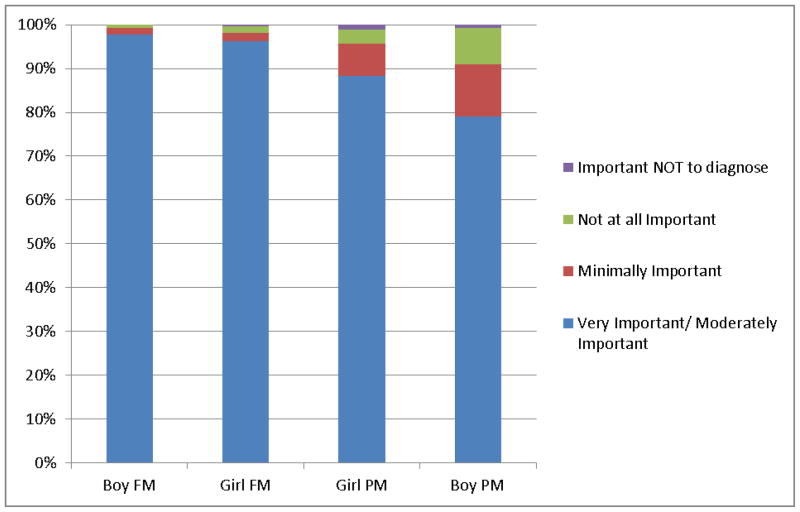
Seventy-four percent of DBP (n=165/223) would support universal NBS for FMR1 mutations. More than twice as many preferred a voluntary (n=107) program over a mandatory (n=58) one. Nearly all endorsed identifying FMR1 full mutations in newborns as at least a moderately important goal of NBS in both male and female infants. (261/267, 98% vs 255/265, 96%; p=NS) (Figure 1). A strong majority also endorsed identifying FMR1 premutation as at least a moderately important goal for NBS in male and female infants. (212/257, 82% vs 229/259; 88%; p=NS) Overall, identifying infants with a full mutation was a relatively more important goal for NBS than identifying premutations independent of the infant’s gender (p<0.001).
Figure 1. Importance of Diagnosing Fragile X in NBS.
FM= Full Mutation
PM= Premutation
* p<0.001 between FM and PM identification for both girls and boys
Regarding reasons to perform NBS, DBP deemed preparing for disability and access to treatment for an affected infant as most important. (Figure 2). Yet, 84% and 75% of DBP also endorsed reproductive planning and utility for research, respectively, as at least moderately important reasons to perform FMR1 NBS.
Figure 2. Reasons for Newborn Screening.
Treatment = To allow for treatment and intervention
Prepare= To help family prepare for disability
Reproductive Choice= To provide parents with reproductive decision making information
Research = To facilitate research
Approximately 50% of DBP would agree to test a sibling at any age independent of the child’s gender. (Table 3) DBP were less supportive of testing only in adolescence. DBP were more likely to test a typically developing female sibling of a boy with FXS compared to a male sibling (p = <0.01). There were no demographic differences between those that supported and those that did not support testing the sibling.
Table 3.
Would you test the typically developing sibling of an 8-year-old with Fragile X?
| Yes, at any Age n (%) | Yes, only in adolescence n (%) | No n (%) | |
|---|---|---|---|
| Male sibling (n=274)*# | 132 (48) | 78 (28) | 64 (23) |
| Female sibling (n=276)*# | 148 (54) | 93 (34) | 35 (13) |
percentages do not add up to 100% due to rounding
p<0.01 between testing a male and female sibling
In addition to being asked if they would test a typically developing 8-year-old brother or sister of an individual with FXS, DBP were asked why they would or would not perform this testing. One hundred and fifty-seven DBP provided optional comments, mainly from those who supported testing. Although 20% mentioned they would defer the decision to a genetic health professional (physician geneticist or genetic counselor) irrespective of the sibling’s gender, the most popular explanations of why to test the brother were for reproductive information (20%), carrier testing (18%), and because of clinical variability (15%). The most popular reasons to test the sister were for carrier testing (32%) and reproductive information (31%).
Discussion
In 1968, Wilson and Junger published criteria about which conditions are appropriate for population screening (Wilson & Junger, 1968). In brief, the criteria state early diagnosis of a condition appropriate for screening should directly benefit the child being tested by reducing morbidity and mortality. Based on these strict criteria, FXS would not be a suitable condition for NBS because there is no cure and the information gained from testing that can help parents make reproductive decisions about future pregnancies does not directly benefit the newborn. When the American College of Medical Genetics created a uniform NBS panel in 2005, a broader definition of “benefit” was used to judge conditions. Benefit beyond the individual extending to the family, and society was not valued. Despite the expanded notion of “benefit,” FXS was not recommended for inclusion primarily because a cost-effective population screening test did not exist at that time. (Botkin, 2011; Ross & Acharya, 2008)
Our data support the growing interest in screening for conditions that do not meet traditional criteria (Bailey Jr., Skinner, Davis, Whitmarsh, & Powell, 2008). Although FrX Syndrome has not been recommended for inclusion in the uniform NBS panel, DBP agree there are valid reasons to screen newborns for FMR1 mutations.. Along with access to treatment/intervention, DBP cite helping families prepare for raising a child with disability as the most important reason to screen newborns for FXS. The majority of DBP also support research and reproductive information as important reasons to conduct FMR1 NBS. DBP prefer a FMR1 NBS program that is universally offered to parents but requires consent. For DBP, an ideal FMR1 NBS program should screen both genders and identify both premutations and full mutations. This is consistent with the preferences of other health care providers (Acharya & Ross, 2009; Kemper & Bailey Jr., 2009).
In order to conduct FMR1 population screening of newborns ethically, pre- and post-test counseling for families will be essential. Counseling about the significance of a positive or negative FrX test result should be occurring at minimum at two points in a doctor-patient interaction: 1) before the test is ordered as a part of pre-test education and the informed consent process and 2) after the test is performed to review the results (National Society of Genetic Counselors, 1997). In accordance with professional guidelines (Filipek et al., 2000; Moeschler et al., 2006; Myers et al., 2007; Sandler et al., 2001; Shevell et al., 2003), the majority of DBP are ordering FMR1 testing on their pediatric patients as part of the diagnostic work-up for unexplained developmental delay, ID and autism. Yet, approximately 40% of DBP do not have the knowledge to adequately counsel patients and families about the potential clinical consequences of having a FMR1 premutation.
The fact that many DBP are unaware of all the clinical implications of having a premutation raises doubts about the adequacy of the informed consent for FMR1 testing in childhood as well as the quality of pre- and post-test counseling. In the case of childhood developmental delays, some parents, if informed of the potential risks of premutation status, may choose to not pursue FMR1 testing if the results would identify health conditions in other family members especially if the diagnosis would have no impact on medical management. If individuals were erroneously told that having a premutation had no future personal implications, they might be upset to learn during a work-up for infertility that their premutation placed them at higher risk. DBP knowledge about FXAD is better than knowledge of general pediatricians (Kemper & Bailey Jr., 2009), but worse than that of genetic health professionals (Acharya & Ross, 2009). It will be increasingly important that DBP have this knowledge about FMR1 premutations if FMR1 NBS is implemented because they, along with geneticists, will be called on to counsel families about newborns with FMR1 full mutations and premutations. If FMR1 NBS is implemented, professional education of subspecialists as well as primary care physicians will be a crucial component (Kemper & Bailey Jr., 2009).
Approximately 50% of DBP would agree to carrier test an asymptomatic sibling of a child with FXS at any age and an additional third supported reserving testing for adolescents. Many DBP who supported carrier testing also spontaneously stated that they would refer parents who request sibling carrier testing to a genetic health professional who is more familiar with the FMR1 premutation associated conditions and could thus provide more comprehensive pre- and post-test counseling. However, it is unclear how feasible this would be because of the growing shortage of genetic counseling resources (Korf, Feldman, & Wiesner, 2005). And yet to only refer parents to genetic counseling after a positive screen would be inadequate because it means that families were tested without fully understanding to what they were agreeing.
Consistent with the views of DBP, multiple stakeholder groups support carrier testing for FMR1 before adulthood. McConkie-Rosell has demonstrated that parents and adolescents of children with FXS also want to know the adolescent’s FMR1 status earlier in childhood (McCann et al., 2009; McConkie-Rosell, Del Giorno, & Heise, 2011; McConkie-Rosell, Heise, & Spiridigliozzi, 2009). Knowing one’s status earlier allows more time for and possible smoother psychosocial adaptation. Moreover, 50% of those tested will be reassured they are not carriers. In the setting of a child with FXS, Rosen et al found that 56% of pediatric residents would agree to carrier test asymptomatic sisters in childhood and 68% would order testing on asymptomatic adolescents (Rosen, Wallenstein, & McGovern, 2002). However, only 25% of U.S. genetic health professionals would agree to order FMR1 carrier testing of asymptomatic siblings (Acharya & Ross, 2009) which is similar to a study in Europe which found that 20% of physician geneticists in Europe report acquiescing to parental requests to perform carrier testing of their children (Borry, Stultiens, Goffin, Nys, & Dierickx, 2008).
Current FMR1 NBS pilot programs have been designed to identify both full mutation and premutations in infants, although there are methods to restrict screening to full mutations only (Strom et al., 2007). The design of these pilot programs aligns with the views of parents of children with FXS and some professionals; however, they are contrary to professional guidelines which proscribe carrier testing in childhood for adult-onset conditions (Clarke et al., 1994; Dalby, 1995; Hogben & Boddington, 2005; Nelson et al., 2001; Sherman et al., 2005). The guidelines argue there is no harm in waiting because no medical intervention is needed in childhood. Additionally, the guidelines advocate against this type of testing because testing in childhood undermines the child’s future ability as an adult to make an autonomous and informed decision about whether to be tested and infringes upon the child’s future confidentiality.
As long as there is no treatment for adult-onset premutation associated conditions, the guidelines support deferring testing until the child is an adult who can then decide for him or herself. Screening for premutations in childhood could be justified if there were a well-defined childhood phenotype associated with FMR1 premutation that required diagnosis to implement specific treatment. To-date there is controversy about whether a premutation phenotype exists and there is no specific therapy other than that which would be recommended to any child with behavioral impairments (Seltzer et al, 2012).
Newer screening technology that only identifies those with FMR1 full mutation is being developed for NBS. Not based on DNA PCR, this technology quantifies the level of FMR1 protein, which is absent in individuals with FXS (Lessard, Chouiali, Drouin, Sébire, & Corbin, 2012). Using this technique in NBS, only newborns with full mutations would be identified, which would obviate the controversies surrounding identifying newborns with asymptomatic FMR1 premutations.
In order to understand the true individual level risks and benefits of identifying premutation alleles in children including newborns, further research is necessary. Seltzer et. al (2012) conducted the first study of population prevalence for FMR1 premutations in the US using a cross-sectional sample of men and women, and found the premuation rate to be 1/468 males and 1/151 females. An earlier study by Hantash et al (2011) found a similar population prevalence of 1/178 among a sample of women being tested for cystic fibrosis. In addition to replicating this data, we need to know the neurocognitive profile of and the level of supports required by individuals with FMR1 premutations picked up incidentally through screening. If the FMR1 premutation rate is high in the general population and individuals who have screened positive do not require significant support, then labeling an asymptomatic newborn with a genetic diagnosis which is unlikely to change prognosis could be stigmatizing and be more harmful than currently appreciated. This information would have to be weighed against the benefits of early identification of the childhood-onset premutation phenotype, which is not yet well-characterized.
Our survey had several limitations. We had a 61% response rate which is consistent with published physician survey of this type (Cull, O’Connor, Sharp, & Tang, 2005; Joffe, Anton, & Decaen, 2008; McMahon et al., 2003); however, our findings may not represent the perspectives of DBP who did not respond, those not on the SDBP mailing list and non-members. Our data reflect the current attitude of DBP, but as we have shown, DBP are not fully aware of what is means to have a FMR1 premutation. Therefore, it is unclear how the attitudes of DBP might change after they become aware of the clinical implications and potential risks of having a FMR1 premutation, Additionally, the preference of DBP for carrier testing of female siblings could be explained in part by the added value of providing reproductive information to girls. However, we did not include questions to determine awareness of DBP regarding the childhood premutation phenotype. Whether DBP were aware of the fact could have influenced their responses. Future studies need to examine how knowledge about gender differences in clinical presentation affects opinions and practices about FMR1 testing.
DBP play an important role in the diagnosis and care of children with FXS which will only become greater if FXS is incorporated into state NBS panels. DBP are familiar with FXS, but are not familiar with the adult-onset premutation conditions, FXPOI and FXTAS. DBP support voluntary NBS and FMR1 premutation testing in siblings, despite the lack of knowledge of the spectrum of FXAD. Targeted educational interventions are needed to ensure that all health care providers, who will be confronted with these situations, have the knowledge and competence to consent and to counsel families on all of the FXAD.
Footnotes
References
- Acharya K, Ross LF. Fragile X screening: Attitudes of genetic health professionals. American Journal of Medical Genetics, Part A. 2009;149(4):626–632. doi: 10.1002/ajmg.a.32725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DB, Jr, Skinner D, Davis AM, Whitmarsh I, Powell C. Ethical, legal, and social concerns about expanded newborn screening: Fragile X syndrome as a prototype for emerging issues. Pediatrics. 2008;121(3):e693–e704. doi: 10.1542/peds.2007-0820. [DOI] [PubMed] [Google Scholar]
- Borry P, Stultiens L, Goffin T, Nys H, Dierickx K. Minors and informed consent in carrier testing: A survey of european clinical geneticists. Journal of Medical Ethics. 2008;34(5):370–374. doi: 10.1136/jme.2007.021717. [DOI] [PubMed] [Google Scholar]
- Botkin JR. Newborn screening for fragile X syndrome: Do we care what parents think? Pediatrics. 2011;127(6):e1593–e1594. doi: 10.1542/peds.2011-0677. [DOI] [PubMed] [Google Scholar]
- Brice P. US centres to pilot controversial newborn screening for fragile X syndrome. 2008 Retrieved September 12, 2011, from http://www.phgfoundation.org/news/4342/
- Clarke A, Fielding D, Kerzin-Storrar L, Middleton-Price H, Montgomery J, Payne H, Tyler A. The genetic testing of children. Journal of Medical Genetics. 1994;31(10):785–797. doi: 10.1136/jmg.31.10.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronister A, DiMaio M, Mahoney MJ, Donnenfeld AE, Hallam S. Fragile X syndrome carrier screening in the prenatal genetic counseling setting. Genetics in Medicine. 2005;7(4):246–250. doi: 10.1097/01.gim.0000159898.90221.d3. [DOI] [PubMed] [Google Scholar]
- Cull WL, O’Connor KG, Sharp S, Tang S-S. Response rates and response bias for 50 surveys of pediatricians. Health Services Research. 2005;40(1):213–226. doi: 10.1111/j.1475-6773.2005.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby S. GIG response to the UK clinical genetics society report ‘the genetic testing of children’ [1] Journal of Medical Genetics. 1995;32(6):490–491. doi: 10.1136/jmg.32.6.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipek PA, Accardo PJ, Ashwal S, Baranek GT, Cook EH, Jr, Dawson G, Volkmar FR. Practice parameter: Screening and diagnosis of autism. report of the quality standards subcommittee of the american academy of neurology and the child neurology society. Neurology. 2000;55(4):468–479. doi: 10.1212/wnl.55.4.468. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Tranfaglia M. Advances in the treatment of fragile x syndrome. Pediatrics. 2009;123(1):378–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman PJ, Hagerman RJ. The fragile-X premutation: A maturing perspective. American Journal of Human Genetics. 2004;74(5):805–816. doi: 10.1086/386296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantash MS, Goos DM, Crossley B, Anderson B, Zhang K, Sun W, Strom CM. FMR1 premutation carrier frequency in patients undergoing routine population-based carrier screening: Insights into the prevalence of fragile X syndrome, fragile X-associated tremor/ataxia syndrome, and fragile X-associated primary ovarian insufficiency in the United States. Genetics in Medicine. 2011;13(1):39–45. doi: 10.1097/GIM.0b013e3181fa9fad. [DOI] [PubMed] [Google Scholar]
- Hogben S, Boddington P. Policy recommendations for carrier testing and predictive testing in childhood: A distinction that makes a real difference. Journal of Genetic Counseling. 2005;14(4):271–281. doi: 10.1007/s10897-005-4840-x. [DOI] [PubMed] [Google Scholar]
- Hunter JE, Leslie M, Novak G, Hamilton D, Shubeck L, Charen K, Sherman SL. Depression and anxiety symptoms among women who carry the FMR1 premutation: Impact of raising a child with fragile X syndrome is moderated by CRHR1 polymorphisms. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics. 2012;159 B(5):549–559. doi: 10.1002/ajmg.b.32061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe AR, Anton NR, Decaen AR. Survey of pediatricians’ opinions on donation after cardiac death: Are the donors dead? Pediatrics. 2008;122(5):e967–e974. doi: 10.1542/peds.2008-1210. [DOI] [PubMed] [Google Scholar]
- Kemper AR, Bailey DB., Jr Pediatricians’ knowledge of and attitudes toward fragile X syndrome screening. Academic Pediatrics. 2009;9(2):114–117. doi: 10.1016/j.acap.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Korf BR, Feldman G, Wiesner GL. Report of banbury summit meeting on training of physicians in medical genetics, october 20–22, 2004. Genetics in Medicine. 2005;7(6):433–438. doi: 10.1097/01.gim.0000171324.58121.cf. [DOI] [PubMed] [Google Scholar]
- Lessard M, Chouiali A, Drouin R, Sébire G, Corbin F. Quantitative measurement of FMRP in blood platelets as a new screening test for fragile X syndrome. Clinical Genetics. 2012;82(5):472–477. doi: 10.1111/j.1399-0004.2011.01798.x. [DOI] [PubMed] [Google Scholar]
- McCann S, MacAuley D, Barnett Y, Bunting B, Bradley A, Jeffers L, Morrison PJ. Family communication, genetic testing and colonoscopy screening in hereditary non-polyposis colon cancer: A qualitative study. Psycho-Oncology. 2009;18(11):1208–1215. doi: 10.1002/pon.1487. [DOI] [PubMed] [Google Scholar]
- McConkie-Rosell A, Del Giorno J, Heise EM. Communication of genetic risk information to daughters in families with fragile X syndrome: The parent’s perspective. Journal of Genetic Counseling. 2011;20(1):58–69. doi: 10.1007/s10897-010-9326-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkie-Rosell A, Heise EM, Spiridigliozzi GA. Genetic risk communication: Experiences of adolescent girls and young women from families with fragile X syndrome. Journal of Genetic Counseling. 2009;18(4):313–325. doi: 10.1007/s10897-009-9215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SR, Iwamoto M, Massoudi MS, Yusuf HR, Stevenson JM, David F, Pickering LK. Comparison of e-mail, fax, and postal surveys of pediatricians. Pediatrics. 2003;111(4 Pt 1):e299–303. doi: 10.1542/peds.111.4.e299. [DOI] [PubMed] [Google Scholar]
- Moeschler JB, Shevell M, Schaefer GB, Bull MJ, Enns GM, Gruen JR, Spire P. Clinical genetic evaluation of the child with mental retardation or developmental delays. Pediatrics. 2006;117(6):2304–2316. doi: 10.1542/peds.2006-1006. [DOI] [PubMed] [Google Scholar]
- Myers SM, Johnson CP, Lipkin PH, Cartwright JD, Desch LW, Duby JC, Yeargin-Allsopp M. Management of children with autism spectrum disorders. Pediatrics. 2007;120(5):1162–1182. doi: 10.1542/peds.2007-2362. [DOI] [PubMed] [Google Scholar]
- National Society of Genetic Counselors. Position statement: Genetic testing for adult-onset disorders. Chicago, IL: National Society of Genetic Counselors; 1997. [Google Scholar]
- Nelson RM, Botkin JR, Kodish ED, Levetown M, Truman JT, Wilfond BS, Steinberg D. Ethical issues with genetic testing in pediatrics. Pediatrics. 2001;107(6):1451–1455. doi: 10.1542/peds.107.6.1451. [DOI] [PubMed] [Google Scholar]
- Rosen A, Wallenstein S, McGovern MM. Attitudes of pediatric residents toward ethical issues associated with genetic testing in children. Pediatrics. 2002;110(2 I):360–363. doi: 10.1542/peds.110.2.360. [DOI] [PubMed] [Google Scholar]
- Ross LF, Acharya K. Policy considerations in designing a fragile X population screening program. Genetics in Medicine. 2008;10(10):711–713. doi: 10.1097/GIM.0b013e3181889457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler AD, Brazdziunas D, Cooley WC, González de Pijem L, Hirsch D, Kastner TA, Ruppert ES. The pediatrician’s role in the diagnosis and management of autistic spectrum disorder in children. Pediatrics. 2001;107(5):1221–1226. [Google Scholar]
- Saul RA, Friez M, Eaves K, Stapleton GA, Collins JS, Schwartz CE, Stevenson RE. Fragile X syndrome detection in newborns - pilot study. Genetics in Medicine. 2008;10(10):714–719. doi: 10.1097/GIM.0b013e3181862a76. [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Baker MW, Hong J, Maenner M, Greenberg J, Mandel D. Prevalence of CGG expansions of the FMR1 gene in a US population-based sample. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics. 2012;159 B(5):589–597. doi: 10.1002/ajmg.b.32065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S, Pletcher BA, Driscoll DA. Fragile X syndrome: Diagnostic and carrier testing. Genetics in Medicine. 2005;7(8):584–587. doi: 10.1097/01.GIM.0000182468.22666.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevell M, Ashwal S, Donley D, Flint J, Gingold M, Hirtz D, Sheth RD. Practice parameter: Evaluation of the child with global developmental delay: Report of the quality standards subcommittee of the american academy of neurology and the practice committee of the child neurology society. Neurology. 2003;60(3):367–380. doi: 10.1212/01.wnl.0000031431.81555.16. [DOI] [PubMed] [Google Scholar]
- Skinner D, Choudhury S, Sideris J, Guarda S, Buansi A, Roche M, Bailey DB., Jr Parents’ decisions to screen newborns for FMR1 gene expansions in a pilot research project. Pediatrics. 2011;127(6):e1455–e1463. doi: 10.1542/peds.2010-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A. Screening for fragile X syndrome: A literature review and modelling study. Health Technology Assessment (Winchester, England) 2003;7(16):1–106. doi: 10.3310/hta7160. [DOI] [PubMed] [Google Scholar]
- Strom CM, Huang D, Li Y, Hantash FM, Rooke J, Potts SJ, Sun W. Development of a novel, accurate, automated, rapid, high-throughput technique suitable for population-based carrier screening for fragile X syndrome. Genetics in Medicine. 2007;9(4):199–207. doi: 10.1097/gim.0b013e31803d3ac9. [DOI] [PubMed] [Google Scholar]
- Tassone F, Greco CM, Hunsaker MR, Seritan AL, Berman RF, Gane LW, Hagerman RJ. Neuropathological, clinical and molecular pathology in female fragile X premutation carriers with and without FXTAS. Genes, Brain and Behavior. 2012;11(5):577–585. doi: 10.1111/j.1601-183X.2012.00779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]