Abstract
Introduction
Motor imagery during functional magnetic resonance imaging is commonly used to understand the neural underpinnings of complex movements. This approach has recently been applied to individuals with Parkinson disease (PD) to better understand how brain function may relate to movement dysfunction. However, the ability of individuals with PD to imagine movements when “Off” dopamine replacement medication is poorly understood. Therefore, the primary purpose of the current study is to test the ability of people with PD to imagine movements while “On” and “Off” anti-Parkinson medication.
Methods
Vividness of imagery was assessed in 28 individuals with mild to moderate PD (Hoehn and Yahr stages 1–3) via the Kinesthetic Visual Imagery Questionnaire (KVIQ-20) both “On” and “Off” anti-Parkinson medication. Vividness of imagery of 32 age-matched older adults was also assessed.
Results
No differences in vividness of imagery were observed between “Off” and “On” medication states (p=0.15). Imagery was similar between controls and PD both “Off” (p=0.25) and “On” (p=0.46) anti-Parkinson medication. A significant correlation was observed between imagery and disease severity while “On” anti-Parkinson medication (r= −0.49; p=0.008).
Discussion and Conclusions
Vividness of movement imagery was not different between “Off” and “On” anti-Parkinson medications or between PD and controls. These results suggest that people with PD are able to imagine similarly to older adults both when “On” and “Off” anti-Parkinson medication, and supports the use of motor imagery in the “Off” medication state.
Keywords: Parkinson’s Disease, Motor Imagery, Levodopa, KVIQ
INTRODUCTION
Motor imagery (MI) is “a dynamic state during which representations of a given motor act are internally rehearsed in working memory without any overt motor output”[1]. MI has been used extensively with imaging techniques such as functional magnetic resonance imaging to provide insight into the neural underpinnings of complex motor processes in healthy adults [2–8]. More recent studies have begun to use MI with imaging techniques to better understand how brain pathology in individuals with Parkinson disease (PD) relates to movement dysfunction [7,9–11]. In these investigations, PD subjects are often studied “Off” anti-Parkinson medication (Levodopa replacement). However, the ability of people with PD to imagine movements while “Off” dopamine replacement medication is not well understood. One recent investigation showed that individuals with PD have similar vividness of imagery as healthy adults [12]; however, this study tested the vividness of imagery in people with PD while “On” anti-Parkinson medication. Levodopa has been suggested to normalize brain activity in PD in many regions, including the supplementary motor area (SMA) [13–16]. This region is associated with motor planning [17,18] and has been shown to be active during both overt [15] and imagined [4,7] movements. Therefore, pathological activation of SMA, as well as other regions, may reduce the ability of this group to imagine movement in the “Off” medication state. As imagery studies are often carried out with patients “Off” anti-Parkinson medications, it is important to determine the degree to which people with PD can imagine in this medication state. Further, MI has shown promise as a rehabilitative strategy in both healthy individuals [19], and recently, those with neurological disorders, specifically stroke [20,21]. Though rehabilitative MI has not yet been tested in those with PD, understanding changes in imagery while “Off” and “On” anti-Parkinson medication could provide insight into which medication state is better suited for this potential intervention.
The purpose of the current study was to test vividness of MI in individuals with PD both “On” and “Off” anti-Parkinson medications, as well as how vividness of MI in those with PD compares to healthy older adults. Due to the altered activation of brain regions (including the SMA) thought to be associated with motor planning while “Off” anti-Parkinson medication, our primary hypothesis is that individuals with PD “Off” anti-Parkinson medication would exhibit worse vividness of imagery with respect to “On” medication, and that the normalizing effects of Levodopa would result in similar imagery scores between PD “On” and healthy controls. Our secondary hypothesis is that more severe PD symptoms will be associated with worse MI.
MATERIALS AND METHODS
Participants
Twenty eight individuals with PD (17 male) and 32 age-matched healthy older adults (16 male) participated in the study. Six PD and 2 controls were left handed based on self report. Thirteen of 28 PD presented with unilaterally increased motor symptoms on their left side, 14 were more affected on the right side and 1 was equally affected bilaterally. Among those with PD, four individuals exhibited dyskinesia. Twenty-four of the 28 subjects exhibited tremor. Exclusion criteria included severe orthopedic problems of upper or lower limbs, deep brain stimulation, and any neurological disorders other than PD. Diagnosis of PD was given by a board certified neurologist using the diagnostic criteria for “definite PD” [22] and based on established criteria [23]. All individuals with PD were taking levodopa (Mean ± SD Levodopa Equivalent Daily Dose=928 ± 566; range 300–3000) when enrolled in the study. Written informed consent was provided by all subjects in accordance with the Helsinki Declaration of 1975, and all procedures were reviewed and approved by the Human Research Protection Office at Washington University School of Medicine.
Quantifying Imagery
To assess imagery ability, the Kinesthetic Visual Imagery Questionnaire (KVIQ-20) was administered to all subjects in a similar manner to that described in Malouin et al [24]. The KVIQ-20 was chosen as it was designed specifically to be administered to individuals with movement disorders [24], and has previously been shown to be reliable for individuals with PD [25]. In addition, the ease and speed of administration of this test make it attractive as a potential tool to screen for ability to imagine.
The KVIQ-20 includes 10 motions of the neck, shoulders, upper limb, lower limb, and trunk. To administer the KVIQ-20, each motion is demonstrated by the tester, and then completed by the participant. The participant then imagines the motion and rates the vividness of his visual imagery followed by the vividness of his kinesthetic imagery, each on a 5 point scale (5=image or intensity as vivid as completing the motion; 1= no image or sensation). Visual imagery represents the clarity of the image, and kinesthetic imagery represents the sensation of motion while imagining the task. Each score is recorded by the examiner.
Seven of the 10 motions consist of movement of a single limb. For these motions, imagery of both the left and right sides were assessed. Within each task, the left side was assessed first, followed by the right.
Kinesthetic and visual scores were calculated as the sum of scores from each motion, with scores from bilateral motions averaged across left and right sides giving a minimum possible score of 10 and a maximum possible score of 50 (10 motions x maximum rating of 5). KVIQ-Total scores were determined as the sum of kinesthetic and visual sub-scores (maximum possible score = 100).
To assess within-subject side differences, scores were compared across more and less affected sides (PD) and across dominant and non-dominant sides (PD, control). Differences across more and less affected sides in PD were assessed for 27 subjects, as one individual showed similar dysfunction across sides. These unilateral scores were calculated as the sum of kinesthetic and visual scores for one side (more affected, less affected, dominant, or non-dominant) across all bilateral movements. As there are 7 movements where both sides are measured, with a maximum KVIQ score of 10 for each movement (max kinesthetic score =5; max visual score = 5), the maximum possible score for KVIQ-Unilateral is 70. For individuals with PD, more and less affected side was determined by summing unilateral components of the part III subscale of the Movement Disorders Unified Parkinson Disease Rating Scale (MDS-UPDRS III [26]). The side which accumulated a larger score was deemed the more affected side. No differences were observed in KVIQ-Unilateral scores across dominant or non-dominant (PD, control), or more or less affected (PD) sides (See Results; Figure 1),
Figure 1.
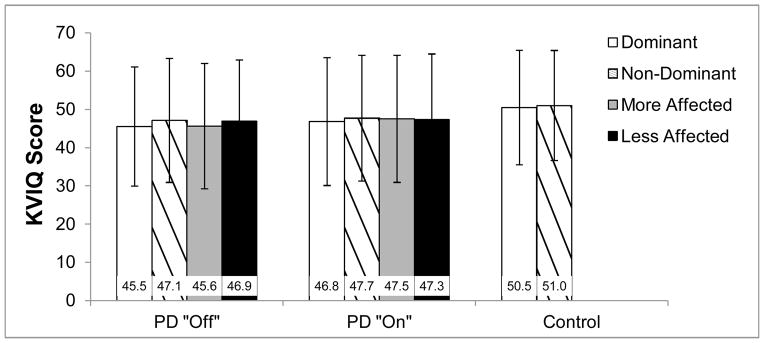
KVIQ-Unilateral scores for more and less affected side, and dominant and non-dominant side. Maximum score = 70 (See Methods). PD “Off” = PD “Off” anti-Parkinson medication, PD “On” = PD “On” anti-Parkinson medication, Control = healthy older adults.
The KVIQ was administered by one of two trained individuals. Both individuals were trained to administer the exam, and given a script to follow to reduce variance in instruction to participants. For an individual with PD, the “Off” and “On” medication testing sessions were always administered by the same tester. Due to the scripted test administration, as well as the fact that ratings reported by the subject are not open to tester interpretation, we are confident that testing was consistent across testers.
Individuals with PD were tested two times, while healthy controls were tested once. Individuals with PD were first tested “Off” anti-Parkinson medication (>12 hours since last dose; a commonly used criterion used to assess PD symptoms “Off” medication [7,9–11,27]). After taking a normal dose of medication, subjects waited for approximately one hour, and the KVIQ-20 was administered again. Approximately 2 hours elapsed between KVIQ-20 testing sessions for individuals with PD.
Subjects’ disease severity was assessed by the MDS-UPDRS III and the Hoehn and Yahr scale [28] both “On” and “Off” medication.
Statistics
Imagery scores for both groups were shown to be normally distributed (non-significant Skewness and Kurtosis) and have approximately equal variance (non-significant Levene’s test) across groups. Therefore, parametric tests were used for all analyses. Paired t-tests were used for all within-subject comparisons: ie: comparison of within-subject kinesthetic and visual scores; effects of medication on KVIQ-Total scores, UPDRS scores, and Hoehn and Yahr staging in those with PD. Independent t-tests were used for across group comparisons: i.e., vividness of MI across groups, and age across groups. To determine whether motor severity predicts one’s ability to imagine, Pearson correlation coefficients were used to assess the relationship between KVIQ scores and motor severity (MDS-UPDRS III and Hoehn & Yahr scale) both “On” and “Off” anti-Parkinson medication. All measures are noted as mean + standard deviation, unless otherwise noted.
RESULTS
Individuals with PD were of similar age as healthy controls (PD=71.0±8.9; Controls=70.3±10.6; p=0.78). Individuals with PD improved MDS-UPDRS III and Hoehn & Yahr scores after administration of anti-Parkinson medication (p<0.001 and p=0.01, respectively; Table 1), suggesting subjects did see significant benefit from their anti-Parkinson medication.
Table 1.
Demographic and imagery results across groups
| Variable | Control (N=32) | PD “Off” (n=28) | PD “On” (n=28) | * P-value: PD “On” vs. PD “Off” | # P-value: PD “Off” vs. Control | # P-value: PD “On” vs. Control |
|---|---|---|---|---|---|---|
| Age | 70.3 (10.6) | 71.0 (8.9) | - | 0.78 | ||
| Disease Duration | - | 6.5 (3.8) | - | - | ||
| UPDRS-MDS III | - | 37.6 (9.9) | 26.6 (9.8) | <0.001 | - | - |
| Hoehn & Yahr Stage | - | 2.4 (0.3) | 2.2 (0.4) | 0.005 | - | - |
| KVIQ - Visual | 38.6 (10.9) | 34.6 (10.9) | 36.3 (11.6) | 0.13 | 0.16 | 0.42 |
| KVIQ - Kinesthestic | 33.6 (12.4) | 31.2 (12.1) | 31.8 (13.0) | 0.42 | 0.45 | 0.59 |
| KVIQ - Total | 72.2 (20.6) | 65.8 (22.0) | 68.1 (23.3) | 0.15 | 0.25 | 0.46 |
Mean (SD). Maximum score of Visual and Kinesthetic sub-components = 50, Maximum score of KVIQ-Total = 100 (See Methods). PD “Off” = PD “Off” anti-Parkinson medication, PD “On” = PD “On” anti-Parkinson medication, Control = healthy older adults.
Paired t-test;
Independent samples t-test
Similarly to previous investigations [12], no differences in KVIQ-Unilateral scores were observed between dominant and non-dominant limbs for control (p=0.34), PD “Off” (p=0.06), or PD “On” anti-Parkinson medication (p=0.22). In addition, within the PD group, no differences were observed in KVIQ-Unilateral between more and less affected limbs “Off” (p=0.13) or “On” (p=0.78) anti-Parkinson medication (Figure 1).
Contrary to our primary hypothesis, there were no statistically significant differences in KVIQ-Total in people with PD when “Off” or “On” anti-Parkinson medication (Table 1). Further, no differences were observed between KVIQ-Total in healthy older adults and individuals with PD “On” or “Off” anti-Parkinson medication. Kinesthetic and visual KVIQ components were also not different between PD “On” and controls or PD “Off” and controls. Across all subjects, the visual component of the KVIQ-20 was significantly higher than kinesthetic component of the KVIQ-20 score (p<0.001). Higher scores on the visual imagery with respect to kinesthetic imagery were also shown in those with PD while both “On” (p=0.006) and “Off” (p=0.013) anti-Parkinson medication.
Six of 32 control subjects and five of 28 PD subjects exhibited scores of <20 on either visual or kinesthetic components of the KVIQ-Total. These PD subjects exhibited KVIQ scores <20 both “On” and “Off” medication. A score of 20 represents an average response of 2 across all tasks, or a “blurred image” and “mildly intense” for visual and kinesthetic imagery, respectively.
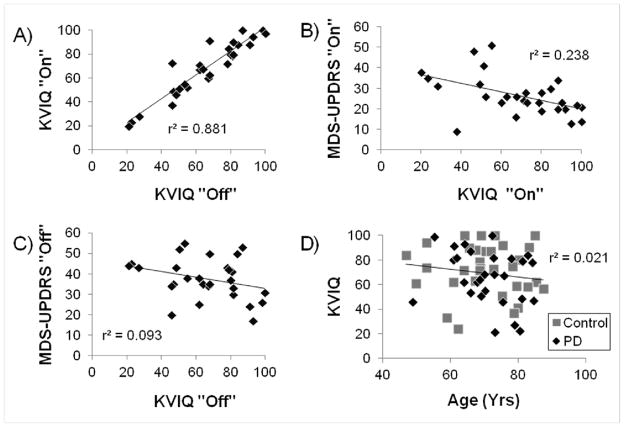
Scores on the KVIQ-Total while “On” anti-Parkinson medication were positively correlated to KVIQ-Total scores “Off” anti-Parkinson medication (r=0.94, p<0.0001; Figure 2a). In partial support of our secondary hypothesis, KVIQ-Total scores “On” medication were negatively correlated to MDS-UPDRS III “On” medication (r=−0.49, p=0.008; Figure 2b) such that increased disease severity predicted worse imagery. MDS-UPDRS III “Off” medication was not, however, correlated to KVIQ-Total “Off” medication (r=−0.31, p=0.11; Figure 2c). Finally, no relationships were observed between KVIQ-Total score and age for PD (r=−.26, p=0.18), control (r=−0.05, p=0.81) or the combination of PD and control subjects together, (r=−0.14, p=0.28; Figure 2d).
Figure 2.
Relationships between: (A) KVIQ “Off” and “On” anti-Parkinson medication, (B) Disease severity (MDS-UPDRS III) and KVIQ “On” anti-Parkinson medication, (C) Disease severity and KVIQ “Off” anti-Parkinson medication, and (D) Age and KVIQ (Regression line and r2 value represents data from all participants; PD data shown is “Off” anti-Parkinson medication).
DISCUSSION
Dopamine replacement therapies have been shown to be beneficial for reducing many of the symptoms of PD [29]. Until now it has been unclear whether dopamine replacement impacts MI in people with PD. As many imagery studies are carried out with individuals “Off” anti-Parkinson medication, it is critical to determine the degree to which individuals with PD can imagine movement while in the “Off” anti-Parkinson medication state. Our results suggest that in both the “Off” and “On” medication states, individuals with PD have similar imagery vividness as healthy older adults. This result provides support for MI testing while people with PD are “Off” anti-Parkinson medication. Further, MI has been suggested as a rehabilitative strategy for individuals with neurological disorders [20,21]. The ability of individuals with PD to imagine “Off” their anti-Parkinson medication suggests this potential rehabilitative strategy may be applicable when subjects are “Off” anti-Parkinson medication state.
Our results generally fit well with previous reports on MI in individuals with PD [12,25] and healthy older adults [24]. Two recent studies have measured imagery vividness among individuals with PD using the KVIQ-20 while “On” anti-Parkinson medication [12,25]. Randhawa and colleagues (2010) reported vividness of MI of individuals with PD were slightly higher (better) than those reported in the current study. However, this may be due to the fact that subjects in the current study exhibited more severe Parkinsonian symptoms (higher MDS-UPDRS III scores) than those of Randhawa and colleagues. Indeed, correlation results from the current study suggest the possibility that worse MDS-UPDRS III scores may predict worse imagery in PD. Heremans et al. (2011) reported KVIQ-20 values for people with PD “On” anti-Parkinson medications as well as healthy adults to be worse than those reported in the current study. However, similarly to Heremans and colleagues, we observed no differences in vividness of imagery between older adults and individuals with PD while “On” anti-Parkinson medication. Our results further extend the findings of both Heremans & Randhawa, showing that even when “Off” medication, individuals with PD seem to retain the ability to imagine movements.
Vividness of imagery “On” and “Off” anti-Parkinson medication was highly correlated, suggesting it was quite consistent across medication states. We also found a medium [30] correlation between MDS-UPDRS III “On” and KVIQ-Total “On” scores, such that individuals with worse MDS-UPDRS III scores had worse KVIQ-Total scores. This correlation suggests that ability to imagine may be related to PD motor symptom disease severity. Our investigation included only individuals with mild or moderate PD. Further studies determining ability of individuals with moderate to severe PD are necessary to better understand how PD severity may be related to vividness of imagery.
Across all subjects, age was not correlated with MI. This is consistent with a previous assessment of vividness of imagery across age groups [31] which also showed age not to have a significant effect on MI. Studies assessing different aspects of imagery, such as timing of imagery, have, however, described age-related differences in the ability to imagine movements [32–34]. For example, Personnier and colleagues showed that when imagining gait, older adults systematically over-estimated the duration of imagined movements with respect to overt motions [33]. Together, these results suggest some aspects of MI (timing of imagery) may be altered across age while others (vividness of imagery) seem to be retained.
Both PD and control subjects in the current study scored higher on visual components of imagery than kinesthetic components. This result is similar to several previous reports [12,24,35,36], and suggests that like healthy controls [24] and individuals who have experienced a stroke [36], individuals with PD more vividly imagine the visual component of movement than the kinesthetic component. Further, results of the current study show that this relationship is maintained while “Off” medication. That is, visual scores tend to be greater than kinesthetic scores both “On” and “Off” anti-Parkinson medication.
Several subjects, both control and PD, demonstrated a marked inability to imagine movement (<20 on either Kinesthetic or Visual components of KVIQ). However, the proportion of individuals who were unable to imagine were similar in control (6/32; 19%) and PD groups (5/28; 18%). This is in conjunction with previous reports, which show a small population of both healthy controls [24] and those with PD [12,25] to exhibit poor imagery ability. Subgroup analyses were conducted to determine if subject characteristics in PD (UPDRS, Hoehn and Yahr, disease duration, and age) and controls (age) were different in those exhibiting low imagery scores. In conjunction with correlational analyses described earlier, UPDRS “On” medication was significantly higher (worse) in the group with low imagery scores with respect to others with PD. However, no other differences were observed in those with low KVIQ scores. Due to the similar proportion of poor imaginers in both groups, data from these subjects were not omitted from analysis. These results underscore the importance of assessing imagery ability in all individuals completing a task requiring MI.
Limitations
In the current study it was not possible to counter-balance the order of testing sessions for individuals with PD. That is, participants with PD were always tested “Off” medication first, then again “On” medication. It is therefore possible that “On” medication scores may have been biased due to a practice effect. If this were the case we may expect an overestimation of imagery vividness on the second administration when “On” medication. Despite the possibility of an overestimation of imagery vividness while “On” medication, we still found no differences between “Off” and “On” medication testing sessions, suggesting that “Off” medication imagery is likely not diminished with respect to imagery in the “On” anti-Parkinson medication state. Though the KVIQ was only administered one time to healthy older adults, the similarity in results from the “On” and “Off” medication PD groups suggests there likely would not have been significant changes across multiple testing sessions. In addition, Moulin et al (2007) also assessed the change in score across multiple testing sessions in controls, showing little change in score across sessions [24].
The lack of significance when “Off” and “On” anti-Parkinson medication in those with PD could be due to an insufficient sample of PD subjects. Our data suggest that on average, those with PD show an approximately 2.3% increase in KVIQ-Total score (2.3 point increase on a 100 point scale) while “On” anti-Parkinson medication with respect to “Off” medication. This corresponds to an effect size of 0.10. Although it is currently unclear what a clinically significant change in KVIQ score may be, we feel that this does not represent a meaningful change in KVIQ score, and therefore are confident that medication did not result in improved MI.
We determined the vividness of imagery in people with mild to moderate PD (Hoehn & Yahr stages 1–3). In addition, we showed that while “On” medication, imagery (KVIQ-20) was negatively correlated to disease severity (MDS-UPDRS III). It is possible that our results do not extrapolate to individuals with more severe PD. It therefore remains to be determined whether individuals with severe PD and/or cognitive deficits are able to effectively imagine movement.
Conclusions
Imagery scores while “On” anti-Parkinson medication and after refraining from medication for a commonly used period of time (12 hrs) were both similar to healthy adults, suggesting anti-Parkinson medication likely does not have a substantial effect on vividness of motor imagery for individuals with mild to moderate PD. Although vividness of imagery does not seem to be affected by medication levels, the negative correlation between UPDRS and KVIQ in the “On” state suggests the possibility that imagery may be degraded in individuals with more severe PD. Further research on individuals with more severe PD is necessary to more fully understand this relationship.
Acknowledgments
The authors thank Ryan Duncan and Marie McNeely for their assistance with data collection. Funding received for this work includes: NIH- TL1RR024995, NIH-RO1HD056051-02, NIH-2T32HD007434-18A, Parkinson’s Disease Foundation, American Parkinson Disease Association Center for Advanced PD Research at Washington University.
Footnotes
The authors declare that they have no conflict of interest, financial or otherwise, related to the submitted manuscript or the associated research.
Reference List
- 1.Decety J. The neurophysiological basis of motor imagery. Behav Brain Res. 1996;77:45–52. doi: 10.1016/0166-4328(95)00225-1. [DOI] [PubMed] [Google Scholar]
- 2.Bakker M, De Lange FP, Helmich RC, Scheeringa R, Bloem BR, Toni I. Cerebral correlates of motor imagery of normal and precision gait. Neuroimage. 2008;41:998–1010. doi: 10.1016/j.neuroimage.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Godde B, Voelcker-Rehage C. More automation and less cognitive control of imagined walking movements in high- versus low-fit older adults. Front Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jahn K, Deutschlander A, Stephan T, Kalla R, Wiesmann M, Strupp M, Brandt T. Imaging human supraspinal locomotor centers in brainstem and cerebellum. Neuroimage. 2008;39:786–792. doi: 10.1016/j.neuroimage.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 5.la Fougere C, Zwergal A, Rominger A, Forster S, Fesl G, Dieterich M, Brandt T, Strupp M, Bartenstein P, Jahn K. Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. Neuroimage. 2010;50:1589–1598. doi: 10.1016/j.neuroimage.2009.12.060. [DOI] [PubMed] [Google Scholar]
- 6.Sacco K, Cauda F, Cerliani L, Mate D, Duca S, Geminiani GC. Motor imagery of walking following training in locomotor attention. The effect of “the tango lesson”. Neuroimage. 2006;32:1441–1449. doi: 10.1016/j.neuroimage.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, Toni I. Gait-related cerebral alterations in patients with Parkinson’s disease with freezing of gait. Brain. 2011;134:59–72. doi: 10.1093/brain/awq324. [DOI] [PubMed] [Google Scholar]
- 8.Wagner J, Stephan T, Kalla R, Bruckmann H, Strupp M, Brandt T, Jahn K. Mind the bend: cerebral activations associated with mental imagery of walking along a curved path. Exp Brain Res. 2008;191:247–255. doi: 10.1007/s00221-008-1520-8. [DOI] [PubMed] [Google Scholar]
- 9.Cunnington R, Egan GF, O’Sullivan JD, Hughes AJ, Bradshaw JL, Colebatch JG. Motor imagery in Parkinson’s disease: a PET study. Mov Disord. 2001;16:849–857. doi: 10.1002/mds.1181. [DOI] [PubMed] [Google Scholar]
- 10.Helmich RC, de Lange FP, Bloem BR, Toni I. Cerebral compensation during motor imagery in Parkinson’s disease. Neuropsychologia. 2007;45:2201–2215. doi: 10.1016/j.neuropsychologia.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Samuel M, Ceballos-Baumann AO, Boecker H, Brooks DJ. Motor imagery in normal subjects and Parkinson’s disease patients: an H215O PET study. Neuroreport. 2001;12:821–828. doi: 10.1097/00001756-200103260-00040. [DOI] [PubMed] [Google Scholar]
- 12.Heremans E, Feys P, Nieuwboer A, Vercruysse S, Vandenberghe W, Sharma N, Helsen W. Motor imagery ability in patients with early- and mid-stage Parkinson disease. Neurorehabil Neural Repair. 2011;25:168–177. doi: 10.1177/1545968310370750. [DOI] [PubMed] [Google Scholar]
- 13.Buhmann C, Glauche V, Sturenburg HJ, Oechsner M, Weiller C, Buchel C. Pharmacologically modulated fMRI--cortical responsiveness to levodopa in drug-naive hemiparkinsonian patients. Brain. 2003;126:451–461. doi: 10.1093/brain/awg033. [DOI] [PubMed] [Google Scholar]
- 14.Ng B, Palmer S, Abugharbieh R, McKeown MJ. Focusing effects of L-dopa in Parkinson’s disease. Hum Brain Mapp. 2010;31:88–97. doi: 10.1002/hbm.20847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rascol O, Sabatini U, Chollet F, Celsis P, Montastruc JL, Marc-Vergnes JP, Rascol A. Supplementary and primary sensory motor area activity in Parkinson’s disease. Regional cerebral blood flow changes during finger movements and effects of apomorphine. Arch Neurol. 1992;49:144–148. doi: 10.1001/archneur.1992.00530260044017. [DOI] [PubMed] [Google Scholar]
- 16.Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, Bozzao L, Berry I, Montastruc JL, Chollet F, Rascol O. Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain. 2000;123 (Pt 2):394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- 17.Makoshi Z, Kroliczak G, van Donkelaar P. Human Supplementary Motor Area Contribution to Predictive Motor Planning. J Mot Behav. 2011 doi: 10.1080/00222895.2011.584085. [DOI] [PubMed] [Google Scholar]
- 18.Tanji J, Shima K. Role for supplementary motor area cells in planning several movements ahead. Nature. 1994;371:413–416. doi: 10.1038/371413a0. [DOI] [PubMed] [Google Scholar]
- 19.Schuster C, Hilfiker R, Amft O, Scheidhauer A, Andrews B, Butler J, Kischka U, Ettlin T. Best practice for motor imagery: a systematic literature review on motor imagery training elements in five different disciplines. BMC Med. 2011;9:75. doi: 10.1186/1741-7015-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu KP, Chan CC, Lee TM, Hui-Chan CW. Mental imagery for promoting relearning for people after stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2004;85:1403–1408. doi: 10.1016/j.apmr.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 21.Page SJ, Levine P, Leonard A. Mental practice in chronic stroke: results of a randomized, placebo-controlled trial. Stroke. 2007;38:1293–1297. doi: 10.1161/01.STR.0000260205.67348.2b. [DOI] [PubMed] [Google Scholar]
- 22.Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson’s disease. Am J Med Genet. 1999;88:539–543. [PubMed] [Google Scholar]
- 23.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malouin F, Richards CL, Jackson PL, Lafleur MF, Durand A, Doyon J. The Kinesthetic and Visual Imagery Questionnaire (KVIQ) for assessing motor imagery in persons with physical disabilities: a reliability and construct validity study. J Neurol Phys Ther. 2007;31:20–29. doi: 10.1097/01.npt.0000260567.24122.64. [DOI] [PubMed] [Google Scholar]
- 25.Randhawa B, Harris S, Boyd LA. The Kinesthetic and Visual Imagery Questionnaire is a reliable tool for individuals with Parkinson disease. J Neurol Phys Ther. 2010;34:161–167. doi: 10.1097/npt.0b013e3181e1aa71. [DOI] [PubMed] [Google Scholar]
- 26.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 27.Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, Watts R. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 28.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 29.Cotzias GC, Van Woert MH, Schiffer LM. Aromatic Amino Acids and Modification of Parkinsonism. New England Journal of Medicine. 1967;276:374–379. doi: 10.1056/NEJM196702162760703. [DOI] [PubMed] [Google Scholar]
- 30.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, N.J: L. Erlbaum Associates; 1988. [Google Scholar]
- 31.Malouin F, Richards CL, Durand A. Normal aging and motor imagery vividness: implications for mental practice training in rehabilitation. Arch Phys Med Rehabil. 2010;91:1122–1127. doi: 10.1016/j.apmr.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Gabbard C, Cacola P, Cordova A. Is there an advanced aging effect on the ability to mentally represent action? Arch Gerontol Geriatr. 2011;53:206–209. doi: 10.1016/j.archger.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Personnier P, Kubicki A, Laroche D, Papaxanthis C. Temporal features of imagined locomotion in normal aging. Neurosci Lett. 2010;476:146–149. doi: 10.1016/j.neulet.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Personnier P, Paizis C, Ballay Y, Papaxanthis C. Mentally represented motor actions in normal aging II. The influence of the gravito-inertial context on the duration of overt and covert arm movements. Behav Brain Res. 2008;186:273–283. doi: 10.1016/j.bbr.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Atienza F, Balaguer I, Garcia-Merita ML. Factor analysis and reliability of the Movement Imagery Questionnaire. Percept Mot Skills. 1994;78:1323–1328. doi: 10.2466/pms.1994.78.3c.1323. [DOI] [PubMed] [Google Scholar]
- 36.Malouin F, Richards CL, Durand A, Doyon J. Clinical assessment of motor imagery after stroke. Neurorehabil Neural Repair. 2008;22:330–340. doi: 10.1177/1545968307313499. [DOI] [PubMed] [Google Scholar]