Abstract
Head and neck squamous cell carcinoma (HNSCC) is one of the most fatal cancers world-wide. Despite advances in the management of HNSCC, the overall survival for patients has not improved significantly due to advanced stages at diagnosis, high recurrence rate after surgical removal, and second primary tumor development, which together underscore the importance of novel strategies for cancer prevention. Cancer chemoprevention, the use of natural or synthetic compounds to prevent, arrest, or reverse the process of carcinogenesis at its earliest stages, aims to reverse premalignancies and prevent second primary tumors. Genomics and proteomics information including initial mutation, cancer promotion, progression and susceptibility has brought molecularly targeted therapies for drug development. The development of preventive approaches using specific natural or synthetic compounds, or both, requires a depth of understanding of the cross-talk between cancer signaling pathways and networks to retain or enhance chemopreventive activity while reducing known toxic effects. Many natural dietary compounds have been identified as multiple molecular targets, effective in the prevention and treatment of cancer. This review describes recent advances in the understanding of the complex signaling networks driving cancer progression using head and neck cancer as a prototype, and of molecularly targeted natural compounds under preclinical and clinical investigation.
Keywords: Head and neck squamous cell carcinoma, Chemoprevention, Molecular target, Natural compound
Introduction
Head and neck squamous cell carcinoma (HNSCC) is one of the most common cancers worldwide and a major cause of significant morbidity and mortality. HNSCC accounts for 3.2% of all cancers in the United States with approximately 48,000 new cases and more than 11,000 expected deaths in 2009 (1). Despite substantial medical advances for HNSCC patients over the past 30 years in treatments, including surgery, radiation, and chemotherapy, the overall survival rate has not been improved significantly due to frequent presentation with advanced stage disease and the development of second primary tumors (SPTs) which remain challenging to control (2–4). Improvements in diagnostic and therapeutic techniques and in chemotherapeutic agents are likely to increase survival rates, and consequently more patients are at risk of developing SPTs, indicating the urgent need to deter HNSCC. Therefore, prevention and early diagnosis of high-risk premalignant lesions should be emphasized rather than directing efforts only at treating end-stage disease. Multiple epigenetic and genetic events, including the dysregulation of many cellular processes such as cell cycle, growth, apoptosis, and angiogenesis are highly associated with pathogenesis of HNSCC. The critical intracellular molecular targets and the mechanisms underlying the variable responses to therapies remain elusive. Damage to numerous regulatory genes ultimately results in the development of invasive and metastatic cancer, providing a strong rationale for a preventive approach to the control of this disease (5, 6).
Cancer chemoprevention is defined as the use of natural or synthetic substances to reverse, suppress or prevent the initiation, promotion, or progression of cancer. To be useful in humans, a chemopreventive agent must have an acceptable safety profile in addition to being effective at a dose low enough to not cause significant toxicity. Natural dietary agents such as fruit and vegetables hold great promise for chemopreventive research due to their potential ability to prevent and suppress cancer (7–10). The great chemical diversity of natural compounds suggests various approaches for cancer chemoprevention. The successes of several recent clinical trials in preventing cancer in high risk populations suggest that chemoprevention is a rationale and appealing strategy. Here, we will discuss recent advances in the understanding of the complex signaling networks driving cancer progression using head and neck cancer as a prototype, and the potency, efficacy and limitations of molecularly targeted natural compounds under preclinical and clinical investigation.
Aspects of chemoprevention for HNSCC
The clinical practice of chemoprevention is still in its early stage, although there is a wealth of data documenting its effectiveness in preclinical and clinical trials. Many cancer incidences could be preventable by consuming a healthy diet including vegetables and fruits, regular physical activity and maintenance of optimum body weight (11). In the planning of clinical trials for primary prevention of cancers, it is important to select agents for which there is a strong mechanistic or experimental basis for inhibition of carcinogenesis. Epidemiological studies into the effects of diet on cancer development are invaluable for giving clues regarding effective dietary components as potential chemopreventive agents (12, 13).
Premalignant lesions of the head and neck is an excellent model for studying chemopreventive approaches, as HNSCC has a high tendency to develop SPTs, which have been reported in over 20% of cases, and up to 47% in patients with previously treated laryngeal cancer (14, 15). Prevention of SPTs in patients who have undergone surgery for removal of a first primary tumor provides an even more meaningful endpoint and these subjects may be at an exceptionally high risk for new cancer. HNSCC well exemplifies the multistep carcinogenesis with stepwise accumulation of genetic alterations, (16, 17) and exhibits clinically defined premalignant lesions such as oral leukoplakia and erythroplakia that can be targeted by a chemopreventive approach. Chromosomal aneuploidy in upper aerodigestive tract cancer indicates the transformation from oral leukoplakia to head and neck cancer (18). Therefore, it is highly desirable to reduce the incidence of HNSCC by developing specific natural compounds as chemopreventive regimens.
Recent approaches for the development of cancer chemopreventive agents
The concept of chemoprevention was initially proposed in the 1920s (19) and was reintroduced with great hope for cancer research by Sporn et. al. (20) in 1976. Proceeding from his concept, modern chemoprevention has achieved enormous progress over 30 years, with hundreds of cancer chemoprevention studies conducted, including over 70 randomized trials (21, 22).
The most extensively studied chemopreventive agents in HNSCC are retinoids (summarized in Table 1), which are structurally and functionally related to vitamin A, to reverse head and neck cancer progression (23–33). The first randomized clinical trial was conducted by Hong et. al. (23), with the vitamin A analog 13-cis-retinoic acid (13-cRA, isotrentinoin) for a short period in patients, which appeared to be an effective treatment for oral leukoplakia. However, substantial toxicity and a high relapse rate upon discontinuation led to the conduct of a maintenance trial with a much lower dose of isotretinoin (26). Long-term maintenance with isotretinoin was more effective than with β-carotene(27). Phase III clinical trials in HNSCC patients who were at high risk for both recurrence and SPTs, low-dose isotretinoin for 3 years or high dose did not show any significant improvements in survival (24, 28, 31). Reduction of dose-related toxicity and durable efficacy are highly desirable for cancer chemoprevention. The high risk of advanced premalignant lesions progressing to malignancy and the resistance to single agent retinoid therapy have elicited the idea of combining retinoids with other agents. It was reported that 13-cRA along with alpha-2a-interferon provides synergistic antitumor effects (34–36). A biochemopreventive trial in patients with advanced premalignant lesions of the upper aerodigestive tract given one year of interferon-α (IFN-α), 13-cRA and α-tocopherol, reported the prevention of laryngeal lesions but no effect on oral cavity lesions (37). Shin et. al. conducted a phase II clinical trial, treating patients with stages III or IV HNSCC with the combination of interferon-α, 13-cRA and α-tocopherol after complete resection, postoperative radiotherapy, or both, and showed that biochemoprevention therapy was promising for the suppression of SPT development and had an excellent survival rate (29, 30). N-4-(hydroxycarbophenyl) retinamide (4-HPR), another retinoid analogue with an encouraging toxicity profile (38), has been studied to evaluate its efficacy in the treatment and prevention of cancer (39). In patients treated after surgical excision of oral leukoplakia, 4-HPR was well-tolerated and was effective in preventing relapses and new localizations during the treatment period (40). 4-HPR has appeared as a promising agent because of its ability to induce apoptosis even in neoplastic cells that are resistant to retinoic acid (41, 42).
Table 1.
Selected chemopreventive trials in HNSCC patients.
| Investigator | Year | Patient No. | Type | Agents | Results |
|---|---|---|---|---|---|
| Hong et al. (23) | 1986 | 44 | Oral leukoplakia | 13-cRA at 1–2mg/kg/d for 3months vs placebo | 13-cRA vs placebo response 67% vs 10% |
| Stich et al. (25) | 1988 | 103 | Oral leukoplakia from tobacco-containing betel quids | Beta-carotene (180mg/wk) with or without vitamin A (100,000 IU/wk) vs placebo | Remission of leukoplakia in beta-carotene (14.8%) vs beta-carotene plus vitamin A (27.5%) vs placebo (3%) |
| Stich et al. (33) | 1988 | 64 | Oral leukoplakia from tobacco- | Vitamin A (200,000 IU/wk) orally for 6months vs placebo | Complete remission vitamin A (57.1%) vs placebo (30%) |
| Hong et al. (24) | 1990 | 103 | Prior HNSCC | 13-cRA at 50–100mg/m2/d for 12 months vs placebo | Second primary tumors 13-cRA (24%) vs placebo (2%) |
| Han et al. (39) | 1990 | 61 | Oral leukoplakia | 4-HPR (40mg/d) vs placebo | Complete remission 4-HPR (87%) vs placebo (17%) |
| Lippman et al. (26) | 1993 | 70 | Oral leukoplakia | 13-cRA at 1.5mg/kg/d for 3months, followed by 13-cRA at 0.5mg/kg/d or beta-carotene 30mg/d for 9 months | Initial response 55% to high dose in 13-cRA and stable lesions 13-cRA (92%) vs beta-carotene (45%). |
| Chiesa et al. (40) | 1993 | 137 | Oral leukoplakia | 4-HPR (200mg/d) for 52wk vs placebo | Local relapses or new localizations occurred 15% in placebo and 6% in 4-HRP group |
| Papdimitrakopoulou et al. (37) | 1999 | 36 | Advanced pre-malignant lesion | 13-cRA (100mg/m2/d) α-tocopherol (1200 IU/d) Interferon-α (3MU/m2) twice per wk for 12 months | Laryngeal lesions 50% complete response vs placebo 0%. |
| Shin et al. (29,30) | 2001 2005 |
44 | Prior HNSCC | 13-cRA (50mg/m2/d) α-tocopherol (1200 IU/d) Interferon-α (3MU/m2 thrice per wk for 12 months | 2 years survival rate 91% and disease free 84%. 5 years overall survival rate 81.3%. |
| Khuri et al. (31) | 2006 | 1190 | Stage I and II HNSCC | 13-cRA (30mg/d) vs placebo for 3 years | Low dose 13-cRA was not effective in reduction of SPTs or death. |
| Papdimitrakopoulou et al. (43) | 2008 | 50 | Oral premalignant lesions (OPLs) | Celecoxib 100mg or 200mg twice daily for 12 weeks | Ineffective in controlling OPLs |
| Papdimitrakopoulou et al. (32) | 2009 | 162 | OPLs | 13-cRA (0.5mg/kg/d) for 1year followed by 0.25mg/kg/d for 2 years or beta-carotene (50mg/d) plus retinyl palmitate (25000U/d) for 3 years. | Beta-carotene plus retinyl palmitate or retinyl palmitate alone are not equivalent to 13-cRA in controlling OPLs |
| Tsao et al. (44) | 2009 | 41 | High risk OPL | Green tea extract (GTE) 500 or 750 or 1000 mg/m2 vs placebo | Two higher dose GTE arms response rate 58.8% vs placebo (18.2%). |
Some promising chemopreventive agents other than retinoids have also been launched into clinical trials. Papdimitrakopoulou et al. conducted a pilot randomized phase II study of celecoxib in oral premalignant lesions (OPLs) (43), but found celecoxib to be ineffective in controlling OPLs. Identification of high risk patients and more active treatments are needed. Recently, Tsao et al. published a phase II randomized trial of short-term green tea extract (GTE) treatment in high risk OPL patients (44). High-dose GTE had a significantly higher response rate than placebo, providing a stong rationale for testing GTE in a longer term clinical trial.
The clinical benefit of chemoprevention was spectacularly demonstrated by the Food and Drug Administration (FDA)’s recent approvals of tamoxifen for breast cancer prevention (45) and celecoxib for the control of familial adenomatous polyposis (46). Efficient chemopreventive intervention requires the further development of molecular prognostic factors and the identification of patients at high risk of cancer who might benefit most from participation in chemoprevention trial.
Molecular targets for chemoprevention
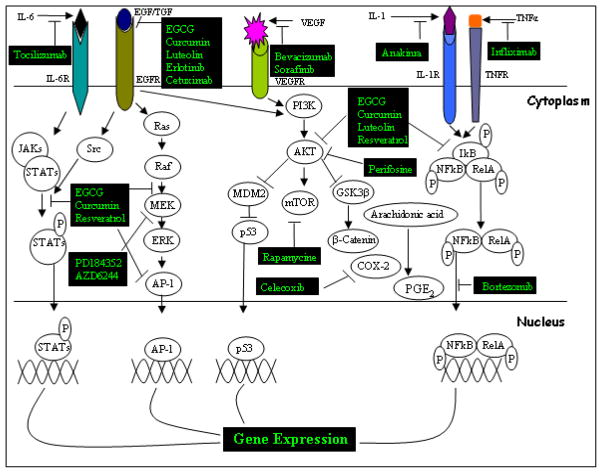
Recent developments in genomics, proteomics and bioinformatics have raised new possibilities for the prevention of cancer. Genetic information regarding initial mutations, cancer promotion, progression and susceptibility has brought about the development of molecularly targeted therapies with high impact on patient outcomes. Designing new therapeutic strategies targeting multiple signaling pathways offers novel approaches to prevent and treat cancer. Several molecular targets, crucial signaling proteins in HNSCC are presented schematically in Figure 1, and their function in HNSCC progression and inhibitory outcome discussed in the following sections.
Figure 1. The consequences of signal cascades frequently dysregulated in HNSCC and the molecular targets of natural or synthetic chemoprevntive agents.
Dysregulation of JAK/STAT, Ras/Raf, PI3K/Akt/mTOR, and the NF-kB signaling pathway contributes to HNSCC cancer progression upon activation of multiple growth factors and cytokine receptors. Several natural or synthetic compounds are summarized as potential inhibitors of these signaling pathways (blocking arrow).
Epidermal Growth Factor Receptor (EGFR)
EGFR, a member of the ErbB family of receptor tyrosine kinases, is overexpressed in 80–90% of HNSCC. It is a widely accepted biomarker for premalignant lesions and is particularly important in pathogenesis of HNSCC, being associated with advanced stages of HNSCC and poor survival (47, 48). Thus, EGFR has become one of the most pursued therapeutic targets for this disease. Mutations, gene amplification and overexpression of EGFR have been implicated as crucial contributors to a variety of cancers. EGFR alterations, specifically increased copy number in the tumor, are correlated with the overall patient survival rate of HNSCC (49). EGFR is activated upon ligand (EGF or TGF-α) binding through receptor dimerization and phosphorylation on multiple tyrosine sites and recruits signaling complexes to activate many downstream intracellular signaling pathways, including Ras/MAP kinase, phosphatidyl inositol-3-kinase (PI3K), phospholipase-Cγ (PLC-γ), protein kinase C (PKC) and signal transducer and activator of transcription (STATs) (50, 51) (Fig 1), which operate many important cellular processes such as tumor growth, survival, invasion, metastasis and angiogenesis (52). EGFR can be translocated to the nucleus and regulate the expression of several genes to drive cell-cycle progression, proliferation and metastasis development (53). EGFR expression is moderately upregulated in normal epithelium adjacent to tumor tissue, and increased when cells transition from dysplasia to HNSCC (54). Upregulated EGFR signaling correlated with growth and metastasis in a wide range of tumors, while downregulation protects HNSCC cellular growth (55). p53 status is crucial for EGFR-mediated cellular growth and apoptosis (56). p53 upregulated modulator of apoptosis (PUMA) is a critical mediator of EGFR inhibitor-induced apoptosis and the p63, p73 and PI3K/AKT pathways play vital roles in HNSCC (57, 58). EGFR is mechanistically involved in the link between tobacco smoke and oral cancer, since tobacco smoke activates EGFR signaling, which increases COX-2 signaling and thus tumor cell growth (52). Inhibition of EGFR by antibodies or selective inhibitors has been shown to induce growth suppression and apoptosis in HNSCC cells and xenograft tumors (52).
Several natural or synthetic compounds have been identified for targeting EGFR-mediated signaling, providing a promising approach to inhibit cancer development and progression. Several EGFR-targeting agents including selective tyrosine kinase inhibitors (TKI), such as gefitinib (ZD1839/Iressa) and erlotinib (OSI774/Tarceva), and the monoclonal antibodies cetuximab (C225/Erbitux) and panitumimab (Vectibix) are approved by the FDA for use in certain types of cancer, and are currently being explored in clinical trials. However, a common challenge to their clinical potential is the development of acquired resistance. The development of preventive approaches using specific natural or synthetic chemical compounds alone or in combination has become highly desirable to reduce the incidence of HNSCC. The EGFR inhibitor erlotinib had synergistic growth inhibitory effects with either green tea-derived EGCG (56, 59) or COX-2 inhibitor (60–62) in cellular and animal models of HNSCC, thus providing an important rationale for chemoprevention or treatment trials. The National Cancer Institute has funded several grants for improving erlotinib efficacy by determining appropriate combination regimens for HNSCC (60, 61). A phase I/II study with a combination of EGFR inhibitor, erlotinib, and an anti-vascular endothelial growth factor (VEGF) antibody, bevacizumab has shown the combination to be well tolerated in recurrent or metastatic HNSCC, with sustained benefit and complete responses (63). A follow up study of a phase III clinical trial in HNSCC patients using the anti-EGFR antibody cetuximab in combination with radiotherapy reported significantly improved overall survival at 5 years (64). The above-mentioned studies suggest that EGFR is a potential candidate to be targeted for chemoprevention purposes in HNSCC.
Cyclooxygenase-2 (COX-2)
Overexpression of COX-2, a key enzyme for the formation of prostaglandins PGE2 from arachidonic acid, an early and central event in carcinogenesis, provides an important target for chemopreventive drug development (65). COX-2 is widely overexpressed in variety of premalignant and malignant lesions including oral leukoplakia and HNSCC (66). Thus COX-2 appears to be a novel molecular target for the prevention and treatment of HNSCC. Treatment with selective COX-2 inhibitors reduces the formation of intestinal, esophageal, tongue, breast, skin, lung and bladder tumors in animals (65, 67–70). COX-2 protect cells from apoptosis by inducing anti-apoptotic Bcl-2 and by suppressing Bax proteins (65). COX-2 inhibitors have been shown to induce apoptosis in HNSCC (71), prostate cancer (72), colorectal cancer (73) and lung cancer (74). COX-2 expression promotes antitumor reactivity by restoring the balance between IL-10 and IL-12 in vivo (74, 75) and inhibitors of COX-2 activity have been shown to attenuate tumor-mediated immune suppression and also regulate invasion through CD44 and matrix metalloproteinase (MMP)-2 (66). COX-2 inhibitors suppress angiogenesis by reduction of VEGF, thus inhibiting growth of HNSCC (71) and lung cancer (74). Celecoxib, a selective COX-2 inhibitor abolished cigarette smoke-induced NF-Kb activity and suppressed cyclin D1 and matrix metalloproteinase-9 (76).
The first human clinical trial of celecoxib in cancer was conducted in familial adenomatous polyposis (FAP) patients treated with celecoxib, which led to a significant reduction in the number of colorectal polyps (46). On the basis of this result, celecoxib was approved by the FDA as an adjunctive therapy for patients with FAP. Combinations of celecoxib with other agents have been sought, given the advantages of combination therapy for cancer treatment and prevention, ie, allowing increased efficacy while reducing toxicity through lowered doses. The combination of celecoxib with the EGFR inhibitor erlotinib was highly effective in HNSCC chemoprevention, as mentioned earlier. The combination of a retinoid and a COX-2 inhibitor could be an effective chemopreventive strategy for HNSCC, since the transcriptional activation of COX-2 is blocked by retinoic acid in oral epithelial cells (77, 78), and retinoic acid has shown promising effects in HNSCC patients in a clinical trial. Cotreatment with a selective COX-2 inhibitor can increase the efficacy of chemotherapy or radiotherapy (79). COX-2 appears to be a novel molecular target and inhibitors need to be given in combination with standard therapy to improve its efficacy in cancer prevention.
p53
To evaluate the efficacy of chemopreventive agents, there is an urgent need to identify novel biomarkers which have predictive value for the clinical disease and risk stratification that can be used for precise chemoprevention. The p53 transcription factor is a known tumor suppressor protein, and one of the most promising biomarkers. p53 has been described as “the guardian of the genome,” referring to its role in conserving stability by preventing genome mutation. Activated p53 regulates genes including p21, 14-3-3, Noxa, Puma, Fas, Bax, and many others to direct cellular processes such as cell cycle inhibition, apoptosis, genetic stability, and inhibition of angiogenesis (7, 80). p53 controls cell cycle arrest in G1 phase in response to DNA damage and thus protects against genomic instability, abnormal DNA replication and chromosome segregation (81, 82). p53 expression is highly associated with increased genetic instability during tumorigenesis in head and neck (81, 82), breast (83), ovarian (84) and renal cancer (85).
Mutations of p53 are commonly found in HNSCC (40–50%), and are strong determinants of more advanced oral cancer and poor survival (44). Restoring normal p53 function would be a potential chemopreventive approach in various types of cancers including head and neck. Many natural chemorpreventive agents have been shown to induce p53 activation. In some clinical trials of HNSCC patients, retinoids have proven to be effective as chemopreventive agents but reduction of toxicity and better efficacy still remains challenging. p53 protein and retinoic acid receptor β (RAR-β) are expressed in most premalignant oral lesions (86) and wild type p53 expression is strongly associated with an increased clinical response to therapy with 13-cRA with or without IFN (80, 87). Thus, confirming p53 activation by suitable natural chemopreventive agents alone or in combination with other agents could be effective in future clinical trials.
Nuclear Factor-Kappa B
NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) is a protein that controls the transcription of DNA, usually in response to stimuli such as stress, cytokines, free radicals, ultraviolet irradiation, carcinogens, tumor promoters and bacterial or viral antigens (88–90). The genes regulated by NF-κB include those controlling apoptosis, cell adhesion, proliferation, the innate- and adaptive-immune responses, inflammation, the cellular-stress response and tissue remodeling (90, 91). The major class of cellular targets of NF-κB are chemokines (IL1α and IFNγ), regulators of apoptosis (Bcl-xL, IAP), transcription factors (G-CSF, GM-CSF), and cell cycle regulators (cyclin D1, mdm2) (91). NF-κB signaling pathways have been targeted for therapeutic applications in many cancers. Several drugs or inhibitors currently in clinical use have significant effects on NF-κB activity and antitumoral activity (91–93) (Fig 1). Constitutively activated NF-kB plays a vital role in pathogenesis and therapeutic resistance in HNSCC by regulating many genes including cyclin D1, Bcl-XL, TRAF1, TRAF2, and mdm2 as cell cycle progressors and regulators of apoptosis; IL-1, -6, -8, TNFα and VEGF as angiogenic factors; MMP-9, uPA, TIMP as metastatic regulators; and P-glycoprotein and GADD45 as therapeutic resistance factors (94). A recent phase I clinical trial was conducted using bortezomib (a proteasome inhibitor that inhibits NF-kB) and cetuximab (EGFR inhibitor), which was found to be moderately effective in non-small cell lung or head and neck cancer patients (95). NF-κB constitutes a potential target for chemoprevention. Thus, different combination approaches including natural chemopreventive agents need to be investigated. Understanding the pathways that regulate NF-κB functions will be useful in developing NF-κB inhibitors for the treatment and prevention of cancers.
Activator protein 1
Activator protein-1 (AP-1) is a transcription factor composed of a group of dimeric proteins belonging to the c-Fos, c-Jun, ATF, Maf and JDP families. AP-1 bind a common DNA sequence (TGAG/CTCA) and a dimeric structure formed by a leucine zipper. Several mechanisms have been shown for AP-1 activity involving growth factor receptor, proinflammatory cytokines and UV radiation (96). AP-1 proteins regulate many target genes such as cyclin D1, p16, p19ARF, p53, p21, Fas L and execute different biological functions including cell proliferation, survival and apoptosis (97).
AP-1 is constitutively activated in HNSCC cell lines and promotes resistance to apoptosis by regulating many proteins including NF-kB and COX-2 (98, 99). Blocking AP-1 activity augmented antitumoral efficacy in HNSCC cells (98). In vitro and in vivo evidence demonstrated that the antitumor effect of retinoid was enhanced by inhibiting AP-1 activity (100, 101). Retinoid has appeared a promising chemopreventive drug in several clinical trials (Table 1). The combination of AP-1 inhibitor with retinoid or other chemopreventive compounds could be a useful tool for cancer chemoprevention.
Signal Transducers and Activator of Transcription (STATs)
STAT proteins are activated by tyrosine phosphorylation, dimerization and nuclear localization, where STATs bind to consensus elements in the promoter regions of targeted genes associated with many aspects of cell growth, survival, differentiation, angiogenesis and apoptosis (102–105). So far, seven mammalian STAT family members have been identified, named STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B and STAT6. Several reports have indicated the important role of STATs in the progression of HNSCC. Autocrine or paracrine activation of TGF-α/EGFR is observed frequently in HNSCC and STAT3 is a vital signaling network downstream of TGF-α/EGFR (106). In primary tumors of HNSCC patients, STAT3 is constitutively activated and induces expression of cyclin D1 and is associated with a lower survival rate (107). Src kinase plays a vital role in EGFR-mediated STAT3 and STAT5 activation in HNSCC (108). Constitutive activation of STAT3 and STAT5 has been implicated in multiple myelomas, lymphomas and several solid tumors (109). Knockout studies have provided evidence that STAT proteins are involved in the development and function of the immune system and play a role in maintaining immune tolerance and tumor surveillance (110). Finding new agents and combination strategies that target STAT3 and STAT5 may be useful in the treatment and prevention of HNSCC.
PI3-K/AKT/mTOR signaling
PI3-K/AKT/mTOR has been reported as a key dysregulated signaling pathway in head and cancer (51). This pathway is an attractive therapeutic target in cancer because it plays a vital role in many growth stimuli, and controls cellular processes through its downstream substrates that contribute to the initiation and maintenance of cancer. Loss of function of the tumor suppressor PTEN, amplification or mutation of PI3K and Akt, activation of growth factor receptors, and exposure to carcinogens are strong contributors to the activation of PI3-K/AKT/mTOR signaling, which confers resistance to treatment and poor prognosis in many types of cancer (111). Constitutive activation of this signaling pathway is frequently found in HNSCC (112, 113) and is also associated with radioresistance (114). In preclinical and clinical studies, targeting this pathway by using several inhibitors is suggested as a promising approach in controlling tumor progression in many cancers including HNSCC (111, 115). Currently many inhibitors, both natural and synthetic, and their combinations are being used and developed to disrupt PI3-K/AKT/mTOR signaling (Fig. 1), and could prove beneficial for cancer prevention and treatment.
Other molecular targets
VEGF and its receptor are overexpressed in HNSCC, and regulate angiogenesis, survival, and tumor growth (116, 117). In a phase I/II study in patients with recurrent or metastatic HNSCC, the combination of the EGFR inhibitor, erlotinib, and an anti-VEGF antibody, bevacizumab, was well tolerated and led to sustained benefit and complete responses (63).
Sorafenib, a bisaryl urea, is a multikinase inhibitor that acts on C-Raf, B-Raf, VEGF, VEGFR-2, VEGFR-3, PDGFR and FLT3. It mainly targets the EGFR-Ras-Raf-MEK-ERK (118) and VEGF-VEGFR (119) signaling pathways to regulate cell growth, differentiation, apoptosis, cellular transformation, angiogenesis and metastasis. A phase II clinical trial of sorafenib in HNSCC patients showed it to be well tolerated with modest anticancer activity (120).
The above-mentioned signaling networks play vital roles in carcinogenesis. It is very important to understand the dysregulated signaling pathways in HNSCC which could be potential targets for chemopreventive strategies.
Potential natural compounds as chemopreventive agents
Successful chemopreventive agents must be effective and safe enough for extended use because short term interventions are not expected to substantially reduce cancer risk over the long term. Several nutritional compounds, including those found in green and yellow vegetables, fruits, and spices belonging to different classes of chemicals, have been shown to prevent the occurrence of cancer in experimental animals. Chemoprevention with a great diversity of natural or synthetic products has already appeared to be a promising approach. The chemopreventive properties and molecular targets of selected promising natural compounds are discussed, and available agents, either natural or synthetic or both, targeting crucial signaling proteins in HNSCC are presented schematically in Figure 1.
Green Tea Polyphenols
Polyphenols (PPs) are most abundant in plant-derived foods such as fruits, seeds, leaves and beverages, and are reactive metabolites characterized by several hydroxylated aromatic groups. PPs have powerful antimicrobial activity and outstanding antioxidant activity, capable of scavenging a wide range of reactive oxygen, nitrogen, superoxide anions and metal ions (121, 122). PPs may derive their preventive effect against oral cavity and oral cancer by coming into direct contact with tissues before being absorbed or metabolized (123), allowing them to deliver the biologically active aglycones at the surface of the epithelial cells to inhibit proliferation of oral cancer cells (124).
One of the most widely consumed beverages in the world is tea, which contains abundant PPs with antioxidant properties. The preventive potential of tea, mainly green tea polyphenols has been reported in many epidemiological and preclinical studies (125–127). Green tea extract (GTE) has been reported to account for the reduction of human cancer risk in multiple organ sites (7, 128). GTE contains four major polyphenols: epicatechin (EC), epigallocatechin (EGC), epicatechin-3-gallate (ECG), and epigallocatechin-3-gallate (EGCG) (6). EGCG, the most abundant and the best studied among tea polyphenols has gained the most attention with respect to anti-carcinogenic activity.
Multiple proteins including EGFR, NF-kB, tumor necrosis factor-α (TNF-α), AKT, mitogen activated protein kinase (MAPK), p53 and its multiple target genes play a critical role in EGCG-driven signal transduction pathways to inhibit cell proliferation, invasion, and angiogenesis (7, 129–131). EGCG has been identified as targeting EGFR-mediated signaling (Fig. 1), providing a promising target to inhibit cancer development and progression. We showed in our earlier study that EGCG synergistically increased the efficacy of EGFR inhibitor in growth inhibition of HNSCC (56, 59). Erlotinib and EGCG synergistically inhibit HNSCC growth via inhibiting NF-kB in a p53-dependent manner (56). EGCG regulates cell cycle progression and drives apoptosis to inhibit overall cancer progression. In preclinical studies, EGCG treatment arrested cells in the G0-G1 phase, downregulated cyclin D1 (132), directed cell cycle progression in oral leukoplakia cell lines (133), and enhanced apoptosis by p53 stabilization upon induction of p14ARF, p16 protein levels and interferon (134, 135). EGCG interrupted angiogenesis by inhibiting phosphorylation of VEGFR (136) and inhibiting VEGF secretion by tumor cells (137). EGCG regulated AP-1 target genes by suppression of AP-1 activity (138). EGCG appears to be a potentially promising agent to prevent oral carcinogenesis (139, 140). EGCG synergistically increased the efficacy of other drugs including curcumin, erlotinib, luteolin, genistein, atorvastin, tamoxifen, celecoxib, cisplatin, sulindac, adriamycin in cell culture and animal models (7).
In vitro and in vivo results of green tea polyphenols have recommended several clinical trials to pursue, and a few trials addressing the efficacy and toxicity of GTE have already been completed. A phase I clinical trial using GTE in patients with advanced cancer showed that 1000mg/m2 three times per day was safe (141). A pilot study in smokers using GTE (2000 to 2500 mg/d) showed that smoking-induced DNA damage was decreased with a reduction in aneuploidy and increased apoptosis (142). Recently, Tsao et al. published the results of a phase II randomized trial of short-term GTE in high risk OPL patients, showed that higher doses had greater response rates (44). A longer treatment time and higher dose were suggested. Nanochemoprevention has also been explored, with nanoparticle-mediated EGCG delivery found to enhance bioavailability and reduce toxicity (143). Currently, several trials involving green tea alone or in combination with other drugs or oral EGCG analogues are ongoing, with the goal of improving bioavailability and efficacy and reducing toxicity (7). A phase I clinical trial using low dose erlotinib and EGCG has been planned at the Emory Winship Cancer Institute to assess the efficacy of this combination in patients with premalignant lesions of the head and neck.
Curcumin
Curcumin, also called diferuloyl methane, is a hydrophobic polyphenol isolated from rhizomes of the plant Curcuma longa L., widely used as spice. In the 1980s, Kuttan et. al. introduced new properties of curcumin as growth inhibition and cytotoxicity in vivo (144, 145). Extensive research over the last 30 years has revealed numerous therapeutic benefits of curcumin, including chemoprevention and therapy in cancer. In vitro and in vivo research revealed that curcumin has versatile properties such as cytotoxicity, anti-inflammation, antioxidant, immunomodulation, anti-angiogenic, cytokine release and apoptosis (128, 146–148). Curcumin has been shown to inhibit all three steps of carcinogenesis, initiation, promotion and progression, in an animal model (148) of skin cancer (149) and oral cancer (150). Curcumin has been shown to regulate the expression and activity of various molecules that play vital roles in cancer progression. As a single agent, curcumin can downregulate several molecular targets including COX-2, HER2, EGFR, AKT and VEGF (151) which are involved in crucial cellular processes discussed earlier in this chapter and in signaling networks summarized in Fig. 1. Curcumin has also been idenfied as an inhibitor of NF-kB and its target genes and thus regulates several cellular processes: it inhibits cell growth and survival by suppressing Bcl-2, cyclin D1, IL-6, COX-2 and MMP-9 protein expression in HNSCC (152) and in colon cancer (153); it reduces cancer cell angiogenesis, invasion and subsequent cell migration by suppressing MMPs (154, 155); and it reduces cell cycle regulation by suppressing cyclin D (148).
The promising preclinical in vitro and in vivo results and the pharmacological safety of curcumin have advanced it into clinical trials. A phase I clinical trial was conducted by Cheng et. al. in patients with high risk or premalignant lesions and demonstrated that orally-administered curcumin taken for 3 months was not toxic up to 8g/day (156), but is not appropriate for clinical utilization due to its rapid metabolism in the liver and intestinal wall. A pharmacodynamic and pharmacokinetic study of oral curcumin in patients with colorectal cancer suggested that curcumin has low oral bioavailability in humans and undergoes intestine metabolism (157). In an attempt to improve curcumin’s medicinal properties, there is a need to increase its potency. Many researchers are currently focusing on developing potent curcumin analogues and combination therapies. Curcumin has synergistic chemopreventive abilities with other diet-derived PPs including genistein (158) and green tea (159), and has been shown to increase the efficacy of some anti-cancer drugs including fluorouracil (160) and gemcitabine (161). Combination treatment of curcumin with quercetin (162) or with piperine (163) has shown positive responses in clinical trials. EF-24 is an analogue of curcumin that has shown promising anticancer activity (164, 165). Several phase I and phase II clinical trials are now searching for improved chemopreventive efficacy of curcumin (7).
Resveratrol
Resveratrol is a phytoalexin produced by several plants including grapes, mulberries, peanuts, vines, and pines. In 1992 it attracted wider interest, when its presence in wine was associated with cardioprotective properties. Since then, several beneficial effects have been documented in many diseases including cancer. Jang et. al (166) showed that resveratrol reduced the incidence of skin tumors in a mouse model by inhibiting all stages of carcinogenesis. Resveratrol interacts with multiple molecular targets and has positive effects on breast, skin, gastric, colon, esophageal, prostate, and pancreatic cancer cells (167). Resveratrol has been shown to induce apoptosis in human breast cancer xenografts in vivo (168), in prostate cancer (169–171) and inhibits angiogenesis in human breast cancer xenografts in vivo (168, 172). Topical application of resveratrol in mice inhibited skin damage and decreased skin cancer incidence but orally given resveratrol was ineffective in treating mice inoculated with melanoma cells, or in leukemia and lung cancer (173, 174). Resveratrol (2.5 or 10 mg/kg via injection) slowed the growth of metastatic lewis lung carcinomas in mice (175) and (1 mg/kg orally) reduced the number and size of esophageal tumors in rats treated with a carcinogen (176). In several studies, small doses (0.02–8 mg/kg) of resveratrol, given prophylactically, reduced or prevented the development of intestinal and colon tumors in rats given different carcinogens (174).
Most of these results have yet to be tested in humans because even high doses of resveratrol are insufficient to achieve the resveratrol concentrations required for the systemic prevention of cancer. This is consistent with animal model studies showing that the effectiveness of resveratrol is limited by its poor systemic bioavailability (174, 177). Recent approaches using nanoparticle-mediated delivery of resveratrol indicate improved bioavailability as evidenced by increased solubility, stability and intracellular delivery (178). The strongest evidence of anti-cancer action of resveratrol exists for tumors it can come into direct contact with, such as skin and gastrointestinal tract tumors. Several clinical trials are ongoing to assess the efficacy of resveratrol in cancer chemoprevention (7).
Lycopene
Lycopene is a red carotene, carotenoid pigment and phytochemical without vitamin A activity, found in tomatoes, fruits and vegetables, such as red carrots, guava, watermelons and papayas. Substantial scientific and clinical research has been devoted to exploring a possible correlation between lycopene consumption and a reduction in the incidence of cancer due to its antioxidant properties. Epidemiological studies have shown dietary intake of lycopene is inversely associated with the risk of many cancers including those of the digestive tract, prostate and cervix (179–182). In different studies, lycopene was found to have an inhibitory effect on different kinds of cancer cells including breast and endometrial cancer cells (183, 184), prostrate carcinoma cells (182) and colon cancer cells (185).
Lycopene is a potent antioxidant and quencher of singlet oxygen (186), resulting in protection against oxidative DNA damage in vitro and in vivo, and is also involved in inhibition of growth and induction of differentiation in cancer cells by modulating the expression of cell cycle regulatory proteins (187, 188). Tomato products may control benign prostate hyperplasia by preventing disease progression (189) and by the induction of apoptosis (190). Two phase II clinical trials suggested that lycopene alone (191) or in combination with soy isoflavones (192) suppresses the growth of prostate cancer.
The molecular mechanisms underlying the biological effects of lycopene as well as its pharmacological properties need to be further elucidated and clinical trials are needed to assess the relevance of lycopene for the prevention of cancer.
Luteolin
Luteolin is a flavonoid of the specific class of flavones, most often found in green vegetables such as celery, green pepper, thyme, broccoli, cabbage, spinach, olive oil, peppermint, rosemary and oregano (193). It plays an important role as an antioxidant, a free radical scavenger, an agent in the prevention of inflammation and in the prevention of cancer (194). Low systemic bioavailability of luteolin due to its metabolism by the liver and intestine is the major obstacle to its clinical application. However, epithelium of the oral cavity can absorb luteolin directly (124) and it has been reported that luteolin induced cell-cycle arrest by inhibiting cyclin dependent kinase and p-retinoblastoma, followed by apoptosis, leading to the growth inhibition of squamous cell cancer cells (195). Topical application also appeared to result in potent antitumor activity against skin papillomas in mice (196). Several studies have reported that luteolin was highly effective in inducing cell cycle arrest, apoptosis and cell growth inhibition in human esophageal adenocarcinoma cells (197), lung carcinoma (198), human colon cancer cells (199) and human hepatoma cells (200). Luteolin was highly effective in chemoprevention or therapy against HER2-overexpressing chemoresistant tumors (201). Recently, it has appeared with a role in inhibition of cell invasion in prostate cancer cells (202). Many in vitro and animal studies have been conducted but have yet to be replicated in human subjects. Thus, clinical trials are recommended to assess the chemoprevention potential of luteolin.
Other promising natural agents
Several other natural compounds besides the above mentioned agents are being extensively investigated to evaluate their chemoprevntive potential. Pomegranate, genistein, ellagic acid, some triterpenes, polyunsaturated fatty acids and ginkolid have appeared as promising chemopreventive agent. Pomegranate has been reported to be a potential chemopreventive agent for breast cancer (203, 204), prostate cancer (205), skin cancer (206) and prostate cancer (207). Genistein is a phytoestrogen mostly found soybeans, and an inverse correlaion has been reported between genistein consumption and the risk of prostate (208), breast (209)and endometrial (210) cancer. Ellagic acid, found in many fruits and vegetables, has chemopreventive ability against skin, lung, esophageal, colon, prostate and breast cancers (211, 212). Triterpenes, polyunsaturated fatty acids and ginkolid have shown their chemopreventive potency in vitro and in vivo in many cancers (7).
Future directions and conclusions
The five year survival rate of HNSCC patients has yet to be significantly improved despite the advances made in therapy including surgery, radiation and chemotherapy. Clinical trials, particularly for the prevention of second primary cancers have already validated the concept of chemoprevention. Multiple deviant or dysregulated signaling pathways involved in growth, survival and apoptosis in HNSCC challenge the clinical efficacy of chemopreventive agents. Thus, it is crucial to better understand these pathways and delineate how they network in the regulation of cancer progression, in order to develop diverse and complex agents that could be more effective in the prevention of HNSCC. Interfering with single steps of these pathways with single agent chemotherapy has been shown to have limitations of both toxicity and potency in patients, mandating the development of new chemopreventive agents that have the potential to act on multiple targets. Alternatively the combination of chemopreventive agents with new targeted agents or with conventional chemotherapy or radiation treatments that target distinct specific pathways is likely to disrupt the malignant programming of the cells and inhibit prosurvival signals; this may also be an attractive approach to clinical therapy and prevention. Natural dietary agents hold particular promise for chemoprevention because of their safety profile. The broad chemical diversity features together with many epidemiological, preclinical and clinical studies suggest a definitive role for selected dietary products in various approaches to cancer chemoprevention. Low potency, poor bioavailabilty and toxicity of dietary agents consititute the major challenges to their clinical development. An important prospect is to standardize the formulation and contents of natural compounds. Many natural or synthetic agents target multiple signal transduction pathways and thus testing combinations could be beneficial approach to enhance efficacy and bioavailability while reducing unexpected toxicities. An anti-proliferative agent and an inducer of apoptosis may be one beneficial combination of natural or synthetic compounds to overcome single agent resistance for more effective chemoprevention, and currently such clinical trials are in progress. For example, the combination of EGCG, a major polyphenol of green tea, with an EGFR-TKI represents a novel synergistic chemopreventive combination for HNSCC (59). To evaluate the efficacy of these chemopreventive agents there is an urgent need to identify novel biomarkers which have predictive value for the clinical disease and risk stratification that can be used for a more disease-specific approach. Natural agents appear to be highly promising molecular targets for chemoprevention and thus, innovative designs for chemopreventive treatment approaches based on appropriate patient selection are critical for meaningful impact.
Acknowledgments
This work was supported by grants from the NIH (P50 CA128613, U01 CA101244, and R01 CA112643).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143–1154. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- 3.Tsao AS, Kim ES, Hong WK. Chemoprevention of cancer. CA Cancer J Clin. 2004;54:150–180. doi: 10.3322/canjclin.54.3.150. [DOI] [PubMed] [Google Scholar]
- 4.Khuri FR, Kim ES, Lee JJ, Winn RJ, Benner SE, et al. The impact of smoking status, disease stage, and index tumor site on second primary tumor incidence and tumor recurrence in the head and neck retinoid chemoprevention trial. Cancer Epidemiol Biomarkers Prev. 2001;10:823–829. [PubMed] [Google Scholar]
- 5.Abbruzzese JL, Lippman SM. The convergence of cancer prevention and therapy in early-phase clinical drug development. Cancer Cell. 2004;6:321–326. doi: 10.1016/j.ccr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 6.Khuri FR, Shin DM. Head and neck cancer chemoprevention gets a shot in the arm. J Clin Oncol. 2008;26:345–347. doi: 10.1200/JCO.2007.14.0913. [DOI] [PubMed] [Google Scholar]
- 7.Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27:2712–2725. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy L, Odhav B, Bhoola KD. Natural products for cancer prevention. a global perspective. Pharmacol Ther. 2003;99:1–13. doi: 10.1016/s0163-7258(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 9.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention. a review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 10.Benetou V, Orfanos P, Lagiou P, Trichopoulos D, Boffetta P, et al. Vegetables and fruits in relation to cancer risk: evidence from the Greek EPIC cohort study. Cancer Epidemiol Biomarkers Prev. 2008;17:387–392. doi: 10.1158/1055-9965.EPI-07-2665. [DOI] [PubMed] [Google Scholar]
- 11.Glade MJ. Food, nutrition, and the prevention of cancer: a global perspective. American Institute for Cancer Research/World Cancer Research Fund, American Institute for Cancer Research, 1997. Nutrition. 1999;15:523–526. doi: 10.1016/s0899-9007(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 12.Prevention of cancer in the next millennium: Report of the Chemoprevention Working Group to the American Association for Cancer Research. Cancer Res. 1999;59:4743–4758. [PubMed] [Google Scholar]
- 13.Greenwald P. Cancer chemoprevention. BMJ. 2002;324:714–718. doi: 10.1136/bmj.324.7339.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184–194. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 15.Gao X, Fisher SG, Mohideen N, Emami B. Second primary cancers in patients with laryngeal cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2003;56:427–435. doi: 10.1016/s0360-3016(02)04613-8. [DOI] [PubMed] [Google Scholar]
- 16.Hunter KD, Parkinson EK, Harrison PR. Profiling early head and neck cancer. Nat Rev Cancer. 2005;5:127–135. doi: 10.1038/nrc1549. [DOI] [PubMed] [Google Scholar]
- 17.O’Shaughnessy JA, Kelloff GJ, Gordon GB, Dannenberg AJ, Hong WK, et al. Treatment and prevention of intraepithelial neoplasia: an important target for accelerated new agent development. Clin Cancer Res. 2002;8:314–346. [PubMed] [Google Scholar]
- 18.Curfman GD, Morrissey S, Drazen JM. Retraction. Sudbo J et al. DNA content as a prognostic marker in patients with oral leukoplakia. N Engl J Med 2001;344:1270–8 and Sudbo J et al. The influence of resection and aneuploidy on mortality in oral leukoplakia. N Engl J Med 2004;350:1405–13. N Engl J Med. 2006;355:1927. doi: 10.1056/NEJMe068247. [DOI] [PubMed] [Google Scholar]
- 19.Wolbach SB, Howe PR. Tissue Changes Following Deprivation of Fat-Soluble a Vitamin. J Exp Med. 1925;42:753–777. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sporn MB, Dunlop NM, Newton DL, Smith JM. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids) Fed Proc. 1976;35:1332–1338. [PubMed] [Google Scholar]
- 21.Lippman SM, Lee JJ, Sabichi AL. Cancer chemoprevention: progress and promise. J Natl Cancer Inst. 1998;90:1514–1528. doi: 10.1093/jnci/90.20.1514. [DOI] [PubMed] [Google Scholar]
- 22.Hong WK, Spitz MR, Lippman SM. Cancer chemoprevention in the 21st century: genetics, risk modeling, and molecular targets. J Clin Oncol. 2000;18:9S–18S. [PubMed] [Google Scholar]
- 23.Hong WK, Endicott J, Itri LM, Doos W, Batsakis JG, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315:1501–1505. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 24.Hong WK, Lippman SM, Itri LM, Karp DD, Lee JS, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323:795–801. doi: 10.1056/NEJM199009203231205. [DOI] [PubMed] [Google Scholar]
- 25.Stich HF, Rosin MP, Hornby AP, Mathew B, Sankaranarayanan R, et al. Remission of oral leukoplakias and micronuclei in tobacco/betel quid chewers treated with beta-carotene and with beta-carotene plus vitamin A. Int J Cancer. 1988;42:195–199. doi: 10.1002/ijc.2910420209. [DOI] [PubMed] [Google Scholar]
- 26.Lippman SM, Batsakis JG, Toth BB, Weber RS, Lee JJ, et al. Comparison of low-dose isotretinoin with beta carotene to prevent oral carcinogenesis. N Engl J Med. 1993;328:15–20. doi: 10.1056/NEJM199301073280103. [DOI] [PubMed] [Google Scholar]
- 27.Papadimitrakopoulou VA, Hong WK, Lee JS, Martin JW, Lee JJ, et al. Low-dose isotretinoin versus beta-carotene to prevent oral carcinogenesis: long-term follow-up. J Natl Cancer Inst. 1997;89:257–258. doi: 10.1093/jnci/89.3.257. [DOI] [PubMed] [Google Scholar]
- 28.Benner SE, Pajak TF, Lippman SM, Earley C, Hong WK. Prevention of second primary tumors with isotretinoin in patients with squamous cell carcinoma of the head and neck: long-term follow-up. J Natl Cancer Inst. 1994;86:140–141. doi: 10.1093/jnci/86.2.140. [DOI] [PubMed] [Google Scholar]
- 29.Shin DM, Khuri FR, Murphy B, Garden AS, Clayman G, et al. Combined interferon-alfa, 13-cis-retinoic acid, and alpha-tocopherol in locally advanced head and neck squamous cell carcinoma: novel bioadjuvant phase II trial. J Clin Oncol. 2001;19:3010–3017. doi: 10.1200/JCO.2001.19.12.3010. [DOI] [PubMed] [Google Scholar]
- 30.Seixas-Silva JA, Jr, Richards T, Khuri FR, Wieand HS, Kim E, et al. Phase 2 bioadjuvant study of interferon alfa-2a, isotretinoin, and vitamin E in locally advanced squamous cell carcinoma of the head and neck: long-term follow-up. Arch Otolaryngol Head Neck Surg. 2005;131:304–307. doi: 10.1001/archotol.131.4.304. [DOI] [PubMed] [Google Scholar]
- 31.Khuri FR, Lee JJ, Lippman SM, Kim ES, Cooper JS, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98:441–450. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]
- 32.Papadimitrakopoulou VA, Lee JJ, William WN, Jr, Martin JW, Thomas M, et al. Randomized trial of 13-cis retinoic acid compared with retinyl palmitate with or without beta-carotene in oral premalignancy. J Clin Oncol. 2009;27:599–604. doi: 10.1200/JCO.2008.17.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stich HF, Hornby AP, Mathew B, Sankaranarayanan R, Nair MK. Response of oral leukoplakias to the administration of vitamin A. Cancer Lett. 1988;40:93–101. doi: 10.1016/0304-3835(88)90266-2. [DOI] [PubMed] [Google Scholar]
- 34.DeLaney TF, Afridi N, Taghian AG, Sanders DA, Fuleihan NS, et al. 13-cis-retinoic acid with alpha-2a-interferon enhances radiation cytotoxicity in head and neck squamous cell carcinoma in vitro. Cancer Res. 1996;56:2277–2280. [PubMed] [Google Scholar]
- 35.Lingen MW, Polverini PJ, Bouck NP. Retinoic acid and interferon alpha act synergistically as antiangiogenic and antitumor agents against human head and neck squamous cell carcinoma. Cancer Res. 1998;58:5551–5558. [PubMed] [Google Scholar]
- 36.Lindner DJ, Borden EC. Synergistic antitumor effects of a combination of interferon and tamoxifen on estrogen receptor-positive and receptor-negative human tumor cell lines in vivo and in vitro. J Interferon Cytokine Res. 1997;17:681–693. doi: 10.1089/jir.1997.17.681. [DOI] [PubMed] [Google Scholar]
- 37.Papadimitrakopoulou VA, Clayman GL, Shin DM, Myers JN, Gillenwater AM, et al. Biochemoprevention for dysplastic lesions of the upper aerodigestive tract. Arch Otolaryngol Head Neck Surg. 1999;125:1083–1089. doi: 10.1001/archotol.125.10.1083. [DOI] [PubMed] [Google Scholar]
- 38.Rotmensz N, De Palo G, Formelli F, Costa A, Marubini E, et al. Long-term tolerability of fenretinide (4-HPR) in breast cancer patients. Eur J Cancer. 1991;27:1127–1131. doi: 10.1016/0277-5379(91)90309-2. [DOI] [PubMed] [Google Scholar]
- 39.Han J, Jiao L, Lu Y, Sun Z, Gu QM, et al. Evaluation of N-4-(hydroxycarbophenyl) retinamide as a cancer prevention agent and as a cancer chemotherapeutic agent. In Vivo. 1990;4:153–160. [PubMed] [Google Scholar]
- 40.Chiesa F, Tradati N, Marazza M, Rossi N, Boracchi P, et al. Fenretinide (4-HPR) in chemoprevention of oral leukoplakia. J Cell Biochem Suppl. 1993;17F:255–261. doi: 10.1002/jcb.240531038. [DOI] [PubMed] [Google Scholar]
- 41.Lotan R. Retinoids and apoptosis: implications for cancer chemoprevention and therapy. J Natl Cancer Inst. 1995;87:1655–1657. doi: 10.1093/jnci/87.22.1655. [DOI] [PubMed] [Google Scholar]
- 42.Oridate N, Lotan D, Xu XC, Hong WK, Lotan R. Differential induction of apoptosis by all-trans-retinoic acid and N-(4-hydroxyphenyl)retinamide in human head and neck squamous cell carcinoma cell lines. Clin Cancer Res. 1996;2:855–863. [PubMed] [Google Scholar]
- 43.Papadimitrakopoulou VA, William WN, Jr, Dannenberg AJ, Lippman SM, Lee JJ, et al. Pilot randomized phase II study of celecoxib in oral premalignant lesions. Clin Cancer Res. 2008;14:2095–2101. doi: 10.1158/1078-0432.CCR-07-4024. [DOI] [PubMed] [Google Scholar]
- 44.Tsao AS, Liu D, Martin J, Tang XM, Lee JJ, et al. Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. Cancer Prev Res (Phila Pa) 2009;2:931–941. doi: 10.1158/1940-6207.CAPR-09-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fisher B, Powles TJ, Pritchard KJ. Tamoxifen for the prevention of breast cancer. Eur J Cancer. 2000;36:142–150. doi: 10.1016/s0959-8049(99)00269-5. [DOI] [PubMed] [Google Scholar]
- 46.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 47.Rubin Grandis J, Melhem MF, Gooding WE, Day R, Holst VA, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 48.Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 49.Temam S, Kawaguchi H, El-Naggar AK, Jelinek J, Tang H, et al. Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. J Clin Oncol. 2007;25:2164–2170. doi: 10.1200/JCO.2006.06.6605. [DOI] [PubMed] [Google Scholar]
- 50.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 51.Matta A, Ralhan R. Overview of current and future biologically based targeted therapies in head and neck squamous cell carcinoma. Head Neck Oncol. 2009;1:6. doi: 10.1186/1758-3284-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24:2666–2672. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 53.Bernier J, Bentzen SM, Vermorken JB. Molecular therapy in head and neck oncology. Nat Rev Clin Oncol. 2009;6:266–277. doi: 10.1038/nrclinonc.2009.40. [DOI] [PubMed] [Google Scholar]
- 54.Shin DM, Ro JY, Hong WK, Hittelman WN. Dysregulation of epidermal growth factor receptor expression in premalignant lesions during head and neck tumorigenesis. Cancer Res. 1994;54:3153–3159. [PubMed] [Google Scholar]
- 55.Nozawa H, Tadakuma T, Ono T, Sato M, Hiroi S, et al. Small interfering RNA targeting epidermal growth factor receptor enhances chemosensitivity to cisplatin, 5-fluorouracil and docetaxel in head and neck squamous cell carcinoma. Cancer Sci. 2006;97:1115–1124. doi: 10.1111/j.1349-7006.2006.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amin AR, Khuri FR, Chen ZG, Shin DM. Synergistic growth inhibition of squamous cell carcinoma of the head and neck by erlotinib and epigallocatechin-3-gallate: the role of p53-dependent inhibition of nuclear factor-kappaB. Cancer Prev Res (Phila Pa) 2009;2:538–545. doi: 10.1158/1940-6207.CAPR-09-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun Q, Ming L, Thomas SM, Wang Y, Chen ZG, et al. PUMA mediates EGFR tyrosine kinase inhibitor-induced apoptosis in head and neck cancer cells. Oncogene. 2009;28:2348–2357. doi: 10.1038/onc.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pernas FG, Allen CT, Winters ME, Yan B, Friedman J, et al. Proteomic signatures of epidermal growth factor receptor and survival signal pathways correspond to gefitinib sensitivity in head and neck cancer. Clin Cancer Res. 2009;15:2361–2372. doi: 10.1158/1078-0432.CCR-08-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang X, Zhang H, Tighiouart M, Lee JE, Shin HJ, et al. Synergistic inhibition of head and neck tumor growth by green tea (−)-epigallocatechin-3-gallate and EGFR tyrosine kinase inhibitor. Int J Cancer. 2008;123:1005–1014. doi: 10.1002/ijc.23585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choe MS, Zhang X, Shin HJ, Shin DM, Chen ZG. Interaction between epidermal growth factor receptor- and cyclooxygenase 2-mediated pathways and its implications for the chemoprevention of head and neck cancer. Mol Cancer Ther. 2005;4:1448–1455. doi: 10.1158/1535-7163.MCT-04-0251. [DOI] [PubMed] [Google Scholar]
- 61.Zhang X, Chen ZG, Choe MS, Lin Y, Sun SY, et al. Tumor growth inhibition by simultaneously blocking epidermal growth factor receptor and cyclooxygenase-2 in a xenograft model. Clin Cancer Res. 2005;11:6261–6269. doi: 10.1158/1078-0432.CCR-04-2102. [DOI] [PubMed] [Google Scholar]
- 62.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53:3579–3584. [PubMed] [Google Scholar]
- 63.Cohen EE, Davis DW, Karrison TG, Seiwert TY, Wong SJ, et al. Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: a phase I/II study. Lancet Oncol. 2009;10:247–257. doi: 10.1016/S1470-2045(09)70002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2009 doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 65.Dannenberg AJ, Altorki NK, Boyle JO, Dang C, Howe LR, et al. Cyclo-oxygenase 2: a pharmacological target for the prevention of cancer. Lancet Oncol. 2001;2:544–551. doi: 10.1016/S1470-2045(01)00488-0. [DOI] [PubMed] [Google Scholar]
- 66.Lin DT, Subbaramaiah K, Shah JP, Dannenberg AJ, Boyle JO. Cyclooxygenase-2: a novel molecular target for the prevention and treatment of head and neck cancer. Head Neck. 2002;24:792–799. doi: 10.1002/hed.10108. [DOI] [PubMed] [Google Scholar]
- 67.Jacoby RF, Seibert K, Cole CE, Kelloff G, Lubet RA. The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the min mouse model of adenomatous polyposis. Cancer Res. 2000;60:5040–5044. [PubMed] [Google Scholar]
- 68.Fischer SM, Lo HH, Gordon GB, Seibert K, Kelloff G, et al. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol Carcinog. 1999;25:231–240. [PubMed] [Google Scholar]
- 69.Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–412. [PubMed] [Google Scholar]
- 70.Buttar NS, Wang KK, Leontovich O, Westcott JY, Pacifico RJ, et al. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett’s esophagus. Gastroenterology. 2002;122:1101–1112. doi: 10.1053/gast.2002.32371. [DOI] [PubMed] [Google Scholar]
- 71.Nishimura G, Yanoma S, Mizuno H, Kawakami K, Tsukuda M. A selective cyclooxygenase-2 inhibitor suppresses tumor growth in nude mouse xenografted with human head and neck squamous carcinoma cells. Jpn J Cancer Res. 1999;90:1152–1162. doi: 10.1111/j.1349-7006.1999.tb00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu XH, Yao S, Kirschenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58:4245–4249. [PubMed] [Google Scholar]
- 73.Hara A, Yoshimi N, Niwa M, Ino N, Mori H. Apoptosis induced by NS-398, a selective cyclooxygenase-2 inhibitor, in human colorectal cancer cell lines. Jpn J Cancer Res. 1997;88:600–604. doi: 10.1111/j.1349-7006.1997.tb00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirsch FR, Lippman SM. Advances in the biology of lung cancer chemoprevention. J Clin Oncol. 2005;23:3186–3197. doi: 10.1200/JCO.2005.14.209. [DOI] [PubMed] [Google Scholar]
- 75.Stolina M, Sharma S, Lin Y, Dohadwala M, Gardner B, et al. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 76.Shishodia S, Aggarwal BB. Cyclooxygenase (COX)-2 inhibitor celecoxib abrogates activation of cigarette smoke-induced nuclear factor (NF)-kappaB by suppressing activation of IkappaBalpha kinase in human non-small cell lung carcinoma: correlation with suppression of cyclin D1, COX-2, and matrix metalloproteinase-9. Cancer Res. 2004;64:5004–5012. doi: 10.1158/0008-5472.CAN-04-0206. [DOI] [PubMed] [Google Scholar]
- 77.Mestre JR, Subbaramaiah K, Sacks PG, Schantz SP, Tanabe T, et al. Retinoids suppress epidermal growth factor-induced transcription of cyclooxygenase-2 in human oral squamous carcinoma cells. Cancer Res. 1997;57:2890–2895. [PubMed] [Google Scholar]
- 78.Mestre JR, Subbaramaiah K, Sacks PG, Schantz SP, Tanabe T, et al. Retinoids suppress phorbol ester-mediated induction of cyclooxygenase-2. Cancer Res. 1997;57:1081–1085. [PubMed] [Google Scholar]
- 79.Kishi K, Petersen S, Petersen C, Hunter N, Mason K, et al. Preferential enhancement of tumor radioresponse by a cyclooxygenase-2 inhibitor. Cancer Res. 2000;60:1326–1331. [PubMed] [Google Scholar]
- 80.Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer. 2004;4:793–805. doi: 10.1038/nrc1455. [DOI] [PubMed] [Google Scholar]
- 81.Shin DM, Charuruks N, Lippman SM, Lee JJ, Ro JY, et al. p53 protein accumulation and genomic instability in head and neck multistep tumorigenesis. Cancer Epidemiol Biomarkers Prev. 2001;10:603–609. [PubMed] [Google Scholar]
- 82.Yin Y, Tainsky MA, Bischoff FZ, Strong LC, Wahl GM. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell. 1992;70:937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- 83.Eyfjord JE, Thorlacius S, Steinarsdottir M, Valgardsdottir R, Ogmundsdottir HM, et al. p53 abnormalities and genomic instability in primary human breast carcinomas. Cancer Res. 1995;55:646–651. [PubMed] [Google Scholar]
- 84.Sood AK, Skilling JS, Buller RE. Ovarian cancer genomic instability correlates with p53 frameshift mutations. Cancer Res. 1997;57:1047–1049. [PubMed] [Google Scholar]
- 85.Uchida T, Wada C, Wang C, Egawa S, Ohtani H, et al. Genomic instability of microsatellite repeats and mutations of H-, K-, and N-ras, and p53 genes in renal cell carcinoma. Cancer Res. 1994;54:3682–3685. [PubMed] [Google Scholar]
- 86.Shin DM, Xu XC, Lippman SM, Lee JJ, Lee JS, et al. Accumulation of p53 protein and retinoic acid receptor beta in retinoid chemoprevention. Clin Cancer Res. 1997;3:875–880. [PubMed] [Google Scholar]
- 87.Lippman SM, Shin DM, Lee JJ, Batsakis JG, Lotan R, et al. p53 and retinoid chemoprevention of oral carcinogenesis. Cancer Res. 1995;55:16–19. [PubMed] [Google Scholar]
- 88.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 89.Brasier AR. The NF-kappaB regulatory network. Cardiovasc Toxicol. 2006;6:111–130. doi: 10.1385/ct:6:2:111. [DOI] [PubMed] [Google Scholar]
- 90.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 91.Tergaonkar V. NFkappaB pathway: a good signaling paradigm and therapeutic target. Int J Biochem Cell Biol. 2006;38:1647–1653. doi: 10.1016/j.biocel.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 92.Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 93.Kim HJ, Hawke N, Baldwin AS. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006;13:738–747. doi: 10.1038/sj.cdd.4401877. [DOI] [PubMed] [Google Scholar]
- 94.Allen CT, Ricker JL, Chen Z, Van Waes C. Role of activated nuclear factor-kappaB in the pathogenesis and therapy of squamous cell carcinoma of the head and neck. Head Neck. 2007;29:959–971. doi: 10.1002/hed.20615. [DOI] [PubMed] [Google Scholar]
- 95.Dudek AZ, Lesniewski-Kmak K, Shehadeh NJ, Pandey ON, Franklin M, et al. Phase I study of bortezomib and cetuximab in patients with solid tumours expressing epidermal growth factor receptor. Br J Cancer. 2009;100:1379–1384. doi: 10.1038/sj.bjc.6605043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 97.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 98.Chen Z, Ricker JL, Malhotra PS, Nottingham L, Bagain L, et al. Differential bortezomib sensitivity in head and neck cancer lines corresponds to proteasome, nuclear factor-kappaB and activator protein-1 related mechanisms. Mol Cancer Ther. 2008;7:1949–1960. doi: 10.1158/1535-7163.MCT-07-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zeng Q, McCauley LK, Wang CY. Hepatocyte growth factor inhibits anoikis by induction of activator protein 1-dependent cyclooxygenase-2. Implication in head and neck squamous cell carcinoma progression. J Biol Chem. 2002;277:50137–50142. doi: 10.1074/jbc.M208952200. [DOI] [PubMed] [Google Scholar]
- 100.Lin F, Xiao D, Kolluri SK, Zhang X. Unique anti-activator protein-1 activity of retinoic acid receptor beta. Cancer Res. 2000;60:3271–3280. [PubMed] [Google Scholar]
- 101.Huang C, Ma WY, Dawson MI, Rincon M, Flavell RA, et al. Blocking activator protein-1 activity, but not activating retinoic acid response element, is required for the antitumor promotion effect of retinoic acid. Proc Natl Acad Sci U S A. 1997;94:5826–5830. doi: 10.1073/pnas.94.11.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leaman DW, Leung S, Li X, Stark GR. Regulation of STAT-dependent pathways by growth factors and cytokines. FASEB J. 1996;10:1578–1588. [PubMed] [Google Scholar]
- 103.Liu KD, Gaffen SL, Goldsmith MA. JAK/STAT signaling by cytokine receptors. Curr Opin Immunol. 1998;10:271–278. doi: 10.1016/s0952-7915(98)80165-9. [DOI] [PubMed] [Google Scholar]
- 104.Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 105.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 106.Song JI, Grandis JR. STAT signaling in head and neck cancer. Oncogene. 2000;19:2489–2495. doi: 10.1038/sj.onc.1203483. [DOI] [PubMed] [Google Scholar]
- 107.Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, et al. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–3355. [PubMed] [Google Scholar]
- 108.Xi S, Zhang Q, Dyer KF, Lerner EC, Smithgall TE, et al. Src kinases mediate STAT growth pathways in squamous cell carcinoma of the head and neck. J Biol Chem. 2003;278:31574–31583. doi: 10.1074/jbc.M303499200. [DOI] [PubMed] [Google Scholar]
- 109.Grandis JR, Drenning SD, Chakraborty A, Zhou MY, Zeng Q, et al. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth In vitro. J Clin Invest. 1998;102:1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Benekli M, Baer MR, Baumann H, Wetzler M. Signal transducer and activator of transcription proteins in leukemias. Blood. 2003;101:2940–2954. doi: 10.1182/blood-2002-04-1204. [DOI] [PubMed] [Google Scholar]
- 111.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moral M, Paramio JM. Akt pathway as a target for therapeutic intervention in HNSCC. Histol Histopathol. 2008;23:1269–1278. doi: 10.14670/HH-23.1269. [DOI] [PubMed] [Google Scholar]
- 113.Amornphimoltham P, Sriuranpong V, Patel V, Benavides F, Conti CJ, et al. Persistent activation of the Akt pathway in head and neck squamous cell carcinoma: a potential target for UCN-01. Clin Cancer Res. 2004;10:4029–4037. doi: 10.1158/1078-0432.CCR-03-0249. [DOI] [PubMed] [Google Scholar]
- 114.Bussink J, van der Kogel AJ, Kaanders JH. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 2008;9:288–296. doi: 10.1016/S1470-2045(08)70073-1. [DOI] [PubMed] [Google Scholar]
- 115.Liu FY, Zhao ZJ, Li P, Ding X, Zong ZH, et al. Mammalian target of rapamycin (mTOR) is involved in the survival of cells mediated by chemokine receptor 7 through PI3K/Akt in metastatic squamous cell carcinoma of the head and neck. Br J Oral Maxillofac Surg. 2009 doi: 10.1016/j.bjoms.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 116.Shang ZJ, Li ZB, Li JR. VEGF is up-regulated by hypoxic stimulation and related to tumour angiogenesis and severity of disease in oral squamous cell carcinoma: in vitro and in vivo studies. Int J Oral Maxillofac Surg. 2006;35:533–538. doi: 10.1016/j.ijom.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 117.Liang X, Yang D, Hu J, Hao X, Gao J, et al. Hypoxia inducible factor-alpha expression correlates with vascular endothelial growth factor-C expression and lymphangiogenesis/angiogenesis in oral squamous cell carcinoma. Anticancer Res. 2008;28:1659–1666. [PubMed] [Google Scholar]
- 118.Beeram M, Patnaik A, Rowinsky EK. Raf: a strategic target for therapeutic development against cancer. J Clin Oncol. 2005;23:6771–6790. doi: 10.1200/JCO.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 119.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 120.Elser C, Siu LL, Winquist E, Agulnik M, Pond GR, et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol. 2007;25:3766–3773. doi: 10.1200/JCO.2006.10.2871. [DOI] [PubMed] [Google Scholar]
- 121.Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Petti S, Scully C. Polyphenols, oral health and disease: A review. J Dent. 2009;37:413–423. doi: 10.1016/j.jdent.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 123.Halliwell B, Zhao K, Whiteman M. The gastrointestinal tract: a major site of antioxidant action? Free Radic Res. 2000;33:819–830. doi: 10.1080/10715760000301341. [DOI] [PubMed] [Google Scholar]
- 124.Walle T, Browning AM, Steed LL, Reed SG, Walle UK. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J Nutr. 2005;135:48–52. doi: 10.1093/jn/135.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bode AM, Dong Z. Epigallocatechin 3-gallate and green tea catechins: United they work, divided they fail. Cancer Prev Res (Phila Pa) 2009;2:514–517. doi: 10.1158/1940-6207.CAPR-09-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cao Y, Cao R. Angiogenesis inhibited by drinking tea. Nature. 1999;398:381. doi: 10.1038/18793. [DOI] [PubMed] [Google Scholar]
- 127.Beltz LA, Bayer DK, Moss AL, Simet IM. Mechanisms of cancer prevention by green and black tea polyphenols. Anticancer Agents Med Chem. 2006;6:389–406. doi: 10.2174/187152006778226468. [DOI] [PubMed] [Google Scholar]
- 128.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 129.Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- 130.Suganuma M, Sueoka E, Sueoka N, Okabe S, Fujiki H. Mechanisms of cancer prevention by tea polyphenols based on inhibition of TNF-alpha expression. Biofactors. 2000;13:67–72. doi: 10.1002/biof.5520130112. [DOI] [PubMed] [Google Scholar]
- 131.Shin DM. Oral cancer prevention advances with a translational trial of green tea. Cancer Prev Res (Phila Pa) 2009;2:919–921. doi: 10.1158/1940-6207.CAPR-09-0207. [DOI] [PubMed] [Google Scholar]
- 132.Ahmad N, Cheng P, Mukhtar H. Cell cycle dysregulation by green tea polyphenol epigallocatechin-3-gallate. Biochem Biophys Res Commun. 2000;275:328–334. doi: 10.1006/bbrc.2000.3297. [DOI] [PubMed] [Google Scholar]
- 133.Khafif A, Schantz SP, al-Rawi M, Edelstein D, Sacks PG. Green tea regulates cell cycle progression in oral leukoplakia. Head Neck. 1998;20:528–534. doi: 10.1002/(sici)1097-0347(199809)20:6<528::aid-hed7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 134.Hastak K, Agarwal MK, Mukhtar H, Agarwal ML. Ablation of either p21 or Bax prevents p53-dependent apoptosis induced by green tea polyphenol epigallocatechin-3-gallate. FASEB J. 2005;19:789–791. doi: 10.1096/fj.04-2226fje. [DOI] [PubMed] [Google Scholar]
- 135.Nihal M, Ahsan H, Siddiqui IA, Mukhtar H, Ahmad N, et al. (−)-Epigallocatechin-3-gallate (EGCG) sensitizes melanoma cells to interferon induced growth inhibition in a mouse model of human melanoma. Cell Cycle. 2009;8:2057–2063. doi: 10.4161/cc.8.13.8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lamy S, Gingras D, Beliveau R. Green tea catechins inhibit vascular endothelial growth factor receptor phosphorylation. Cancer Res. 2002;62:381–385. [PubMed] [Google Scholar]
- 137.Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, et al. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J Exp Ther Oncol. 2002;2:350–359. doi: 10.1046/j.1359-4117.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 138.Kim HS, Kim MH, Jeong M, Hwang YS, Lim SH, et al. EGCG blocks tumor promoter-induced MMP-9 expression via suppression of MAPK and AP-1 activation in human gastric AGS cells. Anticancer Res. 2004;24:747–753. [PubMed] [Google Scholar]
- 139.Fujiki H, Yoshizawa S, Horiuchi T, Suganuma M, Yatsunami J, et al. Anticarcinogenic effects of (−)-epigallocatechin gallate. Prev Med. 1992;21:503–509. doi: 10.1016/0091-7435(92)90057-o. [DOI] [PubMed] [Google Scholar]
- 140.Fujiki H, Suganuma M, Okabe S, Sueoka E, Suga K, et al. Mechanistic findings of green tea as cancer preventive for humans. Proc Soc Exp Biol Med. 1999;220:225–228. doi: 10.1046/j.1525-1373.1999.d01-38.x. [DOI] [PubMed] [Google Scholar]
- 141.Pisters KM, Newman RA, Coldman B, Shin DM, Khuri FR, et al. Phase I trial of oral green tea extract in adult patients with solid tumors. J Clin Oncol. 2001;19:1830–1838. doi: 10.1200/JCO.2001.19.6.1830. [DOI] [PubMed] [Google Scholar]
- 142.Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16:360–367. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 143.Siddiqui IA, Adhami VM, Bharali DJ, Hafeez BB, Asim M, et al. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009;69:1712–1716. doi: 10.1158/0008-5472.CAN-08-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kuttan R, Bhanumathy P, Nirmala K, George MC. Potential anticancer activity of turmeric (Curcuma longa) Cancer Lett. 1985;29:197–202. doi: 10.1016/0304-3835(85)90159-4. [DOI] [PubMed] [Google Scholar]
- 145.Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori. 1987;73:29–31. doi: 10.1177/030089168707300105. [DOI] [PubMed] [Google Scholar]
- 146.Kasinski AL, Du Y, Thomas SL, Zhao J, Sun SY, et al. Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol Pharmacol. 2008;74:654–661. doi: 10.1124/mol.108.046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bar-Sela G, Epelbaum R, Schaffer M. Curcumin as an Anti-Cancer Agent: Review of the Gap between Basic and Clinical Applications. Curr Med Chem. 2009 doi: 10.2174/092986710790149738. [DOI] [PubMed] [Google Scholar]
- 148.Thangapazham RL, Sharma A, Maheshwari RK. Multiple molecular targets in cancer chemoprevention by curcumin. AAPS J. 2006;8:E443–449. doi: 10.1208/aapsj080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Conney AH. Enzyme induction and dietary chemicals as approaches to cancer chemoprevention: the Seventh DeWitt S. Goodman Lecture. Cancer Res. 2003;63:7005–7031. [PubMed] [Google Scholar]
- 150.Li N, Chen X, Liao J, Yang G, Wang S, et al. Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamsters by tea and curcumin. Carcinogenesis. 2002;23:1307–1313. doi: 10.1093/carcin/23.8.1307. [DOI] [PubMed] [Google Scholar]
- 151.Goel A, Jhurani S, Aggarwal BB. Multi-targeted therapy by curcumin: how spicy is it? Mol Nutr Food Res. 2008;52:1010–1030. doi: 10.1002/mnfr.200700354. [DOI] [PubMed] [Google Scholar]
- 152.Aggarwal S, Takada Y, Singh S, Myers JN, Aggarwal BB. Inhibition of growth and survival of human head and neck squamous cell carcinoma cells by curcumin via modulation of nuclear factor-kappaB signaling. Int J Cancer. 2004;111:679–692. doi: 10.1002/ijc.20333. [DOI] [PubMed] [Google Scholar]
- 153.Goel A, Boland CR, Chauhan DP. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001;172:111–118. doi: 10.1016/s0304-3835(01)00655-3. [DOI] [PubMed] [Google Scholar]
- 154.John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res. 2001;7:14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- 155.Woo MS, Jung SH, Kim SY, Hyun JW, Ko KH, et al. Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem Biophys Res Commun. 2005;335:1017–1025. doi: 10.1016/j.bbrc.2005.07.174. [DOI] [PubMed] [Google Scholar]
- 156.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 157.Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- 158.Verma SP, Salamone E, Goldin B. Curcumin and genistein, plant natural products, show synergistic inhibitory effects on the growth of human breast cancer MCF-7 cells induced by estrogenic pesticides. Biochem Biophys Res Commun. 1997;233:692–696. doi: 10.1006/bbrc.1997.6527. [DOI] [PubMed] [Google Scholar]
- 159.Khafif A, Schantz SP, Chou TC, Edelstein D, Sacks PG. Quantitation of chemopreventive synergism between (−)-epigallocatechin-3-gallate and curcumin in normal, premalignant and malignant human oral epithelial cells. Carcinogenesis. 1998;19:419–424. doi: 10.1093/carcin/19.3.419. [DOI] [PubMed] [Google Scholar]
- 160.Koo JY, Kim HJ, Jung KO, Park KY. Curcumin inhibits the growth of AGS human gastric carcinoma cells in vitro and shows synergism with 5-fluorouracil. J Med Food. 2004;7:117–121. doi: 10.1089/1096620041224229. [DOI] [PubMed] [Google Scholar]
- 161.Sen S, Sharma H, Singh N. Curcumin enhances Vinorelbine mediated apoptosis in NSCLC cells by the mitochondrial pathway. Biochem Biophys Res Commun. 2005;331:1245–1252. doi: 10.1016/j.bbrc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 162.Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 163.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 164.Adams BK, Ferstl EM, Davis MC, Herold M, Kurtkaya S, et al. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg Med Chem. 2004;12:3871–3883. doi: 10.1016/j.bmc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 165.Adams BK, Cai J, Armstrong J, Herold M, Lu YJ, et al. EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox-dependent mechanism. Anticancer Drugs. 2005;16:263–275. doi: 10.1097/00001813-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 166.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 167.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 168.Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006;231:113–122. doi: 10.1016/j.canlet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 169.Gill C, Walsh SE, Morrissey C, Fitzpatrick JM, Watson RW. Resveratrol sensitizes androgen independent prostate cancer cells to death-receptor mediated apoptosis through multiple mechanisms. Prostate. 2007;67:1641–1653. doi: 10.1002/pros.20653. [DOI] [PubMed] [Google Scholar]
- 170.Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3′-kinase/Akt pathway and Bcl-2 family proteins. Mol Cancer Ther. 2006;5:1335–1341. doi: 10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- 171.Ma X, Tian X, Huang X, Yan F, Qiao D. Resveratrol-induced mitochondrial dysfunction and apoptosis are associated with Ca2+ and mCICR-mediated MPT activation in HepG2 cells. Mol Cell Biochem. 2007;302:99–109. doi: 10.1007/s11010-007-9431-8. [DOI] [PubMed] [Google Scholar]
- 172.Cao Y, Fu ZD, Wang F, Liu HY, Han R. Anti-angiogenic activity of resveratrol, a natural compound from medicinal plants. J Asian Nat Prod Res. 2005;7:205–213. doi: 10.1080/10286020410001690190. [DOI] [PubMed] [Google Scholar]
- 173.Gao X, Xu YX, Divine G, Janakiraman N, Chapman RA, et al. Disparate in vitro and in vivo antileukemic effects of resveratrol, a natural polyphenolic compound found in grapes. J Nutr. 2002;132:2076–2081. doi: 10.1093/jn/132.7.2076. [DOI] [PubMed] [Google Scholar]
- 174.Athar M, Back JH, Tang X, Kim KH, Kopelovich L, et al. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kimura Y, Okuda H. Resveratrol isolated from Polygonum cuspidatum root prevents tumor growth and metastasis to lung and tumor-induced neovascularization in Lewis lung carcinoma-bearing mice. J Nutr. 2001;131:1844–1849. doi: 10.1093/jn/131.6.1844. [DOI] [PubMed] [Google Scholar]
- 176.Li ZG, Hong T, Shimada Y, Komoto I, Kawabe A, et al. Suppression of N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by resveratrol. Carcinogenesis. 2002;23:1531–1536. doi: 10.1093/carcin/23.9.1531. [DOI] [PubMed] [Google Scholar]
- 177.Niles RM, Cook CP, Meadows GG, Fu YM, McLaughlin JL, et al. Resveratrol is rapidly metabolized in athymic (nu/nu) mice and does not inhibit human melanoma xenograft tumor growth. J Nutr. 2006;136:2542–2546. doi: 10.1093/jn/136.10.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Teskac K, Kristl J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int J Pharm. 2009 doi: 10.1016/j.ijpharm.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 179.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst. 1999;91:317–331. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- 180.Seren S, Lieberman R, Bayraktar UD, Heath E, Sahin K, et al. Lycopene in cancer prevention and treatment. Am J Ther. 2008;15:66–81. doi: 10.1097/MJT.0b013e31804c7120. [DOI] [PubMed] [Google Scholar]
- 181.Franceschi S, Bidoli E, La Vecchia C, Talamini R, D’Avanzo B, et al. Tomatoes and risk of digestive-tract cancers. Int J Cancer. 1994;59:181–184. doi: 10.1002/ijc.2910590207. [DOI] [PubMed] [Google Scholar]
- 182.Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002;94:391–398. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- 183.Wang AH, Zhang LS. Effect of lycopene on the proliferation of MCF-7 and MDA-MB-231 cells. Sichuan Da Xue Xue Bao Yi Xue Ban. 2007;38:958–960. 976. [PubMed] [Google Scholar]
- 184.Fornelli F, Leone A, Verdesca I, Minervini F, Zacheo G. The influence of lycopene on the proliferation of human breast cell line (MCF-7) Toxicol In Vitro. 2007;21:217–223. doi: 10.1016/j.tiv.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 185.Narisawa T, Fukaura Y, Hasebe M, Ito M, Aizawa R, et al. Inhibitory effects of natural carotenoids, alpha-carotene, beta-carotene, lycopene and lutein, on colonic aberrant crypt foci formation in rats. Cancer Lett. 1996;107:137–142. doi: 10.1016/0304-3835(96)04354-6. [DOI] [PubMed] [Google Scholar]
- 186.Conn PF, Schalch W, Truscott TG. The singlet oxygen and carotenoid interaction. J Photochem Photobiol B. 1991;11:41–47. doi: 10.1016/1011-1344(91)80266-k. [DOI] [PubMed] [Google Scholar]
- 187.Amir H, Karas M, Giat J, Danilenko M, Levy R, et al. Lycopene and 1,25-dihydroxyvitamin D3 cooperate in the inhibition of cell cycle progression and induction of differentiation in HL-60 leukemic cells. Nutr Cancer. 1999;33:105–112. doi: 10.1080/01635589909514756. [DOI] [PubMed] [Google Scholar]
- 188.Karas M, Amir H, Fishman D, Danilenko M, Segal S, et al. Lycopene interferes with cell cycle progression and insulin-like growth factor I signaling in mammary cancer cells. Nutr Cancer. 2000;36:101–111. doi: 10.1207/S15327914NC3601_14. [DOI] [PubMed] [Google Scholar]
- 189.Schwarz S, Obermuller-Jevic UC, Hellmis E, Koch W, Jacobi G, et al. Lycopene inhibits disease progression in patients with benign prostate hyperplasia. J Nutr. 2008;138:49–53. doi: 10.1093/jn/138.1.49. [DOI] [PubMed] [Google Scholar]
- 190.Kim HS, Bowen P, Chen L, Duncan C, Ghosh L, et al. Effects of tomato sauce consumption on apoptotic cell death in prostate benign hyperplasia and carcinoma. Nutr Cancer. 2003;47:40–47. doi: 10.1207/s15327914nc4701_5. [DOI] [PubMed] [Google Scholar]
- 191.Kucuk O, Sarkar FH, Sakr W, Djuric Z, Pollak MN, et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol Biomarkers Prev. 2001;10:861–868. [PubMed] [Google Scholar]
- 192.Vaishampayan U, Hussain M, Banerjee M, Seren S, Sarkar FH, et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007;59:1–7. doi: 10.1080/01635580701413934. [DOI] [PubMed] [Google Scholar]
- 193.Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem. 2001;49:3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 194.Shimoi K, Saka N, Kaji K, Nozawa R, Kinae N. Metabolic fate of luteolin and its functional activity at focal site. Biofactors. 2000;12:181–186. doi: 10.1002/biof.5520120129. [DOI] [PubMed] [Google Scholar]
- 195.Yang SF, Yang WE, Chang HR, Chu SC, Hsieh YS. Luteolin induces apoptosis in oral squamous cancer cells. J Dent Res. 2008;87:401–406. doi: 10.1177/154405910808700413. [DOI] [PubMed] [Google Scholar]
- 196.Ueda H, Yamazaki C, Yamazaki M. Inhibitory effect of Perilla leaf extract and luteolin on mouse skin tumor promotion. Biol Pharm Bull. 2003;26:560–563. doi: 10.1248/bpb.26.560. [DOI] [PubMed] [Google Scholar]
- 197.Zhang Q, Zhao XH, Wang ZJ. Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis. Food Chem Toxicol. 2008;46:2042–2053. doi: 10.1016/j.fct.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 198.Ju W, Wang X, Shi H, Chen W, Belinsky SA, et al. A critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor-kappaB pathway and sensitization of apoptosis in lung cancer cells. Mol Pharmacol. 2007;71:1381–1388. doi: 10.1124/mol.106.032185. [DOI] [PubMed] [Google Scholar]
- 199.Lim do Y, Jeong Y, Tyner AL, Park JH. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am J Physiol Gastrointest Liver Physiol. 2007;292:G66–75. doi: 10.1152/ajpgi.00248.2006. [DOI] [PubMed] [Google Scholar]
- 200.Selvendiran K, Koga H, Ueno T, Yoshida T, Maeyama M, et al. Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells. an implication for the antitumor potential of flavonoids. Cancer Res. 2006;66:4826–4834. doi: 10.1158/0008-5472.CAN-05-4062. [DOI] [PubMed] [Google Scholar]
- 201.Chiang CT, Way TD, Lin JK. Sensitizing HER2-overexpressing cancer cells to luteolin-induced apoptosis through suppressing p21(WAF1/CIP1) expression with rapamycin. Mol Cancer Ther. 2007;6:2127–2138. doi: 10.1158/1535-7163.MCT-07-0107. [DOI] [PubMed] [Google Scholar]
- 202.Zhou Q, Yan B, Hu X, Li XB, Zhang J, et al. Luteolin inhibits invasion of prostate cancer PC3 cells through E-cadherin. Mol Cancer Ther. 2009;8:1684–1691. doi: 10.1158/1535-7163.MCT-09-0191. [DOI] [PubMed] [Google Scholar]
- 203.Kim ND, Mehta R, Yu W, Neeman I, Livney T, et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res Treat. 2002;71:203–217. doi: 10.1023/a:1014405730585. [DOI] [PubMed] [Google Scholar]
- 204.Zhang Y, Vareed SK, Nair MG. Human tumor cell growth inhibition by nontoxic anthocyanidins, the pigments in fruits and vegetables. Life Sci. 2005;76:1465–1472. doi: 10.1016/j.lfs.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 205.Malik A, Afaq F, Sarfaraz S, Adhami VM, Syed DN, et al. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc Natl Acad Sci U S A. 2005;102:14813–14818. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hora JJ, Maydew ER, Lansky EP, Dwivedi C. Chemopreventive effects of pomegranate seed oil on skin tumor development in CD1 mice. J Med Food. 2003;6:157–161. doi: 10.1089/10966200360716553. [DOI] [PubMed] [Google Scholar]
- 207.Sartippour MR, Seeram NP, Rao JY, Moro A, Harris DM, et al. Ellagitannin-rich pomegranate extract inhibits angiogenesis in prostate cancer in vitro and in vivo. Int J Oncol. 2008;32:475–480. [PubMed] [Google Scholar]
- 208.Hebert JR, Hurley TG, Olendzki BC, Teas J, Ma Y, et al. Nutritional and socioeconomic factors in relation to prostate cancer mortality: a cross-national study. J Natl Cancer Inst. 1998;90:1637–1647. doi: 10.1093/jnci/90.21.1637. [DOI] [PubMed] [Google Scholar]
- 209.Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 210.Goodman MT, Wilkens LR, Hankin JH, Lyu LC, Wu AH, et al. Association of soy and fiber consumption with the risk of endometrial cancer. Am J Epidemiol. 1997;146:294–306. doi: 10.1093/oxfordjournals.aje.a009270. [DOI] [PubMed] [Google Scholar]
- 211.Jurenka JS. Therapeutic applications of pomegranate (Punica granatum L.): a review. Altern Med Rev. 2008;13:128–144. [PubMed] [Google Scholar]
- 212.Heber D. Multitargeted therapy of cancer by ellagitannins. Cancer Lett. 2008;269:262–268. doi: 10.1016/j.canlet.2008.03.043. [DOI] [PubMed] [Google Scholar]