Abstract
Stem cell therapy has emerged as a promising strategy for cardiac and vascular repair. The ultimate goal is to rebuild functional myocardium by transplanting exogenous stem cells or by activating native stem cells to induce endogenous repair. CS/PCs (cardiac stem/progenitor cells) are one type of adult stem cell with the potential to differentiate into cardiac lineages (cardiomyocytes, smooth muscle cells and endothelial cells). iPSCs (induced pluripotent stem cells) also have the capacity to differentiate into necessary cells to rebuild injured cardiac tissue. Both types of stem cells have brought promise for cardiac repair. The present review summarizes recent advances in cardiac cell therapy based on these two cell sources and discusses the advantages and limitations of each candidate. We conclude that, although both types of stem cells can be considered for autologous transplantation with promising outcomes in animal models, CS/PCs have advanced more in their clinical application because iPSCs and their derivatives possess inherent obstacles for clinical use. Further studies are needed to move cell therapy forward for the treatment of heart disease.
Keywords: cardiac stem/progenitor cell, endogenous activation, induced pluripotent stem cell, heart failure, regeneration, transplantation
INTRODUCTION
Ischaemic heart disease and resultant heart failure are the leading causes of death worldwide [1]. The ongoing loss of cardiomyocytes during and after ischaemic injury and replacement fibrosis are the major reasons for cardiac dysfunction [2]. As adult human hearts have very little ability to make new cardiomyocytes, stem-cell-based cardiac regeneration has attracted a great deal of interest to repair failing hearts resulting from acute MI (myocardial infarction), chronic ischaemia or idiopathic cardiomyopathy [3]. These stem cells have been delivered in diseased hearts via one of the following routes: intramyocardial injection, intracoronary injection, intravenous injection and epicardial placement of cell sheets (patch), as detailed in reviewed animal studies and clinical trials. However, most of the stem cell types implemented in clinical practice yield a modest improvement in cardiac function with a <5% (absolute percentage points) increase in LVEF (left ventricular ejection fraction) [4,5]. These stem cell types include BM-MNCs (bone marrow mononuclear cells), BM-MSCs [bone-marrow-derived MSCs (mesenchymal stem cells)], ADSCs (adipose-derived stem cells) and EPCs (endothelial progenitor cells). Cardiomyogenic differentiation from these stem cells is rare [6].
In contrast, CS/PCs (cardiac stem/progenitor cells) are a most promising source of cells for cardiac repair [7,8]. CS/PCs exhibited greatest cardiac linage differentiation potency, highest angiogenic potential and a balanced profile of paracrine factor production among various adult stem cell types, providing the greatest functional benefit in animal models of MI [9]. A clinical trial with cardiac stem cells [SCIPIO (Stem Cell Infusion in Patients with Ischemic cardiOmyopathy) has shown that CS/PC infusion increased LVEF >8% at 4 months post-treatment and >12% at 1 year post-treatment [10]. Since the advent of iPSCs (induced pluripotent stem cells) [11], they have also attracted much attention and are believed to be another promising cell source for patient-specific cell therapy [12]. iPSCs are easier to generate clinically relevant numbers of cardiac cells compared with adult stem cells, and with a theoretical reduction in risk of immune rejection compared with ESCs (embryonic stem cells) [13]. Both CS/PCs and iPSCs can be generated from adult patients and thus can be autologous. Furthermore, both are able to robustly proliferate and differentiate into functional cardiomyocytes [14,15]. The aim of the present review is to summarize the findings of using these two sources of stem cells for cardiac repair and to discuss the advantages and disadvantages of each source in such an application.
POTENTIAL OF CS/PCs FOR TREATING ISCHAEMIC HEART DISEASE
Cell characteristics
The mammalian heart was viewed as a terminally differentiated post-mitotic organ in which the number of cardiomyocytes was established at birth and no new cardiomyocytes could be regenerated postnatally. This dogma has been challenged [16], and there is now a general consensus that the adult heart contains primitive cells able to regenerate the three main cardiac cell lineages: cardiomyocytes, vascular smooth muscle and endothelial cells [17]. Some of these primitive cells, with self-renewal, multipotency and proliferative potential, were called resident CS/PCs.
Multiple CS/PC subtypes, including c-kit+ cells, sca-1+ cells, side population, CDCs (cardiosphere-derived cells), islet-1+ cells and epicardium-derived cells, have been isolated by different laboratories using diverse methodologies. These CS/PCs have different phenotypic profiles, but are all capable of differentiating into cardiomyocytes, smooth cells and endothelial cells both in vitro and in vivo [18]. Compared with other stem cell types applied in the clinic, the ability to differentiate into cardiomyocytes is an advantage of CS/PCs. A recent study with a comprehensive head-to-head comparison of four different cell types in the same animal model has shown that approximately 9% of CS/PCs underwent spontaneous cardiomyogenic differentiation in vitro, whereas <1% of BM-MNCs, BM-MSCs and ADSCs did so [9].
There are very few CS/PCs in healthy hearts (one stem cell per ≈8000–20000 cardiomyocytes [19]), but the amount of CS/PCs can be increased after cardiac injury [20]. CS/PCs can be cultured and expanded because of their robust proliferative capacity in vitro. To date, the culture of purified c-kit+ CS/PCs or CDCs is able to produce a sufficient number of cells for transplantation into patients [21,22]. CS/PCs are not evenly distributed within the four heart chambers. They are localized mainly in the atria and are more numerous in the subepicardium than in the myocardium [23]. Besides, the right atrium appears to be a reservoir for CS/PCs [24]. The quantity and function, including self-renewal and differentiation efficiency, of endogenous CS/PCs decline with aging [25]. Aging leads to CS/PC senescence and dysfunctional telomeres. The self-renewal of adult myocardium may result from proliferation and differentiation of CS/PCs [7,8]. In an aging heart, with the progressive loss of telomeric DNA in CS/PCs, both CS/PCs and CS/PC-derived new cardiomyocytes may reach the senescent phenotype quickly and their apoptotic rates are markedly increased [26]. Senescence and death of primitive cells and cardiomyocytes lead to premature cardiac aging and heart failure [27]. Disease conditions such as chronic heart failure [28] and diabetes [29,30] also lead to dysfunctional CS/PCs. On the other hand, it is possible that the microenvironment of an aging heart may have an impact on the capability of transplanted CS/PCs to repair injured hearts, although there are no reports on this topic to date.
Strategies for CS/PC therapy
The strategies of CS/PCs and iPSCs-based therapy for ischaemic heart disease are summarized in Figure 1. For both CS/PCs and iPSCs, patient-specific autologous transplantation is the obviously preferred approach because it circumvents the issue of graft immunorejection. For CS/PC-based therapy, the other strategies include allogeneic transplantation and mobilizing the endogenous cardiac regenerative machinery by activating CS/PCs.
Figure 1. Strategies for CS/PC- and iPSC-based therapy for ischaemic heart disease.
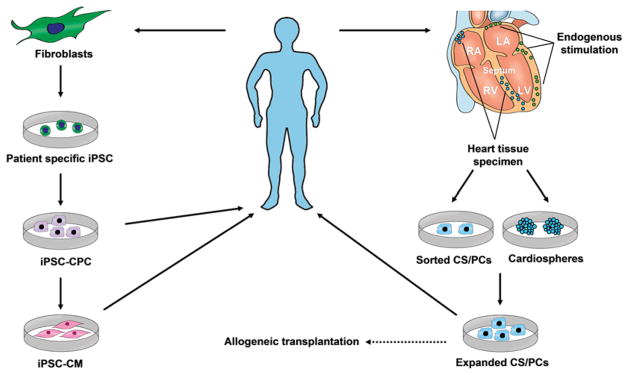
Heart tissues can be obtained from atrial appendage specimens during cardiac surgery or endomyocardial biopsies during cardiac catheterization procedure. These tissues can be used to isolate CS/PCs by sorting with different markers (e.g. c-kit and sca-1) or to yield CDCs. After expansion for 1–2 months in culture, these cells can be used for autologous transplantation or allogeneic transplantation. Endogenous cardiac regeneration may be also stimulated by potentiating putative CS/PCs with drugs or growth factors. iPSCs could be obtained by transducing patient somatic cells (e.g. skin fibroblasts) with a set of exogenous genes (e.g. Yamanaka factors) or gene expression regulators such as proteins and microRNAs. The patient-specific iPSCs can be induced to iPSC-CPCs and then iPSC-CMs, both of which can be used for autologous transplantation.
Autologous transplantation
The methodology of autologous CS/PC transplantation has been well developed in human studies. Efficient isolation and expansion of patient CS/PCs with different markers from cardiac tissue have been established [31]. Biopsy specimens, such as atrial appendage specimens from cardiac surgery or endomyocardial biopsies from cardiac catheterization procedures [32], can be used to yield CDCs, which are cardiogenic in vitro. Using these methods, millions of autologous CS/PCs can be obtained within 1–2 months and then sent back to the bedside and administered for patient-specific therapy [32]. These CS/PCs can promote cardiac regeneration and improve heart function in animal models of heart failure [33].
Allogeneic transplantation
Autologous therapy necessitates patient-specific tissue harvesting and cell processing, with delays to therapy and possible variations in cell potency [34]. CDCs, one subtype of CS/PCs, express neither MHC class II antigens nor B7 co-stimulatory molecules [34], similar to the known low immunogenic profile of MSCs. In a rat MI model, allogeneic CDC transplantation without immunosuppression is safe and effective [34]. In this case, allogeneic CS/PCs might be a readily available product for patients once the transplantation safety is confirmed in humans.
Stimulating native CS/PCs to induce endogenous repair
Cell transplantation has to avoid many unsolved difficulties such as poor graft survival, restricted homing to the site of injury and limited differentiation [35]. Therefore it is an intriguing strategy to stimulate resident CS/PCs in injured hearts instead of cell transplantation.
The mammalian adult heart undergoes some endogenous regeneration, as evidenced by the appearance of a large number of new cardiomyocytes after injury and cardiomyocyte turnover during a normal life span [36]. The self-renewal of adult myocardium may result from proliferation and differentiation of resident CS/PCs, although the process is very slow [7]. Several strategies, such as the applications of growth factors, microRNAs and drugs, may be implemented to potentiate endogenous CS/PCs to repair infarcted hearts [37]. For example, intracoronary administration of IGF-1 (insulin-like growth factor-1)/HGF (hepatocyte growth factor) increases CS/PC number and fosters the generation of new myocardium (cardiomyocytes and microvasculature) in infarcted and peri-infarct/border regions [38].
An alternative path to cardiac regeneration is via reactivating the cell cycle of adult cardiomyocytes, with or without partial dedifferentiation. Activation of cell-cycle-related signalling, including the growth factor neuregulin 1 and its tyrosine kinase receptor (NRG1/ErbB4) [39] and cyclin D2 [40], can promote myocardial regeneration.
However, the uncertainty regarding the contribution of CS/PC-mediated cardiomyocyte regeneration to normal cardiac development and homoeostasis and to cardiac repair after heart injury is the bottleneck restricting the strategy of stimulating native CS/PCs to induce endogenous repair. Stronger evidence is needed to support an unambiguous role for CS/PCs.
Animal studies
Previous studies suggest that, compared with BM-MSCs and ADSCs, CS/PCs are superior in terms of cardiomyogenic differentiation, paracrine factor secretion, angiogenesis, ischaemic tissue preservation, antiremodelling effects and functional benefits in post-MI animal models [9]. However, there is no side-by-side comparison of CS/PC- and iPSC-based therapies to date. The therapeutic effects of CS/PCs in rodent MI models are summarized in Table 1.
Table 1.
Summary of CS/PC and iPSC therapy in rodent models of acute myocardial infarction
| Cell type | Surface marker(s) | Animal species | Number of cells injected | Route of delivery | Timing of cell delivery | LVEF | Infarct size | Follow-up duration | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Control | Stem cell | Control | Stem cell | ||||||||
| CS/PCs | |||||||||||
| Cardiospheres | c-kit+, sca-1+ and KDR+ | Mouse | – | IM | Immediately after MI | 18% | 37% | – | – | 3 weeks | [41] |
| c-kit+ | c-kit+ | Rat | 1×106 | IM | 4 h after MI | 40% | 48% | – | – | 5 weeks | [42] |
| Sca-1+ | Sca-1+ and CD31− | Mouse | 1×106 | IM | Immediately after MI | 31% | 38% | – | – | 3 weeks | [43] |
| SSEA-1+ | SSEA-1+ | Rat | 1×106 | IM | 2 weeks after MI | 28% | 57% | – | – | 5 weeks | [44] |
| CDC (human) | c-kit+, CD90+ and CD34+ | Mouse | 1×105 | IM | Immediately after MI | 26.0 ± 1.8% | 42.8 ± 3.3% | – | – | 3 weeks | [45] |
| CDC | c-kit+ and lin− | Mouse | 1×106 | IV | 1 h after MI | 35% | 43% | 50% | 36% | 4 weeks | [46] |
| Sca-1+ | Sca-1+ | Rat | 1×106 | IM | Immediately after MI | 28.3 ± 4.7% | 43.3 ± 2.8% | 51.9 ± 5.6% | 38.8 ± 1.2% | – | [47] |
| CDC | c-kit+ and nkx2.5+ | Mouse | 1×105 | IM | Immediately after MI | 28% | 43% | – | – | 3 weeks | [48] |
| CDC | c-kit+ | Rat | – | Cell sheet | – | 30 ± 3% | 58 ± 15% | – | – | 3 weeks | [49] |
| CDC | c-kit+ | Mouse | – | IM | – | 40.2 ± 4.2% | 51.5 ± 7.4% | – | – | – | [50] |
| CDC | c-kit+ | Neonatal rat | – | IM | Immediately after MI | 47 ± 2% | 56 ± 3% | 16 ± 2% | 10 ± 1% | 16 weeks | [51] |
| EPDC | WT1+ | Mouse | – | IM | Immediately after MI | 21% | 28% | 35% | 24% | 9 weeks | [52] |
| iPSC/iPSC derivatives | |||||||||||
| iPSC | Mouse | 2×105 | IM | Immediately after MI | 37 ± 4% | 50 ± 5% | 4 weeks | [62] | |||
| iPSC-derived CPC | Flk-1+ | Mouse | 5×105 | IM | Immediately after MI | 23 ± 1% | 32 ± 3% | 43 ± 2% | 19 ± 7% | 2 weeks | [60] |
| iPSC-derived cardiomyocyte | Troponin I+ | Rat | 2×106 | IM | Immediately after MI | 45 ± 9% | 62 ± 4% | 16% | 9% | 10 weeks | [65] |
| iPSC-derived CPC | NCX1+ and Cx43+ | Mouse | – | Cell patch | 7 days after MI | 39.3 ± 1.8% | 50.5 ± 1.9% | – | – | 4 weeks | [63] |
| iPSC | Mouse | 5×104 | IM | Immediately after MI | 65.3 ± 2.4% | 75.7 ± 2.1% | – | – | 2 weeks | [64] | |
Cx43, connexin 43; EPDC, epicardium-derived cell; IM, intramyocardial injection; IV, intravenous injections; KDR, kinase insert domain receptor; NCX, Na+/Ca2+ exchanger.
A series of independent studies have demonstrated that transplantation of CS/PCs improves cardiac function and reduces infarction size in rodent MI models. Twelve CS/PC studies [41–52] have provided data on association between cardiac function and cell therapy. CS/PC therapy induced an absolute increase of 7% to ~29% in LVEF compared with control after MI injury. Some of these studies measured the change of infarction size and showed that CS/PC therapy reduced it by 25–38% (percentage of control).
Clinical applications
Three clinical trials have been conducted to test the therapeutic efficacy of CS/PCs for heart failure resulting from ischaemic heart disease, including SCIPIO (ClinicalTrials.gov Identifier NCT00474461) [10], CADUCEUS (CArdiosphere-Derived aUtologous Stem CElls to Reverse ventricUlar dySfunction; ClinicalTrials.gov Identifier NCT00893360) [22] and ALCADIA (AutoLogous Human CArdiac-Derived Stem Cell to Treat Ischemic cArdiomyopathy; ClinicalTrials.gov Identifier NCT00981006) (results unpublished).
Both SCIPIO and CADUCEUS studies have shown that intracoronary infusion of autologous CS/PCs after MI is safe [10,22]. No adverse effects attributable to CS/PCs were observed in the SCIPIO study. Specifically, none of the 16 CS/PC-treated patients had non-fatal MI (immediately after CS/PC infusion or during follow-up), death, tumour formation, ventricular arrhythmias, systemic infection, stroke, allergic reactions, coronary revascularization or tachyarrhythmias [10]. In the CADUCEUS study, none of the 17 CDC-treated patients died due to ventricular tachycardia, ventricular fibrillation or sudden unexpected death, or had MI after cell infusion, new cardiac tumour formation, or a major adverse cardiac event [22].
The initial results in patients of the SCIPIO study were very encouraging. A total of 1 million autologous CS/PCs were administered by intracoronary infusion in post-MI patients at a mean of 4 months after tissue harvesting during cardiac surgery. In eight CS/PC-treated patients, the absolute percentage points of LVEF was increased by 9% at 4 months and by 12% at 12 months after stem cell infusion (baseline 30%) [10] compared with patients receiving standard pharmacotherapy alone, which is significantly higher than most of the previous reports using other stem cell types [5]. Infarct size was significantly decreased by 24% at 4 months and by 30% at 12 months [10]. In the CADUCEUS trial, at 6 months after treatments MRI (magnetic resonance imaging) analysis of MI patients treated with CS/PCs showed reductions in scar mass, and increases in viable heart mass and regional contractility, and regional systolic wall thickening compared with MI patients receiving no CS/PCs. Scar mass was decreased in patients treated with CDCs by 28% at 6 months and 42% at 12 months, but remained unchanged in controls [22]. However, the CADUCEUS trial did not show increases in LVEF. The discrepancy in the change of LVEF between these two trials could be due to the fact that CADUCEUS used unsorted CDCs with a mixture of cardiac stem cells and supporting cells, whereas SCIPIO used the c-kit+ subpopulation purified from CDCs.
The initial results of the trials of CS/PC-based therapy for ischaemic heart disease are promising and warrant the expansion of such therapy to Phase II/III clinical trials. Besides, the differences in cardiac function improvement after stem cell transplantation may depend on the extent of baseline infarction (quite unexpectedly, better results have been observed after a massive infarct as compared with small infarct size), time of therapy or method of ejection fraction evaluation. CS/PCs should not be considered a better cell source only because of the results from several clinical trials. Longer follow-up and multi-centre clinical studies are required, and many remaining basic science questions need to be addressed [10].
POTENTIAL OF iPSCs FOR TREATING ISCHAEMIC HEART DISEASE
Cell characteristics
iPSCs can be established by epigenetically reprogramming somatic mammalian cells by expressing exogenous genes (e.g. Yamanaka factors) or gene expression regulators such as proteins and microRNAs [53]. iPSCs were first established in 2006 by Takahashi and Yamanaka [11] by retroviral expression of exogenous transcription factors OCT4 (octamer-binding transcription factor 4), SOX2 [SRY (sex determining region Y)-box], KLF4 (Krüppel-like factor 4) and c-MYC [11]. These cells resemble ESCs that have pluripotency and unlimited proliferative capacity. However, for cardiac disease therapy, iPSCs possess several advantages over ESCs: first, iPSCs are derived from adult somatic cells and thus circumvent the ethical issue; and secondly, iPSCs can be made from the same patient and thus are individualized and autologous, reducing the risk of graft rejection.
The remaining issue with iPSCs for clinical application is the formation of teratoma from undifferentiated stem cells, which is the same limitation for ESCs. Therefore the use of partially or fully differentiated iPSCs has attracted a great deal of attention. iPSCs can differentiate into various cardiovascular cells, including cardiomyocytes and vessel cells [54]. The differentiation properties of iPSCs are almost completely identical with those of ESCs. iPSC-derived cardiomyocyte-like cells possess the complete molecular machinery necessary for proper contractile function, including the contractile apparatus, intercellular communication structures, ion channels and receptors for hormonal regulation of heart function [55].
Strategy for iPSC therapy
Autologous transplantation of iPSC derivatives is the major approach for iPSC therapy (Figure 1). As there is a risk of teratoma formation by direct transplantation of undifferentiated iPSCs [56], iPSC derivatives, including partially committed progenitor-like cells [iPSC-CPCs (iPSC-derived cardiovascular progenitor cells)] and differentiated cells (e.g. cardiomyocytes and assembly of iPSC-derived cardiac populations), seem to be the preferred cells for cardiac repair. iPSC-CMs (iPSC-derived cardiomyocytes) have been the most intensely studied cell type and have been demonstrated to be effective after transplantation into ischaemic hearts [57], and many efforts have focused on how to improve the efficiency of iPSC differentiation into cardiomyocytes [58,59].
However, transplanting cardiomyocytes into the injured heart is not sufficient for cardiac repair as angiogenesis also plays an important role. Compared with iPSC-CM transplantation, iPSC-CPC transplantation not only restores myocardium, but also contributes to new vessel formation to increase blood supply. In addition, progenitors may survive better in the hostile graft environment [60]. iPSC-CPCs have recently been identified in early iPSC derivatives with surface markers flk-1 (fetal liver kinase-1) [60], or expression of OCT4, SSEA1 (stage-specific embryonic antigen 1) and MESP1 (mesoderm posterior 1) [61]. These iPSC-CPCs possess most features of conventional CS/PCs. For example, iPSC-derived flk-1+ cells can give rise to cardiomyocyte, endothelial and vascular smooth muscle lineages [60]. Thus the established strategies of employing conventional CS/PCs for cardiac repair can be applied to iPSC-CPCs to improve cardiomyogenesis and angiogenesis. Conversely, the knowledge obtained on iPSC-CPCs induction, proliferation and differentiation might also be applied to conventional CS/PCs.
Another strategy to generate cardiac tissue as a whole is to apply multiple iPSC-derived cell types for cardiac repair. Cardiomyocytes, endothelial cells, smooth muscle cells and mural cells can be systematically induced and purified from iPSCs. These cells can then be assembled into cell sheets for transplantation. Such transplantation has generated satisfactory therapeutic effects in animals [12], which might be a new direction for iPSC-based therapy.
Animal studies
Transplantation of iPSC-derivatives also improves cardiac function and reduces infarction size in rodent MI models. Five iPSC studies [60–65] have provided data including cardiac function (Table 1). iPSC therapy induced an absolute increase in LVEF of 9% to ~17% and reduced infarct size by 44–56%. Apparently, both CS/PCs and iPSC-derivatives exert significant beneficial effects, and the underlying mechanisms include differentiation into the cardiomyocyte lineage commitment [48,60,63], the formation of new blood vessels [51,60] and paracrine effects [49,52]. Because of the limited number of studies, as well as the diversity of cell delivery methods, injected cell numbers, timing of cell delivery and follow-up durations, we cannot judge which cell source is more effective. Future studies to evaluate these two strategies in comparable experimental conditions are needed. In addition, studies of the therapeutic efficacy of the two cell sources in large animals are necessary.
Clinical applications
Both CS/PCs and iPSCs are promising cell sources for clinical applications to treat ischaemic heart disease. The advantages and limitations of both strategies are summarized in Table 2. Clinical trials with CS/PCs have been performed with promising results [10], but, owing to safety concerns, there are no clinical trials to date with iPSCs or iPSC derivatives for cardiac repair. However, potential applications of iPSC technology in cardiovascular medicine include myocardial regeneration and biological pacemakers [53,66]. iPSC derivatives have also been used for drug screening and disease modelling [67].
Table 2.
Advantages and disadvantages of CS/PCs and iPSC-based therapy for ischaemic heart disease
| Cell type | Advantages | Disadvantages |
|---|---|---|
| CS/PCs | Patient-specific multipotent cells | Small number of resident CS/PCs |
| Cardiac-specific differentiation | No specific marker | |
| Possible allogeneic transplantation | Weak proliferation capacity | |
| Possible cell target for stimulating endogenous self-repair | Declining function with aging | |
| Proved therapeutic effect in animal models and in pioneering clinical trials | More invasive because of biopsy obtained from heart tissue | |
| iPSC derivatives | Patient-specific pluripotent cells | Tumorigenic contamination |
| Strong proliferation capacity | Possible immunogenicity | |
| Robust myocardiogenic capacity | Potential genomic instability | |
| Proved therapeutic effect in animal models | Longer time required to derive and characterize iPSCs from patient | |
| Less invasive because of easy accessibility from skin |
The very first safety issue is that iPSC derivatives must not be contaminated with potentially tumorigenic cells. To avoid the tumorgenicity risk of transplanting iPSC derivatives, non-viral and non-integrating methods have been established, including the transient expression of reprogramming factors (e.g. episomal plasmid vectors, minicircle vectors, RNA and protein delivery) without genomic integration [68]. Despite these advances, a complete depletion of tumorigenicity is still difficult to achieve because the contamination of a single iPSC in the iPSC-derived cells could result in a teratoma. Furthermore, the safety of iPSCs faces new challenges. In contrast with derivatives of ESCs, abnormal gene expression in some cells differentiated from iPSCs can induce a T-cell-dependent immune response in syngeneic recipients [69]. Additionally, a recent report has suggested a worrisome presence of genomic instability in iPSCs [70]. For these reasons, the application of iPSCs and their derivatives for cardiac repair has a long way to go.
DIRECT REPROGRAMMING SOMATIC CELLS INTO CARDIOMYOCYTES AS A COMPETITIVE APPROACH FOR CARDIAC REPAIR
Direct reprogramming of fibroblasts into cardiomyocytes has recently emerged as an exciting and potential substitution of stem/progenitor-cell based cardiac repair [71]. Without first becoming an iPSC or stem/progenitor cell, a combination of three developmental transcription factors {GMT [Gata4, Mef2c (myocyte-specific enhancer factor 2C) and Tbx5 (T-box transcription factor 5)]} rapidly and efficiently reprogrammed adult cardiac fibroblasts directly into differentiated cardiomyocyte-like cells [71]. By using this strategy, resident non-cardiomyocytes in the murine heart infarct border zone can be reprogrammed into cardiomyocyte-like cells in vivo by local delivery of GMT after MI [72]. Another group also demonstrated that forced expression of GMT and Hand2 (heart and neural crest derivatives-expressed protein 2) reprogrammed cardiac fibroblasts into cardiomyocytes thereby improving cardiac function following MI [2]. These preliminary data were very exciting, but oncogenes were directly injected into a host in this therapy. Besides, cardiomyocytes produced in this way express only the atrial isoform of myosin [72]. Thus caution must be exercised before it can be seriously considered as a viable option for cellular therapy for cardiac disease.
CONCLUSION AND PERSPECTIVES
Autologous transplantation is the shared methodology of CS/PC and iPSC-based patient-specific therapy of ischaemic heart disease resulting from the loss of cardiomyocytes. Transplantation of either CS/PCs or iPSC derivatives ameliorates cardiac function and reduces infarction size in animal models of ischaemic heart disease. These therapeutic effects can be improved by optimizing (such as genetic modification) the survival and function of CS/PCs and iPSC derivatives in the transplantation microenvironment.
The pioneering clinical trials of CS/PC transplantation have produced promising results with significantly higher efficiency than previous cell types. Larger scale Phase II/III clinical trials with CS/PCs will further define the safety and efficacy of these cells for treating ischaemic heart disease. In addition, activating CS/PCs might be a potential approach for cardiac regeneration by mobilizing endogenous cardiac repair mechanisms.
Despite many safety obstacles, iPSCs may still be an important option for cardiac repair in ischaemic heart disease. Owing to the strong proliferative capacity of iPSCs compared with CS/PCs, iPSCs are apparently a preferable source to produce sufficient number of functional cardiomyocytes to replace lost cardiomyocytes in ischaemic heart disease. Moreover, iPSCs are a robust tool to generate patient-specific engineered cardiac tissue by assembling its cardiovascular derivatives. However, new methods avoiding tumorigenicity, immunogenicity and genomic instability are needed before iPSC derivatives can be applied to clinical trials. Novel strategies such as directly reprogramming cardiac fibroblasts into cardiomyocytes without involving a pluripotent intermediate may be a shortcut to make new myocardium.
Acknowledgments
FUNDING
Our own work was supported by the National Natural Science Foundation of China [grant numbers 30925018, 81100111, 31130029], the National Basic Research Program of China (973 Program) [grant number 2008CB517308, 2012CB517801, 2013CB531104], and the Natural Science Foundation Project of CQ CSTC [grant numbers 2009BA5044, 2011jjzt0118].
Abbreviations
- ADSC
adipose-derived stem cell
- BM-MNC
bone marrow mononuclear cell
- CADUCEUS
CArdiosphere-Derived aUtologous Stem CElls to Reverse ventricUlar dySfunction
- CDC
cardiosphere-derived cell
- CPC
cardiovascular progenitor cell
- CS/PC
cardiac stem/progenitor cell
- ESC
embryonic stem cell
- flk-1
fetal liver kinase-1
- GMT
Gata4, Mef2c (myocyte-specific enhancer factor 2C) and Tbx5 (T-box transcription factor 5)
- iPSC
induced pluripotent stem cell
- iPSC-CM
iPSC-derived cardiomyocyte
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- MSC
mesenchymal stem cell
- BM-MSC
bone marrow-derived MSC
- OCT4
octamer-binding transcription factor 4
- SCIPIO
Stem Cell Infusion in Patients with Ischemic cardiOmyopathy
- SSEA1
stage-specific embryonic antigen 1
References
- 1.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christoffels V. Regenerative medicine: muscle for a damaged heart. Nature. 2011;474:585–586. doi: 10.1038/474585a. [DOI] [PubMed] [Google Scholar]
- 4.Tongers J, Losordo DW, Landmesser U. Stem and progenitor cell-based therapy in ischaemic heart disease: promise, uncertainties, and challenges. Eur Heart J. 2011;32:1197–1206. doi: 10.1093/eurheartj/ehr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29:1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 6.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50:280–289. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unno K, Jain M, Liao R. Cardiac side population cells: moving toward the center stage in cardiac regeneration. Circ Res. 2012;110:1355–1363. doi: 10.1161/CIRCRESAHA.111.243014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan M, Mohsin S, Avitabile D, Siddiqi S, Nguyen J, Wallach K, Quijada P, McGregor M, Gude N, Alvarez R, et al. β-Adrenergic regulation of cardiac progenitor cell death versus survival and proliferation. Circ Res. 2013;112:476–486. doi: 10.1161/CIRCRESAHA.112.280735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59:942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Masumoto H, Matsuo T, Yamamizu K, Uosaki H, Narazaki G, Katayama S, Marui A, Shimizu T, Ikeda T, Okano T, et al. Pluripotent stem cell-engineered cell sheets reassembled with defined cardiovascular populations ameliorate reduction in infarct heart function through cardiomyocyte-mediated neovascularization. Stem Cells. 2012;30:1196–1205. doi: 10.1002/stem.1089. [DOI] [PubMed] [Google Scholar]
- 13.Ptaszek LM, Mansour M, Ruskin JN, Chien KR. Towards regenerative therapy for cardiac disease. Lancet. 2012;379:933–942. doi: 10.1016/S0140-6736(12)60075-0. [DOI] [PubMed] [Google Scholar]
- 14.Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosoda T, Zheng H, Cabral-da-Silva M, Sanada F, Ide-Iwata N, Ogorek B, Ferreira-Martins J, Arranto C, D’Amario D, del Monte F, et al. Human cardiac stem cell differentiation is regulated by a mircrine mechanism. Circulation. 2011;123:1287–1296. doi: 10.1161/CIRCULATIONAHA.110.982918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 17.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 18.Sturzu AC, Wu SM. Developmental and regenerative biology of multipotent cardiovascular progenitor cells. Circ Res. 2011;108:353–364. doi: 10.1161/CIRCRESAHA.110.227066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113:1451–1463. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 20.Kubo H, Jaleel N, Kumarapeli A, Berretta RM, Bratinov G, Shan X, Wang H, Houser SR, Margulies KB. Increased cardiac myocyte progenitors in failing human hearts. Circulation. 2008;118:649–657. doi: 10.1161/CIRCULATIONAHA.107.761031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castaldo C, Di Meglio F, Nurzynska D, Romano G, Maiello C, Bancone C, Muller P, Bohm M, Cotrufo M, Montagnani S. CD117-positive cells in adult human heart are localized in the subepicardium, and their activation is associated with laminin-1 and α6 integrin expression. Stem Cells. 2008;26:1723–1731. doi: 10.1634/stemcells.2007-0732. [DOI] [PubMed] [Google Scholar]
- 24.Itzhaki-Alfia A, Leor J, Raanani E, Sternik L, Spiegelstein D, Netser S, Holbova R, Pevsner-Fischer M, Lavee J, Barbash IM. Patient characteristics and cell source determine the number of isolated human cardiac progenitor cells. Circulation. 2009;120:2559–2566. doi: 10.1161/CIRCULATIONAHA.109.849588. [DOI] [PubMed] [Google Scholar]
- 25.Mishra R, Vijayan K, Colletti EJ, Harrington DA, Matthiesen TS, Simpson D, Goh SK, Walker BL, Almeida-Porada G, Wang D, et al. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation. 2011;123:364–373. doi: 10.1161/CIRCULATIONAHA.110.971622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kajstura J, Gurusamy N, Ogorek B, Goichberg P, Clavo-Rondon C, Hosoda T, D’Amario D, Bardelli S, Beltrami AP, Cesselli D, et al. Myocyte turnover in the aging human heart. Circ Res. 2010;107:1374–1386. doi: 10.1161/CIRCRESAHA.110.231498. [DOI] [PubMed] [Google Scholar]
- 27.Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 28.Cesselli D, Beltrami AP, D’Aurizio F, Marcon P, Bergamin N, Toffoletto B, Pandolfi M, Puppato E, Marino L, Signore S, et al. Effects of age and heart failure on human cardiac stem cell function. Am J Pathol. 2011;179:349–366. doi: 10.1016/j.ajpath.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katare R, Oikawa A, Cesselli D, Beltrami AP, Avolio E, Muthukrishnan D, Munasinghe PE, Angelini G, Emanueli C, Madeddu P. Boosting the pentose phosphate pathway restores cardiac progenitor cell availability in diabetes. Cardiovasc Res. 2013;97:55–65. doi: 10.1093/cvr/cvs291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delucchi F, Berni R, Frati C, Cavalli S, Graiani G, Sala R, Chaponnier C, Gabbiani G, Calani L, Del Rio D, et al. Resveratrol treatment reduces cardiac progenitor cell dysfunction and prevents morpho-functional ventricular remodeling in type-1 diabetic rats. PLoS ONE. 2012;7:e39836. doi: 10.1371/journal.pone.0039836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smits AM, van Vliet P, Metz CH, Korfage T, Sluijter JP, Doevendans PA, Goumans MJ. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: an in vitro model for studying human cardiac physiology and pathophysiology. Nat Protoc. 2009;4:232–243. doi: 10.1038/nprot.2008.229. [DOI] [PubMed] [Google Scholar]
- 32.D’Amario D, Fiorini C, Campbell PM, Goichberg P, Sanada F, Zheng H, Hosoda T, Rota M, Connell JM, Gallegos RP, et al. Functionally competent cardiac stem cells can be isolated from endomyocardial biopsies of patients with advanced cardiomyopathies. Circ Res. 2011;108:857–861. doi: 10.1161/CIRCRESAHA.111.241380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 34.Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, Galang G, Zhang Y, Schoenhoff F, Van Eyk J, et al. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125:100–112. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanashiro-Takeuchi RM, Schulman IH, Hare JM. Pharmacologic and genetic strategies to enhance cell therapy for cardiac regeneration. J Mol Cell Cardiol. 2011;51:619–625. doi: 10.1016/j.yjmcc.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angert D, Berretta RM, Kubo H, Zhang H, Chen X, Wang W, Ogorek B, Barbe M, Houser SR. Repair of the injured adult heart involves new myocytes potentially derived from resident cardiac stem cells. Circ Res. 2011;108:1226–1237. doi: 10.1161/CIRCRESAHA.110.239046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wen Z, Mai Z, Zhang H, Chen Y, Geng D, Zhou S, Wang J. Local activation of cardiac stem cells for post-myocardial infarction cardiac repair. J Cell Mol Med. 2012;16:2549–2563. doi: 10.1111/j.1582-4934.2012.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellison GM, Torella D, Dellegrottaglie S, Perez-Martinez C, Perez de Prado A, Vicinanza C, Purushothaman S, Galuppo V, Iaconetti C, Waring CD, et al. Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J Am Coll Cardiol. 2011;58:977–986. doi: 10.1016/j.jacc.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 39.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 40.Zhu W, Hassink RJ, Rubart M, Field LJ. Cell-cycle-based strategies to drive myocardial repair. Pediatr Cardiol. 2009;30:710–715. doi: 10.1007/s00246-009-9408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 42.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J, et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci USA. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH, Zhang J. The role of the sca-1+/CD31− cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24:1779–1788. doi: 10.1634/stemcells.2005-0386. [DOI] [PubMed] [Google Scholar]
- 44.Ott HC, Matthiesen TS, Brechtken J, Grindle S, Goh SK, Nelson W, Taylor DA. The adult human heart as a source for stem cells: repair strategies with embryonic-like progenitor cells. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S27–S39. doi: 10.1038/ncpcardio0771. [DOI] [PubMed] [Google Scholar]
- 45.Smith RR, Barile L, Messina E, Marban E. Stem cells in the heart: what’s the buzz all about?–Part 1: preclinical considerations. Heart Rhythm. 2008;5:749–757. doi: 10.1016/j.hrthm.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu G, Haider HK, Jiang S, Ashraf M. Sca-1+ stem cell survival and engraftment in the infarcted heart: dual role for preconditioning-induced connexin-43. Circulation. 2009;119:2587–2596. doi: 10.1161/CIRCULATIONAHA.108.827691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, Marban E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zakharova L, Mastroeni D, Mutlu N, Molina M, Goldman S, Diethrich E, Gaballa MA. Transplantation of cardiac progenitor cell sheet onto infarcted heart promotes cardiogenesis and improves function. Cardiovasc Res. 2010;87:40–49. doi: 10.1093/cvr/cvq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonios M, Chang CY, Terrovitis J, Pinheiro A, Barth A, Dong P, Santaularia M, Foster DB, Raman V, Abraham TP, Abraham MR. Constitutive HIF-1α expression blunts the beneficial effects of cardiosphere-derived cell therapy in the heart by altering paracrine factor balance. J Cardiovasc Transl Res. 2011;4:363–372. doi: 10.1007/s12265-011-9265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carr CA, Stuckey DJ, Tan JJ, Tan SC, Gomes RS, Camelliti P, Messina E, Giacomello A, Ellison GM, Clarke K. Cardiosphere-derived cells improve function in the infarcted rat heart for at least 16 weeks – an MRI study. PLoS ONE. 2011;6:e25669. doi: 10.1371/journal.pone.0025669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, Lin RZ, Melero-Martin JM, Dolmatova E, Duffy HS, et al. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest. 2011;121:1894–1904. doi: 10.1172/JCI45529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oh Y, Wei H, Ma D, Sun X, Liew R. Clinical applications of patient-specific induced pluripotent stem cells in cardiovascular medicine. Heart. 2012;98:443–449. doi: 10.1136/heartjnl-2011-301317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Narazaki G, Uosaki H, Teranishi M, Okita K, Kim B, Matsuoka S, Yamanaka S, Yamashita JK. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118:498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11:268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 57.Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, Shimizu T, et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126:S29–S37. doi: 10.1161/CIRCULATIONAHA.111.084343. [DOI] [PubMed] [Google Scholar]
- 58.Lin B, Kim J, Li Y, Pan H, Carvajal-Vergara X, Salama G, Cheng T, Lo CW, Yang L. High-purity enrichment of functional cardiovascular cells from human iPS cells. Cardiovasc Res. 2012;95:327–335. doi: 10.1093/cvr/cvs185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zwi-Dantsis L, Huber I, Habib M, Winterstern A, Gepstein A, Arbel G, Gepstein L. Derivation and cardiomyocyte differentiation of induced pluripotent stem cells from heart failure patients. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs096. [DOI] [PubMed] [Google Scholar]
- 60.Mauritz C, Martens A, Rojas SV, Schnick T, Rathert C, Schecker N, Menke S, Glage S, Zweigerdt R, Haverich A, et al. Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. Eur Heart J. 2011;32:2634–2641. doi: 10.1093/eurheartj/ehr166. [DOI] [PubMed] [Google Scholar]
- 61.Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B, Bellamy V, Rucker-Martin C, Barbry P, Bel A, et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest. 2010;120:1125–1139. doi: 10.1172/JCI40120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dai B, Huang W, Xu M, Millard RW, Gao MH, Hammond HK, Menick DR, Ashraf M, Wang Y. Reduced collagen deposition in infarcted myocardium facilitates induced pluripotent stem cell engraftment and angiomyogenesis for improvement of left ventricular function. J Am Coll Cardiol. 2011;58:2118–2127. doi: 10.1016/j.jacc.2011.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan B, Abdelli LS, Singla DK. Transplanted induced pluripotent stem cells improve cardiac function and induce neovascularization in the infarcted hearts of db/db mice. Mol Pharm. 2011;8:1602–1610. doi: 10.1021/mp2003576. [DOI] [PubMed] [Google Scholar]
- 65.Carpenter L, Carr C, Yang CT, Stuckey DJ, Clarke K, Watt SM. Efficient differentiation of human induced pluripotent stem cells generates cardiac cells that provide protection following myocardial infarction in the rat. Stem Cells Dev. 2012;21:977–986. doi: 10.1089/scd.2011.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mandel Y, Weissman A, Schick R, Barad L, Novak A, Meiry G, Goldberg S, Lorber A, Rosen MR, Itskovitz-Eldor J, Binah O. Human embryonic and induced pluripotent stem cell-derived cardiomyocytes exhibit beat rate variability and power-law behavior. Circulation. 2012;125:883–893. doi: 10.1161/CIRCULATIONAHA.111.045146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee P, Klos M, Bollensdorff C, Hou L, Ewart P, Kamp TJ, Zhang J, Bizy A, Guerrero-Serna G, Kohl P, et al. Simultaneous voltage and calcium mapping of genetically purified human induced pluripotent stem cell-derived cardiac myocyte monolayers. Circ Res. 2012;110:1556–1563. doi: 10.1161/CIRCRESAHA.111.262535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Narsinh K, Narsinh KH, Wu JC. Derivation of human induced pluripotent stem cells for cardiovascular disease modeling. Circ Res. 2011;108:1146–1156. doi: 10.1161/CIRCRESAHA.111.240374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 70.Hussein SM, Batada NN, Vuoristo S, Ching RW, Autio R, Narva E, Ng S, Sourour M, Hamalainen R, Olsson C, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471:58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- 71.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]


