Abstract
We developed a questionnaire to assess motor imagery (MI) of gait and administered it to 34 controls and 28 individuals with PD. Our goals were: 1) compare gait MI in individuals with and without PD, 2) determine whether walking performance relates to gait MI and 3) compare gait MI in individuals with PD with and without freezing of gait. Gait MI was not different between PD and controls. There was no correlation between walking performance and gait MI, and no difference in gait MI between freezers and nonfreezers. The gait imagery questionnaire may be useful for imaging studies involving imagined gait.
Keywords: Parkinson disease, motor imagery, gait, freezing of gait
INTRODUCTION
Gait and balance dysfunction are frequently associated with the progression of Parkinson disease (PD) [1]. However the neurophysiologic etiology of gait impairments in this population is not well understood, due in part to the multifactorial and unpredictable nature of symptoms [1–3]. Neural imaging has been used to investigate the neurophysiological underpinnings of gait disruptions, but motor imagery (MI) of gait within the studied population was not assessed [4]. We have developed a four-item gait imagery questionnaire (GIQ) for neural imaging paradigms requiring gait MI tasks. The four items of the GIQ are forward walking, backward walking, turning to the right and turning to the left. Forward walking was selected as it is a common activity of daily living. Backward walking and turning were selected as tasks which are particularly difficult for individuals with PD and are associated with freezing and falls [5,6]. The purpose of the GIQ is to assess visual and kinesthetic vividness of complex task imagery beyond the single joint movements covered in the Kinesthetic and Visual Imagery Questionnaire (KVIQ) [7].
The aims of this study were to 1) compare gait MI in individuals with and without PD, 2) determine whether walking performance relates to gait MI and 3) compare gait MI in individuals with PD with and without freezing of gait.
METHODS
Participants
Twenty-eight individuals with PD (11 female; mean age 71.0 ± 8.9 yrs) and 33 healthy age-matched individuals (16 female; mean age 69.9 ± 10.7 yrs) participated. Individuals with PD had a diagnosis of idiopathic PD from a board certified neurologist using diagnostic criteria for “definite PD” [8] and established criteria [9]. Inclusion criteria for all participants included independent ambulatory ability, no evidence of dementia (MMSE ≥ 24), normal or corrected to normal vision and hearing, and absence of any orthopedic condition, neurologic disorder (aside from PD), psychiatric condition or other confounding comorbidity. MI was assessed in all individuals with PD after abstaining from anti-Parkinson medication for at least 12 hours. All participants provided informed written consent prior to participation in accord with the procedures approved by the Human Research Protection Office of the Washington University School of Medicine.
Imagery Tasks
The KVIQ was administered by a trained tester following established procedures [7]. Participants were observed to verify the absence of motion while imagining. Immediately after imagining the task, the participant was asked to rate the vividness of visual and kinesthetic sensations associated with imagining the motor task using a five point Likert scale [7]. Following the KVIQ, the GIQ was administered. The GIQ tasks were forward and backward walking as well as turning in 1m diameter circles to the right and left. The additional tasks were selected for their varying level of complexity, multi-joint motor requirements and to allow for imagery assessment across different gait tasks. Additionally, turning has been associated with increased freezing episodes for individuals with PD [5] and was intentionally selected to: 1) test for the presence of freezing while imagining turning and 2) examine the relationship between freezing and vividness of MI.
Following the methods for administration of the KVIQ [7], participants were asked to first listen to the instructions for the task; second, watch the tester demonstrate the task; third, physically perform the task, fourth, imagine the task while seated and finally rate their visual and kinesthetic vividness using the five point Likert scale used for the KVIQ [7]. Performance of forward and backward walking tasks was self-paced and performed over a distance of 5m. The circle walking task consisted of 3 revolutions around the perimeter of a 1m diameter circle, first to the left, then to the right.
Clinical and gait measures in PD
The motor subsection of the Movement Disorder Society Unified Parkinson Disease Rating Scale (MDS-UPDRS) and the Freezing of Gait Questionnaire (FOG-Q) were administered to all individuals with PD [10,11]. The PD group was subdivided into “freezers” (FOG+) and “non-freezers” (FOG-Q) based on their response to question 3 on the FOG-Q. Individuals with a score of 1 or greater, indicating that they experience a freezing event at least one time each month, were classified as FOG+.
Spatiotemporal measures of overground gait were collected on a 4.8m GAITRITE walkway (CIR systems, Inc., Havertown, PA). Each individual with PD completed three forward walking trials at his preferred walking pace.
Data Analysis
Scores for the KVIQ and GIQ were summed separately and the mean score for each component (visual and kinesthetic) was calculated. One-way analysis of variance tests were used to examine between group effects. Linear regression analyses compared KVIQ and GIQ scores as well GIQ to gait velocity and clinical measures. OriginPro v8.0 (OriginLab Corporation, Northampton, MA) was used for statistical analysis. An a priori level of p<0.01 was set for determining statistical significance. All measures are reported as mean ± standard deviation, unless otherwise noted.
RESULTS
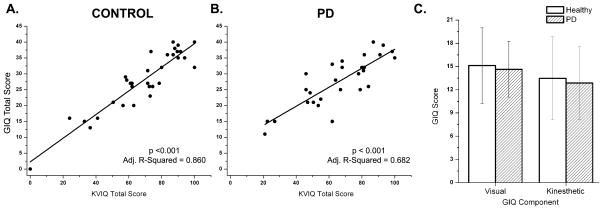
PD and controls did not differ by age (p = 0.66). GIQ scores were highly correlated with KVIQ scores for both healthy and PD groups (Figure 1). Kinesthetic and visual subsections scores of the GIQ were not different between groups (Figure 1).
Figure 1.
Summed scores for the KVIQ compared to summed scores for the GIQ. KVIQ scores were significantly positively correlated with GIQ total scores for both (A) healthy and (B) PD OFF. (C) Visual and Kinesthetic component scores of the GIQ were not significantly different between healthy individuals and individuals with PD.
Within the PD group there were no significant correlations between GIQ total scores and disease duration (adj r2 = 0.036, p = 0.17), age (adj r2 = 0.176, p = 0.015), Hoehn & Yahr (adj r2 = −0.017, p = 0.466) or MDS-UPDRS motor subsection score (adj r2 = 0.036, p = 0.17). Within the control group there was no correlation between age and GIQ total scores (adj r2 = 0.006, p = 0.280).
Overground forward gait velocity was assessed for the PD group only. Velocity did not relate to total imagery score on either the GIQ (p = 0.94, adjusted r2 = −0.038) or the KVIQ (p = 0.90, adjusted r2 = −0.038).
Freezing of Gait Subgroups
The FOG+ group had a significantly greater disease duration than the FOG− group (p = 0.004) but did not differ in age (p = 0.055), gait velocity (p = 0.260), MDS-UPDRS scores (p = 0.071) or H&Y scores (p = 0.757). No freezing events occurred in the FOG+ group during data collection. GIQ scores did not differ between the FOG− and FOG+ groups (Figure 2).
Figure 2.
Imagery questionnaire scores for the KVIQ and GIQ for individuals with PD with freezing of gait (FOG+, grey squares) and without freezing of gait (FOG−, white squares). FOG+ and FOG− imagery scores were not significantly different within any of the four tested conditions. square = mean, line in box = median, bars = 5th and 95th percentiles, box ends = 25th and 75th percentiles.
DISCUSSION
The primary findings of this study were: (1) GIQ and KVIQ scores were highly correlated for both the PD and healthy groups, (2) age, disease duration, gait velocity, H&Y and MDS-UPDRS scores were not significantly correlated to GIQ scores and (3) freezers were not statistically different from non-freezers in their ability to imagine.
Gait imagery during neural imaging is a commonly used technique to better understand locomotor control networks in healthy [12] and diseased [4] populations. However, as has been reported previously, not all individuals are able to imagine motor tasks [13]. Our data support previous findings indicating a broad spectrum of imagery ability across individuals [7,13,14]. These findings indicate an MI screening protocol is a necessary component of any neural imaging study using an MI task. Additionally, recent reports indicate PD affects the supplementary motor area [4,15], a region of overlapping activation during MI and executed motor tasks [16,17]. Therefore, it is particularly important to screen individuals with PD for MI vividness before conducting imaging studies. The GIQ offers a rapidly administered assessment of visual and kinesthetic vividness during imagined gait tasks, providing a useful tool for participant screening.
No relationship was present between walking performance and gait MI, indicating that a deficit in motor ability does not preclude an individual from imagery of the task. Similarly, no deficit in GIQ performance was seen in the FOG+ group, indicating that while gait function may be impacted by FOG, gait imagery is not. Thus MI during functional magnetic resonance imaging may offer a plausible means of comparing gait-related neural function without the confound of differing motor performance between groups of freezers and non-freezers. Similarly, Thobois et al. [15] used PET to compare an imagined task involving both the affected and unaffected hand in people with PD and healthy controls. The use of an MI task allowed for comparison between akinetic and nonakinetic hands within the PD group as well as comparison across groups, as motor task success was not a limiting factor. As MI ability is not impeded in individuals with FOG, MI may offer a unique means of exploring the etiology of FOG, which has remained elusive. In addition, as disease severity did not impact GIQ scores, gait MI research is possible across disease severity levels as the motor performance differences are not a factor. Additionally, the GIQ may be a useful tool in assessing MI of gait in other conditions such as dystonia or stroke.
CONCLUSIONS
The four-question GIQ can be administered to individuals with PD and healthy individuals as a means of assessing vividness of imagined gait tasks. Individuals presenting with freezing of gait are able to visually and kinesthetically imagine the four tested gait tasks as vividly as individuals with PD who do not report freezing of gait.
Acknowledgments
The authors thank Ryan Duncan and Marie McNeely for their help with data collection for this study.
Support for this work was provided by the Parkinson’s Disease Foundation, NIH grants R01HD056015-02, 2T32HD007434-18A, and TL1RR024995, the American Parkinson Disease Association (APDA) Advanced Center for PD Research at Washington University School of Medicine and the St. Louis Chapter of the APDA.
References
- 1.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in parkinson’s disease: A review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 2.Giladi N, Nieuwboer A. Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Mov Disord. 2008;23(Suppl 2):S423–425. doi: 10.1002/mds.21927. [DOI] [PubMed] [Google Scholar]
- 3.Nieuwboer A, De Weerdt W, Dom R, Lesaffre E. A frequency and correlation analysis of motor deficits in parkinson patients. Disabil Rehabil. 1998;20:142–150. doi: 10.3109/09638289809166074. [DOI] [PubMed] [Google Scholar]
- 4.Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, Toni I. Gait-related cerebral alterations in patients with parkinson’s disease with freezing of gait. Brain. 2011;134:59–72. doi: 10.1093/brain/awq324. [DOI] [PubMed] [Google Scholar]
- 5.Horak FB, Dimitrova D, Nutt JG. Direction-specific postural instability in subjects with parkinson’s disease. Exp Neurol. 2005;193:504–521. doi: 10.1016/j.expneurol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff JM. Gait dynamics in parkinson’s disease: Relationship to parkinsonian features, falls and response to levodopa. J Neurol Sci. 2003;212:47–53. doi: 10.1016/s0022-510x(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 7.Malouin F, Richards CL, Jackson PL, Lafleur MF, Durand A, Doyon J. The kinesthetic and visual imagery questionnaire (kviq) for assessing motor imagery in persons with physical disabilities: A reliability and construct validity study. J Neurol Phys Ther. 2007;31:20–29. doi: 10.1097/01.npt.0000260567.24122.64. [DOI] [PubMed] [Google Scholar]
- 8.Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of parkinson’s disease. Am J Med Genet. 1999;88:539–543. [PubMed] [Google Scholar]
- 9.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N. Movement disorder society-sponsored revision of the unified parkinson’s disease rating scale (mds-updrs): Scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 11.Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD. Construction of freezing of gait questionnaire for patients with parkinsonism. Parkinsonism Relat Disord. 2000;6:165–170. doi: 10.1016/s1353-8020(99)00062-0. [DOI] [PubMed] [Google Scholar]
- 12.Jahn K, Deutschlander A, Stephan T, Kalla R, Wiesmann M, Strupp M, Brandt T. Imaging human supraspinal locomotor centers in brainstem and cerebellum. NeuroImage. 2008;39:786–792. doi: 10.1016/j.neuroimage.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 13.Malouin F, Richards CL, Durand A, Doyon J. Reliability of mental chronometry for assessing motor imagery ability after stroke. Arch Phys Med Rehabil. 2008;89:311–319. doi: 10.1016/j.apmr.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Randhawa B, Harris S, Boyd LA. The kinesthetic and visual imagery questionnaire is a reliable tool for individuals with parkinson disease. J Neurol Phys Ther. 2010;34:161–167. doi: 10.1097/npt.0b013e3181e1aa71. [DOI] [PubMed] [Google Scholar]
- 15.Thobois S, Dominey PF, Decety J, Pollak PP, Gregoire MC, Le Bars PD, Broussolle E. Motor imagery in normal subjects and in asymmetrical parkinson’s disease: A pet study. Neurology. 2000;55:996–1002. doi: 10.1212/wnl.55.7.996. [DOI] [PubMed] [Google Scholar]
- 16.Kim SG, Jennings JE, Strupp JP, Andersen P, Ugurbil K. Functional mri of human motor cortices during overt and imagined finger movements. International Journal of Imaging Systems and Technology. 1995;6:271. [Google Scholar]
- 17.Lotze M, Montoya P, Erb M, Hulsmann E, Flor H, Klose U, Birbaumer N, Grodd W. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: An fmri study. Journal of Cognitive Neuroscience. 1999;11:491–501. doi: 10.1162/089892999563553. [DOI] [PubMed] [Google Scholar]