Abstract
Borderline personality disorder (BPD) and substance use disorders often co-occur. Both disorders are heritable and family studies showed that there are familial factors that increase the risk for BPD as well as substance use/abuse. This is the first study that investigates whether the association of borderline personality traits (BPT) with substance use reflects an underlying genetic vulnerability or nongenetic familial influences. To this end we analyzed data of 5,638 Dutch and Belgian twins aged between 21–50 years from 3,567 families. Significant associations between BPT and high alcohol consumption (r = .192), regular smoking (r = .299), and ever use of cannabis (r = .254) were found. Bivariate genetic analyses showed that the associations of BPT and substance use had different etiologies. For regular smoking and for ever use of cannabis, the correlation with BPT was explained by common genetic factors. Interestingly, for high alcohol consumption and BPT the association was explained by unique environmental factors that influence both traits rather than common genetic factors.
The presentation of borderline personality disorder (BPD) with a comorbid substance use disorder (SUD) is common in clinical and population samples (Feske, Tarter, Kirisci, & Pilkonis, 2006; Tomko, Trull, Wood, & Sher, in press; Trull, Sher, Minks-Brown, Durbin, & Burr, 2000; Trull, Jahng, Tomko, Wood, & Sher, 2010). Also, higher rates of alcohol and drug abuse and dependence and a higher rate of new onsets of SUD are found in patients with BPD than in patients with other personality disorders (McGlashan et al., 2000; Walter et al., 2009; Zanarini et al., 2011). In a comprehensive review, Trull et al. (2000) report that almost 60% of the individuals diagnosed with BPD meets criteria for a general category of substance use disorder, almost 50% meets criteria for an alcohol use disorder (abuse or dependence) and almost 40% meets criteria for a drug use disorder (abuse or dependence). The co-occurrence of BPD and SUD is associated with an increased risk for suicide attempts, poorer school performance, a higher level of unemployment, more promiscuity, and more early termination from treatment (Karterud, Arefjord, Andresen, & Pedersen, 2009; Miller, Abrams, Dulit, & Fyer, 1993; van den Bosch, Verheul, & van den Brink, 2001).
It is well established that genetic factors contribute to the liability for substance use (Agrawal & Lynskey, 2008). Heritability estimates between 36% and 72% were reported for several measures of alcohol use (Grant et al., 2009; Kendler, Myers, Dick, & Pescott, 2010; Sartor et al., 2010; Whitfield et al., 2004). Li, Cheng, Ma, and Swan (2003) conducted a meta-analysis for tobacco use and report a heritability estimate of 59%. A meta-analysis for cannabis initiation estimated the heritability to be 48% for males and 40% for females although the confidence intervals around these estimates for males and females overlapped (Verweij et al., 2010).
For BPD and related traits, large scale twin and twin family studies report heritability estimates around 40% for BPD and borderline personality traits (BPT; Bornovalova, Hicks, Iacono, & Mcgue, 2009; Distel et al., 2008; Distel et al., 2009; Kendler et al., 2008; Torgersen et al., 2008) and no evidence of environmental influences that are shared by family members.
First degree relatives of individuals with BPD have a significantly higher lifetime prevalence of substance use disorders (alcohol abuse/dependence, drug abuse/dependence, and any substance use disorder) than first degree relatives of individuals with other personality disorders such as histrionic, narcissistic, sadistic, and antisocial personality disorders (Zanarini, Barison, Frankenburg, Reich, & Hudson, 2009). This suggests that there are familial factors that increase the risk for BPD as well as substance use/abuse. However, family studies do not permit conclusions regarding the nature of these familial factors; the association of BPD with substance use and abuse may reflect an underlying genetic vulnerability to both disorders or may reflect environmental familial influences. Twin studies can distinguish genetic from environmental familial influences. By measuring BPT and substance use in monozygotic and dizygotic twin pairs, it can be investigated whether BPT in one twin is predictive for substance use in the co-twin. Furthermore, it can be tested whether this cross-trait resemblance is stronger in monozygotic twin pairs than in dizygotic twin pairs. In this this case, common genetic risk factors (partly) explain the association between BPT and substance use. We conducted such a twin study and examined whether common genetic risk factors can explain the co-occurrence of BPT and high alcohol consumption, regular smoking, and ever use of cannabis.
METHODS
SAMPLE
The data for this study were collected by the Netherlands Twin Register (Boomsma et al., 2006) and the East Flanders Prospective Twin Survey (EFPTS; Derom & Derom, 2005). In the Netherlands, every two to three years from 1991 onwards, twin families were invited to complete questionnaires on physical and mental health, personality, and lifestyle. The data for this study are from the seventh and eight surveys carried out 2004/2005 and 2009/2010 (ongoing). Dutch-speaking Belgian twins only participated in 2004/2005. The data collection procedure is explained in more detail elsewhere (Distel et al., 2007). For individuals who completed the survey twice, data from the most recent survey in which both twins took part were included. When there was no survey in which both twins participated we selected the most recent data for an individual. The total sample for analysis consisted of 5,638 twins from 3,567 families. There were 2,875 monozygotic twins (MZ; 1,177 complete twin pairs) and 2,763 dizygotic twins (DZ; 894 complete twin pairs). All twins were between 21 and 50 years old (mean age = 32.1, SD = 6.8). Same-sex twin pairs’ zygosity was determined by DNA polymorphisms (n = 2,697) or questionnaire items from self or parental reports (n = 1,687).
MEASURES
Borderline personality traits (BPT) were assessed with the Personality Assessment Inventory-Borderline Features Scale (PAI-BOR; Morey, 1991, 2003), a 24 item questionnaire designed to assess features of severe personality pathology that are clinically associated with BPD. The items have a four-point scale (0–3; false, slightly true, mainly true and very true), and were scored according to Morey’s test manual. Data on BPT were available for 5,548 individuals.
Current high alcohol consumption was assessed by the following question in 2004/2005: “How many glasses of alcohol a week do you drink on average (including the weekend)?” with seven response categories: less than 1 glass, 1–5 glasses a week, 6–10 glasses a week, 11–15 glasses a week, 16–20 glasses a week, 21–40 glasses a week, and more than 40 glasses a week. In 2009/2010 the survey included the question “How many glasses of beer, wine and/or spirits (e.g., liquor or mixed drinks) did you drink per day in a regular week?” Participants rated for each day of the week how many glasses of the three sorts of alcoholic drinks they consumed. The number of glasses on each day was summed to calculate the average number of drinks per week. Following the definition of regular drinking of the Netherlands Mental Health Survey and Incidence Study and the Dutch National Health Compass (Verdurmen, Monshouwer, & van Dorsselaer, 2003), high alcohol consumption is defined as drinking more than 14 glasses of alcoholic drinks per week for women and more than 21 glasses for men. Based on these cutoff points the number of alcoholic drinks was recoded into a dichotomous no high alcohol consumption (0) vs. high alcohol consumption (1) variable. Data on current regular alcohol use were available for 5,446 individuals (69% females).
In both surveys, current regular smoking was assessed with the question “How often do you smoke now?” The response categories in the 2004/2005 survey were: (1) I’ve quit smoking and haven’t smoked since (date), (2) I smoke once a week or less, (3) I smoke several times a week, not every day, (4) I smoke once or several times a day. In the 2009/2010 survey, response categories were: (0) I’ve never been a regular smoker, (1) I used to smoke, but I’ve quit, (2) I smoke once a week or less, (3) I smoke several times a week, not every day, (4) I smoke daily. Categories 1–2 were recoded into currently not regular smoking (0) and categories 3 and 4 into currently regular smoking (1). Data on current regular smoking were available for 5,418 individuals (70% females).
Information on cannabis use came from the 2009/2010 survey in which participants were asked whether they ever experimented with cannabis. The response categories yes and no were coded as 0, when a subject never used cannabis; and 1, when a subject had ever used cannabis. Data on ever use of cannabis were available for 2,983 individuals (71% females).
STATISTICAL ANALYSES
DESCRIPTIVE STATISTICS
Because the BPT data showed a somewhat skewed distribution a square root data transformation was applied. For each substance use variable, a multiple regression analysis was carried out in STATA to test for differences in mean BPT scores between subjects who do (score = 1) and do not (score = 0) use the substance. Sex and age were included as covariates in the regression analyses. STATA’s robust cluster option was used to account for the nonindependence of observations of family members.
GENETIC MODELING
In the classical twin design, liability to a phenotype is considered as a function of additive genetic factors (A), common environmental factors (C) shared by family members, and unique environmental factors (E). Based on a design that includes MZ and DZ twins the influence of A, C, and E can be estimated. The contribution of A, C, and E to variance in a trait is estimated from the data collected in MZ twins, who share (nearly) all their genes, and DZ twins, who share on average half of their segregating genes. When the correlation in DZ twin pairs for a trait is more than half the MZ correlation, there is evidence for C. When the correlation in DZ twins is about half the MZ correlation, there is evidence for A only. The contribution of A, C, and E to the covariance between two or more traits can be estimated by comparing the cross-twin cross-trait-correlation (CCR) of MZ twins to that of DZ twins. If the DZ CCR is about half that of the MZ twins, the influence of A on the covariance is indicated. A DZ CCR larger than half that of MZ twins’ correlation suggests the influence of C on the covariance. An MZ CCR which is less than the within-individual phenotypic correlation between the traits suggests that nonshared environmental factors influence the co-occurrence of two traits.
The influence of genetic and nongenetic factors was estimated with MPlus version 5 (Muthén & Muthén, 2005) which allows for modeling a combination of categorical and continuously scored data (Prescott, 2004). The substance use variables were dichotomous. For these variables underlying liabilities (or vulnerabilities) were assumed, which are normally distributed with unit variance and zero mean. Resemblance between relatives for this underlying liability distribution can be assessed with tetrachoric correlations. A threshold divides the sample into unaffected and affected individuals. The proportion of the distribution above the threshold reflects the prevalence. Thresholds were estimated separately for men and women. The BPT variable was treated as a continuous variable. Resemblance between relatives for BPT can be summarized with Pearson product-moment correlations. The resemblance within and between relatives across BPT and the liability for substance use variables was also summarized with Pearson product-moment correlations. Since earlier analyses showed that women have higher BPT scores than men (Distel et al., 2008), scores were adjusted for sex before the genetic analyses using linear regression.
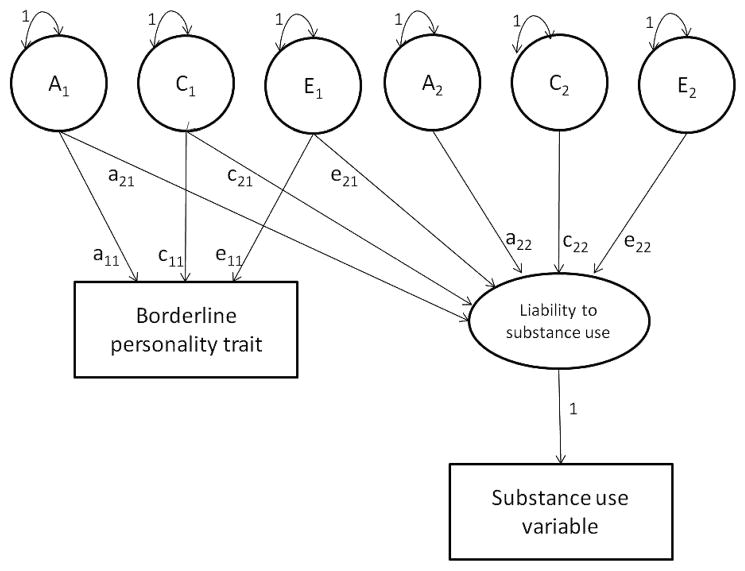
Figure 1 shows the bivariate genetic model. In this model, the BPT variable is influenced by three latent factors (A1, C1, and E1), while the liability to the substance use is influenced by latent variables A1, C1, and E1, which are shared with BPT, and by latent variables A2, C2, and E2, which influence substance use only. This way, the total variance in BPT can be written as (a211) + (c211) + (e211). The broad-sense heritability of borderline personality traits can be calculated as (a211)/[(a211) + (c211) + (e211)]. Total variance in substance use can be written as (a221) + (a222) + (c221) + (c222) + (e221) + (e222). The broad-sense heritability of substance use may be written as (a221) + (a222)/(a221) + (a222) + (c221) + (c222) + (e221) + (e222). In the full model, the total covariance between BPT and substance use may be written as (a11 × a21) + (c11 × c21) + (e11 × e21).
FIGURE 1.
Graphical representation of the full bivariate genetic model. Latent factors are symbolized in circles, observed phenotype as squares. A1 and A2 = latent additive genetic factors, C1 and C2 = latent shared environmental factors, E1 and E2 = latent unique environmental factors, a = factor loading of A, c = factor loading of C, e = factor loading of E. Latent factors can be correlated within families and influence BPT and the liability to substance use.
RESULTS
PREVALENCE OF SUBSTANCE USE
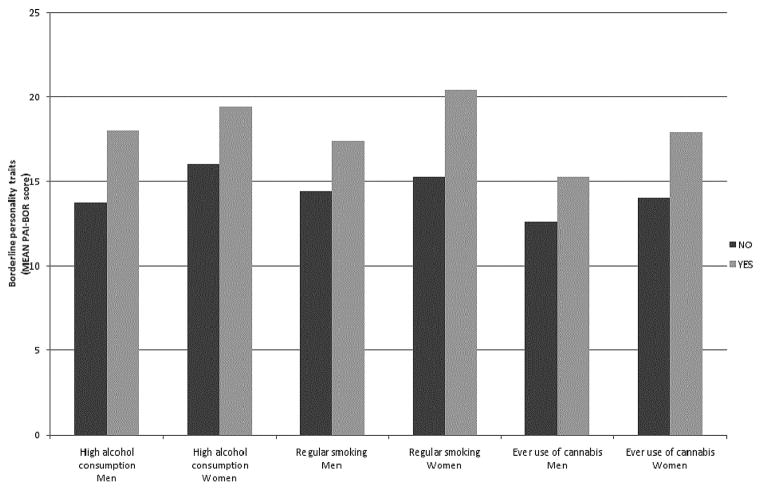
The prevalence rates of high alcohol consumption, regular smoking, and ever use of cannabis, are given in Table 1, separately for men and women. For each substance the prevalence rate is higher for men than for women. Figure 2 shows the mean BPT scores for men and women who do and do not use these substances. Regression analyses showed that mean BPT scores were significantly higher for those who have a high alcohol consumption level, F(1, 3500) = 46.75, p < .001, regularly smoke, F(1, 3468) = 223.59, p < .001, and ever used cannabis, F(1, 2018) = 90.78, p < .001, than for those who do not use these substances.
TABLE 1.
Prevalence Rates for High Alcohol Consumption, Regular Smoking, And Ever Use of Cannabis In Men And Women
| Men
|
Women
|
||||
|---|---|---|---|---|---|
| 0 | 1 | 0 | 1 | ||
| High alcohol consumption | N (%) | 1541 (91.7%) | 140 (8.3%) | 3607 (95.8%) | 158 (4.2%) |
| Regular smoking | N (%) | 1326 (80.3%) | 326 (19.7%) | 3135 (83.2%) | 631 (16.8%) |
| Ever use of cannabis | N (%) | 518 (59.4%) | 354 (40.6%) | 1510 (71.5%) | 601 (28.5%) |
FIGURE 2.
Mean borderline personality trait scores for men and women who do and do not fall in the high alcohol consumption, regular smoking, and ever use of cannabis category.
A total of 47.3% of the sample did not score positive on any substance use variable. A total of 46.6%, 5.7%, and 0.4% of the sample scored positive on one, two, or all of the substance use variables, respectively. Having more BPT symptoms was significantly associated with a higher number of different substances used, F(1,3534) = 173.2, p < .001.
The correlations between BPT and the substance use variables were .192 (95% CI .116–.269) for high alcohol consumption, .299 (95% CI .250–.348) for regular smoking, and .254 (95% CI .195–.313) for ever use of cannabis.
GENETIC ANALYSES
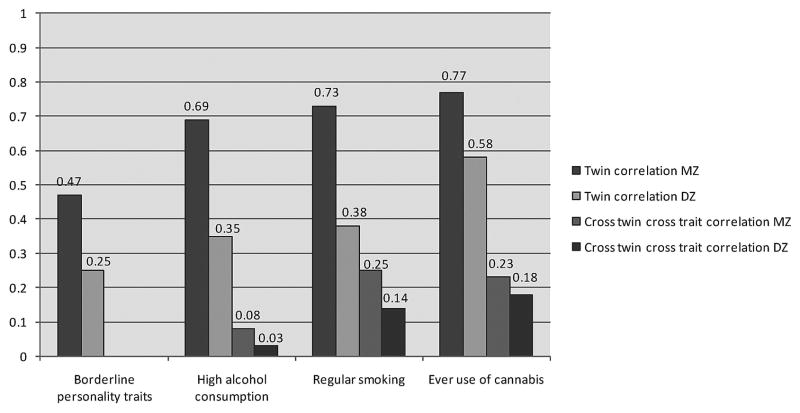
Figure 3 presents the MZ and DZ twin correlations for BPT and the three substance use variables as well as the cross-twin cross-trait correlations between BPT and each substance use variable. For BPT, high alcohol consumption, and regular smoking, the DZ twin correlation is about half the MZ twin correlation indicating the influence of A and no influence of C. For ever use of cannabis the DZ correlation was more than half of the MZ correlation indicating the influence of A and C.
FIGURE 3.
Monozygotic (MZ) and dizygotic (DZ) twin correlations and cross-twin cross-trait correlations.
The estimates of A, C, and E from the full bivariate genetic models are shown in the top part of Table 2. The shared environmental factor did not significantly explain variance in BPT and variance in the liability for high alcohol consumption and regular smoking. The shared environmental factor did explain variance in the liability for ever use of cannabis. The bottom part of Table 2 shows the estimates of A, C, and E and the genetic and environmental correlations from the models in which the nonsignificant C parameter was constrained to zero. The variance in BPT and the liability to high alcohol consumption and regular smoking could be explained by A (47%, 76%, and 85%, respectively) and E (52%, 24%, and 15%, respectively). The variance in the liability for ever use of cannabis could be explained by A (51%), C (38%), and E (11%). The genetic risk factors for the liability to BPT and regular smoking and to BPT and ever use of cannabis were significantly correlated, Genetic correlation may be written as a11 × a21/√(a211) × √(a221 + a222). while the unique environmental influences were uncorrelated, Unique environmental correlation may be written as e11 × e21/√(e211) × √(e221 + e222). The phenotypic correlation between BPT and these two substance use variables can thus entirely be explained by shared genetic risk factors. The genetic correlation between BPT and high alcohol consumption was not significant. The phenotypic correlation between BPT and the liability for high alcohol consumption was explained by unique environmental factors that are shared between the traits.
TABLE 2.
Proportion of Variance in Borderline Personality Traits and Variance in the Liability for High Alcohol Consumption, Regular Smoking, and Ever Use of Cannabis Accounted For By A, C, and E from the Full and Constrained Models, and the Genetic and Unique Environmental Correlation Between Borderline Personality Traits and the Three Substance Use Variables from the Constrained Model (95% Confidence Intervals)
| Borderline personality traits | High alcohol consumption | Regular smoking | Ever use of cannabis | |
|---|---|---|---|---|
| Full model | ||||
| A | .444 (.292–.595) | .758 (.579–.937) | .811 (.593–1.029) | .466 (.136–.796) |
| C | .031 (−.103–.164) | .012 (−.195–.219) | .046 (−.085–.178) | .383 (.127–.639) |
| E | .526 (.484–.567) | .314 (.070–.391) | .164 (.086–.242) | .151 (.003–.299) |
| Constrained model | ||||
| A | .479 (.439–.520) | .758 (.619–.897) | .851 (.760–.942) | .513 (.226–.800) |
| C | — | — | — | .377 (.121–.632) |
| E | .521 (.480–.561) | .242 (.103–.381) | .149 (.080–.218) | .110 (.035–.186) |
| Genetic correlation | — | .132 (−.014–.278) | .400 (.308–.492) | .513 (.312–.715) |
| Unique environmental correlation | — | .317 (.117–.516) | .157 (−.027–.340) | −.002 (−.250–.245) |
Note. A = additive genetic factor; C = shared environmental factor; E = unique environmental factor. Significant parameter estimates are printed in bold.
DISCUSSION
The present study is the first to investigate the genetic etiology of the co-occurrence of BPT and high alcohol consumption, regular smoking, and ever use of cannabis. Data on BPT and substance use from 5,638 twins aged between 21 and 50 years were analyzed.
Consistent with previous research, men more frequently fell into the high alcohol consumptiuon category, smoked more often, and had more often ever used cannabis than women (Hasin, Stinson, Ogburn, & Grant, 2007; Nolen-Hoeksema, 2004). Heritability estimates for the substance use variables ranged from 51% to 85%. In line with previous studies we found shared envrionmental influences for ever use of cannabis but not for high alcohol consumption or regular smoking (Kendler et al., 2010; Maes et al., 2004; Sartor et al., 2010; Verweij et al., 2010). For BPT the heritability was estimated at 48%. In an earlier study, which included twins as well as their parents and nontwin siblings, we found that the heritability of BPT was explained by both additive genetic (21%) and dominant genetic (24%) factors (Distel et al., 2009). In the present study we did not model dominant genetic effects for two reasons. First, there is no evidence that dominant genetic effects influence the liability for the three substance use measures and therefore dominant genetic effects will not explain the covariance between BPT and the substance use measures. Second, the present study only includes twins which do not provide enough power to detect dominant genetic effects (Posthuma & Boomsma, 2000).
BPT were significantly associated with the use of substances. For regular smoking and for ever use of cannabis, the correlation with BPT was explained by common genetic factors. Unique environmental influences did not contribute to the association. For high alcohol consumption, the association with BPT could entirely be explained by unique environmental influences that are shared between the traits.
The personality traits affective instability (i.e., emotional dysregulation) and impulsivity are associated with both BPD and SUD and therefore proposed to reflect the genetic liability to develop both BPD and SUD (Bornovalova, Lejuez, Daughters, Rosenthal, & Lynch, 2005; Jahng, Trull et al., 2011; Sher & Trull, 2002; Trull et al., 2000). First, regarding affective instability, several studies suggest that substance use may serve to decrease feelings of negative affect or increase feelings of positive affect (Baker, Brandon, & Chassin, 2004; Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Jahng, Solhan et al., 2011; Trull et al., 2000). Using ecological momentary assessment methods, Jahng, Solhan et al. (2011) examined the relationship between affect and alcohol use in a sample of BPD outpatients. Consistent with the affect regulation hypothesis, they found that BPD drinkers (versus nondrinkers), in general, were distinguished by larger within-person variability (between-day and within-day) in negative but not positive affect scores. Further, the variances of negative affect scores were significantly larger on drinking versus nondrinking days for BPD drinkers. Overall, their findings can be interpreted as consistent with the negative affect regulation hypothesis: Individuals who are experiencing variability or dysregulation in affect may be more prone to use alcohol in order to regulate their mood state (i.e., a tension-reduction or negative reinforcement model). Although the present study also found an association between BPT and alcohol use, it does not provide evidence for shared genetic etiology between BPT and high alcohol consumption, as was found for regular smoking and ever use of cannabis.
Second, several studies state that substance use in BPD patients should be viewed as a manifestation of impulsivity (Bornovalova et al., 2005; Jahng, Trull et al., 2011). Jahng, Trull, et al. (2011) found using hierarchical factor models that BPD was uniquely related to various forms of substance dependence (alcohol, nicotine, and other drug) above and beyond what could be accounted for by a general personality disorder factor. The authors posit that this unique cluster B factor may be represented by the personality trait impulsivity. Impulsivity has been frequently been linked to substance use, abuse, and dependence (Ball, 2005; Kruedelbach, McCormick, Schulz, & Grueneich, 1993; Mitchell, 1999; O’Boyle & Barratt, 1993).
It is unknown which genes increase the risk for BPT and substance use. Although speculative, recent theory suggests that BPD symptoms and associated features (e.g., mood lability, drug and alcohol addiction) may reflect an underlying dysregulation of the endogenous opioid system (Bandelow, Schmahl, Falkai, & Wedekind, 2010). The endogenous opioid system plays an important role in the brain reward system and in the modulation of response to stressors. Such an account can explain the experience of emotion dysregulation and use of substances in this patient group, mediated by reduced sensitivity of endorphin receptors or low levels of endogenous opioids. In the present study, common genetic risk factors were not the explanation for the cooccurrence of BPT and high alcohol consumption. Further research thus needs to clarify for which substance use measures this biological system may explain the association between BPD and substance use.
Recently, several studies reported that the genetic factors influencing the liability for substance use were significantly correlated to the genetic risk factors for substance dependence (Grant et al., 2009; Kendler et al., 2010; Sartor et al., 2010). In combination with the results of the present study these results underscore the clinical significance of monitoring substance use during the treatment of BPD.
Acknowledgments
Funding was obtained from the following grants: “Psychometric and genetic assessments of substance use” (PI Neale; NIH DA-18673, DA-026119); “Spinozapremie” (NWO/SPI 56-464-14192); Genetics of Mental Illness: European Research Council (ERC-230374); “Twin-family database for behavior genetics and genomics studies” (NWO 480-04-004); and “Causes and consequences of smoking behaviour: a twin-family study” (NWO-VENI 451-06-004 Vink). M. Bartels is financially supported by a senior fellowship of the EMGO+ Institute for Health and Care Research. M. de Moor (VENI-016-115-035) is financially supported by the Netherlands Organization for Scientific Research (NWO).
References
- Agrawal A, Lynskey MT. Are there genetic influences on addiction: Evidence from family, adoption and twin studies. Addiction. 2008;103:1069–1081. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annual Review of Psychology. 2004a;55:463–491. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004b;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Ball SA. Personality traits, problems, and disorders: Clinical applications to substance use disorders. Journal of Research in Personality. 2005;39:84–102. [Google Scholar]
- Bandelow B, Schmahl C, Falkai P, Wedekind D. Borderline personality disorder: A dysregulation of the endogenous opioid system? Psychological Review. 2010;117:623–636. doi: 10.1037/a0018095. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, de Geus EJC, Vink JM, Stubbe JH, Distel MA, Hottenga JJ, et al. Netherlands Twin Register: From twins to twin families. Twin Research and Human Genetics. 2006;9:849–857. doi: 10.1375/183242706779462426. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Hicks BM, Iacono WG, Mcgue M. Stability, change, and heritability of borderline personality disorder traits from adolescence to adulthood: A longitudinal twin study. Development and Psychopathology. 2009;21:1335–1353. doi: 10.1017/S0954579409990186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Lejuez CW, Daughters SB, Rosenthal MZ, Lynch TR. Impulsivity as a common process across borderline personality and substance use disorders. Clinical Psychology Review. 2005;25:790–812. doi: 10.1016/j.cpr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Derom C, Derom R. The East Flanders prospective twin survey. In: Blickstein I, Keith LG, editors. Multiple pregnancy: Epidemiology, gestation and perinatal outcome. 2. Oxford: Taylor and Francis; 2005. pp. 39–47. [Google Scholar]
- Distel MA, Ligthart L, Willemsen G, Nyholt DR, Trull TJ, Boomsma DI. Personality, health and lifestyle in a questionnaire family study: A comparison between highly cooperative and less cooperative families. Twin Research and Human Genetics. 2007;10:348–353. doi: 10.1375/twin.10.2.348. [DOI] [PubMed] [Google Scholar]
- Distel MA, Rebollo-Mesa I, Willemsen G, Derom CA, Trull TJ, Martin NG, et al. Familial resemblance of borderline personality disorder features: Genetic or cultural transmission? PLoS ONE. 2009;4:e5334. doi: 10.1371/journal.pone.0005334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel MA, Trull TJ, Derom CA, Thiery EW, Grimmer MA, Martin NG, et al. Heritability of borderline personality disorder features is similar across three countries. Psychological Medicine. 2008;38:1219–1229. doi: 10.1017/S0033291707002024. [DOI] [PubMed] [Google Scholar]
- Feske U, Tarter RE, Kirisci L, Pilkonis PA. Borderline personality and substance use in women. American Journal on Addictions. 2006;15:131–137. doi: 10.1080/10550490500528357. [DOI] [PubMed] [Google Scholar]
- Grant JD, Agrawal A, Bucholz KK, Madden PAF, Pergadia ML, Nelson EC, et al. Alcohol consumption indices of genetic risk for alcohol dependence. Biological Psychiatry. 2009;66:795–800. doi: 10.1016/j.biopsych.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States—Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Jahng S, Solhan MB, Tomko RL, Wood PK, Piasecki TM, Trull TJ. Affect and alcohol use: An EMA study of outpatients with borderline personality disorder. Journal of Abnormal Psychology. 2011;120:572–584. doi: 10.1037/a0024686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng S, Trull TJ, Wood PK, Tragesser SL, Tomko RL, Grant JD, et al. Distinguishing general and specific personality disorder features and implications for substance dependence comorbidity. Journal of Abnormal Psychology. 2011;120:656–669. doi: 10.1037/a0023539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karterud S, Arefjord N, Andresen NE, Pedersen G. Substance use disorders among personality disordered patients admitted for day hospital treatment. Implications for service developments. Nordic Journal of Psychiatry. 2009;63:57–63. doi: 10.1080/08039480802298705. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Czajkowski N, Roysamb E, Tambs K, Torgersen S, et al. The structure of genetic and environmental risk factors for DSM-IV personality disorders a multivariate twin study. Archives of General Psychiatry. 2008;65:1438–1446. doi: 10.1001/archpsyc.65.12.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Dick D, Prescott CA. The relationship between genetic influences on alcohol dependence and on patterns of alcohol consumption. Alcoholism-Clinical and Experimental Research. 2010;34:1058–1065. doi: 10.1111/j.1530-0277.2010.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruedelbach N, Mccormick RA, Schulz SC, Grueneich R. Impulsivity, coping styles, and triggers for craving in substance-abusers with borderline personality-disorder. Journal of Personality Disorders. 1993;7:214–222. [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Maes HH, Sullivan PF, Bulik CM, Neale MC, Prescott CA, Eaves LJ, et al. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychological Medicine. 2004;34:1251–1261. doi: 10.1017/s0033291704002405. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Grilo CM, Skodol AE, Gunderson JG, Shea MT, Morey LC, et al. The Collaborative Longitudinal Personality Disorders Study: Baseline Axis I/II and II/II diagnostic co-occurrence. Acta Psychiatrica Scandinavica. 2000;102:256–264. doi: 10.1034/j.1600-0447.2000.102004256.x. [DOI] [PubMed] [Google Scholar]
- Miller FT, Abrams T, Dulit R, Fyer M. Substance-abuse in borderline personality-disorder. American Journal of Drug and Alcohol Abuse. 1993;19:491–497. doi: 10.3109/00952999309001637. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Morey LC. The personality assessment inventory: Professional manual. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- Morey LC. Essentials of PAI assessment. Hoboken, NJ: Wiley; 2003. [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 3. Los Angeles, CA: Muthén & Muthén; 2005. [Google Scholar]
- Nolen-Hoeksema S. Gender differences in risk factors and consequences for alcohol use and problems. Clinical Psychology Review. 2004;24:981–1010. doi: 10.1016/j.cpr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- O’Boyle M, Barratt ES. Impulsivity and DSM-III-R personality-disorders. Personality and Individual Differences. 1993;14:609–611. [Google Scholar]
- Posthuma D, Boomsma DI. A note on the statistical power in extended twin designs. Behavior Genetics. 2000;30:147–158. doi: 10.1023/a:1001959306025. [DOI] [PubMed] [Google Scholar]
- Prescott CA. Using the Mplus computer program to estimate models for continuous and categorical data from twins. Behavior Genetics. 2004;34:17–40. doi: 10.1023/B:BEGE.0000009474.97649.2f. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Grant JD, Bucholz KK, Madden PAF, Heath AC, Agrawal A, et al. Common genetic contributions to alcohol and cannabis use and dependence symptomatology. Alcoholism: Clinical and Experimental Research. 2010;34:545–554. doi: 10.1111/j.1530-0277.2009.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Substance use disorder and personality disorder. Current Psychiatry Reports. 2002;4:25–29. doi: 10.1007/s11920-002-0008-7. [DOI] [PubMed] [Google Scholar]
- Tomko RL, Trull TJ, Wood PK, Sher KJ. Characteristics of borderline personality disorder in a community sample: Comorbidity, treatment utilization, and general functioning. Journal of Personality Disorders. doi: 10.1521/pedi_2012_26_093. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen S, Czajkowski N, Jacobson K, Reichborn-Kjennerud T, Roysamb E, Neale MC, et al. Dimensional representations of DSM-IV cluster B personality disorders in a population-based sample of Norwegian twins: A multivariate study. Psychological Medicine. 2008;38:1617–1625. doi: 10.1017/S0033291708002924. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Jahng S, Tomko RL, Wood PK, Sher KJ. Revised NESARC personality disorder diagnoses: Gender, prevalence, and comorbidity with substance dependence disorders. Journal of Personality Disorders. 2010;24:412–426. doi: 10.1521/pedi.2010.24.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trull TJ, Sher KJ, Minks-Brown C, Durbin J, Burr R. Borderline personality disorder and substance use disorders: A review and integration. Clinical Psychology Review. 2000;20:235–253. doi: 10.1016/s0272-7358(99)00028-8. [DOI] [PubMed] [Google Scholar]
- van den Bosch LMC, Verheul R, van den Brink W. Substance abuse in borderline personality disorder: Clinical and etiological correlates. Journal of Personality Disorders. 2001;15:416–424. doi: 10.1521/pedi.15.5.416.19201. [DOI] [PubMed] [Google Scholar]
- Verdurmen J, Monshouwer K, van Dorsselaer S. Bovenmatig drinken in Nederland: Uitkomsten van de Netherlands Mental Health Survey and Incidence Study (Nemesis) [Excessive drinking in the Netherlands: Results of the Netherlands Mental Health Survey and Incidence Study (Nemesis)]. Utrecht: Bureau NDM; 2003. [Google Scholar]
- Verweij KJH, Zietsch BP, Lynskey MT, Medland SE, Neale MC, Martin NG, et al. Genetic and environmental influences on cannabis use initiation and problematic use: A meta-analysis of twin studies. Addiction. 2010;105:417–430. doi: 10.1111/j.1360-0443.2009.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Gunderson JG, Zanarini MC, Sanislow CA, Grilo CM, McGlashan TH, et al. New onsets of substance use disorders in borderline personality disorder over 7 years of follow-ups: Findings from the Collaborative Longitudinal Personality Disorders Study. Addiction. 2009;104:97–103. doi: 10.1111/j.1360-0443.2008.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield JB, Zhu G, Madden PA, Neale MC, Heath AC, Martin NG. The genetics of alcohol intake and of alcohol dependence. Alcoholism: Clinical and Experimental Research. 2004;28:1153–1160. doi: 10.1097/01.alc.0000134221.32773.69. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Barison LK, Franken-burg FR, Reich DB, Hudson JI. Family history study of the familial coaggregation of borderline personality disorder with Axis I and nonborderline dramatic cluster Axis II disorders. Journal of Personality Disorders. 2009;23:357–369. doi: 10.1521/pedi.2009.23.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanarini MC, Frankenbur FR, Weinger-off JL, Reich DB, Fitzmaurice GM, Weiss RD. The course of substance use disorders in patients with borderline personality disorder and Axis II comparison subjects: A 10-year follow-up study. Addiction. 2011;106:342–348. doi: 10.1111/j.1360-0443.2010.03176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]