Abstract
Spore formation by Bacillus subtilis is a primitive form of development. In response to nutrient starvation and high cell density, B. subtilis divides asymmetrically, resulting in two cells with different sizes and cell fates. Immediately after division, the transcription factor σF becomes active in the smaller prespore, which is followed by the activation of σE in the larger mother cell. In this report, we examine the role of the mother cell-specific transcription factor σE in maintaining the compartmentalization of gene expression during development. We have studied a strain with a deletion of the spoIIIE gene, encoding a DNA translocase, that exhibits uncompartmentalized σF activity. We have determined that the deletion of spoIIIE alone does not substantially impact compartmentalization, but in the spoIIIE mutant, the expression of putative peptidoglycan hydrolases under the control of σE in the mother cell destroys the integrity of the septum. As a consequence, small proteins can cross the septum, thereby abolishing compartmentalization. In addition, we have found that in a mutant with partially impaired control of σF, the activation of σE in the mother cell is important to prevent the activation of σF in this compartment. Therefore, the activity of σE can either maintain or abolish the compartmentalization of σF, depending upon the genetic makeup of the strain. We conclude that σE activity must be carefully regulated in order to maintain compartmentalization of gene expression during development.
Sporulation in Bacillus subtilis has become a paradigm for studying prokaryotic development. In response to nutrient starvation and high cell density, a developmental program that results in the formation of environmentally resistant endospores is initiated. An early hallmark event in sporulation is an asymmetric division that divides the cell into two compartments, the smaller prespore and the larger mother cell. The prespore will eventually form a mature spore, whereas the mother cell will assist in the developmental process but will eventually lyse and die, releasing the spore into the environment (29). Gene expression is tightly compartmentalized in these two cells during sporulation, with the RNA polymerase σ factor σF becoming active exclusively in the prespore and σE becoming active only in the mother cell (32). The mechanisms by which this compartmentalization process occurs have been a major focus of study. However, how compartmentalization is maintained throughout development has not been as thoroughly examined. We have utilized two separate lines of investigation in order to explore this aspect of sporulation.
At the time of asymmetric division, only the origin-proximal one-third of a chromosome is present in the prespore (44). The spoIIIE gene encodes a DNA translocase that pumps the rest of the chromosome destined for the prespore into this compartment after asymmetric division (5, 42). In addition to their defects in chromosome partitioning, spoIIIE mutants display two different phenotypes with regard to σF activity. Class I mutants that produce wild-type levels of mutant protein exhibit compartmentalized σF activity that is approximately five times higher than that of the wild type (5). In contrast, class II mutants, which include null mutants, exhibit uncompartmentalized σF activity (23, 42-44). Since SpoIIIE is targeted to the center of the asymmetric septum (38, 43), it has been suggested that in the absence of SpoIIIE, a hole that allows the contents of the prespore and the mother cell to mix is present in the septum (43). In contrast, other studies have demonstrated that β-galactosidase cannot diffuse across the septum of a class II spoIIIE mutant (22, 34), and it has been proposed that a protein phosphatase required for σF activation, SpoIIE (3, 10), persists in the mother cell and activates σF in this compartment (34).
In addition, it has been reported that mutations in the spoIIG operon, encoding the precursor of the mother cell-specific transcription factor σE and its inferred cognate-processing enzyme (16, 17, 25, 27), restore compartmentalization of σF activity to a class II spoIIIE mutant (22, 34, 45). Since mutants deficient in σE activation undergo asymmetric division at both poles of the cell (15), the SpoIIE protein, which was thought to be largely confined to the prespore face of the asymmetric septum when a single septum was formed, was proposed to be confined to the two prespore compartments since no SpoIIE was present in the central compartment of the organisms with two polar septa (22, 45). However, the second septum is formed sometime after the first (29), making it hard to account for sequestration in both prespores without an intermediate stage of monoseptate organisms with some SpoIIE in the mother cell. Moreover, a separate study has since strongly challenged the finding that SpoIIE is largely confined to the prespore face of the asymmetric septum (18). Therefore, another explanation is required as to why the interruption of σE activation restores compartmentalization of σF activity to a null mutant of spoIIIE.
We have revisited these problems and have found that green fluorescent protein (GFP) can diffuse across the septum of a null mutant of spoIIIE, indicating that the loss of compartmentalization of σF observed in this mutant results from the mixing of the contents of the two compartments. In addition, we have found that the abolition of σE activity restores compartmentalization of σF activity to a spoIIIE mutant because it prevents the synthesis of SpoIID and/or SpoIIP, two putative peptidoglycan hydrolases produced in the mother cell and required for the engulfment of the prespore by the mother cell (13, 29, 35). These results indicate that in class II spoIIIE mutants, σE activity can result in a loss of compartmentalization of σF activity. In contrast, we found that under other conditions of increased σF activity, the activation of σE is important for maintaining compartmentalization of σF activity. Therefore, σE can have contrasting effects during sporulation: the degradation of the asymmetric septum that can result in a loss of compartmentalization of gene expression in spoIIIE mutants and the inhibition of σF activity in the mother cell in order to maintain compartmentalization. We conclude that precise regulation of σE activity is important for efficient sporulation.
MATERIALS AND METHODS
Media.
B. subtilis was grown in modified Schaeffer's sporulation medium (MSSM) or on Schaeffer's sporulation agar (30, 36). When required, the medium contained chloramphenicol at a concentration of 5 μg/ml, erythromycin at 1.5 μg/ml, neomycin at 3.5 μg/ml, spectinomycin at 100 μg/ml, or tetracycline at 10 μg/ml. Escherichia coli was grown on Luria-Bertani agar containing ampicillin at a concentration of 100 μg/ml.
Strains and plasmids.
B. subtilis 168 strain BR151 (trpC2 metB10 lys-3) was used as the parent strain. Other B. subtilis strains and plasmids used in this study are listed in Table 1. E. coli strain DH5α (Gibco-BRL) was used to maintain plasmids. To generate a spoIIQ-gfp fusion that would integrate at the thrC locus, we modified pVK208, encoding a spoIIQ-lacZ fusion in the vector pDG793, designed to integrate at the thrC locus by double crossover (a gift from P. Stragier, Institut de Biologie Physico Chimique, Paris, France). pVK208 was digested with BamHI, releasing the lacZ gene, and the linearized vector was then ligated with a BamHI-BamHI fragment from the pGreenTIR vector (26), carrying the gfpmut1 gene with an enhanced ribosome-binding site; gfpmut1 encodes the GFPmut1 protein. Restriction digestion was used to confirm that the spoIIQ promoter and the gfpmut1 gene were in the same orientation. The resulting plasmid, named pDH8, was used to insert the spoIIQ-gfpmut1 transcriptional fusion into the thrC locus by double crossover.
TABLE 1.
B. subtilis strains and plasmids used
| Plasmid or strain | Relevant characteristic(s) | Origin or reference |
|---|---|---|
| Plasmids | ||
| pDH6 | spoIIQ-gfpmut1 neo in pGEM-T vector (Promega) | 14 |
| pDH8 | thrC::spoIIQ-gfpmut1 | This study |
| pDH9 | spoIIE-uvGFP | This study |
| Strains | ||
| BR151 | trpC2 metB10 lys-3 | Laboratory stock |
| SL10206 | trpC2 metB10 lys-3 spoIIQ::pDH6b,c | |
| SL10257 | trpC2 metB10 lys-3 thrC::spoIIQ-gfpmut1 | This study |
| SL10260 | trpC2 metB10 lys-3 spoIIIE::spc thrC::spoIIQ-gfpmut1 | This study |
| SL10566 | trpC2 metB10 lys3 spoIIQ::pDH6b,c | This study |
| SL11811 | trpC2 metB10 lys-3 spoIIE::pDH9 spoIIQ::pDH6b | This study |
| SL11812 | trpC2 metB10 lys-3 spcaspoIIEV697A::pDH9bspoIIQ::pDH6b | This study |
| SL11818 | trpC2 metB10 lys-3 spcaspoIIEV697A spoIIQ::pDH6b | This study |
| SL11939 | trpC2 metB10 lys-3 spoIIGB::erm spcaspoIIEV697A::pDH9bspoIIQ::pDH6b | This study |
| SL11940 | trpC2 metB10 lys-3 spoIIGB::erm spoIIE::pDH9bspoIIQ::pDH6b | This study |
| SL11954 | trpC2 metB10 lys-3 spoIIGB::erm spoIIQ::pDH6b | This study |
| SL11955 | trpC2 metB10 lys-3 spoIIGB::erm spcaspoIIEV697A spoIIQ::pDH6b | This study |
| SL11975 | trpC2 metB10 lys-3 spoIIIE36 thrC::spoIIQ-gfpmut1 | This study |
| SL11978 | trpC2 metB10 lys-3 spoIIQ::pDH6bspoIIIE::spc | This study |
| SL11979 | trpC2 metB10 lys-3 spoIIQ::pDH6bspoIIIE36 | This study |
| SL12003 | trpC2 metB10 lys-3 spoIIGB::neo spoIIIE::spc spoIIQ::pDH6b | This study |
| SL12005 | trpC2 metB10 lys-3 spoIIGB::neo spoIIQ::pDH6b | This study |
| SL12035 | trpC2 metB10 lys-3 spoIIP::tet spoIIIE::spc spoIIQ::pDH6b | This study |
| SL12065 | trpC2 metB10 lys-3 spoIID::neo spoIIP::tet spoIIIE::spc spoIIQ::pDH6b | This study |
| SL12070 | trpC2 metB10 lys-3 spoIID::neo spoIIIE::spc spoIIQ::pDH6b | This study |
Gene is directly upstream of spoIIE.
Plasmid has been inserted by Campbell (single-crossover) integration.
SL10206 and SL10566 have different resistance genes associated with pDH6: erm and cat, respectively.
To generate a spoIIE-uvgfp translational fusion, pSG1902, encoding a C-terminal SpoIIE-GFP translational fusion (45) (a gift from J. Errington, Oxford University, Oxford, United Kingdom), was digested with XhoI and PstI, releasing the 3′ end of the spoIIE gene. This fragment was ligated into pSG1156, encoding uvGFP with an upstream multiple-cloning site and an in-frame linker (21) (a gift from P. Lewis, University of Newcastle, Newcastle, New South Wales, Australia), cut with the same enzymes. The resulting plasmid, named pDH9, integrated into the 3′ end of the spoIIE gene by single crossover and generated a fusion gene encoding an in-frame C-terminal fusion of SpoIIE with uvGFP. uvGFP is a spectral variant of GFP (9).
Fluorescence microscopy.
Cultures used for visualization of GFP and FM4-64 staining were grown in MSSM at 37°C. Two hundred microliters of culture was mixed with 2 μl of FM4-64 (Molecular Probes) that had been previously diluted to 1 mg/ml in phosphate-buffered saline (Gibco-BRL). Samples were incubated at 37°C for 30 min, and 1 μl of unfixed sample was transferred to a slide and visualized essentially as described previously (33). Images were captured by using a FluoView 300 confocal scanning laser microscope with an UPLAPO 100× oil immersion objective and FluoView imaging software (Olympus America Inc.). The fluorographs shown are projection images generated from stacks of 8 to 10 images in the Z plane, with each one separated by 0.3 μm.
Other methods.
B subtilis transformation, sporulation by exhaustion in MSSM, and all other methods were performed essentially as described previously (30, 31, 46).
RESULTS
GFP can diffuse through the septum of a spoIIIE null mutant.
Class II spoIIIE mutants of B. subtilis have previously been reported to exhibit uncompartmentalized σF activity (23, 42-44). Since it has been reported that SpoIIIE localizes to the center of the asymmetric septum (38, 43), it has been suggested that the uncompartmentalized σF activity occurred because a hole present in the septum allowed the contents of the prespore and mother cell to mix (43). Other studies have found that β-galactosidase could not diffuse through the septa of class II spoIIIE mutant cells (22, 34), and it was concluded that the persistence of the protein phosphatase SpoIIE in the mother cell was responsible for the loss of compartmentalization (34).
In order to distinguish between these possibilities, we generated a series of strains bearing different alleles of spoIIIE and σF-dependent spoIIQ-gfpmut1 (referred to as spoIIQ-gfp hereinafter) transcriptional fusions at different sites in the chromosome (Table 1). Previous work has indicated that transcription of spoIIQ is under the control of σF and is confined to the prespore (24). Since only the origin-proximal one-third of a chromosome is trapped in the prespore in spoIIIE mutants (44), the spoIIQ-gfp reporter fusion will become active only if it is present in this region of the chromosome. We have integrated a fusion either at the spoIIQ locus, which is present in the prespore of a spoIIIE mutant, or at the thrC locus, which is absent from this compartment (44). In addition, we have used two mutant alleles of spoIIIE, spoIIIE36, a class I mutation that does not affect compartmentalization of σF activity, and spoIIIE::spc, a class II mutation that results in uncompartmentalized σF activity (23, 42-44). The strains were induced to sporulate by nutrient exhaustion in MSSM, and 6 h after the end of exponential growth, samples were stained with FM4-64 to visualize asymmetric septa (33) and cells expressing GFP were scored with respect to both septation and location of the GFP signal; under these conditions, a period of 6 h is required for high levels of σF activity to be observed in the sporulating population.
Strains bearing a wild-type spoIIIE allele and the spoIIQ-gfp fusion at either spoIIQ (SL10566) or thrC (SL10257) exhibited prespore-specific GFP, with at least 95% of the GFP-expressing cells falling into this category (Fig. 1 and Table 2). When a strain bearing the spoIIIE36 class I allele and the spoIIQ-gfp fusion at spoIIQ (SL11979) was analyzed, a similar pattern was observed, with about 95% of the GFP-expressing cells exhibiting a prespore-specific signal (Fig. 1 and Table 2). Analysis of a strain with the spoIIIE36 class I allele and the spoIIQ-gfp fusion at the thrC locus (SL11975) revealed that less than 2% of the cells expressed the fusion, compared to about 35% of the cells with the fusion at this chromosomal location and a wild-type spoIIIE allele (SL10257). These data reinforce previous results showing that class I spoIIIE mutants are not impaired in compartmentalization and that the thrC locus is excluded from the prespore in the mutants (44).
FIG. 1.
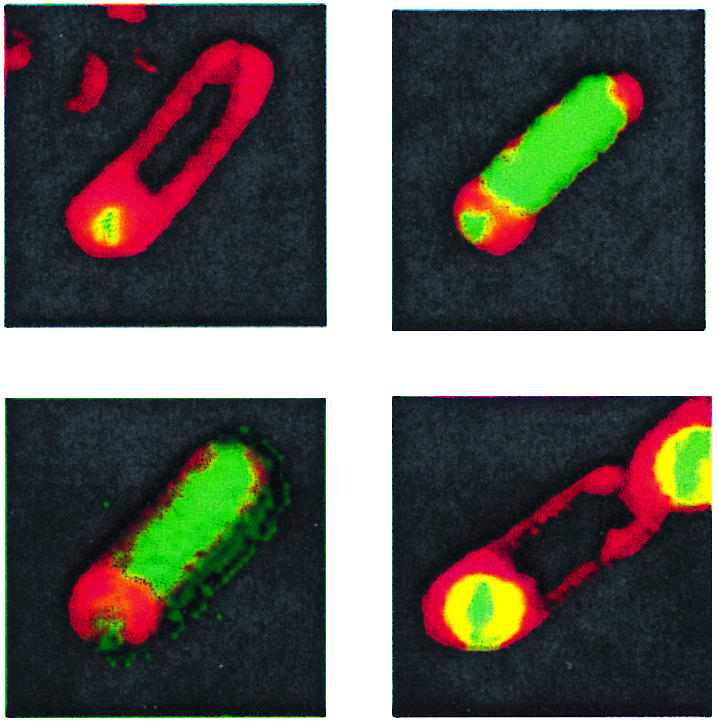
Pattern of σF activation in B. subtilis strains containing different spoIIIE alleles. Bacteria were induced to sporulate in MSSM, and samples were taken 6 h after the start of sporulation and stained with FM4-64 to visualize the cell membrane. The upper left panel depicts a spo+ cell with the spoIIQ-gfp fusion located at the thrC locus (SL10257). The upper right panel depicts a class II spoIIIE mutant (spoIIIE::spc) with the spoIIQ-gfp fusion at spoIIQ (SL11978). The lower left panel depicts a cell with a class II spoIIIE mutation (spoIIIE::spc) and the spoIIQ-gfp fusion at thrC (SL10260). The lower right panel depicts a cell with a class I spoIIIE mutation (spoIIIE36) and the spoIIQ-gfp fusion at spoIIQ (SL11979); also visible is the prespore compartment of a second cell in the upper right portion of the image. In all four images, the prespore compartment is in the lower-left-hand portion of each panel.
TABLE 2.
Compartmentalization of σF activity in spoIIIE mutant strains
| Strain | spoIIIE allele | Location of spoIIQ-gfpa | % Expressionb | Pattern of GFP fluorescencec
|
|||
|---|---|---|---|---|---|---|---|
| % Prespore | % Mother cell | % Prespore and mother cell | % Aseptate cell | ||||
| SL10566 | Wild type | spoIIQ (PS) | 72 ± 18 | 100 | 0 | 0 | 0 |
| SL10257 | Wild type | thrC (MC) | 35 ± 7.0 | 98 ± 3.5 | 0 | 2.0 ± 1.7 | 0 |
| SL11979 | spoIIIE36 | spoIIQ (PS) | 55 ± 13 | 95 ± 2.3 | 0 | 4.7 ± 2.3 | 0 |
| SL11975 | spoIIIE36 | thrC (MC) | 0.67 ± 1.2 | ND | ND | ND | ND |
| SL11978 | spoIIIE::spc | spoIIQ (PS) | 19 ± 1.2 | 7.3 ± 6.1 | 0 | 85 ± 7.6 | 8.0 ± 2.0 |
| SL10260 | spoIIIE::spc | thrC (MC) | 13 ± 3.1 | 1.3 ± 1.2 | 2.3 ± 1.3 | 88 ± 9.2 | 9.3 ± 11 |
MC indicates a mother cell, and PS indicates a prespore. In spoIIIE+ strains, thrC is initially in the mother cell but is transferred to the prespore.
Percentage of the total population expressing the GFP fusion.
The pattern of fluorescence was determined for cells expressing GFP 6 h after the initiation of sporulation in MSSM. Septa were visualized by staining with FM4-64. The data are the means of results from three independent experiments and show the standard deviations (with the qualification that the maximum obtained was 100% and the minimum obtained was 0%). Fifty cells were scored in each experiment. ND, not determined.
When a strain bearing the class II spoIIIE::spc allele and the fusion at spoIIQ (SL11978) was analyzed, it was found that only 7.3% ± 6.1% of the GFP-expressing cells compartmentalized σF activity to the prespore. About 85% of the cells had GFP present on both sides of the asymmetric septum (Fig. 1 and Table 2), indicating that either σF had become active in both compartments or that the GFP made in one compartment had diffused into the other. Again, this result reinforces previous results (23, 42-44). Since the spoIIQ locus is present in both the prespore and the mother cell in a spoIIIE mutant (44), the GFP observed in the two compartments might have originated from the reporter present within them.
If the loss of compartmentalization of spoIIQ-gfp resulted from a persistence of the SpoIIE phosphatase in the mother cell (34), then one could anticipate that when the spoIIQ-gfp reporter was absent from the prespore, GFP would be present only in the mother cell and not in the prespore because it would be produced only in the mother cell and could not cross the asymmetric septum. However, if a hole is present in the septum, then one would expect GFP to be present in the prespore even when no reporter gene was present in this compartment because of diffusion from the neighboring mother cell. A strain having a null allele of spoIIIE and the spoIIQ-gfp reporter at the thrC locus (SL10260) displayed GFP fluorescence in both the prespore and the mother cell in 88% ± 9.2% of the cells in which fluorescence was detected; only 2.3% ± 1.3% of the cells had GFP only in the mother cell (Fig. 1 and Table 2). That is, the GFP produced in the mother cell from the reporter gene integrated at thrC was capable of diffusing across the septum of a class II spoIIIE mutant. We conclude that a small hole is present in the septum and is the most likely cause of the loss of compartmentalization of σF activity in class II spoIIIE mutants, as proposed by Wu and Errington (43).
Blocking σE activation suppresses the spoIIIE compartmentalization phenotype in both monosporic and disporic cells.
During sporulation, B. subtilis has two potential division sites, one near each pole of the cell. SpoIIE, a membrane-bound protein phosphatase critical for σF activation (3, 10), and the essential tubulin homologue FtsZ form ring structures at both of these sites (2, 4, 19, 20). However, normally only one site is used for division. The SpoIIE rings assemble sequentially, and the prespore-distal ring disassembles at the time of, or shortly after, asymmetric division (2, 18, 45). It has been reported that all of the SpoIIE that is present at the asymmetric division site is sequestered onto the prespore face of the septum (22, 45). Although some SpoIIE remains in the mother cell at the other potential asymmetric division site, it was proposed that the sequestration of SpoIIE in the prespore was responsible for confining σF activity to this cell (22, 45). In spoIIIE class II mutants, SpoIIE was found to persist at the second potential asymmetric division site in the mother cell, and this persistence was proposed as the primary cause of the loss of compartmentalization of σF activity in the class II spoIIIE mutants (34). It follows from this proposal that if SpoIIE is removed from the mother cell of spoIIIE null mutant cells, then compartmentalization should be restored.
Certain spo mutants undergo a second asymmetric division in the mother cell, resulting in a three-chambered structure consisting of two prespores separated by a larger central mother cell compartment (29). This abortively disporic phenotype has been found to be the consequence of the blocking of σE activation (15), which in turn prevents the expression of proteins that inhibit division in the mother cell (11, 33). If the act of asymmetric division sequesters the SpoIIE that is located at the asymmetric division site into the prespore (22, 45), then SpoIIE should be completely depleted from the central compartment of abortively disporic cells that have undergone asymmetric division at both poles. Therefore, one could predict that the introduction of a mutation that generates the abortively disporic phenotype in a spoIIIE null (class II) mutant might increase compartmentalization of σF activity because the offending SpoIIE has been removed from the central compartment. Indeed, it has been reported that mutations in the spoIIG operon that prevent σE activation restore compartmentalization of σF activity to class II spoIIIE mutants (22, 34, 45).
However, our finding that GFP can diffuse across the septum of spoIIIE null mutants (Table 2) is at odds with this model and strongly supports a much simpler model in which the contents of the prespore and mother cell mix. Therefore, we investigated the relationship between σE activity and compartmentalization in light of our new findings. We generated strains bearing either wild-type or null alleles of spoIIGB (encoding pro-σE) (16) and spoIIIE and containing spoIIQ-gfp at either spoIIQ or thrC (Table 1). The cells from these populations were analyzed in the same fashion as described for the previous experiment.
We found that a strain bearing wild-type spoIIIE, a deletion of spoIIGB, and the spoIIQ-gfp reporter at spoIIQ (SL12005) exhibited prespore-specific GFP in 96.6% ± 2.4% of the GFP-fluorescent cells (Table 3). When the isogenic strain bearing the spoIIIE::spc allele (SL12003) was analyzed, we found a similar pattern, with 91.3% ± 8.4% of the cells exhibiting a prespore-specific pattern of expression (Table 3). This pattern stands in stark contrast to that of the isogenic spoIIIE::spc strain with a wild-type spoIIGB allele (SL11978) in which only 7.3% ± 6.1% of the GFP-expressing cells had prespore-specific GFP (Table 2). Therefore, the inhibition of σE activation restored compartmentalization of σF activity to a class II spoIIIE mutant, as reported previously (22, 34, 45).
TABLE 3.
Compartmentalization of σF activity in strains with mutant spoIIIE and spoIIGB alleles
| Strain | Relevant genotype | Pattern of GFP fluorescencea
|
|||
|---|---|---|---|---|---|
| % Prespore
|
% Prespore and mother cell | % Aseptate cell | |||
| Monosporic | Disporic | ||||
| SL12005 | spoIIGB::neo wild-type spoIIIE | 54 ± 6.9 | 43 ± 6.1 | 0.67 ± 1.2 | 2.7 ± 1.2 |
| SL12003 | spoIIGB::neo spoIIIE::spc | 26 ± 4.0 | 65 ± 7.6 | 4.7 ± 3.1 | 4.0 ± 5.3 |
The pattern of fluorescence was determined for cells expressing GFP 6 h after the initiation of sporulation in MSSM. Septa were visualized by staining with FM4-64. The data are the means of results from three independent experiments and show the standard deviations (with the qualification that the maximum obtained was 100% and the minimum obtained was 0%). Fifty cells were scored in each experiment.
Strains with spoIIG disrupted first from one septum, and then some but not all of that population formed a second septum (15, 29, 33). Consequently, a sporulating culture of spoIIG mutants contains both monosporic and disporic cells blocked in sporulation after asymmetric septation. We scored GFP-expressing cells as being either monosporic or disporic based on FM4-64 staining. We found that 26% ± 4% of the GFP-fluorescent cells in the spoIIGB spoIIIE double mutant population that had activated σF had done so in the prespore of a monosporic cell; 65% ± 7.6% had GFP present in both prespores of a disporic cell and 4.7% ± 3.1% of the population was monosporic with GFP present in both the prespore and the mother cell (Table 3). If the mechanism of suppression were dependent upon the disporic phenotype, we would expect to find most monosporic cells with uncompartmentalized σF activity. This is not the case, since about 26% of the population was monosporic with compartmentalized σF activity, and only about 4.7% of the cells were monosporic with uncompartmentalized σF activity. In addition, we would expect all of the cells exhibiting compartmentalized σF activity to be disporic. Although most cells exhibiting compartmentalized σF activity were indeed disporic (65% ± 7.6%), a substantial proportion were monosporic (26% ± 4%) (Table 3). Therefore, there must be some other explanation for why the prevention of σE activity restores compartmentalization of σF activity to a class II spoIIIE mutant (22, 34, 45).
The expression of σE-controlled engulfment proteins disrupts compartmentalization of σF activity in class II spoIIIE mutant cells.
Our results indicate that, together, the absence of SpoIIIE and the presence of σE activity disrupt the compartmentalization of σF activity through degeneration of the asymmetric septum (Tables 2 and 3). One plausible explanation for these results is that the septum of class II spoIIIE mutants is altered in some way so that although it is initially intact, it is especially sensitive to some σE-dependent activity that results in degradation. During sporulation, the asymmetric septum is thinned by the removal of peptidoglycan (28), which presumably increases its flexibility so that it can migrate around the prespore during engulfment, the next stage of sporulation. There are three proteins essential for engulfment that are thought to be directly involved in septal thinning: SpoIID, SpoIIM, and SpoIIP (1, 7, 13, 29, 40). They are all expressed in the mother cell under the control of σE (13, 35, 41) and localize to the engulfing septum (1, 11). Recently, SpoIID has been reported to degrade the bacterial cell wall in vitro (1). The properties associated with these proteins make them candidates to be the σE-dependent cause of septum permeability in spoIIIE null mutants.
In order to test our hypothesis that one or more of these proteins permeabilized the septum of spoIIIE null mutant cells, we generated strains containing the spoIIIE::spc allele, a σF-directed spoIIQ-gfp reporter at the spoIIQ locus, and either wild-type or null alleles of spoIID and/or spoIIP. We then analyzed these strains as in the previous experiments. In the spoIIIE spoIIP double mutant (SL12035), we found that 71% ± 11% of the GFP-expressing cells had a prespore-specific GFP signal (Table 4), which was a dramatic increase over the 7.3% ± 6.1% of cells with a prespore-specific signal observed in a spoIIIE single mutant (SL11978) (Table 2). Analysis of the spoIID spoIIIE double mutant (SL12070) revealed an even greater increase, with 88% ± 6.9% of the GFP-expressing cells displaying a prespore-specific signal (Table 4). Finally, the spoIIIE spoIID spoIIP triple mutant (SL12065) exhibited almost total compartmentalization of σF activity, with greater than 97% of the fluorescent cells exhibiting prespore-specific GFP (Table 4). These results indicate that the action of SpoIID and/or SpoIIP in the mother cell is the direct cause of septum permeability and the resulting uncompartmentalized σF activity observed in class II spoIIIE mutants.
TABLE 4.
Compartmentalization of σF activity in strains with different spoIIIE spoIID and spoIIP alleles
| Strain | Relevant genotype | Pattern of GFP fluorescencea
|
||
|---|---|---|---|---|
| % Prespore | % Prespore and mother cell | % Aseptate cell | ||
| SL12035 | spoIIP::tet spoIIIE::spc | 71 ± 11 | 28 ± 11 | 0.67 ± 1.2 |
| SL12070 | spoIID::neo spoIIIE::spc | 88 ± 6.9 | 12 ± 6.9 | 0 |
| SL12065 | spoIID::neo spoIIP::tet spoIIIE::spc | 99 ± 2.3 | 0.67 ± 1.2 | 0.67 ± 1.2 |
The pattern of fluorescence was determined for cells expressing GFP 6 h after the initiation of sporulation in MSSM. Septa were visualized by staining with FM4-64. The data are the means of results from three independent experiments and show the standard deviations (with the qualification that the maximum obtained was 100% and the minimum obtained was 0%). Fifty cells were scored in each experiment.
σE activity can be important for the maintenance of compartmentalization of σF activity in spoIIIE+ strains.
Based upon the above results, we concluded that in the absence of SpoIIIE, the activity of σE could disrupt compartmentalization of σF activity (Table 4). We next wanted to broaden our investigation to determine if there were other situations in which σE activation affected compartmentalization of σF activity. Because of redundancies in determinants of compartmentalization (32), we examined conditions under which compartmentalization of σF activity was partly impaired.
Previous work in our laboratory had resulted in the isolation and characterization of the spoIIEV697A mutation that causes excessive, uncompartmentalized σF activity, a severe impairment of asymmetric division, and impaired spore formation (14). Ongoing studies revealed that by fusing GFP to the C terminus of SpoIIEV697A, sporulation could be partially restored (data not shown). In order to investigate how this modification affected the compartmentalization of σF activity, we generated a translational, C-terminal fusion of SpoIIE to uvGFP, a spectral variant of GFP (9) (see Materials and Methods). Although both GFPmut1 and uvGFP emit at the same wavelength (508 nm), they can be excited independently because of different excitation peaks (488 nm for GFPmut1 and 395 nm for uvGFP) (21). Therefore, we could utilize the spoIIQ-gfp transcriptional fusion to study compartmentalization of σF activity in strains expressing the SpoIIE-uvGFP fusion without an interfering signal from the fusion protein.
Strains containing a spoIIQ-gfp fusion and expressing either native SpoIIE, native SpoIIEV697A, SpoIIE-uvGFP, or SpoIIEV697A-uvGFP were induced to sporulate, and samples were analyzed as in previous experiments. The strain expressing native SpoIIE (SL10206) or SpoIIE-uvGFP (SL11811) exhibited almost exclusively prespore-specific spoIIQ-gfp expression (100 and 98 to 100%, respectively) (Table 5). In contrast, only 6.0% ± 3.5% of the cells from the strain expressing native SpoIIEV697A (SL11818) had a prespore-specific signal, with 86% ± 4% of the cells exhibiting GFP but no septa (Table 5). This result is consistent with that of a previous report in which the spoIIEV697A mutation severely impaired asymmetric division and caused uncompartmentalized σF activity (14). However, the strain expressing SpoIIEV697A-uvGFP (SL11812) exhibited a prespore-specific signal in 66% ± 10% of the cells (Table 5); this strain still exhibited a substantial proportion of cells with no septum and hence, uncompartmentalized σF activity (30% ± 9%) (Table 5). Although the molecular basis for this partial suppression remains under investigation, we thought that examination of such a “leaky” mutant might provide insight into the nature of compartmentalization of gene expression.
TABLE 5.
Compartmentalization of σF activity in strains with different spoIIE and spoIIGB alleles
| Strain | Relevant genotype | Pattern of GFP fluorescencea
|
||
|---|---|---|---|---|
| % Prespore | % Prespore and mother cell | % Aseptate cell | ||
| SL10206 | Wild-type spoIIE wild-type spoIIGB | 100 | 0 | 0 |
| SL11954 | Wild-type spoIIE spoIIGB::erm | 100 | 0 | 0 |
| SL11811 | spoIIE-uvgfp wild-type spoIIGB | 99 ± 1.2 | 0.67 ± 1.2 | 0 |
| SL11940 | spoIIE-uvgfp spoIIGB::erm | 99 ± 1.2 | 0 | 0.67 ± 1.2 |
| SL11818 | spoIIEV697A wild-type spoIIGB | 6.0 ± 3.5 | 8.0 ± 2.0 | 86 ± 4.0 |
| SL11955 | spoIIEV697A spoIIGB::erm | 2.7 ± 4.6 | 13 ± 4.2 | 85 ± 1.2 |
| SL11812 | spoIIEV697A-uvgfp Wild-type spoIIGB | 66 ± 10 | 4.0 ± 3.5 | 30 ± 9.2 |
| SL11939 | spoIIEV697A-uvgfp spoIIGB::erm | 28 ± 11 | 31 ± 6.1 | 41 ± 5.0 |
The pattern of fluorescence was determined for cells expressing GFP 6 h after the initiation of sporulation in MSSM. Septa were visualized by staining with FM4-64. The data are the means of results from three independent experiments and show the standard deviations (with the qualification that the maximum obtained was 100% and the minimum obtained was 0%). Forty-nine or 50 cells were scored in each experiment.
One plausible scenario is that the activity of σE in the mother cell helps to prevent the activation of σF in this compartment in the spoIIEV697A-uvgfp mutant. In order to test this possibility, we generated strains that contained different alleles of spoIIE in the spoIIGB::erm background along with a spoIIQ-gfp transcriptional fusion. We found that preventing σE activity in strains containing either wild-type spoIIE (SL11954) or spoIIE-uvgfp (SL11940) had no effect on compartmentalization, with 100 and 98 to 100% of the population exhibiting a prespore-specific GFP signal, respectively (Table 5). However, a substantial effect was observed in the strain expressing SpoIIEV697A-uvGFP when the proportion of fluorescing cells showing activation of σF in both compartments rose from 4% ± 3.5% in the wild-type spoIIGB background (SL11812) to 31% ± 6% in the spoIIGB::erm background (SL11939) (Table 5). At the same time, the proportion of the GFP-expressing population exhibiting a prespore-specific signal decreased from 66% ± 10% to 28% ± 11% when spoIIGB was inactivated in the spoIIEV67A-uvgfp background (Table 5). We conclude that, under these circumstances, σE action is important to confine σF activity to the prespore.
DISCUSSION
Compartmentalization of σF activity during sporulation has been a major focus of study (32). However, mutants that are defective in compartmentalization of σF activity have rarely been reported. Deletion of the anti-σ factor SpoIIAB causes uncompartmentalized σF activation that prevents asymmetric division and severely impairs sporulation (8, 37). Certain mutations in the protein phosphatase SpoIIE cause a similar, although less severe, phenotype (12, 14; K. Carniol and R. Losick, personal communication). However, only one class of mutation has been reported to cause the activation of σF in both the prespore and the mother cell following asymmetric division, that being the class II spoIIIE mutants, which include null mutants (23, 44-44). SpoIIIE is a DNA translocase (5, 42) that localizes to the center of the asymmetric septum (38, 43), and it has been postulated that in its absence, a small hole is present in the septum that allows the contents of the prespore and mother cell to mix (43). However, other studies have reported that β-galactosidase cannot diffuse across the septum of a class II spoIIIE mutant, thereby casting the leaky septum hypothesis in doubt (22, 34). An alternate explanation was that the SpoIIE protein, which is critical for σF activation (3, 10), persists in the mother cells of those mutants and as a result, activates σF in this compartment (34).
In order to gain greater insight into how σF activity is compartmentalized and to distinguish between the two models, we analyzed the expression of σF-dependent transcriptional GFP fusions in sporulating cells that had been stained with the fluorescent membrane stain FM4-64 (33). We found that when such a fusion was integrated into a site in the chromosome that is excluded from the prespore in a class II spoIIIE mutant, GFP could be detected in both the prespore and the mother cell. Since no reporter gene was present in the prespore, we concluded that GFP could diffuse across the septa of these mutant cells (Table 2). The most plausible explanation for the loss of compartmentalization in this class of mutants is that the contents of the prespore can leak into the mother cell, in accordance with the interpretation of Wu and Errington (43). In light of this evidence, the alternate explanation (34), that the persistence of SpoIIE in the mother cell results in σF activation, appears unlikely. It is important to note that in the studies that did not detect a hole in the septum, compartmentalization was assayed by immunostaining for β-galactosidase (22, 34), which is tetrameric and relatively large (540 kDa) (39). In contrast, we utilized GFP, which is monomeric and relatively small (only 31 kDa) (6). We infer that the hole in the septum of class II spoIIIE mutant cells is large enough to permit the transit of GFP and also σF and/or SpoIIAA, but not β-galactosidase.
The loss of compartmentalization of σF activity in class II spoIIIE mutants requires an intact spoIIG locus, which includes the structural gene for pro-σE (22, 34, 45). We have shown that the expression of the σE-directed spoIID and spoIIP loci is critical for this effect. The SpoIID and SpoIIP proteins are thought to be responsible for the removal of peptidoglycan from the spore septum during engulfment (28). SpoIID and SpoIIP are produced in the mother cell and have been shown to degrade spore septum peptidoglycan and partial septa and to impair asymmetric division when they are expressed prematurely (7, 11, 13, 34, 35, 40, 41). In addition, SpoIID has recently been shown to degrade the bacterial cell wall in vitro (1). We think that it is likely that the SpoIID- and SpoIIP-directed peptidoglycan degradation, which occurs normally during engulfment, resulted in holes in the septa of class II spoIIIE mutants. Our interpretation is that because SpoIIIE is absent, a very small hole is initially present in the septum of a class II spoIIIE mutant cell, but that GFP, or a factor critical for σF activity, cannot diffuse through it. However, the action of SpoIID and SpoIIP (and possibly that of other engulfment proteins) results in the enlargement of the hole until GFP, and presumably either active σF or the anti-anti-σ factor SpoIIAA, or both, can diffuse through it. Consistent with this interpretation, we have observed that the loss of compartmentalization of σF activity in class II spoIIIE mutant cells is progressive, in that the defect becomes more pronounced as a function of time (unpublished data).
The observation that σE activity might disrupt compartmentalization of σF activity in spoIIIE null mutants led us to search for other circumstances where gene expression in one compartment can affect the compartmentalization of a σ factor in the other. We have found that, when compartmentalization of σF activity is slightly compromised in a SpoIIEV697A-uvGFP mutant, the inactivation of σE greatly exacerbates the loss of compartmentalization (Table 5). We think that the most likely explanation for this phenomenon is that after asymmetric division in the spoIIEV697A-uvgfp cells, the action of σE helps to prevent the activation of σF in the mother cell. We propose two different explanations for this phenomenon. The first is that σE activation prevents σF activation in the mother cell by simply absorbing RNA core polymerase. The second is that there is a specific mechanism by which the product(s) of the σE-dependent gene(s) impairs σF activation or activity. Proteolysis of SpoIIAA, SpoIIE, and/or σF is a likely possibility.
In summary, we have examined two long-standing questions in the literature and provided answers to both of them. In the first case, we have demonstrated that GFP can cross the septa of class II spoIIIE mutant cells, providing evidence for a hole, or pore, in the septum. Such a pore is the most likely explanation for the uncompartmentalized σF activity observed in these cells. In the second case, we have addressed why the inhibition of σE activity restores compartmentalization of σF activity to class II spoIIIE mutant cells. We have shown that in the absence of one or more engulfment proteins, SpoIID and SpoIIP, the septa of these mutant cells retain their integrity. We have also identified quite a different situation where the activation of σE in the mother cell is important to prevent the activation of σF in this compartment. Therefore, we conclude that σE has two roles that can lead to opposite effects on compartmentalization during spore formation: the degradation of peptidoglycan during engulfment, which disrupts the septa of class II spoIIIE mutant cells and leads to uncompartmentalized σF activity, and, in the spoIIE mutant strain, the prevention of σF activation in the mother cell through an as-yet-uncharacterized mechanism (Fig. 2). We conclude that σE activity must be carefully regulated in order to maintain compartmentalization of σF activity during sporulation.
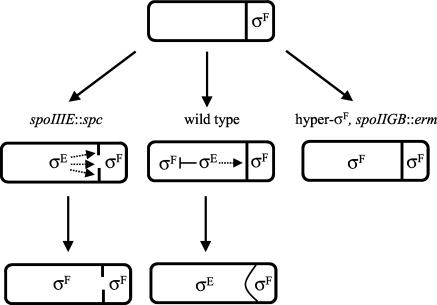
FIG. 2.
Diagram of the conflicting roles of σE activity on σF compartmentalization during sporulation. The top cell shows σF becoming active in the prespore following asymmetric division. Depicted directly below the top cell is a spo+ (wild type) organism in which σE activity results in the inhibition of σF in the mother cell and the regulated degradation (represented by a single dotted arrow) of the asymmetric septum, thereby triggering engulfment. Depicted to the left of the spo+ organism is a class II spoIIIE mutant (spoIIIE::spc) in which σE activation results in excessive degradation (represented by multiple dotted arrows) of the asymmetric septum. This degradation leads to the formation of a hole through which either SpoIIAA and/or σF travels, resulting in uncompartmentalized σF activity. Although not depicted in this diagram, σE remains active in this mutant, albeit at a low level (22), and this activity is partly uncompartmentalized as well (34). Depicted on the right-hand side of the diagram is a spoIIGB mutant strain (hyper-σF, spoIIGB::erm) that is unable to activate σE. When σF activity is partly deregulated in this background, the result is the activation of σF in the mother cell.
Acknowledgments
We thank J. Errington, A. Grossman, M. Karow, P. Lewis, R. Losick, and P. Stragier for kindly providing plasmids and strains.
This work was supported by Public Health Service grants GM43577 (to P.J.P.) and T32AI07101 (to D.W.H.) from the National Institutes of Health.
REFERENCES
- 1.Abanes-De Mello, A., Y. L. Sun, S. Aung, and K. Pogliano. 2002. A cytoskeleton-like role for the bacterial cell wall during engulfment of the Bacillus subtilis forespore. Genes Dev. 16:3253-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arigoni, F., K. Pogliano, C. D. Webb, P. Stragier, and R. Losick. 1995. Localization of protein implicated in establishment of cell type to sites of asymmetric division. Science 270:637-640. [DOI] [PubMed] [Google Scholar]
- 3.Arigoni, F., L. Duncan, S. Alper, R. Losick, and P. Stragier. 1996. SpoIIE governs the phosphorylation state of a protein regulating transcription factor σF during sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 93:3238-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barák, I., J. Behari, G. Olmedo, P. Guzman, D. P. Brown, E. Castro, D. Walker, J. Westpheling, and P. Youngman. 1996. Structure and function of the Bacillus SpoIIE protein and its localization to sites of sporulation septum assembly. Mol. Microbiol. 19:1047-1060. [DOI] [PubMed] [Google Scholar]
- 5.Bath, J., L. J. Wu, J. Errington, and J. C. Wang. 2000. Role of Bacillus subtilis SpoIIIE in DNA transport across the mother cell-prespore division septum. Science 290:995-997. [DOI] [PubMed] [Google Scholar]
- 6.Chalfie, M. 1995. Green fluorescent protein. Photochem. Photobiol. 62:651-656. [DOI] [PubMed] [Google Scholar]
- 7.Coote, J. G. 1972. Sporulation in Bacillus subtilis. Characterization of oligosporogenous mutants and comparison of their phenotypes with those of asporogenous mutants. J. Gen. Microbiol. 71:1-15. [DOI] [PubMed] [Google Scholar]
- 8.Coppolecchia, R., H. DeGrazia, and C. P. Moran, Jr. 1991. Deletion of spoIIAB blocks endospore formation in Bacillus subtilis at an early stage. J. Bacteriol. 173:6678-6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crameri, A., E. A. Whitehorn, E. Tate, and W. P. Stemmer. 1996. Improved green fluorescent protein by molecular evolution using DNA shuffling. Nat. Biotechnol. 14:315-319. [DOI] [PubMed] [Google Scholar]
- 10.Duncan, L., S. Alper, F. Arigoni, R. Losick, and P. Stragier. 1995. Activation of cell-specific transcription by a serine phosphatase at the site of asymmetric division. Science 270:641-644. [DOI] [PubMed] [Google Scholar]
- 11.Eichenberger, P., P. Fawcett, and R. Losick. 2001. A three-protein inhibitor of polar septation during sporulation in Bacillus subtilis. Mol. Microbiol. 42:1147-1162. [DOI] [PubMed] [Google Scholar]
- 12.Feucht, A., L. Abbotts, and J. Errington. 2002. The cell differentiation protein SpoIIE contains a regulatory site that controls its phosphatase activity in response to asymmetric septation. Mol. Microbiol. 45:1119-1130. [DOI] [PubMed] [Google Scholar]
- 13.Frandsen, N., and P. Stragier. 1995. Identification and characterization of the Bacillus subtilis spoIIP locus. J. Bacteriol. 177:716-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilbert, D. W., and P. J. Piggot. 2003. Novel spoIIE mutation that causes uncompartmentalized σF activation in Bacillus subtilis. J. Bacteriol. 185:1590-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Illing, N., and J. Errington. 1991. Genetic regulation of morphogenesis in Bacillus subtilis: roles of σE and σF in prespore engulfment. J. Bacteriol. 173:3159-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonas, R. M., E. A. Weaver, T. J. Kenney, C. P. Moran, Jr., and W. G. Haldenwang. 1988. The Bacillus subtilis spoIIG operon encodes both σE and a gene necessary for σE activation. J. Bacteriol. 170:507-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenney, T. J., and C. P. Moran, Jr. 1987. Organization and regulation of an operon that encodes a sporulation-essential sigma factor in Bacillus subtilis. J. Bacteriol. 169:3329-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King, N., O. Dreesen, P. Stragier, K. Pogliano, and R. Losick. 1999. Septation, dephosphorylation, and the activation of σF during sporulation in Bacillus subtilis. Genes Dev. 13:1156-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin, P. A., and R. Losick. 1996. Transcription factor Spo0A switches the localization of the cell division protein FtsZ from a medial to a bipolar pattern in Bacillus subtilis. Genes Dev. 15:478-488. [DOI] [PubMed] [Google Scholar]
- 20.Levin, P. A., R. Losick, P. Stragier, and F. Arigoni. 1997. Localization of the sporulation protein SpoIIE in Bacillus subtilis is dependent upon the cell division protein FtsZ. Mol. Microbiol. 25:839-846. [DOI] [PubMed] [Google Scholar]
- 21.Lewis, P. J., and A. L. Marston. 1999. GFP vectors for controlled expression and dual labelling of protein fusions in Bacillus subtilis. Gene 227:101-110. [DOI] [PubMed] [Google Scholar]
- 22.Lewis, P. J., L. J. Wu, and J. Errington. 1998. Establishment of prespore-specific gene expression in Bacillus subtilis: localization of SpoIIE phosphatase and initiation of compartment-specific proteolysis. J. Bacteriol. 180:3276-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, Z., and P. J. Piggot. 2001. Development of a two-part transcription probe to determine the completeness of temporal and spatial compartmentalization of gene expression during bacterial development. Proc. Natl. Acad. Sci. USA 98:12538-12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Londõno-Vallejo, J. A., C. Fréhel, and P. Stragier. 1997. spoIIQ, a forespore-expressed gene required for engulfment in Bacillus subtilis. Mol. Microbiol. 24:29-39. [DOI] [PubMed] [Google Scholar]
- 25.Masuda, E. S., H. Anaguchi, T. Sato, M. Takeuchi, and Y. Kobayashi. 1990. Nucleotide sequence of the sporulation gene spoIIGA from Bacillus subtilis. Nucleic Acids Res. 18:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, W. G., and S. E. Lindow. 1997. An improved GFP cloning cassette designed for prokaryotic transcriptional fusions. Gene 191:149-153. [DOI] [PubMed] [Google Scholar]
- 27.Peters, H. K., III, and W. G. Haldenwang. 1994. Isolation of a Bacillus subtilis spoIIGA allele that suppresses processing-negative mutations in the pro-σE gene (sigE). J. Bacteriol. 176:7763-7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piggot, P. J., J. E. Bylund, and M. L. Higgins. 1994. Morphogenesis and gene expression during sporulation, p. 113-137. In P. J. Piggot, C. P. Moran, Jr., and P. Youngman (ed.), Regulation of bacterial differentiation. American Society for Microbiology, Washington, D.C.
- 29.Piggot, P. J., and J. G. Coote. 1976. Genetic aspects of bacterial endospore formation. Bacteriol. Rev. 40:908-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piggot, P. J., and C. A. M. Curtis. 1987. Analysis of the regulation of gene expression during Bacillus subtilis sporulation by manipulation of the copy number of spo-lacZ fusions. J. Bacteriol. 169:1260-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piggot, P. J., C. A. Curtis., and H. de Lencastre. 1984. Use of integrational plasmid vectors to demonstrate the polycistronic nature of a transcriptional unit (spoIIA) required for sporulation of Bacillus subtilis. J. Gen. Microbiol. 130:2123-2136. [DOI] [PubMed] [Google Scholar]
- 32.Piggot, P. J., and R. Losick. 2002. Sporulation genes and intercompartmental regulation, p. 483-518. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 33.Pogliano, J., N. Osborne, M. D. Sharp, A. Abanes-De Mello, A. Perez, Y. L. Sun, and K. Pogliano. 1999. A vital stain for studying membrane dynamics in bacteria: a novel mechanism controlling septation during Bacillus subtilis sporulation. Mol. Microbiol. 31:1149-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pogliano, K., A. E. M. Hofmeister, and R. Losick. 1997. Disappearance of the σE transcription factor from the forespore and the SpoIIE phosphatase from the mother cell contributes to establishment of cell-specific gene expression during sporulation in Bacillus subtilis. J. Bacteriol. 179:3331-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rong, S., M. S. Rosenkrantz, and A. L. Sonenshein. 1986. Transcriptional control of the Bacillus subtilis spoIID gene. J. Bacteriol. 165:771-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaeffer, P., J. Millet, and J. P. Aubert. 1965. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. USA 54:704-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt, R., P. Margolis, L. Duncan, R. Coppolecchia, C. P. Moran, Jr., and R. Losick. 1990. Control of developmental transcription factor sigma F by sporulation regulatory proteins SpoIIAA and SpoIIAB in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 87:9221-9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharp, M. D., and K. Pogliano. 1999. An in vivo membrane fusion assay implicates SpoIIIE in the final stages of engulfment during Bacillus subtilis sporulation. Proc. Natl. Acad. Sci. USA 96:14553-14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shuman, H. A., and T. J. Silhavy. 2003. The art and design of genetic screens: E. coli. Nat. Rev. Genet. 4:419-431. [DOI] [PubMed] [Google Scholar]
- 40.Smith, K., M. E. Bayer, and P. Youngman. 1993. Physical and functional characterization of the Bacillus subtilis spoIIM gene. J. Bacteriol. 175:3607-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith, K., and P. Youngman. 1993. Evidence that the spoIIM gene of Bacillus subtilis is transcribed by RNA polymerase associated with σE. J. Bacteriol. 175:3618-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu, L. J., and J. Errington. 1994. Bacillus subtilis SpoIIIE protein required for DNA segregation during asymmetric cell division. Science 264:572-575. [DOI] [PubMed] [Google Scholar]
- 43.Wu, L. J., and J. Errington. 1997. Septal localization of the SpoIIIE chromosome partitioning protein in Bacillus subtilis. EMBO J. 16:2161-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu, L. J., and J. Errington. 1998. Use of asymmetric cell division and spoIIIE mutants to probe chromosome orientation and organization in Bacillus subtilis. Mol. Microbiol. 27:777-786. [DOI] [PubMed] [Google Scholar]
- 45.Wu, L. J., A. Feucht, and J. Errington. 1998. Prespore-specific gene expression in Bacillus subtilis is driven by sequestration of SpoIIE phosphatase to the prespore side of the asymmetric septum. Genes Dev. 12:1371-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, L., M. L. Higgins, P. J. Piggot, and M. L. Karow. 1996. Analysis of the role of prespore gene expression in the compartmentalization of mother cell-specific gene expression during sporulation of Bacillus subtilis. J. Bacteriol. 178:2813-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]