Abstract
Background
The classic definition of MT, ≥10 units red blood cells (RBCs) in 24 hours, has never been demonstrated as a valid surrogate for severe hemorrhage and can introduce survival bias. In addition, the definition fails to capture other products that the clinician may have immediately available during the initial resuscitation. Assuming that units of resuscitative fluids reflect patient illness, our objective was to identify a rate of resuscitation intensity (RI) that could serve as an early surrogate of sickness for patients with substantial bleeding post-injury.
Methods
Adult patients surviving at least 30 minutes post-admission and receiving ≥1 RBC within 6 hours of admission from ten US Level 1 trauma centers were enrolled in the PRospective Observational Multicenter Major Trauma Transfusion study. Total fluid units were calculated as the sum of the number of crystalloid units (1 L=1 unit), colloids (0.5 L=1 unit) and blood products (1 RBC=1 unit, 1 plasma=1 unit, 6 pack platelets=1 unit). Univariable and multivariable logistic regressions were used to evaluate associations between RI and 6-hour mortality, adjusting for age, center, penetrating injury, weighted Revised Trauma Score, and Injury Severity Score.
Results
1096 eligible patients received resuscitative fluids within 30 minutes, including 620 transfused with blood products. Despite varying products utilized, the total fluid RI was similar across all sites (3.2±2.5 units). Patients who received ≥4 units of any resuscitative fluid had a 6-hour mortality rate of 14.4% vs. 4.5% in patients who received <4 units. The adjusted odds ratio of 6-hour mortality for patients receiving ≥4 units within 30 minutes was 2.1 (95% Confidence Interval: 1.2–3.5).
Conclusions
Resuscitation with ≥4 units of any fluid was significantly associated with 6-hour mortality. This study suggests that early RI regardless of fluid type can be used as a surrogate for sickness and mortality in severely bleeding patients.
Level of Evidence
PROMMTT is a prospective observational study, Level II.
Keywords: rate of transfusion, mortality, plasma, crystalloid, colloid
BACKGROUND
Trauma is the leading cause of death in people under the age of 45 years with hemorrhage accounting for nearly half of these deaths.1–2 Of deaths due to hemorrhage, most occur within the first six hours of admission and many will receive a massive transfusion (MT).3–5 The classical definition of MT is the replacement of a patient’s blood volume within a 24-hour period, commonly considered as transfusion of ≥10 packed red blood cell (RBC) units within 24 hours.6 This definition is based on the approximated blood volume of a 70 kg male and is flawed on multiple levels as it assumes standardized weight, gender and achievement of hemostasis. In addition, this definition fails to capture exsanguinating patients who die before receiving their tenth unit of RBCs and is thus subject to survival bias.7 A recent international forum on the treatment of trauma coagulopathy in patients receiving MT highlighted twelve different definitions for MT.8 In Australia, Mitra et al. (2011) redefined MT to at least 5 units of RBCs in 4 hours based on a cohort of 387 patients to capture patients who were immediate MT candidates as well as patients who develop a need for MT later during the course of their surgical and intensive care management.9 Similarly, several investigators have advocated for MT to be redefined as ≥10 units of RBCs in 6 hours.10–12 These authors have argued that patients receiving ≥10 units in the first 6 hours are quite different in presentation, injury severity, and physiology from the those receiving the same number over a 24-hour time frame.
Regardless, even these definitions of MT remain to be poor surrogates for hemorrhage and bleeding severity. They assume standardized care and do not take into account current resuscitation practices that employ a variety of products, including crystalloids, colloids, RBCs, plasma, platelets and cryoprecipitate. Since MT has been used to target the treatment of acute traumatic coagulopathy with early administration of blood products, this requires having blood products readily available in the Emergency Department (ED) and/or en-route to the hospital via ambulance or helicopter. However, all Level 1 Trauma Centers do not have the same amount or types of resuscitation products immediately available, in particular blood product availability varies between centers. A potential solution is to evaluate all resuscitative fluids administered in the ED.
Another challenge with trauma research is the issue of survival bias, which is often ignored in clinical observational studies.13–14 With respect to hemorrhage and resuscitation research, the survival bias debate centers on two questions: did the treatment (higher ratios) cause patients to survive longer or did patients receive specific treatment only because they survived long enough? Treatment is extremely time sensitive and without compelling evidence to guide uniform transfusion practice, considerable variation persists across all Level 1 trauma centers.6,15–16
Unlike previous papers, which analyzed the effects of specific blood products and resuscitative fluids, we proposed to evaluate all crystalloids, colloids and blood products as group totals, termed as resuscitation intensity (RI), to avoid variability in product availability. Furthermore, since patients had to survive at least 30 minutes to be enrolled in PROMMTT, we evaluated the total RI within the first 30 minutes to eliminate survival bias. We hypothesized that RI in the first 30 minutes of arrival would provide a more generalized definition for sickness and potentially serve as a surrogate for bleeding severity and early mortality in severely injured trauma patients.
METHODS
Study Design
PRospective Observational Multicenter Major Trauma Transfusion (PROMMTT) was an observational clinical study conducted at ten Level-1 trauma centers aimed to identify practices leading to improved survival for trauma patients who require massive blood transfusions. The local institutional review board at each study site, including the Data Coordinating Center (DCC), approved the study. The US Army Human Research Protections Office also provided a second level review and approval.17–18
The PROMMTT study design has been previously described in detail.17–18 Briefly, adult trauma patients (age 16 years or older) with the highest level of trauma activation were enrolled in PROMMTT if they survived for at least 30 minutes and received at least one unit of RBCs within 6 hours of admission. Patients were excluded if: 1) transferred from an outside facility; 2) received more than five minutes of CPR prior to or within 30 minutes of admission; 3) they had a burn injury > 20% of total body surface area; 4) inhalation injury diagnosed by bronchoscopy; 5) pregnant; 6) prisoner; or 7) declared dead within 30 minutes of admission. If ineligibility was identified sometime after enrollment, the patient was withdrawn from the study and post-enrollment samples and data were destroyed.
Data Collection
Standard operating procedure manuals were developed and provided to site coordinators during their training. Research assistants screened and enrolled patients 24/7, recording exact times of all infused crystalloids, colloids and blood products, as well as patient outcomes during direct observation. Direct bedside observation continued until the end of active resuscitation; defined as the time the center transfusion protocol was discontinued, occurrence of death, or two hours after the last blood product transfusion, whichever came first. Following the end of direct observation, new interventions, complications, and outcomes were recorded daily while the patient was in the intensive care unit (ICU) and weekly thereafter during hospitalization. Individual site clinicians ascribed cause of in-hospital death without confirmation or central adjudication and data collectors ascertained sites of bleeding. The DCC audited study data for missing values and outliers.
Description of Variables
Patient demographics including age, gender and race were documented and reported. In addition, the following characteristics were collected: admission vital signs, Glasgow Coma Scale (GCS) score, admission laboratory tests, mechanism and types of injuries. Admission vital signs were used to calculate the weighted Revised Trauma Score (w-RTS) and Injury Severity Scores (ISS). The w-RTS is a product of initial GCS, systolic blood pressure, and respiratory rate, using coded and weighted values ranging from 0 to 4 (poor to normal) for each physiologic variable (yielding range of 0–7.841), thereby representing the physiologic state of injury. Given that many patients were intubated upon arrival or in the ED, the respiratory rate was coded as 0 to account for the poor respiratory state. We also evaluated the w-RTS using a code of 4 instead of 0 to account for the mechanical ventilation of 20 breaths per minute for such cases. Since the respiratory component is the least weighted in calculating w-RTS, using a code of 0 instead of 4 did not change our results. Real-time data related to volume, and types of resuscitative fluid administered within the first 30 minutes of the ED were used in the analysis. Patients were followed and reports on 6-hr and 24-hr mortality, trips to the operating room (OR), and prevalence of co-morbidities were documented.
Total RI was defined as the number of “units” of crystalloid, colloid and blood products administered in the ED. Specifically, Total Crystalloid = Normal Saline (NS) + Lactated Ringer’s Solution (LR) + Plasmalyte + Other crystalloids; Total Colloid = Albumin + Hespan + Hextend + Other colloids; Total Blood = RBCs + FFP + platelets; and Total Units = Total Crystalloid + Total Colloid + Total Blood received in the first 30 minutes. The conversion of crystalloid and colloid fluid volumes to “units” is defined in Table 1. Please note that although hypertonic saline (HTS) was documented in the study, it was not included in total crystalloid calculations for the reason that HTS is used mostly for the treatment of head trauma and not plasma expansion via resuscitation.
Table 1.
Conversion of crystalloid and colloid fluid volumes into units for total resuscitation intensity calculation.
| 1 Unit = | 1000 mL Normal Saline |
| 1000 mL Lactated Ringer’s solution | |
| 1000 mL Plasmalyte | |
| 250 mL Hypertonic Saline | |
| 1000 mL Other Crystalloids | |
| 500 mL Albumin | |
| 500 mL Hespan | |
| 500 mL Hextend | |
| 500 mL Other Colloids |
Statistical Analysis
Univariable and multivariable logistic regressions were used to evaluate associations between RI and 6-hour and 24-hour mortality, adjusting for age, center, penetrating injury, w-RTS, and ISS. The primary outcome of interest was in-hospital mortality at 6 and 24 hours. We first performed logistical regressions to investigate associations between RI and in-hospital mortality. Mixed models with site as the random intercept were also performed for comparison. Secondary and sub-group analyses of associations between Total Crystalloid, Total Colloid and Total Blood and early mortality were also evaluated. Additionally, we were interested in assessing the correlation between RI and emergent OR (i.e. OR within the first 30 and 60 minutes). It should be noted that RI was only calculated for the resuscitation administered within the first 30 minutes to avoid survival bias since patients had to survive at least 30 minutes to be enrolled in PROMMTT. Odds ratio and 95% confidence levels are reported for all significant associations. No interactions (RI multiplied by a primary covariate) were significant at the 0.05 level.
All analyses were performed using SAS Enterprise 4.3 (Cary, North Carolina). Manuscript preparation was guided by the STROBE statement for the reporting of cohort studies in epidemiology.19
RESULTS
There were 34,362 total trauma admissions across ten centers over a period of 58 weeks. 12,560 patients were screened and had data collection initiated. Of these, 11,315 became ineligible and were withdrawn from the study, leaving 1,245 that met all PROMMTT eligibility criteria (Supplemental Digital Content 1). 1235 patients had complete transfusion records and were included in the statistical analysis. Nearly 75% were male, 55% white and 84% were under the age of 60 years. A full breakdown and description of patient demographics and admission characteristics are illustrated in Table 2.
Table 2.
Demographics and admission characteristics of patients with a total RI<4 and RI≥4 units.
| Total RI < 4 units (n=762) | Total RI ≥ 4 units (n=473) | p | |||
|---|---|---|---|---|---|
|
| |||||
| Median(IQR) | No. missing | Median(IQR) | No. missing | ||
| Age, years | 38 (24–55) | 1 | 37 (24–52) | 0 | 0.44 |
| Male, No. (%) | 554 (73%) | 0 | 362 (77%) | 0 | 0.13 |
| Penetrating, No. (%) | 254 (33%) | 0 | 182 (38%) | 0 | 0.07 |
| GCS | 14 (3–15) | 63 | 5 (3–15) | 42 | <0.0001 |
| ISS | 23 (14–34) | 0 | 27 (16–38) | 0 | 0.0003 |
| w-RTS | 7.26 (4.09–7.84) | 67 | 4.5 (4.09–7.11) | 64 | <0.0001 |
| SBP, mmHg | 113 (94–134) | 11 | 91 (73–115) | 23 | <0.0001 |
| DBP, mmHg | 70 (58–84) | 111 | 60 (46–77) | 106 | <0.0001 |
| Heart rate, bpm | 103 (85–120) | 17 | 110 (88–130) | 12 | 0.0007 |
| Respiratory rate | 20 (18–25) | 304* | 21.5 (18–27) | 251* | 0.34 |
| Base Deficit | 5 (2–9) | 185 | 8 (5–12.8) | 98 | <0.0001 |
| OR within first 30 min | 110 (14%) | 0 | 144 (30%) | 0 | <0.0001 |
| OR within first 60 min | 198 (26%) | 0 | 216 (46%) | 0 | <0.0001 |
| Massive Transfusion | 117 (15%) | 0 | 178 (38%) | 0 | <0.0001 |
| Substantial Bleeding | 145 (19%) | 0 | 252 (53%) | 0 | <0.0001 |
| 6 hour mortality | 34 (4.5%) | 0 | 68 (14.4%) | 0 | <0.0001 |
| 24 hour mortality | 57 (7.5%) | 0 | 90 (19%) | 0 | <0.0001 |
Univariable Analysis
Admission characteristics were similar to previously published PROMMTT data.17–18 Of note, patients were significantly acidotic with high base deficits, high lactate levels and low pH values (Table 2). AIS and ISS distributions were fairly similar across the ten centers. Given the severe injury scores and poor admission vitals, it is not surprising that majority of enrolled patients received pre-hospital resuscitation. In fact, 83% of the enrolled patients were resuscitated in the field, leaving 191 patients receiving no resuscitative fluids (Table 3). Pre-hospital resuscitation was predominantly crystalloid-based using NS or LR; only 27 patients received blood products. Upon arrival in the ED, 90% of patients received resuscitation in the first 30 minutes. Of these, 50% were transfused with blood products. Colloid use was minimal during this time (Table 3). This means that 10% of patients did not receive any resuscitative fluid pre-hospital or within the first 30 minutes of admission, despite receiving blood products at later time points. These patients with RI=0, had 11.5% incidence of head trauma which was not statistically different from the total population. Median crystalloid use was 1100 ml (IQR=700–2100 ml) and median blood use was 1 unit (IQR=0–2 units) resulting in a median RI of 3 units (IQR=1–5 units) within the first 30 minutes of ED arrival.
Table 3.
Summary of early fluid resuscitation characteristics.
| Frequency (%)* | Median Volume (IQR) | Mean Volume (SD) | |
|---|---|---|---|
| Pre-hospital fluids given? | |||
| Yes = | 1033(83%) | ||
| No = | 191(15%) | ||
| If yes, type of fluid: | |||
| Normal Saline | 903(73%) | 600 ml (735 ml) | |
| Lactated Ringer’s | 160 (13%) | 159 ml (586 ml) | |
| RBCs | 27(2%) | 1.58 units(0.5 U) | |
| Fluid given in the first 30 minutes in ED? | |||
| Yes = | 1096 (89%) | ||
| No = | 139 (11%) | ||
| If yes, type of fluid: | |||
| Normal Saline | 749 (61%) | 600 ml (0–1100 ml) | 836 ml (998.8 ml) |
| Lactated Ringer’s | 385 (31%) | 0 ml (0–950 ml) | 422.9 ml (780.3 ml) |
| Plasmalyte | 120 (9.7%) | 0 ml (0-0 ml) | 136.8 ml (484.9 ml) |
| Hypertonic Saline | 8 (0.6%) | 0 ml (0-0 ml) | 2.51 ml (35.75 ml) |
| Other Crystalloid | 126 (10%) | 0 ml (0-0 ml) | 107.5 ml (384.7 ml) |
| Albumin | 7 (0.6%) | 0 ml (0-0 ml) | 2.83 ml (37.55 ml) |
| Hespan | 2 (0.2%) | 0 ml (0-0 ml) | 1.13 ml (29.3 ml) |
| Hextend | 1 (0.1%) | 0 ml (0-0 ml) | 0.4 ml (14.2 ml) |
| Other Colloid | 5 (0.4%) | 0 ml (0-0 ml) | 2.02 ml (34.6 ml) |
| RBCs | 611 (49%) | 0 units (0–2 U) | 1.13 units (1.46 U) |
| Plasma | 164 (13%) | 0 units (0-0 U) | 0.28 units (0.78 U) |
| Platelets | 4 (0.3%) | 0 units (0-0 U) | 0.02 units (0.45 U) |
| Cryoprecipitate | 2 (0.2%) | 0 units (0-0 U) | 0.002 units (0.04 U) |
| Cell Saver | 3 (0.2%) | 0 units (0-0 U) | 0.003 units (0.07 U) |
|
| |||
| Total Crystalloid | 1029 (83%) | 1100 ml (700–2100 ml) | 1502.6 ml (1227.6 ml) |
| Total Colloid | 15 (1%) | 0 ml (0-0 ml) | 6.4 ml (60.35 ml) |
| Total Blood | 620 (50%) | 1 unit (0–2 U) | 1.43 units (2 U) |
|
| |||
| Total Fluids | 1096 (89%) | 3 units (1–5 U) | 3.24 units (2.6 U) |
column percentages may total over 100% because a patient could have received multiple types of resuscitative fluids. (n=1,224 pre-hospital, n=1,235 in ED).
Definitions for Total variables:
Total crystalloid = NS + LR + Plasmalyte + Other crystalloids,
Total colloid = Albumin + Hespan + Hextend + Other colloids,
Total blood = RBCs + plasma + platelets.
Univariable analysis of 6-hour and 24-hour mortality and total RI exhibited a strong exponential trend with a R2=0.80 and 0.78, respectively (Figure 1). We observed a two-fold increase in mortality in patients receiving 3–4 units of any resuscitative fluid (compared to 1–2 units) and another doubling with patients receiving >6 units of fluids. This led us to create two groups: total RI<4 units and total RI ≥4 units for our multivariable analysis.
Figure 1.
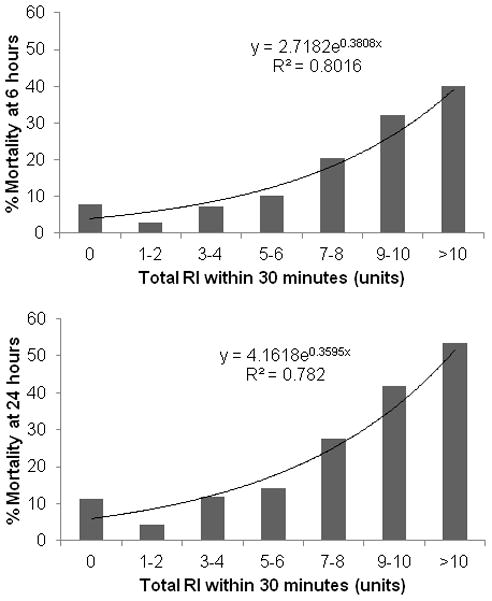
Univariable analysis of total RI and mortality at 6 and 24 hours.
Multivariable Analysis
Total RI was dichotomized into RI<4 units and RI ≥4 units for multivariable analysis based on our observations from the univariable analysis on mortality. Multiple logistic regression revealed a greater than two-fold increase in mortality at 6 hours (i.e. mortality rate of 14.4% vs. 4.5%) and 76% increase in mortality at 24 hours in patients receiving ≥4 units, controlling for age, site, penetrating injury, w-RTS and ISS (Table 4); head AIS did not contribute beyond ISS and w-RTS. We observed no significant site effect in the analyses of associations between mortality and RI. The mixed model with site as a random intercept revealed similar results.
Table 4.
A) Multivariable logistic regressions for 6 and 24-hour mortality when controlling for site; B) Multivariable logistic regressions for 6 and 24-hour mortality excluding site since the effect was not statistically significant.
| A.
| |||
|---|---|---|---|
| For 6-hour mortality | |||
| Variable | Odds Ratio | 95% CI | p-value |
| Age | 1.01 | 0.99–1.03 | 0.0714 |
| Total RI ≥4 units vs. <4 units | 2.09 | 1.2–3.5 | 0.0056 |
| RTS | 0.74 | 0.63–0.87 | 0.0002 |
| ISS | 1.03 | 1.02–1.05 | <0.0001 |
| Penetrating injury | 2.67 | 1.5–4.7 | 0.0008 |
| Site* | 0.56–1.74 | 0.18–8.9 | 0.7509 |
|
| |||
| For 24-hour mortality | |||
| Variable | Odds Ratio | 95% CI | p-value |
|
| |||
| Age | 1.01 | 1.0–1.03 | 0.0184 |
| Total RI ≥4 units vs. <4 units | 1.54 | 1.0–2.375 | 0.0481 |
| RTS | 0.65 | 0.57–0.75 | <0.0001 |
| ISS | 1.03 | 1.02–1.04 | <0.0001 |
| Penetrating injury | 2.19 | 1.3–3.6 | 0.002 |
| Site* | 0.82–2.99 | 0.36–10.3 | 0.5511 |
|
| |||
|
B.
| |||
| For 6-hour mortality | |||
| Variable | Odds Ratio | 95% CI | p-value |
|
| |||
| Age | 1.01 | 0.98–1.03 | 0.6507 |
| Total RI ≥4 units vs. <4 units | 2.21 | 1.09–4.51 | 0.0286 |
| RTS | 0.79 | 0.67–0.93 | 0.0049 |
| ISS | 1.05 | 1.03–1.07 | <0.0001 |
| Penetrating injury | 4.11 | 1.80–9.38 | 0.0008 |
|
| |||
| For 24-hour mortality | |||
| Variable | Odds Ratio | 95% CI | p-value |
|
| |||
| Age | 1.01 | 0.99–1.03 | 0.4245 |
| Total RI ≥4 units vs. <4 units | 1.76 | 0.96–3.22 | 0.0662 |
| RTS | 0.73 | 0.64–0.84 | <0.0001 |
| ISS | 1.05 | 1.03–1.07 | <0.0001 |
| Penetrating injury | 3.16 | 1.56–6.40 | 0.0014 |
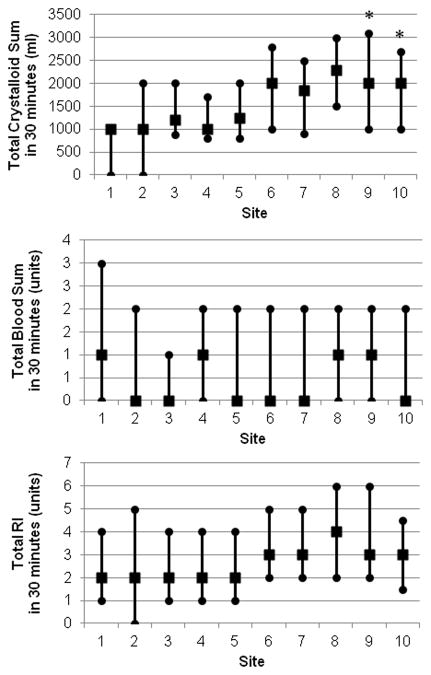
Despite similar RI across sites, we suspected varying crystalloid and blood product use. Sub-group analysis of total RI split into “Total Crystalloid” and “Total Blood” use within the first 30 minutes was evaluated while controlling for the same aforementioned parameters. Total blood use was shown to have a significant effect on 6-hour mortality (OR: 1.27, 95% CI: 1.14–1.42, p-value<0.0001) and 24-hour mortality (OR: 1.25, 95% CI: 1.13–1.37, p-value<0.0001). We also observed a significant site effect on 24-hour mortality; 2 out of 10 centers had significantly higher 24-hour mortality rates compared to the referent site (Site 9: OR: 2.4, 95% CI:1.05–5.48, p-value=0.037, and Site 10: OR: 3.9, 95% CI:1.1–13.65, p-value=0.035). These centers had the greatest discrepancy between choice of products in the first 30 minutes, with a relatively increased crystalloid use (Figure 2). Note that site 1 was used as the referent site, since it had the highest number of enrolled patients.
Figure 2.
Distribution of resuscitative fluids across ten centers. A) Total crystalloid use, B) Total Blood use, and C) Total RI. The total RI was fairly similar across sites, however crystalloid and blood use varied between centers. Sites 9 and 10 were had statistically
When considering emergent operation as the outcome of interest, patients with a total RI ≥4 units were twice as likely to go to the operating room; with an odds ratio of 2.4 (95% CI: 1.78–3.34, p<0.0001) and there was a significant site effect (p=0.046). However, some patients died prior to reaching the operating room.
DISCUSSION
In-hospital mortality at 6 hours post-admission was increased more than two-fold in patients receiving ≥4 units of resuscitative fluids in the first 30 minutes of arrival. The major findings of this study were 1) total RI was similar across centers despite varying crystalloid and blood use; 2) centers that used more crystalloid than blood products in the first 30 minutes had significantly higher mortality rates at 24 hours post-admission, during which 77% of the hemorrhagic deaths had occurred18; and, possibly, 3) that use of ≥4 units of any resuscitative product within 30 minutes of arrival may serve as a surrogate for sickness and bleeding severity in trauma patients.
In trauma patients with substantial bleeding and rapid blood loss, inadequate transfusion of blood products is associated with early death. However, actual transfusion of blood products is complicated by recognizing the need for blood, ordering of appropriate products, having products immediately availability in the blood bank and/or ED, and obtaining and finally transfusing these products in a quick and appropriate fashion. Clinicians need to rapidly identify patients who are severely hemorrhaging, and several predictive algorithms have been developed to do this.18,20–25 Once bleeding patients have been identified, heterogeneous transfusion and resuscitation practice persists (Figure 2). This is demonstrated by not only the different types of resuscitative fluids used but also the differences in plasma:RBC:platelet ratios.15,18,26–30 In this paper, we proposed to use total RI as an indicator for mortality and bleeding severity that would not be biased by survival or affected by availability of blood products across sites and showed that RI was indeed a strong predictor for early mortality at 6 and 24 hours. This was based on the hypothesis that, regardless of product availability or a physician’s resuscitation strategy, the intensity of early resuscitation would be similar.
Finding reliable and immediate indicators for bleeding severity and continuing hemorrhage rates remains a challenge in trauma transfusion practice and research.31 Cumulative counts of patients’ total RBC units received within the first 6 to 24 hours, based on the classical definition of MT, is the standard, though poor, surrogate. We therefore sought an exploratory approach to analyze total RI inclusive of all resuscitative fluids, along with the appropriate statistical analysis that would incorporate the requirements for time-dependent and multi-level techniques and thereby reduce the potential for bias. We strongly believe that we must revise the manner in which we approach trauma research to plan and account for heterogeneity among patients (e.g., variations in the severity of blood loss and rates of continuing hemorrhage) and competing risks of different injuries (heads vs. hemorrhage vs. combined) and trauma centers (e.g., variations in blood product availability, MT definitions, and blood bank-bedside transit times).32–35 As observed in our patient population, an overall 7–8% of patients had head injuries and these patients generally received less fluid volumes than other patients.
The strengths of this study are its prospective, multicenter design and teaming a dedicated DCC (epidemiologists, informatics experts, and biostatisticians) with a group of Level 1 trauma centers. By evaluating all resuscitative fluids in the first 30 minutes, instead of blood products only, or MT patients only, we reduced the impact or likelihood of availability and survival bias. Direct-observational recording of the timing of crystalloid, colloid and blood product infusions, combined with appropriate data analysis strategies, strengthened the quality of the data set. Limitations of this study include missing values on potentially important covariates, such as base deficit, which are unavoidable in observational clinical studies of severely injured trauma patients, and other unmeasured but potentially important confounders and effect modifiers.
In summary, we have shown that total RI in the first 30 minutes is strongly associated with early mortality at 6 and 24 hours post-admission. Patients who received ≥4 units of fluids within the first 30 minutes were 2.2 times more likely to die within 6 hours than patients receiving <4 units, regardless of fluid type. The data from this study also highlighted the uniformity of RI across ten Level 1 trauma centers, but that disparities in crystalloid and blood use affected mortality. In particular, two centers with increased crystalloid use and decreased blood use in the first 30 minutes had significantly higher 24-hour mortality rates while controlling for injury severity. Although, there will always be heterogeneity in trauma research, this study suggests that RI in the first 30 minutes can potentially serve as a surrogate for sickness. We admit that this is a first attempt at developing a new and much more generalized definition for sickness and severe hemorrhage; this is not to be confused with the replacement of MT. Further work needs to be done to develop more reliable definitions for MT. Some attempts have been made by Rahbar et al. (2012) and del Junco et al. (2012) using latent class models and other statistical manipulations to aid in the recognition of bleeding severity.36–37 Such new approaches and techniques are necessary for the future of clinical care and improved trauma research.
Supplementary Material
CONSORT flow diagram for PROMMTT study
Acknowledgments
Study concept and design: Holcomb, del Junco, MH Rahbar, Fox
Acquisition of data: Alarcon, Brasel, Bulger, Cohen, Cotton, Holcomb, Muskat, Myers, Phelan, Schreiber
Analysis and interpretation of data: E Rahbar, del Junco, Fox, Cotton, Holcomb, Wade
Drafting of the manuscript: E Rahbar, Wade, Cotton, Holcomb, Fox, del Junco
Critical revision of the manuscript for important intellectual content: del Junco, Holcomb, Fox, E Rahbar, MH Rahbar, Wade, Bulger, Cotton, Schreiber, Alarcon, Brasel, Cohen, Muskat, Myers, Phelan
Statistical analysis: E Rahbar, del Junco, Fox, MH Rahbar
Obtained funding: MH Rahbar
Administrative, technical, or material support: MH Rahbar, Holcomb, Fox, del Junco, Bulger, Cotton, Schreiber, Wade, Myers, Phelan, Brasel, Alarcon, Muskat, Cohen
Study supervision: MH Rahbar, Wade, Cotton, Holcomb
Footnotes
Previous Presentation of the Information Reported in the Manuscript: Portions of these data were presented at the PROMMTT Symposium held at the 71st Annual Meeting of the American Association for the Surgery of Trauma (AAST) on September 10-15, 2012 in Kauai, Hawaii.
Disclaimer: The views and opinions expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Army Medical Department, Department of the Army, the Department of Defense, or the United States Government.
Conflict of Interest Disclosures: Dr. Holcomb reported serving on the board for Tenaxis, the Regional Advisory Council for Trauma, and the National Trauma Institute; providing expert testimony for the Department of Justice; grants funded by the Haemonetics Corporation, and KCI USA, Inc. and consultant fees from the Winkenwerder Company. Dr Wade reported serving on the Science Board for Resuscitation Products, Inc. and the Advisory Board for Astrazeneca. Dr. Cotton reported grant funding from Haemonetics Corporation. No other disclosures were reported.
Disclosures of funding: This project was funded by the U.S. Army Medical Research and Materiel Command subcontract W81XWH-08-C-0712. Infrastructure for the Data Coordinating Center was supported by CTSA funds from NIH grant UL1 RR024148.
Contributor Information
Elaheh Rahbar, Email: Elaheh.Rahbar@uth.tmc.edu.
Erin E. Fox, Email: Erin.e.Fox@uth.tmc.edu.
Deborah J. del Junco, Email: Deborah.j.DelJunco@uth.tmc.edu.
John A. Harvin, Email: jaharvin@gmail.com.
John B. Holcomb, Email: John.Holcomb@uth.tmc.edu.
Charles E. Wade, Email: Charles.e.Wade@uth.tmc.edu.
Martin A. Schreiber, Email: schreibm@ohsu.edu.
Mohammad H. Rahbar, Email: Mohammad.h.Rahbar@uth.tmc.edu.
Eileen M. Bulger, Email: ebulger@u.washington.edu.
Herb A. Phelan, Email: herb.phelan@utsouthwestern.edu.
Karen J. Brasel, Email: kbrasel@mcw.edu.
Louis H. Alarcon, Email: AlarconL@ccm.upmc.edu.
John G. Myers, Email: myersjg@uthscsa.edu.
Mitchell J. Cohen, Email: mcohen@sfghsurg.ucsf.edu.
Peter Muskat, Email: muskatp@uc.edu.
Bryan A. Cotton, Email: Bryan.a.Cotton@uth.tmc.edu.
References
- 1.Cothren CC, Moore EE, Hedegaard HB, Meng K. Epidemiology of urban trauma deaths: a comprehensive reassessment 10 years later. World J Surg. 2007;31:1507–1511. doi: 10.1007/s00268-007-9087-2. [DOI] [PubMed] [Google Scholar]
- 2.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 suppl):S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 4.Demetriades D, Murray J, Charalambides K, et al. Trauma fatalities: time and location of hospital deaths. J Am Coll Surg. 2004;198:20–26. doi: 10.1016/j.jamcollsurg.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 6.Malone DL, Hess JR, Fingerhut A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J Trauma. 2006;60:S91–96. doi: 10.1097/01.ta.0000199549.80731.e6. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, Cocanour CS, Balldin BC, Mckinley BA. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62:112–119. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 8.Levi M, Fries D, Gombotz H, et al. Prevention and treatment of coagulopathy in patients receiving massive transfusions. Vox Sang. 2011;101:154–174. doi: 10.1111/j.1423-0410.2011.01472.x. [DOI] [PubMed] [Google Scholar]
- 9.Mitra B, Cameron PA, Gruen RL, Mori A, Fitzgerald M, Street A. The definition of massive transfusion in trauma: a critical variable in examining evidence for resuscitation. Eur J Emerg Med. 2011;18:137–142. doi: 10.1097/MEJ.0b013e328342310e. [DOI] [PubMed] [Google Scholar]
- 10.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, et al. Post-injury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65:261–270. doi: 10.1097/TA.0b013e31817de3e1. discussion 270–271. [DOI] [PubMed] [Google Scholar]
- 11.Moore FA, Nelson T, McKinley BA, et al. Massive transfusion in trauma patients: tissue hemoglobin oxygen saturation predicts poor outcome. J Trauma. 2008;64:1010–1023. doi: 10.1097/TA.0b013e31816a2417. [DOI] [PubMed] [Google Scholar]
- 12.Nunez TC, Dutton WD, May AK, Holcomb JB, Young PP, Cotton BA. Emergency department blood transfusion predicts early massive transfusion and early blood component requirement. 2010. Transfusion. 50(9):1914–1920. doi: 10.1111/j.1537-2995.2010.02682.x. [DOI] [PubMed] [Google Scholar]
- 13.van WC, Davis D, Forster AJ, Wells GA. Time-dependent bias was common in survival analyses published in leading clinical journals. J Clin Epidemiol. 2004;57(7):672–682. doi: 10.1016/j.jclinepi.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Austin PC, Platt RW. Survivor treatment bias, treatment selection bias, and propensity scores in observational research. J Clin Epidemiol. 2010;63(2):136–138. doi: 10.1016/j.jclinepi.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 16.Wade CE, del Junco DJ, Holcomb JB, et al. Variations between level I trauma centers in 24-hour mortality in severely injured patients requiring a massive transfusion. J Trauma. 2011;71(2 Suppl 3):S389–S393. doi: 10.1097/TA.0b013e318227f307. [DOI] [PubMed] [Google Scholar]
- 17.Rahbar MH, Fox EE, del Junco DJ, et al. Coordination and management of multicenter clinical studies in trauma: Experience from the PRospective Observational Multicenter Major Trauma (PROMMTT) Study. Resuscitation. 2011 doi: 10.1016/j.resuscitation.2011.09.019. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holcomb JB, del Junco DJ, Fox EE, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: Comparative effectiveness of a time-varying treatment with competing risks. Arch Surg. 2012 doi: 10.1001/2013.jamasurg.387. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Von EE, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Yucel N, Lefering R, Maegele M, et al. Trauma Associated Severe Hemorrhage (TASH)-Score: probability of mass transfusion as surrogate for life threatening hemorrhage after multiple trauma. J Trauma. 2006;60(6):1228–1236. doi: 10.1097/01.ta.0000220386.84012.bf. [DOI] [PubMed] [Google Scholar]
- 21.Schreiber MA, Perkins J, Kiraly L, Underwood S, Wade C, Holcomb JB. Early predictors of massive transfusion in combat casualties. J Am Coll Surg. 2007;205(4):541–545. doi: 10.1016/j.jamcollsurg.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Nunez TC, Voskresensky IV, Dossett LA, Shinall R, Dutton WD, Cotton BA. Early prediction of massive transfusion in trauma: simple as ABC (assessment of blood consumption)? J Trauma. 2009;66(2):346–352. doi: 10.1097/TA.0b013e3181961c35. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin DF, Niles SE, Salinas J, et al. A predictive model for massive transfusion in combat casualty patients. J Trauma. 2008;64(2 Suppl):S57–S63. doi: 10.1097/TA.0b013e318160a566. [DOI] [PubMed] [Google Scholar]
- 24.Rainer TH, Ho AM, Yeung JH, et al. Early risk stratification of patients with major trauma requiring massive blood transfusion. Resuscitation. 2011;82(6):724–729. doi: 10.1016/j.resuscitation.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Borgman MA, Spinella PC, Holcomb JB, et al. The effect of FFP:RBC ratio on morbidity and mortality in trauma patients based on transfusion prediction score. Vox Sang. 2011;101(1):44–54. doi: 10.1111/j.1423-0410.2011.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stinger HK, Spinella PC, Perkins JG, Grathwohl KW, Salinas J, Martini WZ, Hess JR, Dubick MA, Simon CD, Beekley AC, Wolf SE, Wade CE, Holcomb JB, Park MS. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma. 2008;64:S79–85. doi: 10.1097/TA.0b013e318160a57b. discussion S. [DOI] [PubMed] [Google Scholar]
- 27.Riskin DJ, Tsai TC, Riskin L, et al. Massive transfusion protocols: the role of aggressive resuscitation versus product ratio in mortality reduction. J Am Coll Surg. 2009;209(2):198–205. doi: 10.1016/j.jamcollsurg.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Gunter OL, Jr, Au BK, Isbell JM, Mowery NT, Young PP, Cotton BA. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma. 2008;65(3):527–534. doi: 10.1097/TA.0b013e3181826ddf. [DOI] [PubMed] [Google Scholar]
- 29.Wafaisade A, Maegele M, Lefering R, et al. High plasma to red blood cell ratios are associated with lower mortality rates in patients receiving multiple transfusion (4</=red blood cell units<10) during acute trauma resuscitation. J Trauma. 2011;70(1):81–88. doi: 10.1097/TA.0b013e3182032e0b. [DOI] [PubMed] [Google Scholar]
- 30.Holcomb JB, Weiskopf R, Champion H, et al. Challenges to effective research in acute trauma resuscitation: consent and endpoints. Shock. 2011;35(2):107–113. doi: 10.1097/SHK.0b013e3181f7fd01. [DOI] [PubMed] [Google Scholar]
- 31.Snyder CW, Weinberg JA, McGwin G, Jr, et al. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma. 2009;66(2):358–362. doi: 10.1097/TA.0b013e318196c3ac. [DOI] [PubMed] [Google Scholar]
- 32.Magnotti LJ, Zarzaur BL, Fischer PE, et al. Improved survival after hemostatic resuscitation: does the emperor have no clothes? J Trauma. 2011;70(1):97–102. doi: 10.1097/TA.0b013e3182051691. [DOI] [PubMed] [Google Scholar]
- 33.Scalea TM, Bochicchio KM, Lumpkins K, et al. Early aggressive use of fresh frozen plasma does not improve outcome in critically injured trauma patients. Ann Surg. 2008;248(4):578–584. doi: 10.1097/SLA.0b013e31818990ed. [DOI] [PubMed] [Google Scholar]
- 34.Ho AM, Dion PW, Yeung JH, et al. Prevalence of Survivor Bias in Observational Studies on Fresh Frozen Plasma: Erythrocyte Ratios in Trauma Requiring Massive Transfusion. Anesthesiology. 2012 doi: 10.1097/ALN.0b013e318245c47b. [DOI] [PubMed] [Google Scholar]
- 35.Rahbar MH, et al. A latent class model for defining severe hemorrhage: Experience from the Prospective, Observational, Multicenter, Major Trauma Transfusion (PROMMTT) study. J Trauma. 2012 In press. [Google Scholar]
- 36.Del Junco D, et al. Resuscitate early with plasma and platelets or balance blood products gradually: Findings from the Prospective, Observational, Multicenter, Major Trauma Transfusion (PROMMTT) Study. J Trauma. 2012 doi: 10.1097/TA.0b013e31828fa3b9. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT flow diagram for PROMMTT study