Abstract
The multifunctional enzyme tissue transglutaminase (TG2) contributes to the development and progression of several cardiovascular diseases. Extracellular rather than intracellular TG2 is enzymatically active, however, the mechanism by which it is exported out of the cell remains unknown. Nitric oxide (NO) is shown to constrain TG2 externalization in endothelial and fibroblast cells. Here, we examined the role of both exogenous and endogenous (endothelial cell-derived) NO in regulating TG2 localization in vascular cells and tissue. NO synthase inhibition in endothelial cells (ECs) using N-nitro l-arginine methyl ester (l-NAME) led to a time-dependent decrease in S-nitrosation and increase in externalization of TG2. Laminar shear stress led to decreased extracellular TG2 in ECs. S-nitrosoglutathione treatment led to decreased activity and externalization of TG2 in human aortic smooth muscle and fibroblast (IMR90) cells. Co-culture of these cells with ECs resulted in increased S-nitrosation and decreased externalization and activity of TG2, which was reversed by l-NAME. Aged Fischer 344 rats had higher tissue scaffold-associated TG2 compared to young. NO regulates intracellular versus extracellular TG2 localization in vascular cells and tissue, likely via S-nitrosation. This in part, explains increased TG2 externalization and activity in aging aorta.
Keywords: Aging, Tissue transglutaminase, Nitric oxide, S-nitrosation
Introduction
An increase in central vascular stiffness is pathognomonic of vascular aging and occurs as a result of complex changes that occur in all components of the blood vessel wall, including the endothelium, smooth muscle cells, and matrix (Lakatta and Levy 2003). Increased stiffness of the typically low resistance conduit vessels can lead to diverse vascular pathologies such as atherosclerosis and isolated systolic hypertension, which is associated with age-related adverse outcomes, such as stroke, myocardial infarction (Lakatta and Levy 2003), and renal failure (London and Drueke 1997).
We have recently shown that increased tissue transglutaminase (TG2) cross-linking activity contributes to age-related increases in vascular stiffness (Santhanam et al. 2010). TG2 is highly expressed in all cellular components of the vasculature including endothelial cells, smooth muscle cells, fibroblasts, and monocytes/macrophages (Sane et al. 2007; Bakker et al. 2008). NO is known to inhibit TG2 protein cross-linking function via S-nitrosation (Lai et al. 2001). Indeed, NO reverses TG2-dependent small-artery remodeling in response to vasoconstrictive stimuli (Bakker et al. 2005, 2008; Pistea et al. 2008). We have shown that TG2 is typically S-nitrosated and its cross-linking activity held latent in the young aorta (Santhanam et al. 2010). Diminished NO bioavailability in aging vasculature leads to decreased TG2 S-nitrosation, and consequently, increased cross-linking activity in the extracellular matrix (ECM). An interesting aspect of TG2 biology is that it is predominantly a cytosolic protein; over 80% of total TG2 resides inside the cell in GTP bound form (Begg et al. 2006; Zemskov et al. 2006; Lorand and Graham 2003). However, TG2 is externalized to the cell surface and exported to the ECM through a non-conventional and as yet undefined mechanism, where it catalyzes the classical protein cross-linking (transamidation) reaction (Bakker et al. 2008; Zemskov et al. 2006; Bergamini et al. 2005). Previously, we have shown that NO constrains TG2 externalization in human aortic endothelial cells (HAEC) (Santhanam et al. 2010). A similar phenomenon has been shown in fibroblasts (Telci et al. 2009). Thus, NO can directly inhibit TG2 cross-linking function via S-nitrosation (Lai, et al. 2001) and regulate TG2 by constraining it to the cytosol. In this study, we demonstrate that NOS3-derived NO S-nitrosates and constrains TG2 externalization in HAEC. Moreover, in co-culture studies, NO from HAEC regulates TG2 activity and localization in human aortic smooth muscle cells (HASMCs) and fibroblasts (IMR90). Importantly, aged rats have higher levels of ECM-associated TG2, and attenuating NO bioavailability ex vivo leads to increased TG2 deposition in the ECM and increased cross-linking activity. We thus describe a novel mechanism by which TG2 externalization, and thus its cross-linking function, is regulated in the vasculature.
Materials and methods
Animals
The animal protocols used have been approved by the Institutional Animal Care and Use Committee at the Johns Hopkins University School of Medicine. Eight young rats (3- to 6-month old) and eight old (22- to 24-month old) Fischer 344 rats (National Institutes of Aging) were used in this study. All animals were fed and watered ad libitum.
Cell cultures
HAEC (Invitrogen) were cultured in endothelial cell media [ScienCell Research Labs; ECM media supplemented with 5% fetal bovine serum (FBS), endothelial cell growth supplement, and penicillin/streptomycin]. HASMC (Invitrogen), were cultured in smooth muscle cell media (ScienCell Research Labs; SMCM media supplemented with 2% serum, SMC growth supplement, and penicillin/streptomycin). The human fibroblast cell-line, IMR90, was purchased from ATCC, cultured in DMEM with 10% FBS and penicillin/streptomycin. All cells types were used between passages 7 and 10. For co-culture studies, HASMC or IMR90 cells were seeded in 6-well plates with HAECs in transwell inserts (Millipore). Cells were co-cultured in the presence and absence of l-NAME (100 μM; in both upper and lower chambers) for 18 h.
Shear stress
HAEC were grown to ~80% confluence and subjected to laminar shear stress (LSS) of 18 dynes/cm2 (~400 rpm) for 16 h using a cone-and-plate viscometer as described previously (Santhanam et al. 2010).
TG2 expression
Expression was determined by western blotting using mouse-monoclonal TGase2 antibody (TG 100, Ab-2, Neomarkers; 1:5,000) for all cell and tissue samples. GAPDH was determined as loading control using anti-GAPDH antibody (1D4, Neomarkers; 1:5,000). HRP conjugated goat-antimouse secondary (BioRad; 1:5,000) was used for detection. Blots were developed using HyGlo Chemiluminescent HRP detected reagent (Denville Scientific), scanned, and analyzed by densitometry using ImageJ software.
Transglutaminase activity assay
A dot blot assay was used to determine transglutaminase activity as previously described (Santhanam et al. 2010). The assay is based on the incorporation of the TG substrate 5-(biotinamido)pentylamine (BPA) (Pierce, USA) into proteins. In brief, intact cells/tissues were incubated with 0.1 mM BPA and 1 mM Ca2+ at 37°C for 4 h in culture media (phenol red-free DMEM supplemented with 2% FBS and penicillin/streptomycin) and then rinsed to free of unreacted BPA using PBS (3 × 5 min washes). Samples were then homogenized/lysed to recover proteins. Proteins (0.5–1 μg) were loaded onto nitrocellulose membrane (BioDot Dot Blot apparatus; BioRad). The membrane was rinsed and blocked in 3% BSA overnight and probed with HRP-conjugated streptavidin (Amersham Bioscience; 1:10,000 dilution in 3% BSA) to determine BPA incorporation. Blots were then stripped using Restore Plus stripping buffer (Pierce) and reprobed with GAPDH to determine protein loading. BPA incorporation and GAPDH levels were determined by densitometry analysis using ImageJ software (National Institutes of Health). For each sample, activity was calculated as a ratio of BPA/GAPDH.
Isolation of cell surface proteins
Surface proteins were enriched using sulfo-NHS-LC biotin (Pierce) (Santhanam et al. 2010). Briefly, cells were cultured to confluence in 100 mm dishes and treated with indicated drugs/reagents. Plates were then rinsed in cold PBS and surface proteins were biotinylated using sulfo-NHS-LC biotin (Pierce) following manufacturer's protocol. At the end of labeling, cells were trypsinized, recovered in PBS, and counted. 1.5 × 105 cells from each sample were pelleted and lysed in 100 μl 1× RIPA buffer (Upstate) containing protease inhibitors (Roche). Biotinylated proteins were enriched using streptavidin-coated agarose. TG2 was determined by western blotting.
Recovery of ECM proteins
Cells were grown to confluence and treated as indicated. ECM was recovered following established methods (Santhanam et al. 2010). In brief, cells were rinsed twice with PBS. Cellular and nuclear materials were extracted by incubation with cell removal solution (0.05% Triton X-100, 50 mM NH4OH, in PBS) until the cells were floating. The matrix was then briefly washed once with rinse buffer (50 mM NH4OH in PBS) and then three times with PBS (Quality Biological Inc, Gaithersburg, MD, USA). ECM was scraped into 100 μl lysis buffer and TG2 was determined by western blotting.
Rat aorta decellularization
The aortas of rats were dissected out and cleaned. Rings of approximately 5 mm were cut from the aortas and treated as indicated. Samples were then decellularized following methods described in the literature (Gui et al. 2009) with minor modifications. In brief, samples were placed in decellularization solution 1 (8 mM CHAPS, 1 M NaCl, 25 mM EDTA/PBS) for 24 h at room temperature with continuous shaking, and then rinsed once for 15 min in PBS. The samples were then incubated in decellularization solution 1 again for 48 h and subjected to three 15 min washes with PBS. Next, samples were transferred to decellularization buffer 2 (1.8 mM SDS, 1 M NaCl, 25 mM EDTA in PBS) followed by 3 PBS washes of 1 h each. The aorta were then incubated for 1 day in endothelial cell culture media (ScienCell) followed by three 15-min washes with PBS to obtain decellularized vessels. Removal of cells was confirmed by absence of DNA assayed using the Pico Green assay kit (Invitrogen), and absence of GAPDH (cytosolic protein) by western blotting.
Statistics
Bar graphs represent mean ± standard error. Statistical analyses were performed using GraphPad Prism software. 1-Way ANOVA with Tukey post hoc analysis was used to compare three or more treatment groups; unpaired Student's t test was used to compare 2 groups. The minimum level of significance was set at p < 0.05.
Results
Endogenous NO regulates TG2 externalization in HAEC
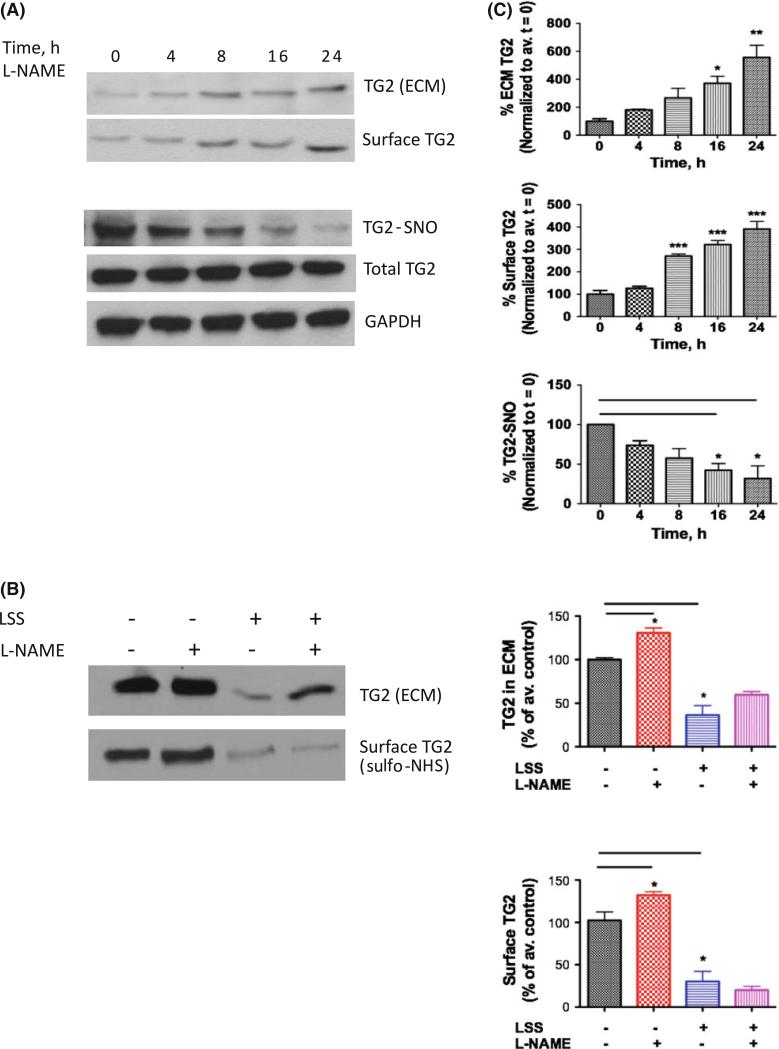
The role of NOS3 in regulating TG2 location in HAECs was first investigated. Intact HAEC were treated with NOS inhibitor l-NAME (100 μM) for 0–24 h and TG2 S-nitrosation and externalization were determined. There was a time-dependent decrease in TG2 S-nitrosation and increased surface/ECM-associated TG2 (Fig. 1a) with the changes reaching significance at 16 h. Thus, a 16–24 h treatment period was used for all subsequent experiments. Next, HAECs were subjected to LSS at 18 dynes/cm2 for 16 h, in the absence or presence of the NOS inhibitor N-nitro-l-arginine methyl ester (l-NAME, 100 μM, Sigma) and TG2 externalization was determined (Fig. 1b). LSS led to decreased ECM-associated TG2 as compared to static controls. l-NAME treatment tended to reverse the effect of LSS, but the data did not reach significance. Surface TG2 levels decreased in cells exposed to LSS, compared to unsheared controls, however, l-NAME treatment did not restore surface bound TG2 toward baseline.
Fig. 1.
Endogenous NO regulates TG2 externalization in HAECs. a NOS inhibition using l-NAME (100 μM; 0–24 h) led to a time-dependent increase in ECM and surface-associated TG2 and decrease in TG2 S-nitrosation, while expression was unaltered; b Laminar shear stress (LSS; 18 dyne/cm2, 16 h) led to decreased ECM and surface TG2; l-NAME (100 μM) partially reversed ECM-associated TG2 but not surface TG2 toward untreated controls; blots are representative of six independent experiments; c Bar graphs of densitometry analyses (n = 6; *p < 0.05 by 1-way ANOVA with Tukey post-test)
NO regulates TG2 localization and activity in HASMC
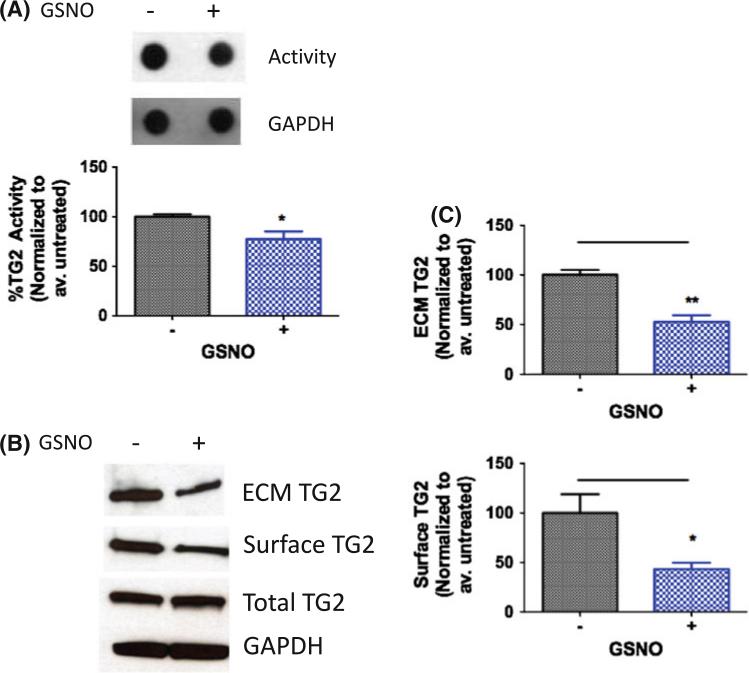
Next, the effect of exogenous and endogenous (endothelial cell-derived) NO on other cell types within the vascular wall was studied. We first examined the effect of exogenous NO by treating confluent monolayers of HASMC with the NO donor S-nitrosoglutathione (GSNO; 200 μM, 1 h). GSNO treatment led to decreased cross-linking activity as measured using BPA incorporation in intact cells (Fig. 2a). In addition, GSNO treatment led to decreased surface and ECM-associated TG2 in HASMCs (Fig. 2b, c). TG2 abundance remained unchanged with GSNO treatment.
Fig. 2.
Exogenous NO regulates TG2 localization and activity in HASMCs. a HASMCs treated with GSNO (200 μM, 1 h) have lower TG activity compared to untreated controls; b ECM-associated and cell surface TG2 levels decrease with GSNO treatment; while TG2 abundance is unchanged; blots are representative of six independent experiments; and c bar graphs of densitometry analyses (n = 6, *p < 0.05; **p < 0.01 by unpaired Student's t test)
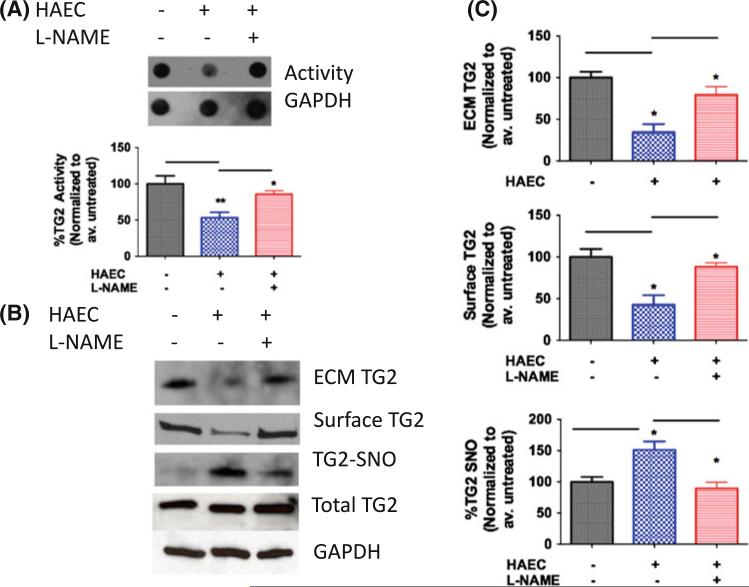
To determine the effect of endogenous NO, a co-culture system was used in which HASMCs were cultured in 6-well plates with HAECs in transwell inserts (Millipore). Samples were either treated with l-NAME (100 μM) or left untreated to determine the role of NOS. After 18 h of co-culture, TG2 activity, expression, S-nitrosation, and localization were determined in the HASMC layer. Co-culture with HAEC led to decreased activity in the HASMC layer (Fig. 3a), and decreased ECM/surface-associated TG2 levels (Fig. 3b, c). NOS inhibition using l-NAME in the HAEC layer restored both activity and localization in the HASMC layer toward untreated controls (Fig. 3a–c). Conversely, TG2 S-nitrosation increased in the presence of HAEC and decreased toward untreated HASMC in the l-NAME treated wells. TG2 abundance remained unaltered in the HASMCs over all conditions.
Fig. 3.
Endothelial cell-derived NO regulates TG2 localization and activity in HASMCs. a HASMCs co-cultured with HAECs have lower TG activity compared to HASMCs alone (baseline); l-NAME restores TG activity in the HASMC layer toward baseline; b ECM-associated and cell surface TG2 decrease and TG2 S-nitrosation increases in HASMCs co-cultured with HAECs, l-NAME partially reverses this; TG2 abundance in the HASMC layer is unchanged; and c Bar graphs of densitometry analyses. (n = 6, *p < 0.05; **p < 0.01; ***p < 0.001 by 1-way ANOVA with Tukey post-test)
NO regulates TG2 localization and activity in fibroblasts
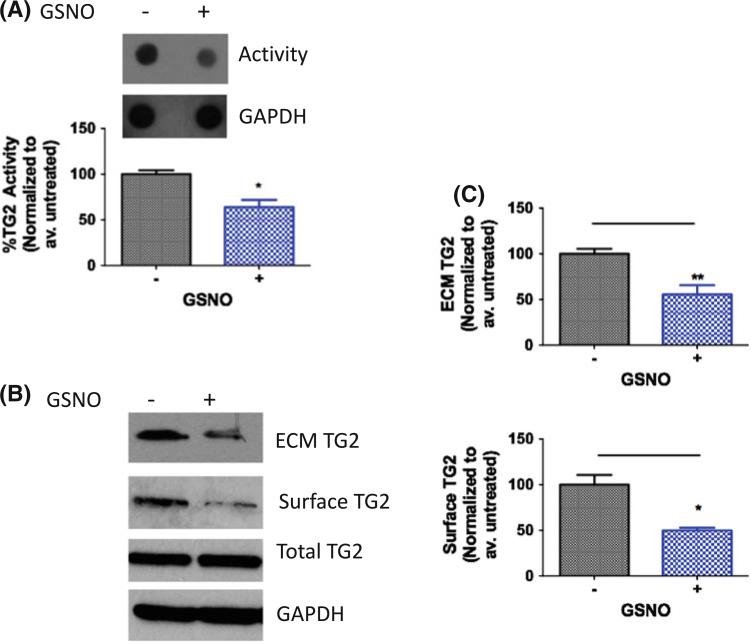
Next, the effect of NO on TG2 localization was examined in human fibroblasts cells (IMR90). The effect of exogenous NO was first determined using GSNO (200 μM; 1 h). TG2 activity, expression, and externalization were measured. As with HASMCs, TG2 activity decreased following GSNO treatment (Fig. 4a). Surface/ECM-associated TG2 also decreased (Fig. 4b, c), while total TG2 remained unchanged.
Fig. 4.
Exogenous NO regulates TG2 localization and activity in IMR90 fibroblasts. a IMR90 fibroblasts treated with GSNO (200 μM, 1 h) have lower TG activity compared to untreated controls; b ECM-associated and cell surface TG2 levels decrease with GSNO treatment; while TG2 abundance is unchanged; blots are representative of six independent experiments; and c bar graphs of densitometry analyses (n = 6, *p < 0.05; **p < 0.01 by unpaired Student's t test)
IMR90 cells were also co-cultured with HAECs in transwell inserts to determine the effect of endogenous NO on TG2 activity, S-nitrosation, and localization. Samples were either treated with l-NAME or left untreated to interrogate the role of NOS in TG2 compartmentalization. Again, TG activity was diminished in the IMR90 layer in the presence of HAEC and NOS inhibition by l-NAME reversed this (Fig. 5a). Co-culture with HAEC also led to decreased cell-surface/ECM-associated TG2 and increased TG2 S-nitrosation in the IMR90 layer (Fig. 5b, c). This was reversed with l-NAME. TG2 abundance was constant through all experiments.
Fig. 5.
Endothelial cell-derived NO regulates TG2 localization and activity in IMR90 fibroblasts. a IMR90 fibroblasts co-cultured with HAECs have lower TG activity compared to IMR90 alone (baseline); l-NAME restores TG activity in the IMR90 layer toward baseline; b ECM-associated and cell surface TG2 decrease and TG2 S-nitrosation increases in IMR90 co-cultured with HAECs, l-NAME partially reverses this; TG2 abundance in the IMR90 layer is unchanged; and c Bar graphs of densitometry analyses. (n = 6, *p < 0.05; **p < 0.01; ***p < 0.001 by 1-way ANOVA with Tukey post-test)
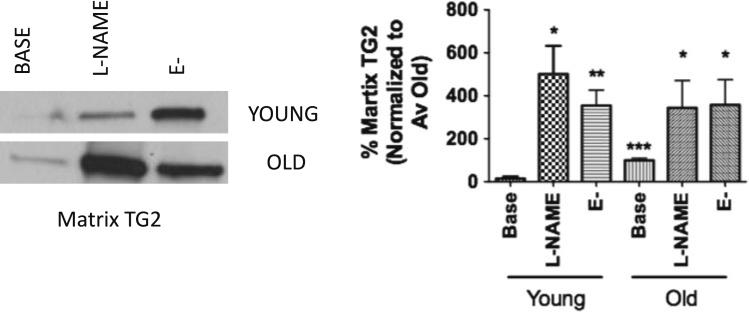
TG2 externalization increases in aorta of aged rats
We next determined whether aging is associated with alterations in TG2 localization in rat aorta. Aorta from four young (3- to 6-month old) and four old (22- to 24-month old) Fischer 344 rats were used. Aortic segments were left intact (baseline), treated with l-NAME (l-NAME; 100 μM), or de-endothelialized (E-) and incubated in media (phenol red-free DMEM with 2% FBS, penicillin/streptomycin) overnight. TG2 externalization was determined by decellularizing the aortic segments to recover the tissue matrix scaffold. Samples were then homogenized and TG2 abundance in the matrix measured by western blotting. Efficiency of decellularization was determined by assaying for DNA (not detected; data not shown) using the PicoGreen assay kit and western blotting for GAPDH (not detected, data not shown). Aged rats have significantly increased matrix-associated TG2 compared to young (Fig. 6). Both NOS inhibition with l-NAME and removal of endothelial layer led to increased deposition of TG2 in the matrix in both young and old rats.
Fig. 6.
TG2 externalization and activity are increased with age in rat aorta. Old rats have higher levels of matrix-associated TG2 compared to young; removal of endothelial layer (E-) and l-NAME treatment lead increased matrix-associated TG2 compared to baseline (n = 4, *p < 0.05; **p < 0.01; ***p < 0.001 vs. Young baseline; 1-way ANOVA with Tukey post-test)
Discussion
TG2 is a multifunctional enzyme that is constitutively expressed at high levels in the vasculature including endothelial cells, smooth muscle cells, and fibroblasts. The role of TG2 in mediating development and progression of cardiovascular disease is emerging (Sane et al. 2007; Bakker et al. 2008). TG2 is shown to be involved in resistance vessel remodeling (Bakker et al. 2005; Pistea et al. 2008), arterial calcification (Johnson et al. 2008; Matlung et al. 2009), and age-associated central aortic stiffening (Santhanam et al. 2010). NO inhibits TG2 cross-linking function in vitro by S-nitrosation of key cysteine residues (Lai et al. 2001) and exogenous NO donors mitigate TG2-mediated resistance vessel remodeling in mice (Bakker et al. 2005). We have recently shown that endothelium-derived NO regulates TG2 cross-linking activity in rat aorta ex vivo (Santhanam et al. 2010). At least 80% of total TG2 is cytosolic and exists in GTP-bound form with its cross-linking activity held latent. TG2 is exported out of the cell via a non-conventional and unknown mechanism and deposited into the ECM where it performs the classical protein cross-linking function of the TGases (Bakker et al. 2008; Zemskov et al. 2006; Bergamini et al. 2005). NO donors lead to decreased externalization of TG2 in fibroblasts (Telci et al. 2009). We have shown that increased NO bioavailability confines TG2 to the cytosol, and decreased NO (NOS3 inhibiton using l-NAME) leads to increased externalization in endothelial cells (Santhanam et al. 2010). Moreover, DTT led to increased externalization of TG2, suggesting a role for a reversible thiol modification such as S-nitrosation in this phenomenon. Thus, while NO is believed to directly inhibit TG2 cross-linking function by S-nitrosation of key cysteines (in particular, the active site cysteine C277), it is possible that NO additionally confines TG2 to the cytosol, thereby holding its cross-linking activity latent.
In this study, we show that NOS3-dependent NO regulates TG2 localization in vascular cells. NOS3 inhibition with l-NAME led to a time-dependent loss of TG2 S-nitrosation and increased externalization TG2 in HAECs. Both treatment with GSNO and co-culture with HAEC led to decreased externalization of TG2 in HASMC and IMR90 fibroblasts. The effects of HAEC were reversed by l-NAME, further supporting a role for NOS3-dependent NO in regulating TG2 localization. Interestingly, while LSS led to decreased TG2 externalization in HAECs, l-NAME did not reverse the effect of LSS. This suggests an involvement of another mechanosensitive, NOS3-independent pathway in regulating TG2 localization in the case of LSS. These remain to be determined. Genetic studies using endothelial cells from wild type and NOS3–/– mice or HAECs treated with NOS3 siRNA will help elucidate the specific role of NOS3 in regulating TG2 localization. These studies are ongoing in our laboratory.
As aging is associated with decreased NO bioavailability, it is important to interrogate whether this leads to increased deposition of TG2 in the matrix of aortic tissue. To this end, we used decellularized aortic segments from young and old Fischer 344 rats, a widely used and well-accepted aging model. We confirmed that aged rats have higher levels of TG2 in the matrix (Fig. 6). We also demonstrate that reducing NO bioavailability ex vivo either by denuding the endothelial layer or by NOS inhibition leads to increased TG2 levels in the matrix. This again supports a role for NO in regulating TG2 localization in the aorta.
The potential role of NO in regulating protein trafficking in the cardiovascular system is emerging (Lowenstein 2007; Matsushita et al. 2003). This is of particular interest in understanding vascular aging, where reduced NO bio-availability is well established. Thus, NO can regulate passive properties of the blood vessel by regulating trafficking of proteins such as TG2 and matrix cross-linking in addition to mediating well-established effects on vascular reactivity through cGMP signaling.
As regards TG2 function, the effects of NO on TG2 externalization provide important insights toward the mechanism by which this trafficking might occur. TG2 nitrosylated in the absence of Ca2+ is about sixfold more susceptible to binding and inhibition by GTP (Lai et al. 2001). Moreover, GTP stabilizes TG2 in its compact conformation (Begg et al. 2006), which in turn, is associated with decreased TG2 externalization (Balklava et al. 2002). Thus, it is possible that S-nitrosation of TG2 encourages GTP binding and compact conformation of TG2 and constrains it to the cytosol. Furthermore, it is well known that TG2 is not exported via the classical ER/Golgi route (Zemskov et al. 2006). Recently, Belkin and co-workers have shown that TG2 is externalized via recycling endosomes in an NO-dependent manner (Zemskov et al. 2011). One of the major components of endosome machinery in vascular systems is N-ethylmaleimide sensitive factor (NSF), which is regulated by NO through S-nitrosation (Matsushita et al. 2003). NO inhibits exocytosis and accelerates endocytosis via S-nitrosation of NSF and dynamin in endothelial cells (Lowenstein 2007; Matsushita et al. 2003). This could represent the pathway by which TG2 trafficking occurs in the cell and remains to be proven. Should NSF be a critical component of TG2 trafficking, an important question arises—is it nitrosation of NSF and/or of TG2 that determines the subcellular fate of TG2? Studies to address this question are ongoing in our laboratory.
In conclusion, we show that endothelial cell-derived NO serves to constrain TG2 to the cytosol in fibroblasts and SMCs. We also show that this is relevant not only in cell culture, but also at an organ level in rat aorta. Importantly, we show that aging is associated with increased deposition of TG2 to the ECM. In this study, we provide an insight into a mechanism that might regulate TG2 externalization in the vasculature, which is critical to our understanding the biology of this complex protein. Moreover, this has important implications for understanding the role of TG2 in vascular disease in which NO bioavailability is decreased.
Acknowledgments
This work was supported by an American Heart Association Grant 09BGIA2220181 to LS and a National Institutes of Health Grant 1R01-HL105296-01 to DEB.
Footnotes
S. K. Jandu and A. K. Webb contributed equally to this work.
Contributor Information
Simran K. Jandu, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross 1150, Baltimore, MD 21205, USA
Alanah K. Webb, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross 1150, Baltimore, MD 21205, USA
Alina Pak, Department of Biomedical Engineering, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross 1150, Baltimore, MD 21205, USA.
Baris Sevinc, Department of Biomedical Engineering, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross 1150, Baltimore, MD 21205, USA.
Daniel Nyhan, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross 1150, Baltimore, MD 21205, USA.
Alexey M. Belkin, Department of Biochemistry and Molecular Biology, University of Maryland School of Medicine, 800 W Baltimore St, Room 213, Baltimore, MD 21201, USA
Nicholas A. Flavahan, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross 1150, Baltimore, MD 21205, USA
Dan E. Berkowitz, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross 1150, Baltimore, MD 21205, USA Department of Biomedical Engineering, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross 1150, Baltimore, MD 21205, USA.
Lakshmi Santhanam, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross 1150, Baltimore, MD 21205, USA.
References
- Bakker EN, Pistea A, VanBavel E. Transglutaminases in vascular biology: relevance for vascular remodeling and atherosclerosis. J Vasc Res. 2008;45:271–278. doi: 10.1159/000113599. [DOI] [PubMed] [Google Scholar]
- Bakker EN, Buus CL, Spaan JA, Perree J, Ganga A, Rolf TM, Sorop O, Bramsen LH, Mulvany MJ, Vanbavel E. Small artery remodeling depends on tissue-type transglutaminase. Circ Res. 2005;96:119–126. doi: 10.1161/01.RES.0000151333.56089.66. [DOI] [PubMed] [Google Scholar]
- Balklava Z, Verderio E, Collighan R, Gross S, Adams J, Griffin M. Analysis of tissue transglutaminase function in the migration of Swiss 3T3 fibroblasts: the active-state conformation of the enzyme does not affect cell motility but is important for its secretion. J Biol Chem. 2002;277:16567–16575. doi: 10.1074/jbc.M109836200. [DOI] [PubMed] [Google Scholar]
- Begg GE, Carrington L, Stokes PH, Matthews JM, Wouters MA, Husain A, Lorand L, Iismaa SE, Graham RM. Mechanism of allosteric regulation of transglutaminase 2 by GTP. Proc Natl Acad Sci USA. 2006;103:19683–19688. doi: 10.1073/pnas.0609283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamini CM, Griffin M, Pansini FS. Transglutaminase and vascular biology: physiopathologic implications and perspectives for therapeutic interventions. Curr Med Chem. 2005;12:2357–2372. doi: 10.2174/0929867054864804. [DOI] [PubMed] [Google Scholar]
- Gui L, Muto A, Chan SA, Breuer CK, Niklason LE. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng A. 2009;15:2665–2676. doi: 10.1089/ten.tea.2008.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102:529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai TS, Hausladen A, Slaughter TF, Eu JP, Stamler JS, Greenberg CS. Calcium regulates S-nitrosylation, denitrosylation, and activity of tissue transglutaminase. Biochemistry. 2001;40:4904–4910. doi: 10.1021/bi002321t. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- London GM, Drueke TB. Atherosclerosis and arteriosclerosis in chronic renal failure. Kidney Int. 1997;51:1678–1695. doi: 10.1038/ki.1997.233. [DOI] [PubMed] [Google Scholar]
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- Lowenstein CJ. Nitric oxide regulation of protein trafficking in the cardiovascular system. Cardiovasc Res. 2007;75:240–246. doi: 10.1016/j.cardiores.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlung HL, Groen HC, de Vos J, van Walsum T, van der Lugt A, Niessen WJ, Wentzel JJ, Vanbavel E, Bakker EN. Calcification locates to transglutaminases in advanced human atherosclerotic lesions. Am J Pathol. 2009;175:1374–1379. doi: 10.2353/ajpath.2009.090012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Morrell CN, Cambien B, Yang SX, Yamakuchi M, Bao C, Hara MR, Quick RA, Cao W, O'Rourke B, Lowenstein JM, Pevsner J, Wagner DD, Lowenstein CJ. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115:139–150. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistea A, Bakker EN, Spaan JA, Hardeman MR, van Rooijen N, VanBavel E. Small artery remodeling and erythrocyte deformability in l-NAME -induced hypertension: role of transglutaminases. J Vasc Res. 2008;45:10–18. doi: 10.1159/000109073. [DOI] [PubMed] [Google Scholar]
- Sane DC, Kontos JL, Greenberg CS. Roles of transglutaminases in cardiac and vascular diseases. Front Biosci. 2007;12:2530–2545. doi: 10.2741/2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam L, Tuday EC, Webb AK, Dowzicky P, Kim JH, Oh YJ, Sikka G, Kuo M, Halushka MK, Macgregor AM, Dunn J, Gutbrod S, Yin D, Shoukas A, Nyhan D, Flavahan NA, Belkin AM, Berkowitz DE. Decreased S-nitrosylation of tissue transglutaminase contributes to age-related increases in vascular stiffness. Circ Res. 2010;107:117–125. doi: 10.1161/CIRCRESAHA.109.215228. [DOI] [PubMed] [Google Scholar]
- Telci D, Collighan RJ, Basaga H, Griffin M. Increased TG2 expression can result in induction of transforming growth factor beta1, causing increased synthesis and deposition of matrix proteins, which can be regulated by nitric oxide. J Biol Chem. 2009;284:29547–29558. doi: 10.1074/jbc.M109.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemskov EA, Janiak A, Hang J, Waghray A, Belkin AM. The role of tissue transglutaminase in cell–matrix interactions. Front Biosci. 2006;11:1057–1076. doi: 10.2741/1863. [DOI] [PubMed] [Google Scholar]
- Zemskov EA, Mikhailenko I, Hsia R-C, Zaritskaya L, Belkin AM. Unconventional secretion of tissue transglutaminase involves phospholipid-dependent delivery into recycling endosomes. PLoS One. 2011;6:19414. doi: 10.1371/journal.pone.0019414. [DOI] [PMC free article] [PubMed] [Google Scholar]