Abstract
Rationale
Transplantation of stem cells into damaged hearts has had modest success as a treatment for ischemic heart disease. One of the limitations is the poor stem cell survival in the diseased microenvironment. Prolyl hydroxylase domain protein 2 (PHD2) is a cellular oxygen sensor that regulates two key transcription factors involved in cell survival and inflammation, hypoxia-inducible factor (HIF) and nuclear factor-κB (NF-κB).
Objective
We studied if and how PHD2 silencing in human adipose-derived stem cells (ADSCs) enhances their cardioprotective effects after transplantation into infarcted hearts.
Methods and Results
ADSCs were transduced with lentiviral shPHD2 to silence PHD2. ADSCs with or without shPHD2 were transplanted after myocardial infarction (MI) in mice. ADSCs reduced cardiomyocyte apoptosis, fibrosis and infarct size and improved cardiac function. shPHD2-ADSCs exerted significantly more protection. PHD2 silencing induced greater ADSCs survival, which was abolished by shHIF-1α. Conditioned medium (CM) from shPHD2-ADSCs decreased cardiomyocyte apoptosis. Insulin-like growth factor 1 (IGF-1) levels were significantly higher in the CM of shPHD2-ADSCs versus ADSCs, and depletion of IGF-1 attenuated the cardioprotective effects of shPHD2-ADSCs-CM. NF-κB activation was induced by shPHD2 to induce IGF-1 secretion via binding to IGF-1 gene promoter.
Conclusions
PHD2 silencing promotes ADSCs survival in MI hearts and enhances their paracrine function to protect cardiomyocytes. The pro-survival effect of shPHD2 on ADSCs is HIF-1α dependent and the enhanced paracrine function of shPHD2-ADSCs is associated with NF-κB-mediated IGF-1 up-regulation. PHD2 silencing in stem cells may be a novel strategy for enhancing the effectiveness of stem cell therapy after MI.
Keywords: Myocardial infarction, stem cell, survival, paracrine effect, cardiomyocyte
INTRODUCTION
Heart disease is a major cause of mortality and morbidity throughout the world. Acute and chronic loss of cardiomyocytes is a main contributor to the poor cardiac function that culminates in heart failure.1 Therefore, myocardium protection and regeneration are considered as important means of heart disease treatment. Stem cell therapy was intended to regenerate myocardium (cardiomyocytes and supporting vasculature and extracellular matrix). It also appears that injected stem cells can salvage myocardium from death through a protective paracrine mechanism.2 Several types of stem cells have been tested for safety and efficacy in animal models of myocardial infarction (MI) and human MI patients.3 Some of these studies showed that stem cell transplantation in MI animals or patients resulted in improvement of cardiac function, but others showed neutral or temporary benefits.3
One of the limitations of stem cell therapy for heart disease is that transplanted stem cells can hardly survive in the harsh ischemic environment,4–6 which is high in inflammation factors and free radicals generated by oxidative stress.7, 8 Overcoming this limitation could improve the effectiveness of stem cell therapy for cardiac disease. One strategy is to genetically manipulate the expression of key genes involved in cell survival. Prosurvival genes such as Akt, Bcl-2, SDF-1 and chemokine (c-c motif) receptor 1 have been tested to improve stem cell viability after transplantation but with little efficacy.4, 9 In the present study we tested a new target gene, oxygen-dependent prolyl hydroxylase domain protein 2 (PHD2) that regulates two key transcription factors involved in cell survival and inflammation, hypoxia-inducible factor-1α (HIF-1α) and nuclear factor-κB (NF-κB).10 Under normoxia, PHD2 hydroxylates HIF-1α leading to its ubiquitination and degradation11 and thus HIF-1α has a short half-life of about 5 minutes. Hypoxia decreases the activity of PHD2 resulting in less hydroxylation and increased HIF-1α abundance. However, elevated HIF-1α is not stable since reoxygenation easily causes its degradation, which hampers HIF-1α induecd cytoprotection.12 HIF-1α is required for survival of stem cells,13, 14 and hypoxic preconditioning promotes stem cell survival through a HIF-1α dependent mechanism.15 Reducing PHD2 activity induced by hypoxia also enhances IκB kinase activity which activates NF-κB.16 In stem cells, it has been shown that the activation of NF-κB promotes not only the survival but also the secretion of cytokines/growth factors.17
This study tests the hypothesis that PHD2 silencing enhances stem cell survival and paracrine function after transplantation, enhancing protection and repair of the damaged heart. Short hairpin RNA (shRNA) was used to silence PHD2 in human adipose-derived stem cells (ADSCs) that were then injected into a mouse MI model. ADSCs were used because they are easily obtainedand abundant so they can be used without expansion,18–20 and they have the potential of autologous administration or low immunogenicity when used heterologously.21 The experiments performed showed that ADSCs administration promoted angiongenesis and reduced myocardial inflammation, infarct size and fibrosis, leading to improved cardiac function. PHD2 silencing in ADSCs enhanced cardioprotection by promoting ADSCs survival through a HIF-1α dependent pathway and ADSCs secretion of insulin-like growth factor (IGF)-1 via an NF-κB mediated mechanism. These results suggest that PHD2 silencing in ADSCs enhances their therapeutic efficacy in the infarcted heart.
METHODS
Human ADSCs were isolated and cultured as previously described.20 ADSCs of passage 3 were used for this study. ADSCs were infected with lentiviruses containing shRNA-encoding sequences for PHD2 or HIF-1α at the MOI of 10 and stably transduced ADSCs were enriched by cell sorting with FACS Aria II (BD Biosciences) for transplanting into infarcted hearts or further culture. PHD2 silencing and its effect on HIF-1α abundance were confirmed by Western blotting. Permanent ligation of left anterior descending coronary artery and cell injection were performed in male C57 mice (8 weeks old). Immediately after coronary ligation, 1×105 ADSCs suspended in 50μL PBS were injected at 5 sites in the anterior and posterior infarct border zones of the ischemic myocardium. FK506 (3mg/kg/day, i.p., Sigma) was administered to ADSCs-transplanted and sham-operated animals daily from 1 week before the operation until the end of the study. Animal care and all experimental procedures were performed in strict accordance with the approved protocols and animal welfare regulations of the Animal Care and Use Committee at Third Military Medical University. Echocardiography was used to measure cardiac function and immunohistology (Masson's trichrome, TUNEL) was performed to determine cardiac remodeling. TTC staining was done to evaluate infarction size. Cultured ADSCs with or without PHD2 silencing or neonatal rat ventricular myocytes (NRVMs) were challenged with H2O2. To test the protective effect of paracrine factors secreted by ADSCs against H2O2 induced cell death, conditioned medium (CM) from ADSCs cultures was added to NRVMs. Western blotting of activated caspase-3 and TUNEL and annexin V staining were used to determine anti-apoptotic effects of PHD2 silencing in ADSCs and CM on NRVMs. CCK-8 assay and [3H]-thymidine assay were performed to detect the proliferation of ADSCs. Fluorescence activated cell sorting (FACS) analysis was used to determine the percentage of GFP+ cells isolated from ADSCs-injected hearts and annexin V positive cells. To indentify cytokines/growth factors secreted by ADSCs, Human Cytokine Antibody Array C (series 2000 membranes, Ray Biotech) targeting 79 secreted factors was executed. ELISA was done to further measure the concentrations of identified cytokines/growth factors. A chromatin immunoprecipitation (ChIP) assay was performed to detect the association between IGF-1 promoter and NF-κB. Student t-test or one or two-way ANOVA with the Bonferroni post-hoc test were done to determine statistical significance (p<0.05). An expanded Methods section is available in the Supplemental Material.
RESULTS
Expression of PHD2 in ADSCs transduced with lentiviral shPHD2-GFP
Primary cultured ADSCs of passage 3 were characterized by multipotent differentiation potential and surface markers. Similar to a previous study,20 ADSCs maintained the potential to differentiate into adipocytes and osteoblasts, demonstrating their multi-lineage differentiation potential. As shown in Online Figure IA, ADSCs treated with lipogenic medium for 3 weeks were stained with oil red O to mark neutral lipids in the cytoplasm; ADSCs treated with osteogenic medium for 3 weeks were stained with Alizarin red to mark mineralized matrix. Flow cytometry demonstrated that these cells were positive for stem cell markers CD90+, CD73+, CD44+, CD105+, but negative for haematopoietic lineage markers CD45− and CD34− (Online Figure I B). These results indicated that these cells maintain a typical ADSC phenotype.20
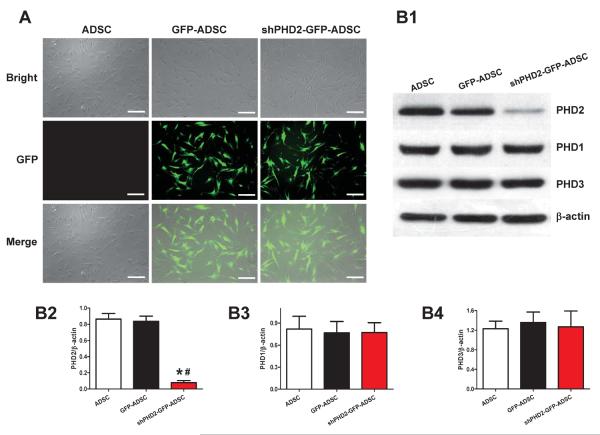
ADSCs were transduced with lentiviruses containing GFP or shPHD2-GFP constructs. Transduced ADSCs were enriched by FACS and then expanded in culture (Figure 1A). shPHD2 significantly decreased PHD2 protein abundance by 90.7%±6.9% (p<0.05) compared withGFP-ADSCs, but did not alter PHD1 and PHD3 protein abundance, documenting the specificity of the approach (Figure 1B).
Figure 1.
Lentiviral transduction of ADSCs with shPHD2-GFP specifically reduced PHD2 expression. A: ADSCs with lentiviral transduction of ADSCs with GFP or shPHD2-GFP constructs. B: Representative blots (B1) and quantification (B2–4) of western blotting analysis of PHD2, PHD1 and PHD3 expression in ADSCs with or without shPHD2. N=6. *p<0.05 vs. ADSCs; # p<0.05 vs. GFP-ADSCs. Scale bar = 200 μm.
Effects of shPHD2-GFP-ADSCs transplantation on myocardial infarct size and cardiac function
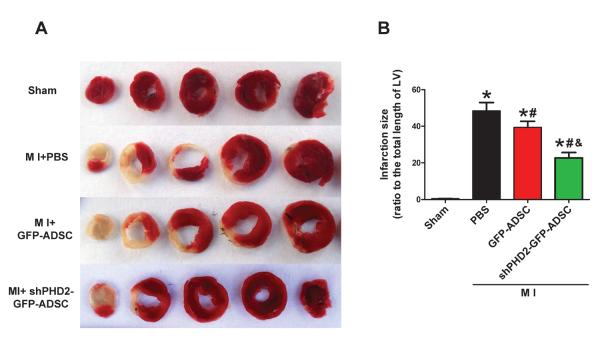
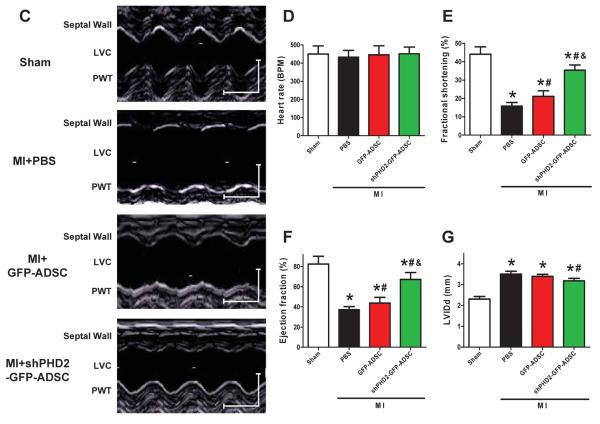
To determine whether silencing of PHD2 generates an enhanced therapeutic effect of stem cells, we evaluated the effects of ADSCs transplantation on infarct size and cardiac function in post-MI mouse hearts. The initial areas at risk (AAR, i.e., the area of ischemia) of infarcted hearts in different groups are comparable (data not shown). Myocardial infarct size was significantly smaller in mice treated with shPHD2-GFP-ADSCs than those with PBS and GFP-ADSCs at 4 weeks post-MI (Figures 2A–B). These data suggest that fewer cardiomyocytes in the AAR die after shPHD2-GFP-ADSCs transplantation. Left ventricle fractional shortening (FS) and ejection fraction (EF) were greater in post-MI hearts at 4 weeks treated with shPHD2-GFP-ADSCs than in post-MI hearts treated with GFP-ADSCs or PBS. MI significantly decreased FS and EF in all groups of post-MI hearts compared to sham-operated hearts (Figures 2C, E and F). Diastolic left ventricle internal diameter (LVIDd) of post-MI hearts at 4 weeks was significantly larger than that of sham hearts, indicating LV dilation induced by MI injury. shPHD2-GFP-ADSCs but not GFP-ADSCs transplantation significantly limited the increase of LVIDd induced by MI (Figure 2G). These results show that ADSCs with shPHD2 reduce the post-MI deterioration of cardiac function, and this cardioprotective effect is significantly greater than that of ADSCs without shPHD2.
Figure 2.
Transplantation of ADSCs with PHD2 silencing reduces infarct size and improves cardiac function in MI mice. A: Representative images of TTC-stained heart sections obtained from Sham, MI+PBS, MI+GFP-ADSCs, and MI+shPHD2-GFP-ADSCs groups at 4 weeks after transplantation. B: LV infarct sizes expressed as the ratio of the length of the infarct band to the total length of LV (N=14). C: Representative M-mode images of hearts with sham surgery or MI at 4 weeks after PBS, GFP-ADSCs and shPHD2-GFP-ADSCs injection. Scale bar: X axis: 0.1 second; Y axis: 0.2 cm. D: Heart rates were controlled to be similar in different groups. E–G: LV fraction shortening (E), LV ejection fraction (F) and diastolic left ventricle internal diameter (LVIDd, G) at 4 weeks after ADSCs transplantation. N=11. * p<0.05 vs. Sham; # p<0.05 vs. MI+PBS; & p<0.05 vs. MI+GFP-ADSCs.
Effects of shPHD2-GFP-ADSCs transplantation on post-MI myocardial remodeling and inflammation
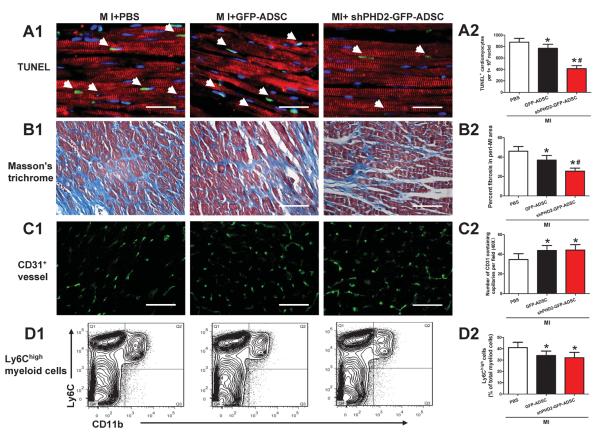
To determine the mechanisms underlying the protective effects of shPHD2-GFP-ADSCs transplantation, TUNEL assay was used to measure apoptotic cell death in the infarct border zone. As shown in Figure 3A and Online Figure II, TUNEL+ cardiomyocyte nucleus number per 106 nuclei was moderately reduced in GFP-ADSCs treated hearts and more significantly reduced in shPHD2-GFP-ADSCs treated hearts at 48 hours post-MI. Masson's trichrome staining showed that there were increased islands of viable cardiac muscle in the peri-infarct regions at 4 weeks after ADSCs treatment. The percentage of fibrotic area in total peri-infarct zone was significantly reduced in ADSCs treated hearts, and shPHD2-GFP-ADSCs further reduced it (Figure 3B and Online Figure III).
Figure 3.
Transplantation of ADSCs with PHD2 silencing reduces cardiomyocyte loss and fibrosis. A: Representative images (A1) and quantification (A2) of TUNEL+ cardiomyocytes in infarct border zone at 48 hours post-MI. TUNEL+ nuclei are stained green, tropomyosin is red and DAPI is blue. B: Representative images (B1) and quantification (B2) of fibrotic area in infarct border zone by Masson's trichrome staining at 4 weeks post-MI. For A and B, scale bar = 50 μm. C: Representative images (C1) and quantification (C2) of capillary density in infarct border zone at 4 weeks post-MI analyzed by CD31 staining. Scale bar = 30 μm. D: Representative contour plots (D1) and quantification (D2) of infiltrating monocyte population (Ly6Chigh/CD11b+) in cells isolated from infracted hearts at 48 hours post-MI analyzed by FACS analysis. N=6. * p<0.05 vs. MI+PBS; # p<0.05 vs. MI+GFP-ADSCs.
We also investigated the effects of shPHD2-GFP-ADSCs transplantation on angiogenesis and inflammation in the post-MI hearts. At 4 weeks after transplantation, immunostaining showed more CD31-expressing capillaries being present in peri-infarct regions of ADSCs versus PBS-treated hearts (Figure 3C). However, there was no difference between GFP-ADSCs and shPHD2-GFP-ADSCs groups. Inflammatory cell infiltration was analyzed by flow cytometry at 48 hours after MI. ADSCs transplantation reduced the percentage of CD11b+/Ly6Chigh cells and shPHD2-GFP-ADSCs and GFP-ADSCs exerted comparable effects (Figure 3D and Online Figure IV). These data suggest that the enhanced therapeutic effect of shPHD2-GFP-ADSCs treatment for MI is associated with reduced cardiomyocyte apoptosis along with a decrease in fibrosis but not related to angiogenesis and inflammatory responses.
Effect of PHD2 silencing on ADSCs survival
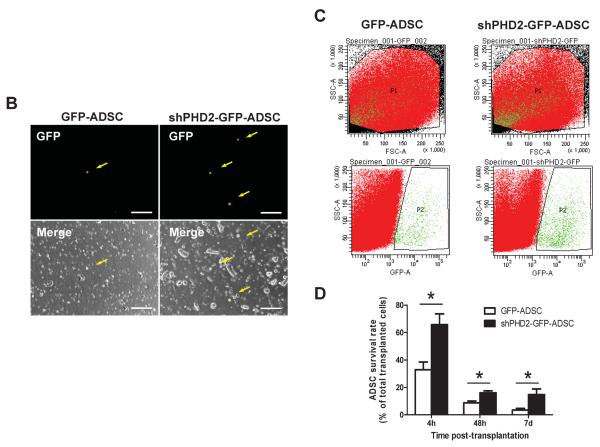
As shown in Figure 4A, the transplanted GFP+ cells were found in vivo by immunostaining. To quantify the survival of transplanted ADSCs, MI hearts were digested at 4 hours, 48 hours and 7 days post-transplantation. There were significantly more GFP+ cells in the myocardium in the shPHD2-GFP-ADSCs group compared with the GFP-ADSCs group at 7 days after transplantation (Figures 4B–C). The better survival rate of shPHD2-GFP-ADSCs was confirmed with FACS analysis of isolated GFP+ cells at multiple time points post-transplantation (Figure 4D). At 4 weeks post-transplantation, more shPHD2-GFP-ADSCs were engrafted in the myocardium than GFP-ADSCs (engraftment rate: 0.65±0.09% vs. 0.12±0.04%, N=4, p<0.05). The increased % of shPHD2-GFP-ADSCs found in the MI heart does not appear to be due to enhanced cell proliferation, assessed with CCK-8 and [3H]-thymidine assays (Online Figure V).
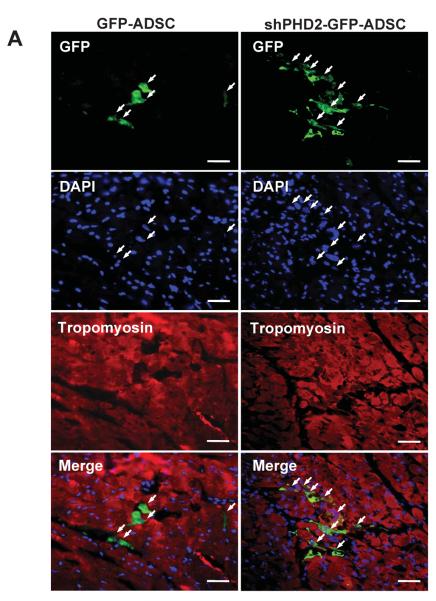
Figure 4.
PHD2 silencing increased ADSCs survival after transplantation to post-MI hearts. A: Representative images of survived GFP-ADSCs and shPHD2-GFP-ADSCs in hearts at 7 days after transplantation. Heart tissue was immunostained for Tropomyosin (red), GFP (green) and DAPI (blue). Scale bar = 50 μm. White arrows indicate surviving ADSCs and their nuclei. B: Post-MI hearts with ADSCs treatment were enzymatically digested, and small cells from the heart (<30 μm diameter) were collected after depletion of cardiomyocytes. As indicated with yellow arrow, GFP labeled cells represent surviving ADSCs under fluorescence microscope. Scale bar = 100 μm. C: Representative flow cytometric plots of surviving GFP+ ADSCs counted by FACS. Gate P1 indicates all the isolated cells from the heart for flow cytometric analysis. Gate P2 indicates the GFP+ cells out of gate P1. D: The percentage of survived ADSCs out of total transplanted ADSCs at different time points. N=4. *p<0.05 vs. GFP-ADSCs.
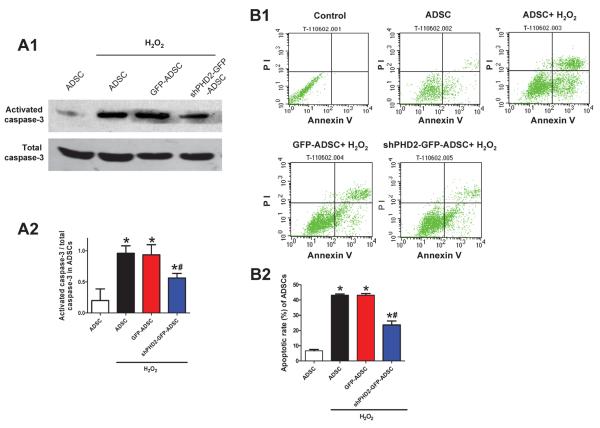
Hypoxia and oxidative stress in the ischemic area of the post-MI heart are thought to be the main reasons for the death of resident cardiomyocytes and transplanted ADSCs.7, 8 We used H2O2 to treat ADSCs and cardiomyocytes to simulate oxidative stress in vitro. ADSCs apoptosis was assessed by Western blotting of activated caspase-3 and FACS of Annexin V staining. H2O2 increased the ratio of activated caspase-3/total caspase-3 and annexin V positive cells in all groups of ADSCs but these increases were significantly blunted by PHD2 silencing (Figures 5A–B).
Figure 5.
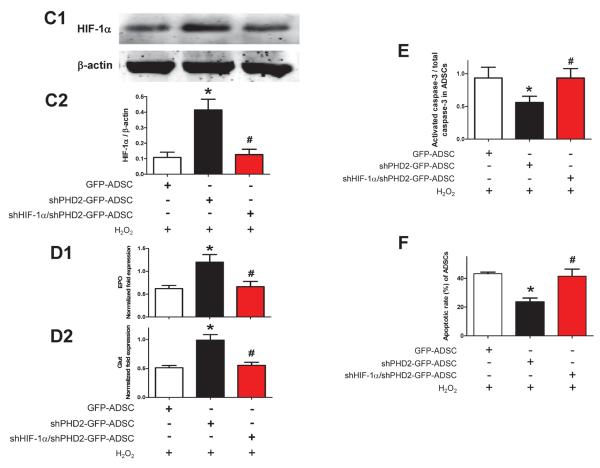
Silencing of PHD2 reduces ADSCs apoptosis induced by oxidative injury via a HIF-1α dependent pathway. A, Representative blots (A1) and quantification (A2) of Western blotting analysis of activated caspase-3 expression in ADSCs subjected to 200 μM H2O2 for 6 hours. B: Flow cytometric analysis with Annexin V-PI double staining assay of ADSCs treated with H2O2. B1, Representative density plots of apoptotic ADSCs (Annexin V+). B2, Average percentages of apoptotic ADSCs. For A–B: N=6. *p<0.05 vs. ADSCs without H2O2; # p<0.05 vs. GFP-ADSCs with H2O2. C: Representative blots (C1) and quantification (C2) of Western blotting analysis of HIF-1α protein expression in ADSCs subjected to H2O2. D: The mRNA expression of canonical HIF downstream targets EPO (D1) and GLUT (D2) in ADSCs subjected to H2O2 analyzed by qPCR. E–F: Western blotting analysis of activated caspase-3 expression (E) and apoptotic rates measured by flow cytometric analysis with Annexin V-PI double staining (F) in ADSCs treated with H2O2 in vitro. For C–F: N=5. *p<0.05 vs. GFP-ADSCs; # p<0.05 vs. shPHD2-GFP-ADSCs.
The mechanism involved in shPHD2-mediated cell protection against H2O2 likely involves HIF-1α stabilization because HIF-1α prolyl hydroxylation by PHDs triggers proteasome targeting and protein degradation.11 In the presence of H2O2, we found that HIF-1α protein abundance was increased 3.8-fold in shPHD2-GFP-ADSCs compared with that in GFP-ADSCs (Figure 5C). Concomitantly, the mRNA levels of canonical HIF downstream targets EPO and GLUT were found to be significantly increased in shPHD2-GFP-ADSCs (Figure 5D). Both the increases of HIF-1α and HIF-1α target mRNAs in shPHD2-GFP-ADSCs could be reduced by shHIF-1α treatment (Figures 5C–D). When HIF-1α was silenced in ADSCs, the anti-apoptotic effect of shPHD2 on ADSCs was lost (Figures 5E–F).
PHD2 silencing does not enhance differentiation of ADSCs into cardiac lineages
We determined the phenotypes of injected cells at 4 weeks after transplantation. A few ADSCs expressed von Willebrand factor or α-smooth muscle actin, indicating that some injected cells differentiated into endothelial cells and smooth muscle cells (Online Figures VIA-B). The contribution of ADSCs to neoangiogenesis was demonstrated by the co-localization of vascular marker CD31 with GFP, indicating the vessels incorporated with cells derived from transplanted ADSCs (Online Figure VII). shPHD2 does not change the potential of ADSCs to form new vessels in the infarcted heart (rate of ADSCs expressed CD31: GFP-ADSCs: 9.54±2.23%; shPHD2-GFP-ADSCs: 9.91±2.08%. N=4, p>0.05). Because of the low survival rate of ADSCs at 4 weeks post-transplantation, the new vessels formed by ADSCs differentiation were still rare, which amounted to a very few of the angiogenesis.
In all treatment groups, no ADSC was found to express sarcomeric tropomyosin or α-actinin, suggesting no mature cardiomyocyte differentiated from ADSCs (Online Figure VIC). Therefore, the enhanced beneficial effects on infarct size and cardiac function by shPHD2-GFP-ADSCs transplantation were not due to ADSC-derived new cardiomyocytes, rather this could be achieved by other mechanisms including cardioprotective paracrine mechanisms.
PHD2 silencing enhances the secretion of anti-apoptotic ADSC paracrine factors to protect cardiomyocytes
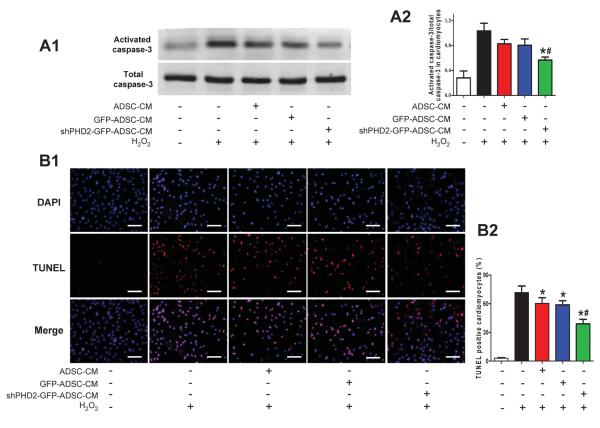
PHD2 silencing in ADSCs could protect the post-MI heart via a paracrine mechanism. This idea was tested by defining the effects of CM from ADSCs with or without shPHD2 on H2O2-induced cardiomyocyte apoptosis. Treatment of NRVMs with 100μM H2O2 for 6 hours caused significant activation of caspase-3. GFP-ADSCs-CM moderately decreased activated caspase-3 of NRVMs but it was significantly deceased by shPHD2-GFP-ADSCs-CM (Figure 6A). GFP-ADSCs-CM decreased TUNEL+ cells compared with NRVMs treated with H2O2 alone, and shPHD2-GFP-ADSCs-CM further decreased TUNEL+ cardiomyocytes (Figure 6B). These data suggest that CM of ADSCs exerts anti-apoptotic effects on injured cardiomyocytes, and this beneficial effect is significantly enhanced by PHD2 silencing.
Figure 6.
The anti-apoptotic effect of ADSCs conditioned medium (CM) on cardiomyocytes subjected to oxidative injury. A: Representative blots (A1) and quantification (A2) of Western blotting analysis of activated caspase-3 of NRVMs treated with H2O2 (N=7). B: Representative images (B1) and quantification (B2) of TUNEL staining in cardiomyocytes subjected to H2O2 treatment (N=16). Cell nuclei were stained with DAPI (blue) and TUNEL+ nuclei were labeled with TMR-red. TUNEL positive rate= (TUNEL positive nuclei / DAPI positive nuclei)×100%. *p<0.05 vs. Control with H2O2; # p<0.05 vs. H2O2+GFP-ADSC-CM.
PHD2 silencing enhances protective paracrine effects of IGF-1 via a NF-κB dependent mechanism in ADSCs
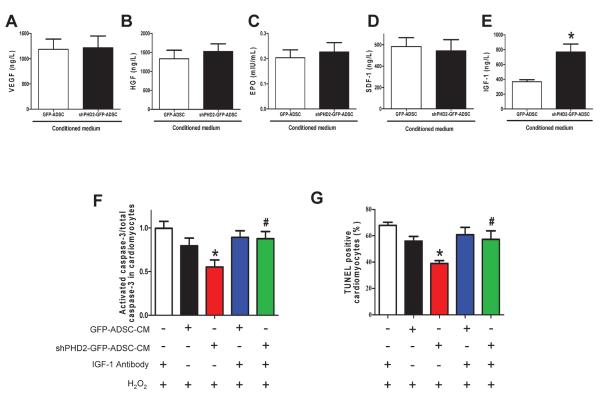
An antibody array detecting 79 cytokines/growth factors was used to identify cardioprotective factors secreted from shPHD2-GFP-ADSCs and GFP-ADSCs. Nine secreted factors were induced by shPHD2 with a >1.3-fold increase in 2 independent array analyses (Online Table I). IGF-1 was increased by 3.30-fold and 4.32-fold in these 2 experiments, which was a greater increase than any other factor. ELISA analysis confirmed that IGF-1 was significantly up-regulated in shPHD2-GFP-ADSCs-CM but the levels of other pro-survival cytokines VEGF, HGF, SDF-1 and EPO did not differ between the two groups of ADSCs (Figures 7A–E).
Figure 7.
PHD2 silencing enhances ADSCs paracrine effect by increasing IGF-1 secretion via the NF-κB pathway. A–E: Levels of pro-survival cytokines, VEGF (A), HGF (B), EPO(C), SDF-1 (D) and IGF-1 (E) in ADSCs-CM determined by ELISA assay. N=10, * p<0.05 vs. GFP-ADSCs-CM. F–G: The effect of IGF-1 neutralization and shHIF-1α on ADSC paracrine mediated anti-apoptotic effects on cardiomyocytes. The cardiomyocyte apoptosis was analyzed by Western blotting of activated caspase-3 expression (F) and TUNEL+ rate (G). N=3. * p<0.05 vs. H2O2+IGF-1 antibody; #p<0.05 vs. H2O2+shPHD2-GFP-ADSC-CM. H–I: The anti-apoptotic effect of shPHD2/shHIF-1α-ADSCs-CM on cardiomyocytes, analyzed by Western blotting of activated caspase-3 expression (H) and TUNEL+ rate (I). N=4. * p<0.05 vs. H2O2+GFP-ADSC-CM. J: The effect of HIF-1α and NF-κB inhibition on NF-κB activity in different groups of ADSCs. * p<0.05 vs. GFP-ADSCs; # p<0.05 vs. shPHD2-GFP-ADSCs. K: The effect of HIF-1α silencing and NF-κB inhibition on IGF-1 secretion of ADSCs. For J–K: N=6. * p<0.05 vs. GFP-ADSCs; # p<0.05 vs. shPHD2-GFP-ADSCs. L–M: The effect of NF-κB inhibition on ADSC paracrine mediated anti-apoptotic effect on cardiomyocytes subjected to H2O2. NF-κB activity is inhibited by BAY11-7082 or IκBα dominant negative. The cardiomyocyte apoptosis was analyzed by Western blotting of activated caspase-3 expression (L) and TUNEL+ rate (M). N=3. * p<0.05 vs. H2O2+GFP-ADSC-CM; # p<0.05 vs. H2O2+shPHD2-GFP-ADSC-CM. VEGF: vascular endothelial growth factor; HGF: hepatocyte growth factor; SDF-1: stromal cell-derived factor 1; IGF-1: insulin-like growth factor 1.
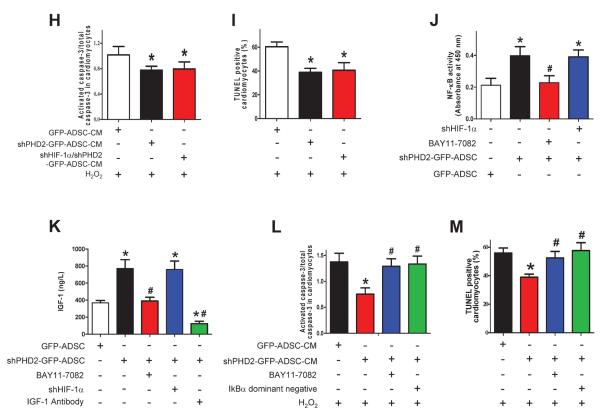
To determine if the enhanced cardioprotective effect of shPHD2-GFP-ADSCs-CM is mediated by IGF-1, CM was treated with a neutralizing IGF-1 antibody. This treatment abrogated the decrease in activated caspase-3 and reduction of the TUNEL+ NRVMs induced by shPHD2-GFP-ADSCs-CM (Figures 7F–G). The IGF-1 antibody alone neither influenced the activation of caspace-3 nor did it change the TUNEL+ rate in cardiomyocytes. These data suggest that IGF-1 is an important pro-survival cytokine associated with shPHD2-GFP-ADSCs. To determine if enhanced IGF-1 secretion in shPHD2-GFP-ADSCs was mediated by HIF-1α stabilization, we prevented the increase in HIF-1α abundance in shPHD2-GFP-ADSCs with shHIF-1α. Double silencing of PHD2 and HIF-1α did not alter the IGF-1 concentration in CM (Figure 7K). Nor did it change the capability of shPHD2-GFP-ADSCs-CM to attenuate cardiomyocyte apoptosis (caspase-3 activation and TUNEL+ rate, Figures 7H–I and Online Figure VIII). These results indicate that HIF-1 signaling may not be involved in the anti-apoptotic effect of the shPHD2-GFP-ADSCs-CM.
The NF-κB pathway is reported to be regulated by PHD2,16, 22, 23 and regulates the expression of IGF-1 independent of HIF-1α signaling.24, 25 Therefore we tested if NF-κB activation accounts for the enhanced IGF-1 secretion of shPHD2-GFP-ADSCs. shPHD2 increased NF-κB activity (Figure 7J) and induced nuclear translocation of NF-κB in ADSCs (Online Figure IX). These effects were blocked by NF-κB inhibitor BAY11-7082. We demonstrated that PHD2 silencing increased the phosphorylation of IKKβ, and further increased the phosphorylation and degradation of IκBα thereby activating NF-κB in ADSCs (Online Figure X), similar to the previous finding.16 BAY11-7082 blocked the elevation of IGF-1 secretion from shPHD2-GFP-ADSCs (Figure 7K). The decrease of cardiomyocyte apoptosis (caspase-3 activation and TUNEL+ rate) induced by shPHD2-GFP-ADSCs-CM was eliminated by BAY11-7082 or the IκBα dominant negative mutant (Figures 7L–M and Online Figure XI).
The molecular bases of NF-κB mediated secretion of IGF-1 have not clearly defined. We demonstrated that the IGF-1 mRNA was significantly increased by NF-κB stimulator lipopolysaccharide (Online Figure XIIA). Three NF-κB binding sites in IGF-1 promoter were predicted using TFSEARCH program. A ChIP assay showed that the predicted site 2 of the IGF-1 promoter binded to NF-κB p50 (Online Figure XIIB). These results indicate that NF-κB signaling can be activated by PHD2 silencing, and NF-κB activation may directly up-regulate the transcription of IGF-1, increasing the secretion of IGF-1 in ADSCs.
Effects of HIF-1α silencing and IGF-1 neutralization on the viability of shPHD2-GFP-ADSCs in infarct myocardium and cardiac function
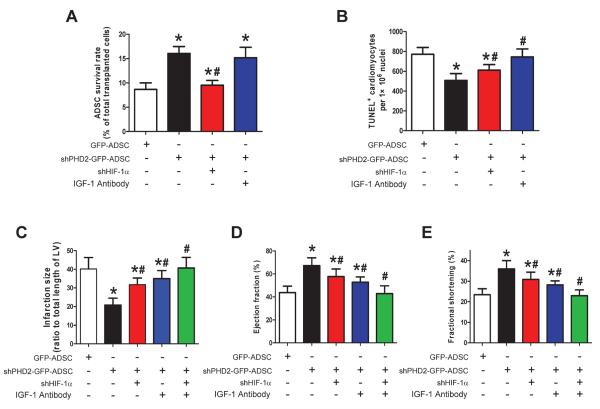
Our in vitro data suggest that PHD2 silencing promotes the survival of ADSCs via a HIF-1α dependent pathway and that IGF-1 secreted by shPHD2-GFP-ADSCs protects cardiomyocytes from apoptosis. We further determined the roles of HIF-1α and IGF-1 in the beneficial effects of shPHD2-GFP-ADSCs in vivo. HIF-1α silencing abolished the protection of shPHD2 against ADSCs death after being injected into the myocardium (Figure 8A). However, the beneficial effects of shPHD2-GFP-ADSCs on post-MI infarction size, cardiac function and cardiomyocyte apoptosis were not completely eliminated by HIF-1αsilencing (Figures 8B–E and Online Figure XIII). IGF-1 antibody injected with shPHD2-GFP-ADSCs did not change ADSC survival (Figure 8A), but abolished shPHD2-GFP-ADSCs induced protection on cardiomyocytes (Figure 8B). Similarly, the beneficial effects of shPHD2-GFP-ADSCs on MI heart were not completely eliminated by blocking IGF-1 (Figures 8C–E and Online Figure XIII). The combination of silencing HIF-1α and blocking IGF-1 treatment fully inhibited the therapeutic effects of shPHD2-GFP-ADSCs on MI heart (Figures 8C–E and Online Figure XIII). Collectively, these results suggest that both enhancing ADSCs survival and IGF-1 secretion are central to the beneficial effects of PHD2 silencing in ADSCs.
Figure 8.
HIF-1α silencing and IGF-1 neutralization reduce the therapeutic potential of shPHD2-GFP-ADSCs on infarcted hearts. A: The percentage of survived ADSCs out of total transplanted ADSCs at 48 hours after ADSCs transplantation. B: TUNEL+ cardiomyocytes in infarct border zone at 48 hours after ADSCs transplantation. C–E: LV infarct size (C), EF% (D) and FS% (E) at 4 weeks after MI. For A–E: N=6. * p<0.05 vs. GFP-ADSCs; # p<0.05 vs. shPHD2-GFP-ADSCs.
DISCUSSION
Stem cell therapy to repair damaged myocardium has evolved into a promising treatment for ischemic heart disease. One of the limitations of stem cell therapy for heart disease is the low survival rate of transplanted stem cells.4–6 Apoptosis has been implicated as the main contributor to the massive loss of donor cells.26 Inflammation dose not appear to be a factor accelerating the death of injected ADSCs because human ADSCs have a low immunogenic profile and elicit little inflammatory response.21 Furthermore, an immune suppressant drug was administrated in our study to achieve an equivalence of immune suppression as in allograft transplantation.27 The major factor contributing to transplanted cell death appears to be the limited blood supply in the infarct zone that produces hypoxia and oxidative stress. The present study explored a novel approach to enhance the viability of stem cells injected into this harsh environment.
We tested a novel hypothesis that reducing PHD2 abundance in ADSCs would enhance their survival and protective paracrine function when injected into the hostile environment of a post-MI heart. Transplantation of modified ADSCs with PHD2 silencing reduced infarction size and improved cardiac function in MI mice. In MI hearts, we measured ADSCs survival, ADSC differentiation, cardiomyocyte survival, angiogenesis, fibrosis and inflammatory responses after cell transplantation. We found that those beneficial effects of PHD2 silencing in ADSCs were associated with improved ADSC survival, reduced cardiomyocyte apoptosis and decreased fibrosis. The experiments showed that PHD2 silencing reduced ADSCs death via a HIF-1α dependent pathway, and that PHD2 silencing in ADSCs limited cardiomyocyte apoptosis through a protective paracrine mechanism mediated by enhanced IGF-1 secretion after the activation of NF–κB signaling (summarized in Online Figure XIV). Our study provides strong support for approaches that enhance the survival and function of stem cells injected into hearts with limited blood supply.
PHD proteins were originally identified as oxygen-sensitive enzymes that transduce changes in oxygen availability into changes in the stability of the HIF-1α, a master regulator of genes that promote survival in a low-oxygen environment.28 There are three isoforms of PHD enzymes, PHD1, PHD2, and PHD3.28 PHD2 is considered to be the key oxygen sensor and the primary HIF prolyl hydroxylase in the heart, which maintains low steady-state levels of HIF-1αin normoxia by regulating HIF-1αdegradation.11 Previous studies have shown that PHD2 inhibition can improve cardiomyocyte viability and cardiac function in stressed hearts. Oral administration of a PHD inhibitor increased the microvascular density in infarcted rat hearts by stabilizing HIF-1α29 Direct intramyocardial injection of a shRNA plasmid targeting PHD2 led to significant improvement in angiogenesis and cardiac contractility after MI.30 However, inhibiting PHD2 in all cells in the heart or deficiency of PHD2 in cardiomyocytes has yielded controversial results. Cardiomyocyte specific PHD2 knockout mice ameliorated cardiac disease progression induced by MI for a period of 3 weeks31 but these mice developed more severe cardiac dysfunction when subjected to transverse aortic constriction or MI over a longer time period.32 Long-term inactivation of PHD2 in cardiomyocytes leads to dilated cardiomyopathy and premature mortality.33 These studies suggest that direct inhibition of PHD2 in cardiomyocytes could cause damaging effects and the beneficial effect of PHD2 inhibition in the ischemic heart might be through non-cardiomyocyte mechanisms, e.g., by promoting angiogenesis29,30. In the present study, PHD2 activity was specifically modified in transplanted stem cells, avoiding the above-mentioned risks and promoted cardiomyocyte survival. Our findings suggest that PHD2 silencing in transplanted ADSCs promotes cardiomyocyte survival via a protective paracrine mechanism mediated by IGF-1.
Mechanisms of cardioprotection of ADSCs and shPHD2-GFP-ADSCs
A previous study showed that a non-specific PHD inhibitor, dimethyloxalylglycine, significantly attenuated apoptosis of cultured mesenchymal stem cells (MSCs) induced by serum deprivation.34 The exact mechanism mediating the beneficial effects of PHD2-mediated regulation of cell survival, however, is still not clear. We demonstrated that silencing of PHD2 significantly increased HIF-1α abundance thereby promoting ADSC survival in the injured heart in vivo and ADSCs challenged with H2O2 in vitro. These pro-survival effects on ADSCs were fully abolished with simultaneous HIF-1α silencing.
Since ADSCs did not differentiate into cardiomyocytes and ADSC differentiation into vascular cells was rare, it seems that the paracrine function was the major mechanism for the beneficial effects of injected ADSCs. Transplanted ADSCs with or without PHD2 silencing increase capillary density in post-MI hearts, likely by secreting angiogenic factors (VEGF, EGF and HGF) that may protect cardiomyocytes. PHD2 silencing did not further increase the secretion of these angiogenic factors and capillary density. Thus the cardiomyocyte protection exerted by PHD2 silencing in ADSCs cannot be ascribed to increased capillary density. Our results suggest that PHD2 silencing increases the secretion of IGF-1 in ADSCs. IGF-1 provides the cardioprotective effects via reduction of cardiomyocyte apoptosis both in vitro and in vivo. IGF-1 is thought to reduce apoptosis of ischemic cardiomyocytes,35 through up-regulating anti-apoptotic protein Bcl-2.36 It was reported that exogenous stem cell transplantation might activate resident cardiac stem cells (CSCs) via a paracrine action.37 Some paracrine factors secreted by stem cells including IGF-1 may stimulate CSCs-mediated endogenous cardiac regeneration.38 Thus it is also possible that PHD2 silencing enhanced the ADSC-stimulated endogenous cardiac repair through increasing the secretion of pro-regenerative factors such as IGF-1.
Silencing HIF-1α or neutralizing secreted IGF-1 only partially attenuated the therapeutic effect of shPHD2-GFP-ADSCs in vivo. Thus, stem cell survival and paracrine factors regulated by PHD2 silencing appear to function synergistically to promote the cardioprotective effects of transplanted ADSCs.
How is IGF-1 induced by PHD2 silencing?
Our data shows that HIF-1α silencing does not block the increase of IGF-1 induced by shPHD2, indicating that the enhancement of the paracrine function by shPHD2 is HIF-1α independent. These results are consistent with those showing that constitutive expression of HIF-1α in bone marrow stromal cells does not increase IGF-1.39 The activation of NF-κB in MSCs leads to higher expression of pro-survival paracrine factors including IGF-1.24 Our data demonstrate that shPHD2 induces NF-κB activation probably by enhancing IKKβ activity and thus reducing IκBα, the native NF-κB inhibitor. We show that inhibition of NF-κB blocks the increase of IGF-1 secretion in shPHD2-GFP-ADSCs and NF-κB activation increases the mRNA expression of IGF-1. Also, ChIP assays suggest that NF-κB p50 can bind to IGF-1 promoter. Collectively, these data indicate that activation of NF-κB can directly up-regulate the transcription of IGF-1, ultimately increasing the secretion of IGF-1. This can, at least partially, explain the increased HIF-1α–independent paracrine effects that we observed.
Will PHD2 silencing effects work in other types of stem cells?
PHD2, HIF-1 and NF-κB are ubiquitously expressed in various types of stem cells. As a cellular oxygen sensor, the regulatory effects of PHD2 on HIF-1 are well known in various cell types.11, 28, 29 The association between PHD2 and NF-κB signaling was also reported in cancer cells16,23 besides ADSCs. In addition, non-specific PHD inhibition attenuated apoptosis of cultured bone marrow-MSCs induced by serum deprivation.34 These studies indicate that the effect of PHD2 silencing is not specific to ADSCs and may work in other types of stem cells.
CONCLUSIONS
Our study effectively combines beneficial properties of PHD2 inhibition and advantages of stem cell therapy for cardiac repair. Silencing of PHD2 enhances the beneficial effects of ADSCs by increasing their viability and paracrine function. These beneficial effects are associated with HIF and NF-κB signaling pathways. PHD2 silencing could have a similar effect in other types of stem cells, and therefore, it could be a novel strategy for enhancing the efficacy of stem cell mediated cardiac repair after MI.
Supplementary Material
Novelty and Significance.
What Is Known?
Poor stem cell survival in the hostile microenvironment limits their efficacy for treating ischemic heart disease.
Paracrine effects appear to be an important mechanism for the therapeutic effects of adult stem cell.
Prolyl hydroxylase domain protein 2 (PHD2) is a cellular oxygen sensor that regulates two key transcription factors involved in cell survival and inflammation, hypoxia-inducible factor (HIF) and nuclear factor-κB (NF-κB).
What New Information Does This Article Contribute?
PHD2 silencing in transplanted adipose-derived stem cells (ADSCs) promotes their therapeutic efficacy in post-myocardial infarction (MI) mouse hearts.
PHD2 silencing promotes the survival of transplanted ADSCs in MI hearts through a HIF-1α dependent pathway.
PHD2 silencing enhances the paracrine function of ADSCs to protect cardiomyocytes, through NF-κB-mediated IGF-1 up-regulation.
Stem cell transplantation to repair damaged myocardium is a promising, novel treatment for ischemic heart disease. However, a major limitation of this approach is poor survival and engraftment of stem cells in the ischemic heart. This study offers a new approach to overcome this limitation by silencing PHD2 in stem cells. PHD2 silencing promoted the survival and beneficial paracrine function of transplanted ADSCs, an abundant and easy-to-acquire source of stem cells. In ADSCs, PHD2 regulates two key transcription factors, HIF-1α and NF-κB, under hypoxic and oxidative stress. PHD2 silencing enhanced stem cell viability by stabilizing HIF-1α, and promoted stem cell paracrine mediated anti-apoptotic effects by inducing NF-κB-regulated IGF-1 expression. Stem cells with PHD2 silencing had significant beneficial effects on structural and functional remodeling in post-MI hearts. Modulating the molecular pathways that determine stem cell survival and function may be critical in implementing stem cell therapy for diseased hearts.
ACKNOWLEDGMENTS
We thank Dr. Rongtuan Lin and Dr. Yingying Le for providing the dominant negative Iκα plasmid.
SOURCES OF FUNDING
This study was supported by the National Natural Science Foundation of China (31130029, 81100111, 30925018, 2013CB531104), the National Basic Research Program of China (973 Program, 2008CB517308, 2012CB517801 and 2013CB531104) and Chongqing Natural Science Foundation (cstc2011jjzt0118, cstc2012jjB10020). Dr. Dayue Darrel Duan's laboratory was supported by the National Heart, Lung, and Blood Institute Grants HL106256, HL113598, and American Heart Association 11GRNT7610161. Dr. Xiongwen Chen' laboratory was supported by NIH HL088243 and American Heart Association 0730347N. Dr. Steven R. Houser's laboratory was supported by grants NIH HL089312, HL033921, HL091799.
Nonstandard Abbreviations and Acronyms
- AAR
area at risk
- ADSC
adipose-derived stem cell
- ChIP
chromatin immunoprecipitation
- CM
conditioned medium
- EF
ejection fraction
- FACS
Fluorescence activated cell sorting
- FS
fractional shortening
- HIF
hypoxia-inducible factor
- IGF-1
insulin-like growth factor 1
- LVIDd
diastolic left ventricle internal diameter
- MI
myocardial infarction
- MSC
mesenchymal stem cell
- NF-κB
nuclear factor-κB
- NRVM
neonatal rat ventricular myocyte
- PHD2
Prolyl hydroxylase domain protein 2
- shRNA
short hairpin RNA
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Christoffels V. Regenerative medicine: Muscle for a damaged heart. Nature. 2011;474:585–586. doi: 10.1038/474585a. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen TL, Raveendran G, Zhang J, Garry DJ. Getting to the heart of myocardial stem cells and cell therapy. Circulation. 2011;123:1771–1779. doi: 10.1161/CIRCULATIONAHA.109.858019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ptaszek LM, Mansour M, Ruskin JN, Chien KR. Towards regenerative therapy for cardiac disease. Lancet. 2012;379:933–942. doi: 10.1016/S0140-6736(12)60075-0. [DOI] [PubMed] [Google Scholar]
- 4.Penn MS, Mangi AA. Genetic enhancement of stem cell engraftment, survival, and efficacy. Circ Res. 2008;102:1471–1482. doi: 10.1161/CIRCRESAHA.108.175174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terrovitis JV, Smith RR, Marban E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res. 2010;106:479–494. doi: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forte G, Pietronave S, Nardone G, Zamperone A, Magnani E, Pagliari S, Pagliari F, Giacinti C, Nicoletti C, Musaro A, Rinaldi M, Ribezzo M, Comoglio C, Traversa E, Okano T, Minieri M, Prat M, Di Nardo P. Human cardiac progenitor cell grafts as unrestricted source of supernumerary cardiac cells in healthy murine hearts. Stem Cells. 2011;29:2051–2061. doi: 10.1002/stem.763. [DOI] [PubMed] [Google Scholar]
- 7.Tongers J, Losordo DW, Landmesser U. Stem and progenitor cell-based therapy in ischaemic heart disease: Promise, uncertainties, and challenges. Eur Heart J. 2011;32:1197–1206. doi: 10.1093/eurheartj/ehr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chacko SM, Ahmed S, Selvendiran K, Kuppusamy ML, Khan M, Kuppusamy P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol. 2010;299:C1562–1570. doi: 10.1152/ajpcell.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Zhang Z, Guo J, Ni A, Deb A, Zhang L, Mirotsou M, Pratt RE, Dzau VJ. Genetic modification of mesenchymal stem cells overexpressing ccr1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circulation Research. 2010;106:1753–1762. doi: 10.1161/CIRCRESAHA.109.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kappab in hypoxic inflammation. J Physiol. 2008;586:4055–4059. doi: 10.1113/jphysiol.2008.157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. Hif prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of hif-1alpha in normoxia. EMBO J. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang M, Nguyen P, Jia F, Hu S, Gong Y, de Almeida PE, Wang L, Nag D, Kay MA, Giaccia AJ, Robbins RC, Wu JC. Double knockdown of prolyl hydroxylase and factor-inhibiting hypoxia-inducible factor with nonviral minicircle gene therapy enhances stem cell mobilization and angiogenesis after myocardial infarction. Circulation. 2011;124:S46–54. doi: 10.1161/CIRCULATIONAHA.110.014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milosevic J, Maisel M, Wegner F, Leuchtenberger J, Wenger RH, Gerlach M, Storch A, Schwarz J. Lack of hypoxia-inducible factor-1 alpha impairs midbrain neural precursor cells involving vascular endothelial growth factor signaling. J Neurosci. 2007;27:412–421. doi: 10.1523/JNEUROSCI.2482-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Li H, Xi HS, Li S. Hif1alpha is required for survival maintenance of chronic myeloid leukemia stem cells. Blood. 2012;119:2595–2607. doi: 10.1182/blood-2011-10-387381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing cxcr4 expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates ikappab kinase-beta, giving insight into hypoxia-induced nfkappab activity. Proc Natl Acad Sci U S A. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao C, Xiu Y, Ashton J, Xing L, Morita Y, Jordan CT, Boyce BF. Noncanonical nf-kappab signaling regulates hematopoietic stem cell self-renewal and microenvironment interactions. Stem Cells. 2012;30:709–718. doi: 10.1002/stem.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667–2677. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Deng J, Tian W, Xiang B, Yang T, Li G, Wang J, Gruwel M, Kashour T, Rendell J, Glogowski M, Tomanek B, Freed D, Deslauriers R, Arora RC, Tian G. Adipose-derived stem cells are an effective cell candidate for treatment of heart failure: An mr imaging study of rat hearts. Am J Physiol Heart Circ Physiol. 2009;297:H1020–1031. doi: 10.1152/ajpheart.01082.2008. [DOI] [PubMed] [Google Scholar]
- 20.Bai X, Yan Y, Song YH, Seidensticker M, Rabinovich B, Metzele R, Bankson JA, Vykoukal D, Alt E. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur Heart J. 2010;31:489–501. doi: 10.1093/eurheartj/ehp568. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Yuan J, Zhou B, Lee AJ, Ghawji M, Jr., Yoo TJ. The therapeutic efficacy of human adipose tissue-derived mesenchymal stem cells on experimental autoimmune hearing loss in mice. Immunology. 2011;133:133–140. doi: 10.1111/j.1365-2567.2011.03421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konisti S, Kiriakidis S, Paleolog EM. Hypoxia--a key regulator of angiogenesis and inflammation in rheumatoid arthritis. Nat Rev Rheumatol. 2012;8:153–162. doi: 10.1038/nrrheum.2011.205. [DOI] [PubMed] [Google Scholar]
- 23.Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter mct1 supports an nf-kappab/il-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- 24.Afzal MR, Haider H, Idris NM, Jiang S, Ahmed RP, Ashraf M. Preconditioning promotes survival and angiomyogenic potential of mesenchymal stem cells in the infarcted heart via nf-kappab signaling. Antioxid Redox Signal. 2010;12:693–702. doi: 10.1089/ars.2009.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. Human mesenchymal stem cells stimulated by tnf-alpha, lps, or hypoxia produce growth factors by an nf kappa b-but not jnk-dependent mechanism. Am J Physiol Cell Physiol. 2008;294:C675–682. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- 26.Bai X, Yan Y, Coleman M, Wu G, Rabinovich B, Seidensticker M, Alt E. Tracking long-term survival of intramyocardially delivered human adipose tissue-derived stem cells using bioluminescence imaging. Mol Imaging Biol. 2011;13:633–645. doi: 10.1007/s11307-010-0392-z. [DOI] [PubMed] [Google Scholar]
- 27.Yuan SH, Martin J, Elia J, Flippin J, Paramban RI, Hefferan MP, Vidal JG, Mu Y, Killian RL, Israel MA, Emre N, Marsala S, Marsala M, Gage FH, Goldstein LS, Carson CT. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS One. 2011;6:e17540. doi: 10.1371/journal.pone.0017540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loinard C, Ginouves A, Vilar J, Cochain C, Zouggari Y, Recalde A, Duriez M, Levy BI, Pouyssegur J, Berra E, Silvestre JS. Inhibition of prolyl hydroxylase domain proteins promotes therapeutic revascularization. Circulation. 2009;120:50–59. doi: 10.1161/CIRCULATIONAHA.108.813303. [DOI] [PubMed] [Google Scholar]
- 29.Bao W, Qin P, Needle S, Erickson-Miller CL, Duffy KJ, Ariazi JL, Zhao S, Olzinski AR, Behm DJ, Pipes GC, Jucker BM, Hu E, Lepore JJ, Willette RN. Chronic inhibition of hypoxia-inducible factor prolyl 4-hydroxylase improves ventricular performance, remodeling, and vascularity after myocardial infarction in the rat. J Cardiovasc Pharmacol. 2010;56:147–155. doi: 10.1097/FJC.0b013e3181e2bfef. [DOI] [PubMed] [Google Scholar]
- 30.Huang M, Chan DA, Jia F, Xie X, Li Z, Hoyt G, Robbins RC, Chen X, Giaccia AJ, Wu JC. Short hairpin rna interference therapy for ischemic heart disease. Circulation. 2008;118:S226–233. doi: 10.1161/CIRCULATIONAHA.107.760785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holscher M, Silter M, Krull S, von Ahlen M, Hesse A, Schwartz P, Wielockx B, Breier G, Katschinski DM, Zieseniss A. Cardiomyocyte-specific prolyl-4-hydroxylase domain 2 knock out protects from acute myocardial ischemic injury. J Biol Chem. 2011;286:11185–11194. doi: 10.1074/jbc.M110.186809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minamishima YA, Moslehi J, Bardeesy N, Cullen D, Bronson RT, Kaelin WG., Jr. Somatic inactivation of the phd2 prolyl hydroxylase causes polycythemia and congestive heart failure. Blood. 2008;111:3236–3244. doi: 10.1182/blood-2007-10-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moslehi J, Minamishima YA, Shi J, Neuberg D, Charytan DM, Padera RF, Signoretti S, Liao R, Kaelin WG. Loss of hypoxia-inducible factor prolyl hydroxylase activity in cardiomyocytes phenocopies ischemic cardiomyopathy. Circulation. 2010;122:1004–1016. doi: 10.1161/CIRCULATIONAHA.109.922427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu XB, Wang JA, Ogle ME, Wei L. Prolyl hydroxylase inhibitor dimethyloxalylglycine enhances mesenchymal stem cell survival. J Cell Biochem. 2009;106:903–911. doi: 10.1002/jcb.22064. [DOI] [PubMed] [Google Scholar]
- 35.Hynes B, Kumar AH, O'Sullivan J, Klein Buneker C, Leblond AL, Weiss S, Schmeckpeper J, Martin K, Caplice NM. Potent endothelial progenitor cell-conditioned media-related anti-apoptotic, cardiotrophic, and pro-angiogenic effects post-myocardial infarction are mediated by insulin-like growth factor-1. Eur Heart J. 2013;34:782–789. doi: 10.1093/eurheartj/ehr435. [DOI] [PubMed] [Google Scholar]
- 36.Mehrhof FB, Muller FU, Bergmann MW, Li P, Wang Y, Schmitz W, Dietz R, von Harsdorf R. In cardiomyocyte hypoxia, insulin-like growth factor-i-induced antiapoptotic signaling requires phosphatidylinositol-3-oh-kinase-dependent and mitogen-activated protein kinase-dependent activation of the transcription factor camp response element-binding protein. Circulation. 2001;104:2088–2094. doi: 10.1161/hc4201.097133. [DOI] [PubMed] [Google Scholar]
- 37.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padin-Iruegas ME, Misao Y, Davis ME, Segers VF, Esposito G, Tokunou T, Urbanek K, Hosoda T, Rota M, Anversa P, Leri A, Lee RT, Kajstura J. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120:876–887. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ben-Shoshan J, Schwartz S, Luboshits G, Maysel-Auslender S, Barzelay A, Polak-Charcon S, Tzahor E, Barshack I, Barak A, Levkovitch-Verbin H, Keren G, George J. Constitutive expression of hif-1alpha and hif-2alpha in bone marrow stromal cells differentially promotes their proangiogenic properties. Stem Cells. 2008;26:2634–2643. doi: 10.1634/stemcells.2008-0369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.