Abstract
Variation in the CYP2A6 gene, that decreases the rate of nicotine metabolic-inactivation, is associated with higher adult smoking cessation rates during clinical trials. We hypothesized that slow metabolism is associated with increased quitting during adolescence. Caucasian adolescent smokers (N=308, aged 12 to 17, 36.3% male) from a cohort study were genotyped for CYP2A6 resulting in 7.8% slow, 14.0% intermediate and 78.2% normal metabolizers. Overall, 144 smokers quit smoking, as indicated by being abstinent for ≥12 months. In logistic regression analyses, the odds ratio for quitting was 2.25 (95% confidence interval 1.05, 4.80; P=0.037) for slow metabolizers relative to normal metabolizers. A linear trend toward increased quitting with decreasing CYP2A6 activity was also observed (odds ratio = 1.44, 95% confidence interval 1.02, 2.01; P=0.034). Thus, CYP2A6 slow metabolism is associated with increased adolescent smoking cessation, indicating that even early in the smoking history genetic variation is influencing smoking cessation.
Keywords: adolescent, longitudinal studies, epidemiology, smoking cessation, genetic association studies
Introduction
Adolescence is a critical period for smoking acquisition [1]. Although a large proportion of adolescents smoke during their teen years, only a fraction progress to regular, dependent smoking as adults, suggesting that many quit smoking before adulthood.
Interindividual variation in the ability to quit smoking in adolescence may be related, in part, to pharmacogenetics. Genetic variation in CYP2A6 is associated with the ability to quit smoking among adults [2]. CYP2A6 plays a major role in the inactivation of nicotine to cotinine, and the conversion of cotinine to 3′-hydroxycotinine [3]. Slow nicotine metabolism, measured by CYP2A6 genotype or the nicotine metabolite ratio (3′-hydroxycotinine to cotinine ratio), is associated in adults with higher spontaneous quit rates as well as increased quitting on placebo and the nicotine patch [2].
In adolescence, slow nicotine metabolism is associated with increased risk and rate of acquisition of nicotine dependence relative to normal metabolizers [4, 5], possibly due to increased initial nicotine reinforcement leading to an enhanced risk of acquiring nicotine dependence. However, once dependent, slow nicotine metabolizers display relatively limited cigarette consumption [4, 6] and reduced escalation in dependence [5, 6] potentially increasing the ability of adolescent slow metabolizers to quit smoking.
We undertook a prospective cohort investigation of the association between CYP2A6 genotype and smoking cessation, defined here as smoking abstinence for ≥12 months, in a cohort of Caucasian adolescent smokers. We hypothesized that being a CYP2A6 slow nicotine metabolizer is associated with an increased likelihood of smoking cessation.
Materials and methods
Participants were selected from the ongoing Nicotine Dependence in Teens (NDIT) study [1], based on ethnicity, smoking status, and provision of a blood or saliva sample for DNA extraction and CYP2A6 genotyping. A total of 610 Caucasian adolescents were genotyped for CYP2A6; 308 Caucasian adolescents who were either smokers at baseline or initiated smoking during follow-up were retained for analysis. Data on sociodemographic variables and smoking behaviors were collected in self-report questionnaires administered every 3-4 months between 1999 and 2004. All participants were asked about their cigarette smoking behaviours using 2 indicators: 1. “Check the one box that describes you best: I have never smoked a cigarette, even just a puff; I have smoked cigarettes, even just a puff, but not at all in the past 12 months; I smoked cigarettes once or a couple of times in the past 12 months; I smoke cigarettes once or a couple of times each month; I smoke cigarettes once or a couple of times each week; I smoke cigarettes everyday”, and 2. A recall of cigarette use in each of the 3 months preceding completion of the survey. Here, the number of days smoked was multiplied by the average number of cigarettes smoked per day on smoking days. Smokers were defined as participants who had ever puffed on a cigarette, and quitting smoking (abstinence for ≥12 months) was defined as subsequently having ≥4 consecutive surveys with a value of 0 for the total number of cigarettes smoked. Among those who quit smoking, measures of nicotine dependence were computed in the survey immediately preceding the onset of quitting smoking, including craving scores, derived from 14 items assessing craving frequency, physical and mental addiction, and ability to refrain from smoking; nicotine withdrawal scores, assessing irritability, restlessness, anxiety, urge to smoke, and difficulty sleeping and concentrating while abstinent; and self-medication scores, where subjects either endorsed or refuted statements that smoking improves their functioning, concentration, affect, energy, and stress.
All participants provided assent and a parent or guardian provided written informed consent at baseline. The study was approved by the Montreal Department of Public Health Ethics Review Committee, the McGill University Faculty of Medicine Institutional Review Board, and the University of Toronto Research Ethics Board.
DNA was extracted from saliva or blood samples using Oragene kits (DNA Genotek Inc., Kanata, ON, Canada) and QIAamp blood kits (Qiagen Inc., Valencia, CA, USA), respectively. Genotyping for CYP2A6 variant alleles was performed using a previously validated two-step gene and allele specific polymerase chain reaction [7], or an allele-specific TaqMan single nucleotide polymorphism genotyping assay (Applied Biosystems) and real-time polymerase chain reaction. Individuals were genotyped for CYP2A6*2, CYP2A6*4, CYP2A6*9, and CYP2A6*12 alleles, which were selected based on their known role in decreasing nicotine metabolism [3]. Subjects were categorized as normal, intermediate, or slow CYP2A6 genotype metabolizers based on the metabolic impact of CYP2A6 genotype [3]. Normal metabolizers (NM) had no variant alleles (100% CYP2A6 activity), while intermediate metabolizers (IM) had 1 copy of a decreased-function variant allele (CYP2A6*9 or CYP2A6*12; ~80% CYP2A6 activity), and slow metabolizers (SM) had 2 copies of a decreased-function allele or 1 or 2 copies of a loss-of-function variant allele (CYP2A6*2 or CYP2A6*4; ≤50% CYP2A6 enzymatic activity).
Bootstrap based multiple imputation was used to manage missing survey data, according to the protocol by Honaker and King, which utilizes the longitudinal nature of the data to improve the imputation process [8]. Ten imputed sets of data were generated, and multiple logistic regression within a generalized estimating equation (GEE) framework was performed to examine the association between CYP2A6 metabolic group (independent variable) and cessation (dependent variable) while accounting for the correlation between measures within individuals. No covariates were included. The additive model assumed a linear relationship between CYP2A6 metabolic group, consistent with genotype group impact on metabolism [3], and the odds of quitting. The statistical programs used to complete the analysis were R (version 2.14.1, available online: http://www.r-project.org/), the Amelia II package for multiple imputation (version 1.5-5, available online: http://gking.harvard.edu/amelia/), and the Zelig package for GEE models (version 3.5.4, available online: http://gking.harvard.edu/zelig).
Results
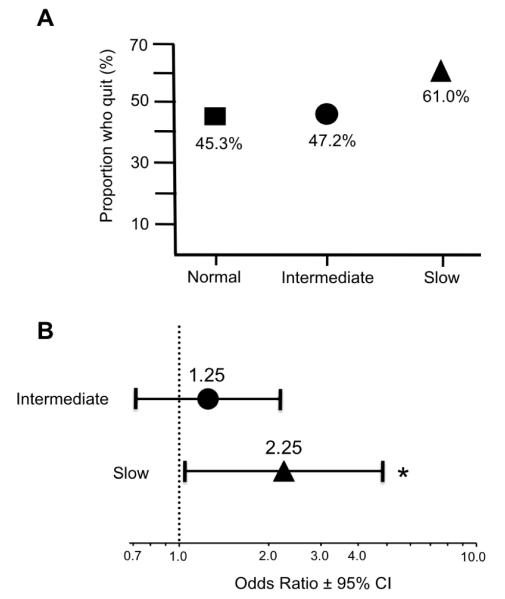
The demographic characteristics of the whole population as well as smoking variables among quitters are reported in Table 1. A total of 144 smokers quit smoking for at least 1 year. A greater proportion of slow metabolizers quit smoking (Fig. 1A), consistent with an increased likelihood of quitting by slow metabolizers relative to normal metabolizers (odds ratio (OR) 2.3, 95% confidence interval (CI) 1.1, 4.8; P=0.037) (Fig. 1B). There was no significant difference in the probability of quitting by intermediate nicotine metabolizers compared to normal nicotine metabolizers (OR 1.3, 95% CI 0.7, 2.2; P=0.436) (Fig. 1B), however a significant linear trend towards increased quitting with decreasing CYP2A6 metabolism (SM>IM>NM) was observed (OR for linear trend across CYP2A6 activity groups = 1.44, 95% CI 1.0, 2.0; P=0.034). In an exploratory analysis restricted to regular smokers (n=188, defined as monthly smokers), we observed a similar direction and size of effect, where slow metabolizers (n=12) were more likely to quit than normal metabolizers (n=154; OR 1.7, 95% CI 0.58, 4.74), although the smaller numbers reduced power.
Table 1.
Selected characteristics of study participants according to CYP2A6 metabolic group
| Characteristic | CYP2A6 Metabolic Group | ||
|---|---|---|---|
| Normal | Intermediate | Slow | |
|
|
|||
| Total sample, % | 78.2 | 14 | 7.8 |
| Male, % (95% CI)b | 33.8 (28.0, 40.0) | 51.0 (36.3, 65.4) | 35.1 (18.4, 55.7) |
| Mean age at baseline, y (95% CI)b | 14.4 (14.2, 14.6) | 14.2 (13.9, 15.4) | 14.0 (13.4, 14.6) |
| Francophone, % (95% CI)b | 22.0 (16.9, 29.2) | 13.0 (4.5, 32.0) | 17.9 (2.7, 62.7) |
| Single parent family, % (95% CI)b | 12.3 (8.4, 17.7) | 7.0 (2.1, 20.9) | 17.7 (6.8, 39.0) |
| Parents smoke, % (95% CI)b | 41.7 (35.6, 48.3) | 43.3 (28.8, 59.1) | 26.7 (11.9, 49.9) |
| Friends smoke, % (95% CI)b | 88.6 (83.7, 92.2) | 92.8 (75.1, 98.2) | 85.4 (62.5, 95.4) |
| Mean monthly cigarettesa (95% CI)b | 17.6 (−4.7, 39.8) | 18.5 (−14.2, 51.3) | 22.0 (−62.0, 106.0) |
| Mean craving scorea (95% CI)b | 5.1 (3.8, 6.5) | 3.3 (1.4, 5.2) | 3.0 (−0.1, 6.1) |
| Mean withdrawal scorea (95% CI)b | 0.8 (0.5, 1.2) | 0.7 (0.2, 1.3) | 0.4 (−0.3, 1.0) |
| Mean self-medication scorea (95% CI)b | 1.7 (1.1, 2.3) | 1.2 (0.03, 2.5) | 0.9 (−0.6, 2.5) |
y, years; CI, confidence interval
Computed in quitters (n = 144) in survey immediately preceding onset of cessation
95% confidence that the population mean will lie in this interval; overlap in 95% CI between CYP2A6 metabolic groups suggests no differences
Figure 1.
Association between CYP2A6 metabolic group and quitting smoking for at least 12 months in adolescence
Three hundred and eight adolescent Caucasian smokers who were genotyped for CYP2A6 variant alleles were grouped according to projected CYP2A6 metabolic activity: CYP2A6 normal metabolizers, CYP2A6 intermediate metabolizers, and CYP2A6 slow metabolizers. A) The proportion in each CYP2A6 metabolic group that quit smoking is shown as a percentage of the total for that CYP2A6 metabolic group. B) The likelihood of quitting smoking for at least 12 months by CYP2A6 intermediate metabolizers and CYP2A6 slow metabolizers relative to CYP2A6 normal metabolizers is shown as odds ratios with 95% confidence intervals. No covariates were used in the model. * Indicates P = 0.037.
Discussion
This is the first prospective cohort investigation of the association between CYP2A6 genotype and quitting smoking in adolescence, and showed that CYP2A6 slow nicotine metabolism is associated with an increased likelihood of quitting smoking. This is similar to the effect of CYP2A6 slow metabolism on increasing quit rates in adults [2], providing further evidence that slow nicotine metabolizers have better quit rates in general.
The current findings add novel information regarding the association between CYP2A6 and the tobacco use continuum in adolescence: while slow nicotine metabolizers convert to dependence faster [4], they smoke fewer cigarettes [4, 6], escalate in dependence slower [5, 6], and, we now show, are more likely to quit. This enhanced quitting ability may be mediated by reduced brain response to smoking-related cues [9]. Among young adult smokers, slow metabolizers (vs. normal metabolizers with similar smoking behaviors) displayed attenuated responses to visual smoking cues in reward and cue processing areas of the brain, as assessed by fMRI [9] which may reduce cravings and potentially improve quitting.
Among adults, slow nicotine metabolizers have higher quit rates on the nicotine patch relative to normal nicotine metabolizers, while bupropion may be more useful for faster metabolizers than slower metabolizers [2]. If these findings extend to younger populations, there may be utility in tailoring smoking cessation treatments based on nicotine metabolism rate in adolescent smokers. For example, the nicotine patch, which is safe and effective in adolescent smokers [10], may be a more effective treatment for adolescent slow nicotine metabolizers.
Strengths of this analysis include the prospective design and repeated sampling, which may minimize recall bias among participants. In addition, multiple imputation was used to manage missing data. Limitations include potential selection bias due to convenience sampling, restriction of the study population to Caucasians (to minimize population stratification), as well as genotyping for prevalent CYP2A6 alleles in Caucasians known to reduce CYP2A6 activity. The presence of undetected CYP2A6 alleles conferring slow nicotine metabolism may misclassify some individuals as normal or intermediate metabolizers, however this would likely reduce, rather than enhance, the likelihood of observing increased quitting among slow metabolizers.
Overall, the findings herein reveal that in adolescence, genetically slow nicotine metabolizers are more likely to quit smoking than normal nicotine metabolizers. A greater understanding of the factors underpinning cessation in adolescence may improve quit rates early in the course of smoking onset, reducing subsequent long-term illnesses and mortality that result from cigarette smoking.
Acknowledgements
The data used in this analysis were drawn from the NDIT study. The authors wish to thank the NDIT research team and participants. This work was supported by a University Endowed Chair in Addictions (R. F. Tyndale) and a Canada Research Chair (J. O’Loughlin), Canadian Institutes of Health Research (CIHR) Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award (M.J. Chenoweth), CIHR (grant TMH-109787 to R.F. Tyndale), NIH (grant DA020830 to R.F. Tyndale), the Canadian Cancer Society (grants 010271 and 017435 to J. O’Loughlin), CAMH, the CAMH foundation, the Canada Foundation for Innovation (#20289 and #16014) and the Ontario Ministry of Research and Innovation.
Footnotes
Conflicts of Interest Dr. Tyndale has been involved in one-day workshops for Novartis and McNeil. For the remaining authors, no conflicts were declared.
References
- 1.O’Loughlin J, et al. Determinants of first puff and daily cigarette smoking in adolescents. Am J Epidemiol. 2009;170(5):585–97. doi: 10.1093/aje/kwp179. [DOI] [PubMed] [Google Scholar]
- 2.Ray R, Tyndale RF, Lerman C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J Neurogenet. 2009;23(3):252–61. doi: 10.1080/01677060802572887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benowitz NL, et al. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin Pharmacol Ther. 2006;80(5):457–67. doi: 10.1016/j.clpt.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 4.O’Loughlin J, et al. Genetically decreased CYP2A6 and the risk of tobacco dependence: a prospective study of novice smokers. Tob Control. 2004;13(4):422–8. doi: 10.1136/tc.2003.007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Koudsi N, et al. The genetic aspects of nicotine metabolism and their impact on adolescent nicotine dependence. Journal of Pediatric Biochemistry. 2010;1(2):105–123. [Google Scholar]
- 6.Audrain-McGovern J, et al. The role of CYP2A6 in the emergence of nicotine dependence in adolescents. Pediatrics. 2007;119(1):e264–74. doi: 10.1542/peds.2006-1583. [DOI] [PubMed] [Google Scholar]
- 7.Lerman C, et al. Genetic variation in nicotine metabolism predicts the efficacy of extended-duration transdermal nicotine therapy. Clin Pharmacol Ther. 2010;87(5):553–7. doi: 10.1038/clpt.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honaker J, King G. What to Do about Missing Values in Time-Series Cross-Section Data. American Journal of Political Science. 2010;54(2):561–581. [Google Scholar]
- 9.Tang DW, et al. Genetic variation in CYP2A6 predicts neural reactivity to smoking cues as measured using fMRI. Neuroimage. 2012;60(4):2136–43. doi: 10.1016/j.neuroimage.2012.01.119. [DOI] [PubMed] [Google Scholar]
- 10.Moolchan ET, et al. Safety and efficacy of the nicotine patch and gum for the treatment of adolescent tobacco addiction. Pediatrics. 2005;115(4):e407–14. doi: 10.1542/peds.2004-1894. [DOI] [PubMed] [Google Scholar]