Abstract
Type 1 diabetes (insulin-dependent, IDDM) results in immune-mediated destruction of pancreatic β cells, which leads to a deficiency in insulin secretion and as a result, to hyperglycaemia. Keeping blood glucose levels under tight control represents the most effective way either to prevent the onset or to reduce the progression of the chronic complications of IDDM. At present, pancreatic islet transplantation is emerging as the most promising clinical modality, which can stop diabetes progression without increasing the incidence of hypoglycaemic events. Although early results of clinical trials using the Edmonton Protocol and its variations are very encouraging, it is still unclear how long the islets will survive and how often the transplantation procedure will be successful. In order to monitor transplantation efficiency and graft survival, reliable non-invasive imaging methods are critically needed. If such methods are introduced clinically, essential information regarding the location, function and viability of transplanted islets can be obtained repeatedly and non-invasively. This review will focus on the latest advancements in the field of in vivo imaging of islet transplantation and describe various islet labelling and imaging techniques. In addition, we will critically look into limitations and obstacles currently present on the way to successful clinical implementation of this approach.
Keywords: contrast agent, in vivo imaging, islet labelling, islet transplantation, magnetic resonance imaging
Islet Transplantation
Islet transplantation has emerged as one of the most promising new treatments for diabetes. For more than 30 years scientists have tried to restore normoglycaemia by transplanting healthy islets into diabetic recipients. The earliest reports of islet transplantation in animal models appeared in 1972 [1]. The first clinical islet allograft was performed in 1974 [2]. Over the next 25 years, numerous attempts were made to achieve normoglycaemia in type 1 diabetic patients using islet transplantation, with limited success. Although some functionality was achieved, as evidenced by C-peptide, the majority of cases failed to demonstrate insulin independence or long-term engraftment [2]. In 1999, a group from the University of Alberta developed an improved protocol for islet transplantation with reproducible success in terms of insulin independence [3]. The success of this new protocol, named the Edmonton protocol, is based mainly on the enhanced immunosupression regimen compared to previous protocols and on the improved islet delivery strategy, namely, intraportal infusion of freshly isolated islets, followed by a second or third infusion of additional islets [2].
At present, intraportal infusion is the only method used in human trials that leads to insulin independence. As of 2003, there were a total of 282 sites throughout the world actively engaged in islet transplantation. The long-term functionality and viability of transplanted islets are unknown. In a 5-year follow-up report from the Edmonton group, it is emphasized that the results, though promising, still point to the need for improving islet engraftment and preserving islet function [4]. Even in optimal conditions and in the absence of graft rejection, approximately 60% of transplanted islet tissue is lost 3 days after transplantation, because of cell death [5,6]. Despite the 80% success rate of the Edmonton protocol at one year post-transplantation, by 2 years, this number may decline to approximately 65% [7]. A more recent international trial using the Edmonton protocol showed that only 44% of the patients receiving islet transplantation remained insulin independent at one year post-transplant [8].
It is not clear at this point to what extent this decline in insulin independence is the result of islet loss and what the mechanisms are behind the deterioration of graft function. Some possibilities include auto- or alloimmune destruction, immunosuppressive toxicity, or a progressive disruption of insulin secretion [9]. It is becoming clear that one of the most critical points that need to be resolved is the viability and long-term functionality of the transplant. Furthermore, because post-transplantation islet mass is a critical element of the potential for glucose stabilization and insulin independence in islet graft recipients, some important relevant questions need to be addressed. For example, it remains unresolved why, even when insulin independence is attained, there are signs of marginal islet mass, and what the time-course is of loss of islet mass in the transplant. Clearly, successful monitoring of the stability and functionality of the graft would also permit testing of the effectiveness of various immunosuppressive regimens, as well as islet delivery strategies and ultimately assist the further optimization of the islet transplantation procedure.
In addition to these questions, almost nothing is known about the structure and function of islets following transplantation. In a normal pancreas, islet microvasculature is complex. It consists of arterioles splitting into capillaries within the islet core, leaving the islet through the islet mantle, and draining into the portal system. During transplantation, the islet microvasculature is severely disrupted. Virtually no information is available concerning islet revascularization and function following transplantation. Besides the observed blood glucose normalization, very little is known about other aspects of islet function, i.e. the participation of glucagon, somatostatin and pancreatic polypeptide in the regulation of metabolism [10].
Functional Assessment of Islet Transplant: Current Status
It is clear by now that an effective approach to islet graft assessment following transplantation is urgently needed. However, currently there are no clinically relevant tools to achieve that goal. The functionality of the graft is evaluated by indirect methods. The major criteria of graft function include indicators of metabolic control, such as fasting and stimulated glucose levels, oral glucose tolerance testing, C-peptide levels, HbA1c levels, mean amplitude glycaemic excursions and insulin secretion. In addition, complications associated with introduction of the graft are assessed both by means of evaluating symptoms of rejection, such as glutamic acid decarboxylase antibodies and islet cell autoantibodies, and signs of toxicity or impairment of liver function [11].
All of these strategies rely on indirect assessment of metabolic parameters. The mechanisms behind islet function, however, represent a fine tuned network of molecular events. Because islets upregulate their insulin production in response to need, abnormalities in glucose, C-peptide and insulin release do not become apparent until most islets have already been destroyed [12–14]. Consequently, these parameters only provide information on the late stage of graft rejection. Therefore, it is critical to develop non-invasive imaging techniques that can determine the fate of the transplant and assess its functional status, viability and ultimately, graft outcome after transplantation.
The only direct functional assessment of transplant fate that is currently available is performed through liver biopsy. As of right now, imaging modalities such as ultrasound, computerized tomography (CT) and magnetic resonance imaging (MRI) have been used to identify abnormalities in liver tissue, following transplantation. Several reports have established the occurrence of hepatic steatosis, or fatty liver, following islet transplantation [15–17]. All of these diagnoses are based on the detection of intrahepatic lesions on CT, MRI and ultrasound scans. The association between graft function and liver steatosis, however, is highly controversial. Markmann et al. concluded that despite the presence of steatosis, graft function at the time of imaging was not compromised, based on the metabolic criteria described above. Because the dissemination of these lesions appears to match the distribution of infused islets, it has been postulated that the appearance of steatosis is the result of local insulin secretion by the graft [17]. It has also been speculated that the appearance of steatosis reflects an abnormal local utilization of insulin, which may indicate or result in graft dysfunction [15,16].
None of these strategies image the islet graft directly and, therefore, their conclusions can be affected by various processes such as excess glycogen accumulation, oedema, inflammation, etc. [18]. It is crucial, therefore, to develop direct methods for imaging of the graft in order to assess its stability over time, as well as to devise novel, non-invasive, reliable strategies for evaluation of graft viability and function.
Optical Imaging of Transplanted Islets
The first reports of non-invasive imaging of transplanted islets utilized optical imaging as a method for detection of islet grafts. In vivo optical imaging methods originated from previously developed in vitro and ex vivo microscopic techniques based on fluorescence, absorption, reflectance, or bioluminescence as a source of contrast. Imaging systems can be based on diffuse optical tomography, surface-weighted imaging (reflectance diffuse tomography), phase-array detection, confocal imaging, multiphoton imaging or microscopic imaging with intravital microscopy. Progress in optical molecular imaging strategies has come from the recent development of targeted bioluminescence probes, near-infrared fluorochromes, activatable near-infrared fluorochromes and red-shifted fluorescent proteins [19]. In addition, the development of the sensitive charged coupled device (CCD) has made it possible to detect light in the visible to near-infrared range. CCD cameras are used for all kinds of optical imaging and have proven to be highly reproducible, whenever the exposure conditions are kept identical [20]. The equipment for optical imaging is overall inexpensive, and space- and time-efficient compared to other imaging modalities. Furthermore, the advantages of optical imaging methods include the use of non-ionizing low energy radiation, high sensitivity with the possibility of detecting micron-sized objects and continuous data acquisition in real time and in an intact biological environment. Fluorescence optical imaging utilizes various fluorochromes (dyes, fluorescent proteins, etc.), whereas bioluminescence imaging (BLI) exploits the reaction between luciferase and its substrate, luciferin. The family of luciferase enzymes consists of proteins that can generate visible light through the oxidation of an enzyme-specific substrate in the presence of oxygen and, usually, ATP as a source of energy. During these reactions, part of the chemical energy is released as visible light. A significant advantage of luciferases as optical indicators in live mammalian cells and tissues is the inherently low background, given the near absence of endogenous light from mammalian cells and tissues. Luciferase has therefore become a frequently used reporter in many optical imaging applications [21].
In contrast to positron-emission tomography (PET) optical imaging has a limited depth penetration but, in the case of bioluminescence optical imaging, high sensitivity. In fluorescence imaging, an excitation light of one wavelength (in the visible light range of 395–600 nm) illuminates the living subject, and a CCD camera collects emission light of a shifted wavelength. Cells tagged with fluorescently labelled ligands or expressing fluorescent proteins can be detected by this technique. The two main advantages of the latter are that florescent proteins (e.g. green fluorescent protein) can be visualized as reporters in both live and fixed cells/tissues and that no substrate is required for their visualization. Certain drawbacks include difficulties in quantitation as well as the surface- weighted nature of the image (objects closer to the surface will appear brighter than deeper structures [19]).
The very first report utilizing fluorescence optical imaging came from Dr. M. Trucco’s laboratory. In this investigation, transgenic mice were engineered to express proinsulin II fused with a live-cell fluorescent reporter protein, Timer [22]. Timer protein is unique in its ability to change colour from green to red in the first 24 h after synthesis. Therefore, insulin synthesis can be easily monitored through the changes in fluorescence over time. Islets expressing this construct were transplanted into the recipient mice, and a body window device was inserted to monitor insulin synthesis as well as the migration and replication of exogenous T cells to these islets. Using optical imaging, the monitoring of both insulin-producing cells and T cells may be carried out repeatedly without recurrent surgery, while preserving the life of the studied animal. The combination of Timer monitoring of insulin production with the body window technique holds promise for future investigations that involve the tracking of T cell graft infiltration, as well as islet behaviour and insulin secretion after transplantation.
The earliest reports of the non-invasive imaging of transplanted islets using the bioluminescence optical imaging modality came independently from several laboratories [23–26]. These studies established proof-of-principle that isolated rodent or human islets can be genetically engineered to express luciferase without affecting their function and be imaged following transplantation in immunocompromized mice using BLI. Because the resulting signal was proportional to the number of transplanted islets, this strategy could be used to non-invasively evaluate islet mass. In addition, the feasibility of long-term monitoring of islets transplanted under the kidney capsule was tested using recombinant lentivirus vectors expressing luciferase under the cytomegalovirus (CMV) promoter. In these studies, luciferase signals emanating from the graft remained stable for at least 140 days, indicating graft stability in this model of transplantation [25]. Dr. A. Powers and his colleagues extended these studies to investigate factors influencing bioluminescence signal [27]. They found that variables such as post-surgical effects, animal positioning, light attenuation and transplant site should be accounted for in BLI. More recently, Fowler et al. [26] extended the application of BLI to tracking the fate of transplanted islets at the hepatic site, which is the only clinically applied location for islet transplantation. The same group has recently reported on the application of BLI for assessing the biocompatibility of alginate-based capsules in islet transplantation [28]. In addition to these feasibility studies, a recent paper described a more comprehensive investigation into the relative effects of immune rejection on functional islet mass [29]. Islets obtained from a transgenic mouse strain, which constitutively expresses firefly luciferase, were transplanted to various implantation sites of syngeneic or allogeneic streptozotocin-induced diabetic recipients. Whereas in isografts stable luminescence intensity signals remained consistent for up to 18 months after transplantation, in allografts, graft bioluminescent intensity progressively decreased several days before the permanent recurrence of rejection-induced hyperglycaemia. As the authors discuss, whereas very valuable from a research perspective, the results of this imaging approach need to be interpreted cautiously, keeping in mind the fact that the BLI signal can be influenced by multiple factors besides functional islet mass, such as serum glucose levels, mouse position, surgical and motion artefacts, etc.
Clearly, BLI, which was initially used for the simple detection of transplanted islets, has now become a useful laboratory tool for evaluating new therapies and for the development of new approaches to improve islet survival. General limitations of this modality include the absence of a clinical equivalent and the fact that it is only appropriate for cells in a superficial locality, as emission is limited from tissue over 0.5 cm deep [20].
PET Imaging of Transplanted Islets
PET imaging has so far been the only modality applied to transplanted islet imaging in humans (described below).
PET is an example of radionuclide imaging, a procedure that records rays emitted from within the subject. A variety of molecules can be labelled with a positron-emitting isotope (15O, 13N, 11C and 18F, etc.). Most of them are produced in a cyclotron and have relatively short halflives. Labelled molecular imaging probes or tracers can be introduced into the subject (animal or human). PET imaging can follow the distribution and concentration of these injected molecules [30]. Gamma-emitting isotopes (e.g., 99mTc, 111In, 123I, 131I) are used in single photon emission computed tomography (SPECT). This technique requires different types of scanners known as gamma cameras, which when rotated around the subject result in the production of tomographic images [31]. After acquiring a signal, the reconstruction software produces an image, which ‘portrays’ the region of interest that was scanned. The sensitivity of PET is relatively high (10−11–10−12 mol/l), which is at least a log order more sensitive than SPECT, and is independent of the location depth of the reporter probe of interest. Because a positron-emitting isotope is capable of producing two γ-rays of the same energy through emission of a positron from its nucleus, it is not possible for PET to distinguish between two probes labelled with different isotopes and used simultaneously. To investigate multiple molecular events, it would be necessary to allow one probe to decay prior to administration of the other. However, multiple isotopes with various energy γ-rays can be used in SPECT. The spatial resolution of most clinical PET scanners is 3–12 mm, resulting from a combination of factors. The number and geometry of detectors in the scanner as well as the number of counts acquired in the image and their statistical imprecision each reduce PET image resolution. These aspects vary between tomographs of different design as well as from study to study, owing to varying image-acquisition times and tissue-radioactivity levels. The ultimate theoretical limit of PET resolution, however, is the distance travelled by the positron in tissue before the annihilation reaction.
The first report regarding the application of radionuclide imaging to monitor transplanted pancreatic islets came from a collaboration between Drs. D. Kaufman and S. Gambhir. In this study, islets were transduced with an Adeno-Tkm adenovirus engineered to express a mutant herpes simplex virus type 1 thymidine kinase driven by the CMV promoter (sr39tk) [32,33]. Mice were subjected to micro-PET imaging after injection of its substrate [18F]FHBG. The signals from sr39tk-expressing islets declined substantially over the first weeks after transplantation and were at the background level 40 days after the procedure. The authors suggest that the loss of the signal could be because of cellular death shortly after transplantation and the transient expression of adenovirally directed reporter genes. Subsequently, the same group has investigated the value of using lentiviral transduction, which is stable and can be used for the long-term monitoring of transplanted islet fate [34]. In islets implanted into the axillary cavity, signal decreased by approximately one-half during the first few weeks after transplantation, followed by stabilization over 90 days, suggesting significant islet cell death in the immediate post-transplant period. A similar technique was later used to follow the expression of a therapeutic gene (interleukin-10), which prolonged the lifetime of islets transplanted under the kidney capsule of diabetic NOD mice [35]. A major issue in applying these imaging methods, however, is that they are based on transgenic modification and represent a highly artificial imaging strategy, lacking an equivalent applicable to human studies, thus preventing direct translation to clinical use [20].
In a clinically relevant approach for the PET imaging of early post-transplant events, rodent islets were labelled with 2-[18F]fluoro-2-deoxy-d-glucose (FDG) and implanted in the livers of syngeneic rats and monitored for up to 6 h [36]. The authors of this study claimed that all infused islets implanted in the liver after intraportal infusion. Because similar clearance in liver radioactivity was observed in animals infused with FDG-labelled islets and in animals injected with FDG alone, the authors concluded that no islet loss could be identified during this 6-h period. However, because it is not clear how long the islets stay labelled with FDG, the observed phenomenon could be because of islets releasing FDG naturally or because of damage. In fact, in a set of more recent preclinical and clinical studies, islet damage was reported as the reason for observing only ~50% of the administered radioactivity in the liver at the end of islet infusion in humans [37] and in non-human primates [38]. As in the previous study, it is not entirely clear whether this is a consequence of islet damage or FDG leakage from the islets. A significant problem associated with this method, however, is the short half-life of 18F (110 min), which considerably limits its applicability for long-term monitoring of post-transplant events in a clinical or research setting.
MRI of Transplanted Islets
MRI is a modality which does not utilize ionizing radiation, has tomographic capabilities, can deliver the highest- resolution images in vivo, and has unlimited depth penetration. To perform MRI, the subject is placed in a strong magnetic field. Visualization is based on the fact that atomic nuclei of certain elements have a magnetic moment that aligns preferentially along the direction of the magnetic field. Applied radio frequency results in excitation with subsequent relaxation of the nuclear spin system, which can be detected and then translated into an MR image. Modern MRI systems are available at near-microscopic resolutions for small animals. Major advantages of MRI are in its high spatial resolution and the ability to acquire physiological and anatomical parameters simultaneously. MRI, however, has intrinsic low sensitivity and various amplification strategies are used to detect the desired molecular or cellular event. Although increasing the field strength of the MR scanner can improve signal-to-noise and resolution, the use of contrast agents can significantly enhance the detection limit even within a relatively low magnetic field. Contrast agents normally act by changing the relaxation rates of neighbouring water molecules and giving positive or negative contrast on T1- or T2-weighted images respectively. A different class of contrast agents relies on magnetization transfer to provide negative contrast. Magnetization transfer and T1- and T2-weighted agents alter some property of water in a catalytic way, but it is still the water that is imaged. Other contrast agents use alternative nuclei such as fluorine or hyperpolarized nuclei such as carbon, helium, or xenon, and these nuclei are imaged directly [39]. T1-weighted contrast agents are typically gadolinium(III) complexes, manganese(II) complexes, or Mn2+ cation. Gd(III) and Mn(II) are used as T1 relaxation agents because their electronic relaxation is slow, the magnetic moment is large and water exchange is typically fast [39]. T2 contrast agents are iron oxide nanoparticles with various coatings (polysaccharide, synthetic polymer, or monomer coating). Superparamagnetic iron oxide (SPIO) nanoparticles have a strong magnetic moment, which makes them an excellent contrast agent. Nanoparticle presence in tissue is evident primarily by a darkening effect on T2-or T2*-weighted MR images. Remarkably, the T2* effect of SPIOs results in a hypointensity footprint many times larger than the labelled entity, in essence constituting a powerful signal amplification tool. SPIOs alone or conjugated to target-specific ligands are widely used in biomedical applications to image cell trafficking [40–42], gene expression [43–46], cancer lesions and their response to therapy [47–50], as well as siRNA delivery [51].
So far, MRI has been the most widely used modality to evaluate the outcome of islet transplantation non-invasively. As mentioned above, native MR imaging has a low overall sensitivity and is not likely to be capable of distinguishing pancreatic islets from surrounding non-pancreatic tissue. However, this drawback can be overcome by the application of contrast agents.
As a first step towards unravelling the reasons behind transplanted islet loss and improving transplantation efficiency and graft survival, our laboratory has recently developed a method for the non-invasive detection and tracking of pancreatic islet grafts by dual-modality fluorescence/MRI [52]. In that study, isolated human pancreatic islets were labelled with SPIO magnetic nanoparticles (MN) modified with the near-infrared fluorescent Cy5.5 dye (MN-NIRF), and transplanted under the kidney capsule in immunocompromized mice. These initial experiments established proof of principle that our method could be used to track labelled transplanted islets for a substantial period of time with the goal of monitoring graft longevity. MN-NIRF-labelled and unlabelled human pancreatic islets were monitored up to 188 days after transplantation under the kidney capsule demonstrating a significant difference in T2 relaxation times between labelled and unlabelled grafts for the duration of the experiment, as well as preservation of graft associated T2 relaxation times, reflecting graft stability and persistence of the label (figure 1). An independent macroscopic confirmation of the presence of the labelled islet graft after transplantation was obtained by near-infrared optical imaging using the same animals. Furthermore, in subsequent experiments we confirmed the functional integrity of the labelled graft in diabetic NOD.scid mice by showing the ability of this graft to restore normoglycaemia. The labelled graft was able to normalize glucose levels as efficiently as its unlabelled counterpart [52].
Fig. 1.
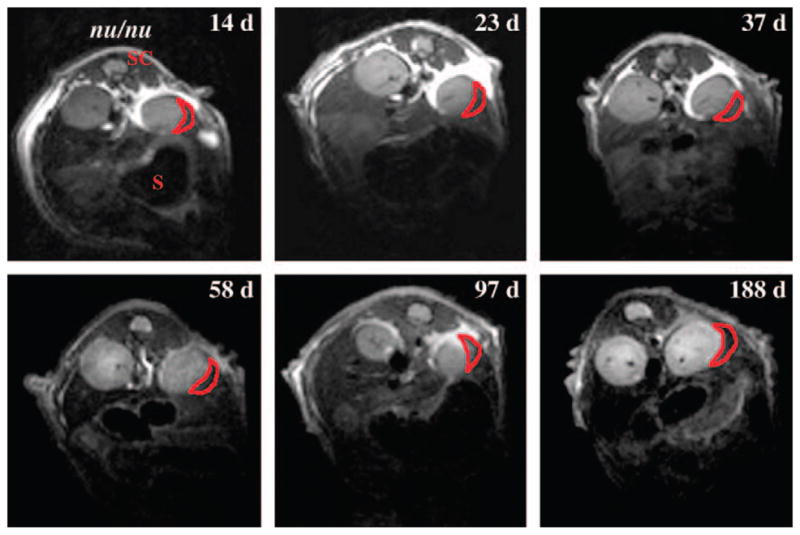
In vivo MRI of islet transplantation under the kidney capsule. Transverse T2-weighted magnetic resonance images of transplanted labeled and non-labelled human islets 14, 23, 37, 58, 97 and 188 days after transplantation under the kidney capsule in nude (nu/nu) mice. The dark area in the left kidney represents a labeled graft (red outline). No darkening was reported for the right kidney with unlabelled graft. S, stomach; SC, spinal cord. Reprinted with permission from Macmillan Publishers Ltd [52].
Because the ultimate goal of these studies is to translate them into clinical trials we further employed a preclinical model of islet transplantation at the hepatic site by intraportal infusion, because currently this is the clinically approved protocol. In addition, for islet labelling we utilized the FDA-approved commercially available contrast agent Feridex, which is routinely used in the clinic for liver imaging. Similar to MN-NIRF, it consists of SPIO covered with a dextran coat. When used for hepatic MRI, the iron is phagocytosed and accumulates in endosomes of Kupffer cells and reticuloendothelial cells [53]. Iron oxide particles are non-toxic, biodegradable and have been used in the clinic as intravenous contrast agents. Human islets labelled with Feridex and transplanted into the liver appeared as dark hypointense foci representing single islets and/or, possibly, islet clusters (figure 2, arrows), whereas nonlabelled islets did not cause any change in signal intensity on T2*-weighted images. Furthermore, we were able to track the fate of intrahepatically transplanted human islets early post-transplantation, which is the most crucial period for islet survival. Interestingly immunocompetent Balb/cmice exhibited a significantly higher rate of islet disappearance on MR images, compared to immunocompromized animals, especially pronounced on day 10 and resulting in a 20% difference in relative islet number by 14 days after transplantation, presumably because of the input from severe immune rejection [54]. Generally, islet loss after transplantation is associated with immune rejection (in allogeneic transplants), mechanical injury, ischaemia, non-alloantigen-specific inflammatory events in the liver after transplantation, and recurrent autoimmunity [55].
Fig. 2.
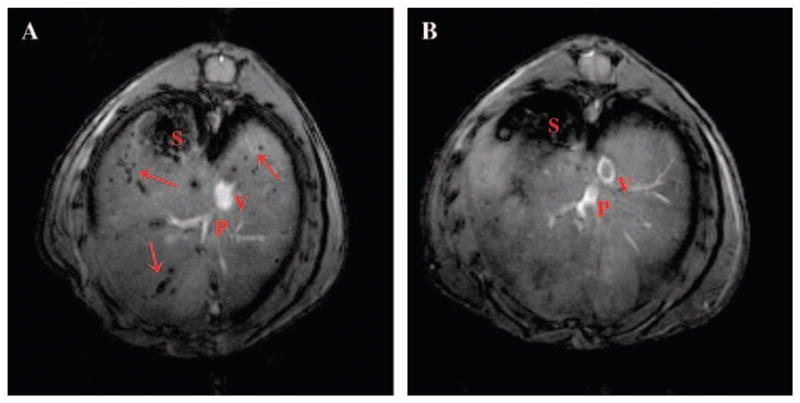
In vivo imaging of intrahepatically transplanted human islets. (A) Representative images of NOD.scid mice with transplanted islets. On in vivo images, Feridex-labeled islets appeared as signal voids scattered throughout the liver. (B) Non-labeled islets were not detectable using the same imaging parameters. Copyright © 2006 American Diabetes Association From Diabetes, Vol. 55, 2006; 2419–2428. Reprinted with permission from The American Diabetes Association.
In order to fully interpret the imaging findings, it is critical to investigate factors normally present during clinical transplantation and influencing MR imaging of transplanted islets. Therefore, in our latest studies we focused on both the effect of hyperglycaemia and the influence of contaminating non-endocrine tissue, which is always present in islet preparations, on MRI of islet grafts. In this study, human pancreatic islets were labelled with Feridex and transplanted in diabetic and healthy animals [56]. Separate groups of animals were transplanted with Feridex-labelled pure and impure (50% islets and 50% non-endocrine tissue) preparations. The fate of the graft in all groups was monitored by in vivo MRI. We found that diabetic animals with transplanted islets showed a significantly higher rate of islet death than their healthy counterparts on in vivo MR images [56]. The most likely reason for this difference is the presence of hyperglycaemia, which is associated with islet cytotoxicity. Previous animal studies have demonstrated that severe hyperglycaemia impairs graft function, and that successful islet transplantation depends on the degree of hyperglycaemia in the recipient [5,57–63]. Interestingly, transplantation of islets contaminated with non-endocrine tissue did not have any significant influence on MR images presumably because of a low labelling rate of this tissue and its rapid clearance after transplantation [56]. In fact, according to our immunohistochemical assessment and morphological results obtained by other investigators, non-islet tissue mostly disappears during the first two weeks post-transplantation, whereas islet tissue remains well preserved [64,65]. This study serves as yet another step on the way to clinical use of in vivo imaging of islet transplantation. Other recent studies relevant to MRI of transplanted pancreatic islets provide further support for the feasibility of the imaging method in the intrahepatic transplantation model, using paramagnetic beads [66], the T1 agent, GdHPDO3A [67] and similar SPIOs at the 1.5-T clinical field strength in a rodent model of islet transplantation [68].
An important prerequisite for islet survival after transplantation includes revasculatization of the islet graft after isolation from the native pancreas. Towards that end the study by Hathout et al. [69] attempted to image neo-vascularization of Feridex-labelled islet grafts under the kidney capsule using dynamic contrast enhanced (DCE) MRI. This type of MR imaging utilizes gadolinium (Gd) as a contrast agent to evaluate dynamic changes in blood flow, blood volume and vascular permeability, among others. Although the idea to monitor vascular changes in transplanted islets deserves special consideration, the technical details of the study contain substantial flaws, which need to be revisited before the results of the study can be taken at face value. As one example, the T2 relaxation times reported for Feridex-labelled islets were longer than those of muscle and kidney, in direct contradiction to the contrast on the actual MR image presented in the study and in conflict with the expected T2-shortening effect of SPIOs. DCE-MRI data of the newly formed blood vessels showed enhancement in the graft on day 3 after transplantation. The authors claim that this is because at this early time the graft vasculature is undeveloped and ‘leaky’. At the same time they stated that ‘no similar enhancement was observed in the surrounding kidney tissue possibly because of rapid clearance of gadolinium from pre-existing intact blood vessels’. However, this is not supported by their experimental data, which show similar enhancement in both regions of interest. Therefore, their statement that systemic injection of gadolinium leads to selective visualization of the graft is unreasonable and unsubstantiated by their own experimental data.
In spite of these issues, the idea of tracking the progress of revascularization in transplanted islets should be investigated further, because clearly the vasculature could be used as a surrogate marker of islet success/failure.
The first study in a large animal model (swine) by Barnett et al. describes the application of immunoptotective magnetocapsules containing ferumoxides, in which human islets are encapsulated [70]. The contrast agent within the capsule permitted the tracking of initial islet-containing magnetocapsule infusion and engraftment into the liver. Though his approach does not permit for direct islet visualization it demonstrated for the first time the utilization of a clinical scanner for detection of single encapsulated islets in large animals facilitated by the higher iron content of the capsules.
Conclusion
The in vivo imaging of islet transplantation is clearly a very rapidly developing field. The introduction of new approaches for the non-invasive visualization, quantitation, and functional assessment of transplanted islet mass would likely provide insight into many of the intricate biological aspects that determine graft fate and its relationship to metabolic control. This technology ultimately holds the unique advantage of being directly translatable to a clinical scenario. The potential benefits that clinical translation of these studies could bring to patients are obvious and include the collection of detailed spatial and temporal information regarding location, quantity, viability and function of transplanted islets. In addition, with the development of new drugs, in vivo imaging would assist in monitoring therapeutic intervention during graft rejection, as well as the design of optimized immunosuppressive regimens and transplantation protocols.
Acknowledgments
This work was supported in part by National Institutes of Health grants (RO1DK072137 and RO1DK078615) to A.M.
Footnotes
Conflict of interest:
The authors declare no conflict of interest.
References
- 1.Hauptman PJ, O’Connor KJ. Procurement and allocation of solid organs for transplantation. N Engl J Med. 1997;336:422–431. doi: 10.1056/NEJM199702063360607. [DOI] [PubMed] [Google Scholar]
- 2.Stock PG, Bluestone JA. Beta-cell replacement for type I diabetes. Annu Rev Med. 2004;55:133–156. doi: 10.1146/annurev.med.55.091902.103539. [DOI] [PubMed] [Google Scholar]
- 3.Bretzel RG, Brandhorst D, Brandhorst H, et al. Improved survival of intraportal pancreatic islet cell allografts in patients with type-1 diabetes mellitus by refined peri-transplant management. J Mol Med. 1999;77:140–143. doi: 10.1007/s001090050322. [DOI] [PubMed] [Google Scholar]
- 4.Ryan E, Paty B, Senior P, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060– 2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 5.Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes. 2002;51:66–72. doi: 10.2337/diabetes.51.1.66. [DOI] [PubMed] [Google Scholar]
- 6.Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Vulnerability of islets in the immediate posttransplantation period: dynamic changes in structure and function. Diabetes. 1996;45:1161–1167. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- 7.Ryan E, Lakey J, Paty B, et al. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51:2148–2157. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 9.Robertson RP. Pancreas and islet transplants for patients with diabetes: taking positions and making decisions. Endocr Pract. 1999;5:24–28. doi: 10.4158/EP.5.1.24. [DOI] [PubMed] [Google Scholar]
- 10.Inverardi L, Kenyon NS, Ricordi C. Islet transplantation: immunological perspectives. Curr Opin Immunol. 2003;15:507–511. doi: 10.1016/s0952-7915(03)00115-8. [DOI] [PubMed] [Google Scholar]
- 11.Ryan E, Lakey J, Rajotte R, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50:710–719. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 12.Pileggi A, Ricordi C, Alessiani M, Inverardi L. Factors influencing islet of Langerhans graft function and monitoring. Clin Chim Acta. 2001;310:3–16. doi: 10.1016/s0009-8981(01)00503-4. [DOI] [PubMed] [Google Scholar]
- 13.Castano L, Eisenbarth G. Type-I diabetes: a chronic autoimmune disease of human, mouse, and rat. Annu Rev Immunol. 1990;8:647–679. doi: 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- 14.Atkinson M, Maclaren N. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1428–1436. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 15.Bhargava R, Senior PA, Ackerman TE, et al. Prevalence of hepatic steatosis after islet transplantation and its relation to graft function. Diabetes. 2004;53:1311–1317. doi: 10.2337/diabetes.53.5.1311. [DOI] [PubMed] [Google Scholar]
- 16.Eckhard M, Lommel D, Hackstein N, et al. Disseminated periportal fatty degeneration after allogeneic intraportal islet transplantation in a patient with type 1 diabetes mellitus: a case report. Transplant Proc. 2004;36:1111–1116. doi: 10.1016/j.transproceed.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 17.Markmann JF, Rosen M, Siegelman ES, et al. Magnetic resonance-defined periportal steatosis following intraportal islet transplantation: a functional footprint of islet graft survival? Diabetes. 2003;52:1591–1594. doi: 10.2337/diabetes.52.7.1591. [DOI] [PubMed] [Google Scholar]
- 18.Garg A, Misra A. Hepatic steatosis, insulin resistance, and adipose tissue disorders. J Clin Endocrinol Metab. 2002;87:3019–3022. doi: 10.1210/jcem.87.7.8736. [DOI] [PubMed] [Google Scholar]
- 19.Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001;219:316–333. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 20.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 21.Contag C, Bachmann M. Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng. 2002;4:235–260. doi: 10.1146/annurev.bioeng.4.111901.093336. [DOI] [PubMed] [Google Scholar]
- 22.Bertera S, Geng X, Tawadrous Z, et al. Body window-enabled in vivo multicolor imaging of transplanted mouse islets expressing as insulin-Timer fusion protein. BioTechniques. 2003;35:718–722. doi: 10.2144/03354st01. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman D, Baker M, Nelson J, Zhang X, Chen X. In vivo, real-time, non-invasive bioluminescent imaging of transplanted islets in a functional murine model. Presented at: Imaging the Pancreatic Beta Cell; Bethesda, MD. 2003. [Google Scholar]
- 24.Powers A, Fowler M, Virostko J, et al. Using bioluminescence to non-invasively image and assess transplanted islet mass. Presented at: Imaging the Pancreatic Beta Cell; Bethesda, MD. 2003. [Google Scholar]
- 25.Lu Y, Dang H, Middleton B, et al. Bioluminescent monitoring of islet graft survival after transplantation. Mol Ther. 2004;9:428–435. doi: 10.1016/j.ymthe.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Fowler M, Virostko J, Chen Z, et al. Assessment of pancreatic islet mass after islet transplantation using in vivo bioluminescence imaging. Transplantation. 2005;79:768–776. doi: 10.1097/01.tp.0000152798.03204.5c. [DOI] [PubMed] [Google Scholar]
- 27.Virostko J, Chen Z, Fowler M, Poffenberger G, Powers AC, Jansen ED. Factors influencing quantification of in vivo bioluminescence imaging: application to assessment of pancreatic islet transplants. Mol Imaging. 2004;3:333–342. doi: 10.1162/15353500200404133. [DOI] [PubMed] [Google Scholar]
- 28.Roth DJ, Jansen ED, Powers AC, Wang TG. Bioluminescent imaging of an NF-kB transgenic mouse model. Transplantation. 2006;81:1185–1190. doi: 10.1097/01.tp.0000203808.84963.13. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Zhang X, Larson CS, Baker MS, Kaufman DB. In vivo bioluminescence imaging of transplanted islets and early detection of graft rejection. Transplantation. 2006;81:1421–1427. doi: 10.1097/01.tp.0000206109.71181.bf. [DOI] [PubMed] [Google Scholar]
- 30.Gambhir S. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 31.Rosenthal M, Cullom J, Hawkins W, Moore S, Tsui B, Yester M. Quantitative SPECT imaging: a review and recommendations by the Focus Committee of the Society of Nuclear Medicine Computer and Instrumentation Council. J Nucl Med. 1995;36:1489–1513. [PubMed] [Google Scholar]
- 32.Lu Y, Dang H, Middleton B, et al. Repetitive microPET imaging of implanted human islets in mice. Presented at: Imaging the Pancreatic Beta Cell; Bethesda, MD. 2003. [Google Scholar]
- 33.Lu Y, Dang H, Middleton B, et al. Noninvasive imaging of islet grafts using positron-emission tomography. Proc Natl Acad Sci USA. 2006;103:11294–11299. doi: 10.1073/pnas.0603909103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu Y, Dang H, Middleton B, et al. Long-term monitoring of transplanted islets using positron emission tomography. Mol Ther. 2006;14:851–856. doi: 10.1016/j.ymthe.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Kim S, Doudet D, Studentov A, et al. Quantitative micro positron emission tomography (PET) imaging for the in vivo determination of pancreatic islet graft survival. Nat Med. 2006;12:1423–1428. doi: 10.1038/nm1458. [DOI] [PubMed] [Google Scholar]
- 36.Toso C, Zaidi H, Morel P, et al. Positron-emission tomography imaging of early events after transplantation of islets of Langerhans. Transplantation. 2005;79:353–355. doi: 10.1097/01.tp.0000149501.50870.9d. [DOI] [PubMed] [Google Scholar]
- 37.Eich T, Eriksson O, Lundgren T. Visualization of early engraftment in clinical islet transplantation by positron- emission tomography. N Engl J Med. 2007;356:2754–2755. doi: 10.1056/NEJMc070201. [DOI] [PubMed] [Google Scholar]
- 38.Eich T, Eriksson O, Sundin A, et al. Positron emission tomography: a real-time tool to quantify early islet engraftment in a preclinical large animal model. Transplantation. 2007;84:893–898. doi: 10.1097/01.tp.0000284730.86567.9f. [DOI] [PubMed] [Google Scholar]
- 39.Caravan P. Physicochemical principles of MR contrast agents. In: Modo M, Bulte J, editors. Molecular and Cellular MR Imaging. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2007. pp. 37–59. [Google Scholar]
- 40.Moore A, Sun P, Cory D, Högemann D, Weissleder R, Lipes M. MR imaging of insulitis in autoimmune diabetes. Magn Reson Med. 2002;47:751–758. doi: 10.1002/mrm.10110. [DOI] [PubMed] [Google Scholar]
- 41.Kraitchman D, Tatsumi M, Gilson W, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1454–1464. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Vries IJ, Lesterhuis WJ, Barentsz JO, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 2005;23:1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 43.Stegman L, Rehemtulla A, Beattie B, et al. Noninvasive quantitation of cytosine deaminase transgene expression in human tumor xenografts with in vivo magnetic resonance spectroscopy. Proc Natl Acad Sci USA. 1999;96:9821–9826. doi: 10.1073/pnas.96.17.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weissleder R, Moore A, Mahmood U, et al. In vivo magnetic resonance imaging of transgene expression. Nat Med. 2000;6:351–354. doi: 10.1038/73219. [DOI] [PubMed] [Google Scholar]
- 45.Loule A, Hyber M, Ahrens E, et al. In vivo visualization of gene expression using magnetic resonance imaging. Nat Biotechnol. 2000;18:321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 46.Moore A, Josephson L, Bhorade R, Basilion J, Weissleder R. Human transferrin receptor gene as a marker gene for MR imaging. Radiology. 2001;221:751–758. doi: 10.1148/radiol.2211001784. [DOI] [PubMed] [Google Scholar]
- 47.Medarova Z, Pham W, Kim Y, Dai G, Moore A. In vivo imaging of tumor response to therapy using a dual-modality imaging strategy. Int J Cancer. 2006;118:2796–2802. doi: 10.1002/ijc.21672. [DOI] [PubMed] [Google Scholar]
- 48.Moore A, Medarova Z, Potthast A, Dai G. In vivo targeting of underglycosylated MUC-1 tumor antigen using a multimodal imaging probe. Cancer Res. 2004;64:1821–1827. doi: 10.1158/0008-5472.can-03-3230. [DOI] [PubMed] [Google Scholar]
- 49.Artemov D, Mori N, Okollie B, Bhujwalla Z. MR molecular imaging of the HER-2/neu receptor in breast cancer cells using targeted iron oxide nanoparticles. Magn Reson Med. 2003;49:403–408. doi: 10.1002/mrm.10406. [DOI] [PubMed] [Google Scholar]
- 50.Artemov D, Mori N, Ravi R, Bhujwalla Z. MR molecular imaging of the HER-2/neu receptor. Cancer Res. 2003;63:2723–2727. [PubMed] [Google Scholar]
- 51.Medarova Z, Pham W, Farrar C, Petkova V, Moore A. In vivo imaging of siRNA delivery and silencing in tumors. Nat Med. 2007;13:372–377. doi: 10.1038/nm1486. [DOI] [PubMed] [Google Scholar]
- 52.Evgenov NV, Medarova Z, Dai G, Bonner-Weir S, Moore A. In vivo imaging of islet transplantation. Nat Med. 2006;12:144–148. doi: 10.1038/nm1316. [DOI] [PubMed] [Google Scholar]
- 53.Ferrucci J, Stark D. Iron oxide-enhanced MR imaging of the liver and spleen: review of the first 5 years. AJR Am J Roentgenol. 1990;1990:943–950. doi: 10.2214/ajr.155.5.2120963. [DOI] [PubMed] [Google Scholar]
- 54.Evgenov NV, Medarova Z, Pratt J, et al. In vivo imaging of immune rejection in transplanted pancreatic islets. Diabetes. 2006;55:2419–2428. doi: 10.2337/db06-0484. [DOI] [PubMed] [Google Scholar]
- 55.Ricordi C, Strom T. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol. 2004;4:259–268. doi: 10.1038/nri1332. [DOI] [PubMed] [Google Scholar]
- 56.Evgenov N, Pratt J, Pantazopoulos P, Moore A. Effects of glucose toxicity and islet purity on in vivo MR imaging of transplanted pancreatic islets. Transplantation. doi: 10.1097/TP.0b013e31816b183e. in press. [DOI] [PubMed] [Google Scholar]
- 57.Davalli A, Scaglia L, Zangen D, Hollister J, Bonner-Weir S, Weir G. Vulnerability of islets in the immediate post-transplantation period. Dynamic changes in structure and function. Diabetes. 1996;45:1161–1167. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- 58.Montana E, Bonner-Weir S, Weir G. Beta-cell mass and growth after syngeneic islet cell transplantation in normal and streptozotocin diabetic C57BL/6 mice. J Clin Invest. 1993;91:780–787. doi: 10.1172/JCI116297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Makhlouf L, Duvivier-Kali VF, Bonner-Weir S, Dieperink H, Weir GC, Sayegh MH. Importance of hyperglycemia on the primary function of allogeneic islet transplants. Transplantation. 2003;76:657–664. doi: 10.1097/01.TP.0000080881.75767.0E. [DOI] [PubMed] [Google Scholar]
- 60.Nacher V, Merino JF, Raurell M, Soler J, Montanya E. Normoglycemia restores beta-cell replicative response to glucose in transplanted islets exposed to chronic hyperglycemia. Diabetes. 1998;47:192–196. doi: 10.2337/diab.47.2.192. [DOI] [PubMed] [Google Scholar]
- 61.Melzi R, Battaglia M, Draghici E, Bonifacio E, Piemonti L. Relevance of hyperglycemia on the timing of functional loss of allogeneic islet transplants: implication for mouse model. Transplantation. 2007;83:167–173. doi: 10.1097/01.tp.0000250659.24286.43. [DOI] [PubMed] [Google Scholar]
- 62.Eizirik DL, Jansson L, Flodstrom M, Hellerstrom C, Andersson A. Mechanisms of defective glucose-induced insulin release in human pancreatic islets transplanted to diabetic nude mice. J Clin Endocrinol Metab. 1997;82:2660–2663. doi: 10.1210/jcem.82.8.4150. [DOI] [PubMed] [Google Scholar]
- 63.Jansson L, Eizirik DL, Pipeleers DG, Borg LA, Hellerstrom C, Andersson A. Impairment of glucose-induced insulin secretion in human pancreatic islets transplanted to diabetic nude mice. J Clin Invest. 1995;96:721–726. doi: 10.1172/JCI118115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Franklin WA, Schulak JA, Reckard CR. The fate of transplanted pancreatic islets in the rat. Am J Pathol. 1979;94:85–95. [PMC free article] [PubMed] [Google Scholar]
- 65.Grotting JC, Rosai J, Matas AJ, et al. The fate of intraportally transplanted islets in diabetic rats. A morphologic and immunohistochemical study. Am J Pathol. 1978;92:653–670. [PMC free article] [PubMed] [Google Scholar]
- 66.Koblas T, Girman P, Berkova Z, et al. Magnetic resonance imaging of intrahepatically transplanted islets using paramagnetic beads. Transpl Proc. 2005;37:3493–3495. doi: 10.1016/j.transproceed.2005.09.142. [DOI] [PubMed] [Google Scholar]
- 67.Biancone L, Crich SG, Cantaluppi V, et al. Magnetic resonance imaging of gadolinium-labeled pancreatic islets for experimental transplantation. NMR Biomed. 2007;20:40–48. doi: 10.1002/nbm.1088. [DOI] [PubMed] [Google Scholar]
- 68.Tai J, Foster P, Rosales A, et al. Imaging islets labeled with magnetic nanoparticles at 1. 5 Tesla. Diabetes. 2006;55:2931–2938. doi: 10.2337/db06-0393. [DOI] [PubMed] [Google Scholar]
- 69.Hathout E, Sowers L, Wang R, et al. In vivo magnetic resonance imaging of vascularization in islet transplantation. Transpl Int. 2007;20:1059–1065. doi: 10.1111/j.1432-2277.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barnett BP, Arepally A, Karmarkar PV, et al. Magnetic resonance-guided, real-time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islet cells. Nat Med. 2007;13:986–991. doi: 10.1038/nm1581. [DOI] [PubMed] [Google Scholar]


