Abstract
Staphylococcus aureus is a potent pathogen of humans exhibiting a broad disease range, in part, due to an extensive repertoire of secreted virulence factors, including proteases. Recently, we identified the first example of an intracellular protease (leucine aminopeptidase - LAP) that is required for virulence in S. aureus. Disruption of pepZ, the gene encoding LAP, had no affect on the growth rate of bacteria, however, in systemic and localized infection models the pepZ mutant was significantly attenuated in virulence. Recently, a contradictory report has been published, suggesting that LAP is an extracellular enzyme and it is required for growth in S. aureus. Here, we investigate these results and confirm our previous findings that LAP is localized to the bacterial cytosol and is not required for growth. In addition we conduct a biochemical investigation of purified recombinant LAP identifying optimal conditions for enzymatic activity and substrate preference for hydrolysis. Our results show that LAP has a broad substrate range, including activity against the dipeptide cysteine-glycine and that leucine is not the primary target of LAP.
Keywords: Leucine, aminopeptidase, M17, cysteinylglycinase, LAP, Staphylococcus
Introduction
Aminopeptidases are a class of proteolytic enzymes that selectively hydrolyse single amino acids or di-peptides from the N-termini of proteins. Best studied for their role in peptide metabolism and protein turnover, recently they have received increased attention as they are frequently linked to virulence in a number of protozoan and bacterial species. Of particular interest, in Plasmodium falciparum two aminopeptidases are involved in the final stages of hemoglobin degradation (Dalal & Klemba, 2007). Loss of aminopeptidase activity, due to the addition of inhibitors such as bestatin, interrupts the parasite life cycle, making this enzyme a promising target for anti-malaria drugs (Skinner-Adams et al., 2009). A concern is that aminopeptidases are ubiquitous in all domains of life. Therefore, in order to develop them further as viable drug targets, a thorough understanding of their mechanisms of action, in particular the differences that exist in specificity of host and pathogen aminopeptidases, is crucial.
Staphylococcus aureus is a versatile Gram-positive bacterial pathogen, and a major public health concern due to the rise of strains that are resistant to most front line antibiotics. As a result there is an urgent need for new approaches to combat and treat S. aureus infections. Recently, we demonstrated for the first time, an aminopeptidase that is required for virulence in a Gram-positive bacteria (Carroll et al., 2012). LAP is an M17 family metallo-aminopeptidase required for virulence in S. aureus. Strains of S. aureus containing a mutation in pepZ (the gene encoding LAP) demonstrated no growth defects, however were severely attenuated for virulence in a number of mouse infection models. Our studies showed expression of pepZ was induced in the host intracellular environment, and that LAP was localized to the bacterial cytosol.
Despite this demonstration that LAP contributes to S. aureus infection, the mechanism by which it contributes to pathogenesis is unknown. S. aureus secrets a number of extracellular proteases that are involved in virulence (Shaw et al., 2004, Kantyka et al., 2011). These proteases are known to contribute to disease casaution by targeting host and/or bacterial proteins in the extracellular milieu, however as an intracellular protease this is not the case for LAP. Rather, it is likely that LAP targets as yet unknown intracellular bacterial proteins, and in this way influences the infectious process. Identification of these intracellular targets of LAP will provide valuable insight into the mechanism of action of this protease, pathogenic mechanisms of S. aureus, and potentially aid in the design of inhibitor molecules as antibacterial therapeutics.
The M17 family of leucine-aminopeptidases were initially named because of their ability to hydrolyze leucine (Matsui et al., 2006). However, most members of this family exhibit a substrate range that is fairly broad and frequently includes the amino acids Met, Ala, Arg, Ile and others, in addition to Leu (Stack et al., 2007, Lin et al., 2004, Morty & Morehead, 2002, Dong et al., 2005). In addition to substrate variation, the conditions required for enzymatic activity can also differ. While in general LAPs are functional at pH 8–9, their activity in the presence of divalent metal ions tends to vary from strain to strain. Typically enzymatic activity is optimal with Mn2+ as a cofactor however frequently activity is also observed with Ni2+ and Co2+, and to a lesser extent with other metals (Jia et al., 2009, Morty & Morehead, 2002, Dong et al., 2005). To understand better the role of S. aureus LAP in infection, and aid in identifying intracellular targets, we undertook an in-depth investigation into the biochemical characteristics and substrate preference of the protease.
While our previous study was in review another investigation reported contradictory findings with regard to S. aureus LAP (Singh et al., 2012). Singh et al. reported that LAP was found in both intracellular protein fractions and extracellular culture supernatants, suggesting that it is secreted. In addition, they reported that inhibition of LAP with bestatin in the culture medium disrupted the ability of bacteria to form biofilms, and also slowed growth rates. In our study the disruption of pepZ (and loss of LAP) had no affect on bacterial viability in a number of different media tested.
Herein, we investigate further the localization of LAP and its role in growth and biofilm formation. We confirm our previous findings that LAP is an intracellular enzyme, and suggest an explanation for the contradictory results recently published. Additionally, to understand the biological function of LAP, we purify a recombinant form of the protein and conduct a biochemical characterization of the optimal reaction conditions and metal cofactors required for activity of the enzyme. We perform an investigation into the substrate preferences of LAP and reveal that it efficiently hydrolyzes a number of amino acids in addition to leucine and also cleaves the dipeptide cysteine-glycine, raising interesting possibilities regarding its role in vivo during the Staphylococcal infectious process.
Materials and Methods
Strains, plasmids, primers and growth conditions
Bacterial strains, plasmids and primers used throughout this study are listed in Table 1. Routine growth of S. aureus and E. coli was carried out as described previously in TSB and LB respectively, at 37 °C with shaking (Miller et al., 2012). Where indicated bestatin was added to cultures at a concentration of 50 μg ml−1. Antibiotics were used at the following concentrations for S. aureus; chloramphenicol 5 μg ml−1, erythromycin 5 μg ml−1, lincomycin 25 μg ml−1 and for E. coli; ampicillin 100 μg ml−1, kanamycin 50 μg ml−1, chloramphenicol 15 μg ml−1.
Table 1.
| Strain | Characteristics | Source |
|---|---|---|
| S. aureus | ||
| USA300 FPR | Sequenced USA300 FPR MRSA isolate cured of pUSA300-FPR-MRSA | (Diep et al., 2006) |
| LNS885 | USA300 FPR pepZ::pAZ106 pepZ− | (Carroll et al., 2012) |
| LNS1141 | LNS885 pTIF1100 pepZ+ complement | This work |
| LNS1143 | USA300 FPR pLES734 pepZ+ | (Carroll et al., 2012) |
| LNS1207 | USA300 FPR pMK4 | (Carroll et al., 2012) |
| NE2 | USA300 LAC JE2 | Nebraska transposon mutant library |
| NE444 | USA300 LAC JE2 pepS− Tn mutant | Nebraska transposon mutant library |
| NE652 | USA300 LAC JE2 pepZ− Tn mutant | Nebraska transposon mutant library |
| E. coli | ||
| DH5a | Routine cloning strain | Lab stocks |
| BL21 (DE3) pLysS | Protein expression strain | Promega |
| pRKC1281 | DH5a pRKC1281 | This work |
| pRKC1282 | BL21 pRKC1281 | This work |
| Plasmids | ||
| pMK4 | Shuttle vector cmR | (Sullivan et al., 1984) |
| pLES734 | pMK4 containing 1.8 kb pepZ-6his fragment | (Carroll et al., 2012) |
| pTIF1100 | pMK4 pepZ complement | This work |
| pET24d | C-terminal 6xHis tag expression vector | Novagen |
| pRKC1281 | pepZ gene cloned into pET24d | This work |
| Primers | ||
| OL558a | 5′-ATGACCATGGATTTTAAATTAAAT AACACACTAAGC-3′ | |
| OL559b | 5′-ATGACTCGAGATTGTTGTTTTAACC ATTGTAC-3′ | |
NcoI site underlined
XhoI site underlined
Leucine-AMC hydrolysis assay with bacterial lysates
Bacterial lysates were prepared as follows: Triplicate replicate cultures of each strain were synchronized and grown for 15 h. Bacterial cells were pelleted by centrifugation, resuspended in 500 μl PBS, and lysed using FastPrep lysing matrix B (MP Biomedicals). Following centrifugation, cleared bacterial lysates were removed and quantified using the Bradford assay (BioRad). Equal amounts of total bacterial protein were incubated with 0.2 mM leucine-AMC (Sigma) and fluorescence measured using a BioTek Synergy 2 spectrophotometer with an excitation wavelength of 360 nm and emission wavelength of 460 nm, following 60 mins incubation at 37 °C.
Purification of recombinant LAP
Recombinant LAP containing a C-terminal 6 histidine tag was generated using the pET24d plasmid (Novagen) as follows: The pepZ gene was amplified by PCR using primers OL558 and OL559, which contained restriction enzyme sites for NcoI and XhoI respectively. The resulting fragment was cloned into pET24d to create plasmid pRKC1281, which contains the pepZ gene followed by 6 histidine codons and a stop codon. Plasmid pRKC1281 was transformed into BL21 (DE3) pLysS and expression of LAP-His induced with 1 mM IPTG for 3 h. Purification of LAP-His was performed using Ni-NTA agarose beads (Qiagen) according to the manufacturers guidelines. Following elution, fractions containing LAP-His were pooled and dialyzed three times against PBS. Glycerol was added to a final concentration of 25% and aliquots stored at −20 °C until needed.
Western immunoblots
Western immunoblots were performed as described previously using strains LNS1143 and LNS1207 (Table 1 and (Carroll et al., 2012). LNS1143 expresses a plasmid encoded, histidine-tagged LAP while LNS1207 contains the empty vector control. Intracellular and extracellular protein fractions from LNS1143 and LNS1207 were collected following 15 h growth in TSB. Lysates containing intracellular protein fractions were prepared as outlined above. Extracellular protein fractions were prepared as described previously (Rivera et al., 2012), by concentrating culture supernatants 1000-fold using Amicon centrifugal filtration units with a 10 KDa molecular weight cut-off.
Biofilm assay
Biofilm assays were performed as described previously (Kolar et al., 2011).
Leucine-AMC hydrolysis assay
Hydrolysis assays were carried out in 200 μl volumes as follows: 20 pmoles of recombinant LAP was pre-incubated in buffer containing divalent metal cations as indicated for 15 min at 37 °C. Leu-AMC was added to a final concentration of 2 mM and reactions incubated in a BioTek Synergy 2 spectrophotometer at 37 °C for 60 mins. At 5 min intervals fluorescence was measured with an excitation wavelength of 360 nm and emission wavelength of 460 nm. Data presented is the average of three replicates and error bars represent standard deviation.
Cysteine-Glycine hydrolysis assay
Cysteine-glycine hydrolysis was measured using the method of Gaitonde to detect L-cysteine using the ninhydrin reagent (Gaitonde, 1967). Reactions were carried out in a 200 μl volume containing 20 pmoles of recombinant LAP, 40 mM TAPS buffer (pH as indicated), 0.2 mM divalent metal (as indicated) and 2 mM Cys-Gly (Sigma). LAP was pre-incubated at 37 °C for 15 mins prior to addition of the substrate. Reactions were incubated at 37 °C for 60 mins and stopped by the addition of 200 μl acetic acid. 200 μl of ninhydrin reagent was added (250 mg ninhydrin dissolved in 10 ml acetic acid/4 M HCl [3:2]) (Gaitonde, 1967) and reactions heated to 100 °C for 15 mins. Reactions were cooled on ice and used for spectrophotometric measurements at 560 nm. Duplicate reactions containing no LAP were used to establish and subtract background values. Data presented is the average of three replicates and error bars represent standard deviation.
Fluorogenic substrate fingerprint assay
Activity of LAP against a library of 60 fluorogenic substrates was performed as previously described (Poreba et al., 2012). This library is composed of 19 proteinogenic and 41 non-proteinogenic moieties coupled to the fluorophore 7-amino-4-carbamoylmethylcoumarin (ACC), using solid phase chemistry (Drag et al., 2010, Poreba et al., 2012). Briefly, 40 pmoles of LAP was incubated in 40 mM TAPS buffer pH 9.0 supplemented with 0.2 mM MnCl2 for 15 min at 37°C. Each substrate was added to a final concentration of 0.2 mM and the fluorescence released was recorded using a SpectraMax M5 spectrofluorimeter plate-reader (λexc = 380 nm; λem = 460 nm). The data presented is from duplicate samples with error bars representing standard deviation.
Determination of Km and kcat
The Km and kcat values for LAP in hydrolysis reactions with Arg-ACC, Leu-ACC, hArg-ACC, Phg-ACC and Cys-Gly were determined by performing hydrolysis assays with increasing concentrations of substrate while keeping the reaction conditions and enzyme concentration constant. Data was analyzed generating Km and kcat values using GraphPad Prism 6.
Results
LAP is an intracellular enzyme
In a previous study we reported that LAP was located intracellularly (Carroll et al., 2012). This result was in contrast to recent data published by Singh et al. where LAP was reported to be found in culture supernatants (Singh et al., 2012). To investigate the discrepancy in results between the two studies we repeated our western analysis. Intracellular and extracellular protein fractions from wild-type S. aureus USA300 expressing His-tagged LAP from a plasmid, and from USA300 containing an empty vector control, were used. As previously reported by us, LAP-his was only detected in the intracellular fraction from the strain expressing the LAP-his plasmid (Fig. 1A) (Carroll et al., 2012). No LAP-his was detected in the culture supernatant fraction from this strain, or from the intracellular/extracellular fractions from the strain containing the empty vector control. Interestingly, when the western blot was overexposed to x-ray film, a weak band appeared in both lanes corresponding to extracellular fractions (Fig. 1B). This band was of equal intensity in both samples, and ran at an apparent molecular weight similar, but slightly larger than, LAP-his. The empty vector control contained no LAP-his, therefore this band does not correspond to secreted LAP. Rather it is likely that this band represents protein A secreted into the culture supernatant. Protein A non-specifically binds antibodies, is approximately the same size as LAP (~54 kDa), and is frequently observed on western blots using S. aureus protein fractions. We suggest that the band observed by Singh et al. in the extracellular fractions of their western blots also corresponds to protein A, and that LAP is only found in the bacterial cytosol (Singh et al., 2012). This result confirms our previous findings that LAP is an intracellular protein and explains the discrepancy in results.
Figure 1. Western immunoblot analysis of LAP-his from intracellular and extracellular protein fractions.
A - Intracellular and extracellular protein fractions from S. aureus expressing histidine tagged LAP, or an empty vector control, were analyzed by western blot using an anti-histidine antibody. LAP-his is only detected in the intracellular fraction from strains containing the LAP-his plasmid. Purified recombinant LAP-his is included as a positive control. B - Overexposure of the western blot in panel A to x-ray film. A band similar in size to LAP-his is visible in the extracellular fractions in both the strain containing the LAP-his plasmid and the empty vector control.
Inhibition of LAP with bestatin does not adversely affect growth of S. aureus
Previously it was reported that inhibition of LAP using the protease inhibitor bestatin led to a decrease in growth rate of S. aureus in TSB (Singh et al., 2012). This result suggests that LAP is required for maximal growth in rich media, which our previous data suggested was not the case (Carroll et al., 2012). To investigate further the affect of LAP on the growth rate of bacteria, we repeated our growth analysis using wild type USA300, a USA300 pepZ mutant and the complemented pepZ strain. Growth was performed with and without bestatin at 50 μg ml−1, a concentration previously shown to impair growth of S. aureus (Singh et al., 2012). As previously observed by us, there was no difference in growth between the wild type and pepZ mutant (Fig. 2A). Furthermore addition of bestatin did not impact the growth of any strain. Together these data support that LAP is not required for growth in TSB, and that the addition of bestatin to culture medium does not affect bacterial growth.
Figure 2. LAP is not required for bacterial growth or biofilm formation.
A - Growth of S. aureus in the presence of bestatin. Wild type USA300, USA300 pepZ mutant and USA300 pepZ complement strains were grown in TSB with and without the aminopeptidase inhibitor bestatin. No difference in growth was observed between any of the strains in any condition tested. B - The wild-type, pepZ mutant and pepZ complemented strains of USA300 and SH1000 were used in biofilm formation assays. The ability of each wild type strain to form biofilms varied, however the biofilm formation capabilities of each strain were not affected in the pepZ mutant or pepZ complemented strains. Assays were performed in duplicate and a representative data set is shown.
LAP is not required for biofilm formation
Singh et al. reported that inhibition of LAP with bestatin led to a decrease in biofilm formation, suggesting a role for LAP in this process (Singh et al., 2012). Biofilms are complex multicellular structures and their formation is known to involve a number of bacterial proteases. Therefore, it is possible that the addition of a protease inhibitor may have pleiotropic affects, and that any differences observed may not be due to the targeted inhibition of one protease. To more specifically determine the role of LAP in biofilm formation we performed biofilm assays with wild type S. aureus, and its pepZ mutant and pepZ complemented strains. As different strains of S. aureus have different biofilm forming capabilities we performed these assays in both USA300 and in SH1000, a strain known to be proficient in biofilm formation (Kolar et al., 2011). Results show that in both backgrounds tested there was no difference in biofilm formation between the wild type, pepZ mutant and pepZ complemented strains (Fig. 2B and C). This indicates that LAP is not required for biofilm formation, and suggests that the defect in biofilm formation observed by Singh et al. upon addition of bestatin is due to pleiotropic affects of this inhibitor on bacterial cells.
LAP is not the primary leucine aminopeptidase of S. aureus
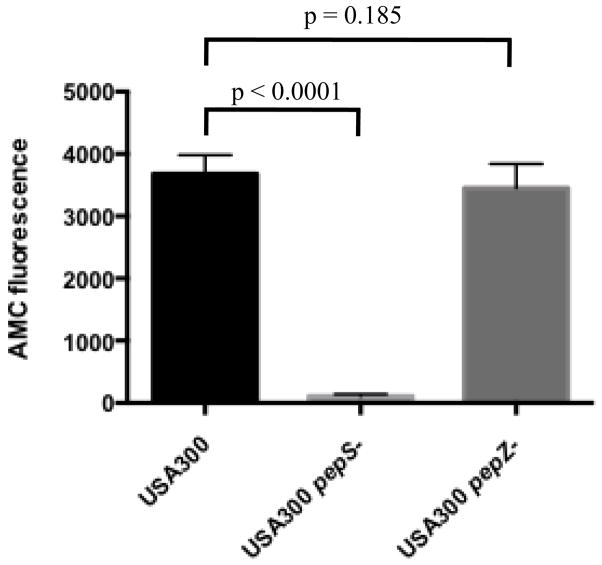
A previous study in our lab demonstrated that S. aureus LAP, an M17 family member, is required for virulence in numerous mouse models of infection (Carroll et al., 2012). To begin to understand how LAP contributes to pathogenesis we investigated the enzymatic properties of this enzyme, beginning with its leucine aminopeptidase activity. Interestingly, there is not one, but two leucine aminopeptidases encoded in the S. aureus genome, LAP and another enzyme referred to as PepS (SACOL1937). PepS is an uncharacterized M29 family member leucine aminopeptidase in S. aureus, whose crystal structure has been solved (Odintsov et al., 2005). The presence of two leucine aminopeptidases in the S. aureus genome suggests that either (i) there is functional redundancy between the two enzymes, or (ii) one or both of these proteases has substrates in addition to leucine. M17 family members are frequently active against a broad range of substrates in addition to leucine, while M29 family members tend to have a narrower substrate range. To test the relative contribution of each enzyme to cellular leucine aminopeptidase activity we performed a series of leucine-AMC hydrolysis assays using whole cell lysates from an S. aureus wild type strain, or its pepZ and pepS mutant derivatives. Equal quantities of whole cell lysates were incubated with leucine-AMC, and subsequent AMC release as a result of cleavage was determined (excitation wavelength 360 nm, emission wavelength 460 nm). Following 60 min incubation, lysates from wild type S. aureus demonstrated high leucine aminopeptidase activity (Fig. 3). In contrast, lysates from pepS mutant cells demonstrated a dramatic reduction in leucine aminopeptidase activity (33-fold decrease compared to the wild type). Interestingly, lysates from pepZ mutant cells showed leucine aminopeptidase activity comparable to that of the wild type strain. These results suggest that the majority of leucine aminopeptidase activity observed in S. aureus cells comes from PepS, and not LAP. Although the pepS lysates demonstrate greatly reduced leucine aminopeptidase activity, some residual proteolysis was still observed in these samples. While it is possible that this activity is due to LAP, these results strongly suggest that cleavage of leucine is not the primary function of LAP within the cell.
Figure 3. Leucine aminopeptidase activity of S. aureus bacterial lysates.
Whole cell lysates from wild type USA300, USA300 pepS and USA300 pepZ mutant strains were used in leucine-AMC hydrolysis assays. Experiments were performed in triplicate and the data shown is the average of three independent experiments. Error bars represent standard deviation. Significance was determined by t-test.
Optimization of LAP enzymatic reaction conditions
Given that the data above suggests that LAP likely has a substrate range beyond leucine, we next set out to explore the cleavage preference of this enzyme. To do this we first identified the optimal reaction conditions required for enzymatic activity. To do this we used leucine-AMC, a substrate that is known to be cleaved by M17 family members, and previously shown to be a substrate for LAP (Singh et al., 2012). Incubating 0.5 mM leucine-AMC with increasing concentrations of purified recombinant LAP-his resulted in a concentration dependent increase in fluorescence over time, as expected for an enzymatic reaction (Fig. 4A). This result confirms that leucine-AMC is hydrolyzed by LAP and that the reaction occurs in a dose-dependent manner.
Figure 4. Hydrolysis of Leu-AMC by recombinant LAP.
A - Purified recombinant LAP-his at increasing concentrations (50 nm - ●; 100 nm - ○; 200 nm - ■; 500 nm - □) was incubated with Leu-AMC and the hydrolysis reaction measured by AMC fluorescence over 35 mins. B - Leu-AMC hydrolysis assays were performed with MnCl2 concentrations in the range 0–10 mM. C - Leu-AMC hydrolysis assays were performed in three different buffers at pH 9. Activity of LAP in these buffers was compared to activity in phosphate buffer at pH 7.4 and is expressed as fold increase.
M17 family aminopeptidases require divalent metal ions for proteolysis (Matsui et al., 2006). For most family members activity is highest with Mn2+ while Ni2+ and Co2+ also promote activity, albeit to a lesser degree (Singh et al., 2012, Chu et al., 2008, Dong et al., 2005). To further optimize reaction conditions for LAP we determined the optimum concentration of MnCl2 required for enzymatic activity. The leucine-AMC hydrolysis assay was performed with a range of concentrations of MnCl2 from 0 to 10 mM. Results show an approx 7-fold increase in activity when the concentration of MnCl2 is increased from 0 to 0.2 mM (Fig. 4B). Activity remains high at MnCl2 concentrations of 0.2 to 0.4 mM and thereafter decreases as the concentration increases. These results show a clear concentration dependent activity of LAP in the presence of MnCl2. Based on this, all subsequent assays were performed with divalent metal ions concentrations of 0.2 mM.
We next performed the leucine-AMC hydrolysis assay in a variety of different buffers to determine the optimal conditions for activity. As M17 family members frequently demonstrate higher activity at pH 8–10 we compared activity of LAP in three buffers at pH 9 to activity in Na-phosphate buffer at pH 7.4. Results show higher activity for LAP in all three buffers at pH 9, with highest levels of activity observed in CAPS and TAPS (Fig. 4C). Due to the greater buffering capacity of TAPS at pH 9, all subsequent enzymatic analyses were performed in this buffer.
Metal and pH activity profiling of LAP
Having determined the optimal reaction conditions for the leucine-AMC hydrolysis assay we next set out to comprehensively determine the divalent metal ion and pH preference of LAP. Previous studies on this enzyme have reported high activity in the presence of Mn2+, Ni2+ and Co2+ at pH 8.5 (Singh et al., 2012). However, this study only examined pH preference in the presence of MnCl2 and divalent metal ion preference at pH 8.5, using a concentration of divalent metal ions (1 mM) found to be sub-optimum in our assays (Fig 4B).
To gain a comprehensive metal/pH activity profile for LAP we performed the leucine-AMC hydrolysis assay in the presence of 7 different divalent ions (Mn2+, Zn2+, Ni2+, Co2+, Cd2+, Ca2+, and Mg2+), and at 4 different pH conditions (pH 7, 8, 9, 10). Assays performed in the presence of Zn2+, Co2+, Ca2+ or Mg2+ demonstrated no activity at any of the pH conditions tested (Fig. 5), suggesting that LAP is not functional in the presence of these metal ions. In contrast activity was observed in the presence of Mn2+, Ni2+ and Cd2+. The pH preference of LAP in the presence of Mn2+ and Cd2+ was similar, with maximal activity around pH 9, while activity in the presence of Ni2+ was maximal at pH 7. These results show that LAP is functional in the presence of multiple divalent ions and at multiple pH conditions. Interestingly the presence of different divalent ions can influence the pH at which the enzyme is most active.
Figure 5. pH and metal ion preference for LAP in Leu-AMC hydrolysis assays.
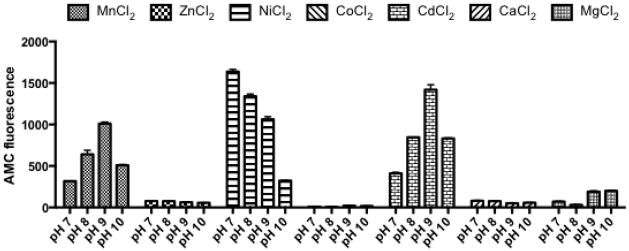
Leu-AMC hydrolysis assays were performed at pH 7, 8, 9 and 10 in the presence of seven different divalent metal cations (Mn2+, Zn2+, Ni2+, Co2+, Cd2+, Ca2+, and Mg2+) to determine the optimal metal/pH conditions for LAP activity. Hydrolysis of Leu-AMC by recombinant LAP-his was measured by increase in AMC fluorescence over 60 mins. Data shown is the average from three replicates with error bars representing standard deviation.
LAP exhibits a broad substrate range
The result above (Fig. 3), suggests that leucine is not the primary substrate of LAP. This is consistent with other M17 family aminopeptidases (Chu et al., 2008). To investigate further the substrate range of LAP we performed hydrolysis assays using a unique library of 60 fluorogenic substrates derived from both naturally occurring and unnatural amino acids (Poreba et al., 2012). This recently described technique generates an enzyme specific “substrate fingerprint”. Upon analysis we determined that LAP has a rather broad, yet varying, substrate range amongst the naturally occurring amino acids (Fig. 6A). Low levels of activity were observed against Thr and Trp, while higher levels were observed against Leu, Lys and Met. Interestingly, the highest levels of activity demonstrated by LAP in this assay were not against Leu, but rather activity against Ala and Arg (approx two-fold higher than for Leu). This demonstrates that the substrate range of LAP is quite complex, and that Leu is not the preferred substrate under the conditions tested.
Figure 6. Profiling the substrate specificity of LAP.
The specificity of LAP was assayed using a collection of 60 fluorogenic substrates. The range in size and diversity of these synthetic compounds represents a large majority of possible substrate specificities for aminopeptidase enzymes. A - Activity of LAP against naturally occurring amino acids. B - Activity of LAP against unnatural amino acids.
Hydrolysis by LAP was also observed for a large number of unnatural amino acids. Highest levels of activity were detected against L-hArg and L-Phg (Fig. 6B). The activity against these substrates was higher than that observed against the naturally occurring amino acids Ala and Arg (approx 1.5-fold higher) and Leu (approx 3.5-fold higher). High levels of activity were also observed against L-hPhe, 4-NH2-L-Phe, L-hLeu, L-hCha and L-Nle. No activity was observed against substrates without α-amines as previously shown for aminopeptidases (Kasperkiewicz et al., 2012).
The results above suggest the substrate preference of LAP in these assays is hArg/Phg > Ala/Arg > Leu/Lys/Met. To confirm this result we determined the kcat/Km ratio for hArg, Phg, Arg and Leu (Table 2). Results from the kinetic analysis confirm the data from the initial screen. Of the two naturally occurring amino acids selected, LAP shows a higher preference for Arg than Leu (176.58 M−1s−1 compared to 87.25 M−1s−1). Activity against the unnatural amino acids hArg and Phg was higher than activity against either Leu or Ala (as previously shown – Fig 6) with Phg being the preferred substrate of the four tested.
Table 2.
| Substrate | Km | kcat | kcat/Km |
|---|---|---|---|
| mM | s−1 | M−1s−1 | |
| Leu-ACC | 1.38 | 0.1204 | 87.25 |
| Arg-ACC | 0.79 | 0.1395 | 176.58 |
| hArg-ACC | 0.82 | 0.1584 | 193.17 |
| Phg-ACC | 1.33 | 0.3952 | 297.14 |
| Cys-Gly | 1.64 | 0.8758 | 534.02 |
LAP possesses cysteinylglycinase activity
Many M17 family members have been shown to demonstrate cysteinylglycinase activity in addition to leucine aminopeptidase activity. Indeed for some M17 members, Cys-Gly is the preferred substrate for the enzyme (Chu et al., 2008). Previously, homology studies have suggested that LAP is a potential cysteinylglycinase in S. aureus (Soutourina et al., 2009). This, in addition to the results above (suggesting leucine aminopeptidase activity is not the primary function of LAP (Fig 3)), led us to hypothesize that LAP may be a cysteinylglycinase. To test this hypothesis we next preformed a cysteine-glycine hydrolysis assay. Our results with the leucine-AMC hydrolysis assay show that LAP has different activity at different pH/metal conditions; therefore we performed the cysteine-glycine hydrolysis assay using the same range of metals and pH conditions employed earlier. In contrast to the results from leucine-AMC hydrolysis, where multiple metal/pH combinations resulted in LAP activity, only one condition tested resulted in cysteine-glycine activity (Fig 7). In the presence of MnCl2 at pH 7 LAP demonstrated strong activity towards the Cys-Gly substrate. LAP was not functional against cysteine-glycine under any of the other conditions tested.
Figure 7. LAP has Cysteine-Glycinase activity.

Cys-Gly hydrolysis assays were performed at pH 7, 8, 9 and 10 in the presence of seven divalent cations (Mn2+, Zn2+, Ni2+, Co2+, Cd2+, Ca2+, and Mg2+). Cys-Gly hydrolysis was measured by the release of free cysteine using the ninhydrin reagent measured at OD560. Data shown is the average from three replicates with error bars representing standard deviation.
We next determined the kinetic parameters for cysteine-glycine activity. The kcat/Km ratio against Cys-Gly is 534.02 M−1s− which is higher that the kcat/Km ratio for any other substrate tested (Table 2). Although the kinetic parameters for cysteine-glycine activity were determined under different reaction conditions than the four other substrates tested (i.e. pH 7 versus pH 9) these results suggest that the highest levels of LAP activity are against Cys-Gly. Together these results show that LAP is functional as a cysteinylglycinase, that the conditions required for LAP cysteinylglycinase activity are more stringent than for leucine aminopeptidase activity, and that Cys-Gly may be the preferred substrate for LAP.
Discussion
S. aureus is a highly versatile pathogen of humans and is a major public health concern due to the continued emergence of drug resistant strains. An in-depth understanding of the organism and its virulence mechanisms is needed to design effective therapies and treatments to combat the rise in antibiotic resistance. Recently we identified an intracellular leucine aminopeptidase that is required for virulence in S. aureus (Carroll et al., 2012). In this study we set out to gain an insight into the in vivo targets of LAP by determining the substrate preference of the purified recombinant protein. In addition to this, to address the contradictory findings of Singh et al. with regard to the localization and function of LAP, we examined the discrepancy in results between the two studies (Singh et al., 2012). Our analyses confirmed that LAP is localized to the bacterial cytosol and demonstrated that LAP is not required for biofilm formation in S. aureus.
While our previous study demonstrated that LAP is required for virulence in S. aureus, the specific mechanism with which it contributes to pathogenesis is not known. Expression of the pepZ gene is induced inside macrophages therefore we hypothesize that LAP is required during infection for N-terminal hydrolysis of some as yet unknown intracellular target (Carroll et al., 2012) In the absence of LAP the loss of aminopeptidase activity towards such a substrate leads to the altered virulence phenotypes observed. To investigate this hypothesis we began by purifying a recombinant form of LAP and determining its optimal reaction conditions and substrate specificity profile in vitro. This substrate specificity profile will help in future investigations into the cellular target of LAP.
Previous studies have only examined a limited number of pH/metal combinations and determined that LAPs leucine aminopeptidase activity is optimal at pH 8.5 in the presence of Mn2+, Ni2+, and Co2+. We performed a broad survey of LAP activity under 4 pH conditions and 7 metals ions. We determined that LAP was active in the presence of Mn2+, Ni2+ and Cd2+ but not with Zn2+, Co2+, Ca2+ or Mg2+. Singh et al. also found LAP to be active in the presence of Mn2+ and Ni2+, however unlike this study they also demonstrated activity with Co2+. The differences in buffer composition and metal ion concentration used may account for these discrepancies. Interestingly, we also demonstrated activity of LAP in the presence of Cd2+. To our knowledge examples of M17 family members activated by Cd2+ are rare with the only other example the PepB protein from E. coli. (Suzuki et al., 2001). It will be interesting to determine if Cd2+ activation is a prokaryotic-specific trend amongst M17 family members.
One interesting observation from our pH/metal analysis was that activity of LAP in the presence of a particular metal varies according to the pH. For example, in the presence of Mn2+ LAP activity is maximal at pH 9 while in the presence of Ni2+ optimal activity was observed at pH 7. These results raise the intriguing prospect that the activity of LAP may be modified by interacting with different metal ions. This idea is supported by previous studies where the introduction of Mn2+ into site 1 of bovine lens LAP causes the enzyme to be active against Cys-Gly (see discussion below) (Cappiello et al., 2006, Cappiello et al., 2004).
Having determined the optimal conditions for LAP activity we next investigated the substrate specificity profile of LAP using a library of 60 fluorogenic substrates. This library consists of 19 naturally occurring amino acids (cysteine is absent due to its susceptibility to oxidation) and 41 unnatural amino acids coupled to the fluorophore 7-amino-4-carbamoylmethylcoumarin (ACC). Among the 19 natural amino acids Leu was not the preferred substrate of LAP. Ala and Arg were the preferred substrates with Leu, Lys and Met demonstrating slightly lower affinity. Low levels of activity were also observed against Thr and Trp. Interestingly, this profile among the naturally occurring amino acids very closely resembles that of the Plasmodium falciparum M1 family aminopeptidase (Poreba et al., 2012). These results demonstrate that LAP, like many other M17 family members, has a broad substrate range and show that Leu is not the primary substrate of LAP. This conclusion is further confirmed by the result of the Leu-AMC hydrolysis assay using bacterial lysates. Lysates from pepZ mutant bacteria demonstrated leucine aminopeptidase activity comparable with wild type lysates. In contrast lysates from pepS mutant cells had significantly lower levels of activity. Together these results suggest that PepS is the primary leucine aminopeptidase of S. aureus and that LAP is active against alternative substrates, most likely Ala and Arg.
An examination of the unnatural amino acid substrates that are hydrolysed by LAP reveals some interesting information regarding substrate preference. As expected for an aminopeptidase, substrates lacking an α-amine group were not hydrolyzed. In addition the substrates most preferred by LAP, in general, contain large hydrophobic side chains (hPhe, hArg, Phg, hCha and Nle). This is similar to results found with human aminopeptidases and suggest that the substrate binding pocket is relatively large and can accommodate bulky sidechains (Kasperkiewicz et al., 2012).
Bovine lens LAP (blLAP) represents one of the best-studied LAP family members. It has been shown to contain two Zn2+ ions in the active site of the enzyme, which are fundamental for activity (Kim & Lipscomb, 1993). The Zn2+ ion at site 2 binds strongly, however the Zn2+ ion at site 1 can be readily exchanged for other divalent metal ions such as Mn2+, Mg2+ or Co2+. Previously it has been show that introduction of Mn2+ into site 1 alters the activity of blLAP, making it active against the dipeptide Cys-Gly (Cappiello et al., 2006, Cappiello et al., 2004). M17 family members from prokaryotes have also been shown to demonstrate cysteinylglycinase activity, and in some cases cysteinylglycinase activity is greater than leucine aminopeptidase activity (Chu et al., 2008, Suzuki et al., 2001).
We investigated the cysteinylglycinase activity of S. aureus LAP using a Cys-Gly hydrolysis assay. Results demonstrated that LAP has the ability cleave Cys-Gly however only under very specific conditions i.e. with MnCl2 at pH 7. Cysteinylglycinase activity was not detected in the presence of metal ions other than Mn2+ suggesting that similarly to blLAP, occupancy of site 1 with Mn2+ in LAP may activate the cysteinylglycinase activity of this enzyme. In contrast the activity of LAP against Leu and other amino acids is evident under a range of different pH and metal conditions.
The activity of LAP against Cys-Gly raises interesting possibilities with regard to its role and potential targets in vivo. Cysteine containing molecules such as glutathione (L-γ-glutamyl-L-cysteinylglycine) are important in cellular defense against low pH, oxidative and osmotic stress; and sulfur metabolism has been shown to influence virulence (Masip et al., 2006, Soutourina et al., 2009). In addition, certain bacterial species secrete glutathione from the cell during exponential growth and subsequently utilize it as a cysteine and glycine source during nutrient limitation (Suzuki et al., 1993). Although S. aureus does not produce glutathione the low-molecular mass thiol bacillithiol (BSH, Cys-GlcN-mal) is thought to substitute for glutathione in this and other low-GC Gram-positive bacteria (Newton et al., 2009). The central role of low-molecular mass thiols in cellular physiology, and the demonstrated cysteinylglycinase activity of LAP suggests that a role in cellular cysteine metabolism may be the mechanism with which LAP influences S. aureus virulence.
The biological significance of an aminopeptidase that can hydrolyze different substrates depending on pH and metal cofactor is intriguing and remains to be fully determined. Previously it has been reported that the intracellular pH of S. aureus cells is pH 7.4 to 7.6 during growth under standard laboratory conditions (Kashket, 1981). Many bacteria (including S. aureus) can tolerate a wide range of extracellular pH conditions but while the intracellular pH can vary, it is unlikely that it undergoes such radical changes in pH (Booth, 1985, Cotter & Hill, 2003). It is tempting to speculate that under certain conditions encountered by the bacteria during infection, the cytosolic pH could shift slightly towards either pH 7 or pH 8 and that this pH shift could influence the activity of LAP by altering its substrate preference. Likewise the in vivo availability of transition metals, which is tightly regulated by both the host and bacteria, could result in altered enzymatic activity under certain conditions (Hood & Skaar, 2012). Sequestration of Mn2+ and Zn2+ by host calprotectin has been demonstrated to adversely affect S. aureus virulence by disrupting activity of bacterial superoxide dismuthase (Kehl-Fie et al., 2011). The broad metal cofactor profile of LAP may allow activity of this peptidase under in vivo conditions where Mn2+ and Zn2+ are limited.
In summary, this study clarifies certain inconsistencies regarding recent contradictory results and generates new insight into the substrate preference and reaction conditions required for LAP activity. We have conclusively demonstrated that LAP is located within the bacterial cell and identified the amino acids for which LAP has highest affinity. The results presented in this study expand our knowledge of LAP and lay the foundation for further investigations to identify its cellular target and determine how it influences S. aureus pathogenesis.
Acknowledgments
This study was supported in part by grant AI080626 (LNS) and DE09761 (JP) from the National Institutes of Health, and by grants to JP from the National Science Center (2011/01/B/NZ6/00268, Kraków, Poland), and the Foundation for Polish Science (TEAM project DPS/424-329/10). The Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University is a beneficiary of structural funds from the European Union (POIG.02.01.00-12-064/08)
References
- Booth IR. Regulation of cytoplasmic pH in bacteria. Microbiological reviews. 1985;49:359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappiello M, Alterio V, Amodeo P, Del Corso A, Scaloni A, Pedone C, Moschini R, De Donatis GM, De Simone G, Mura U. Metal ion substitution in the catalytic site greatly affects the binding of sulfhydryl-containing compounds to leucyl aminopeptidase. Biochemistry. 2006;45:3226–3234. doi: 10.1021/bi052069v. [DOI] [PubMed] [Google Scholar]
- Cappiello M, Lazzarotti A, Buono F, Scaloni A, D’Ambrosio C, Amodeo P, Mendez BL, Pelosi P, Del Corso A, Mura U. New role for leucyl aminopeptidase in glutathione turnover. Biochem J. 2004;378:35–44. doi: 10.1042/BJ20031336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RK, Robison TM, Rivera FE, Davenport JE, Jonsson IM, Florczyk D, Tarkowski A, Potempa J, Koziel J, Shaw LN. Identification of an intracellular M17 family leucine aminopeptidase that is required for virulence in Staphylococcus aureus. Microbes Infect. 2012;14:989–999. doi: 10.1016/j.micinf.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu L, Lai Y, Xu X, Eddy S, Yang S, Song L, Kolodrubetz D. A 52-kDa leucyl aminopeptidase from treponema denticola is a cysteinylglycinase that mediates the second step of glutathione metabolism. J Biol Chem. 2008;283:19351–19358. doi: 10.1074/jbc.M801034200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter PD, Hill C. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiology and molecular biology reviews : MMBR. 2003;67:429–453. doi: 10.1128/MMBR.67.3.429-453.2003. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal S, Klemba M. Roles for two aminopeptidases in vacuolar hemoglobin catabolism in Plasmodium falciparum. J Biol Chem. 2007;282:35978–35987. doi: 10.1074/jbc.M703643200. [DOI] [PubMed] [Google Scholar]
- Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- Dong L, Cheng N, Wang MW, Zhang J, Shu C, Zhu DX. The leucyl aminopeptidase from Helicobacter pylori is an allosteric enzyme. Microbiology. 2005;151:2017–2023. doi: 10.1099/mic.0.27767-0. [DOI] [PubMed] [Google Scholar]
- Drag M, Bogyo M, Ellman JA, Salvesen GS. Aminopeptidase fingerprints, an integrated approach for identification of good substrates and optimal inhibitors. J Biol Chem. 2010;285:3310–3318. doi: 10.1074/jbc.M109.060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitonde MK. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J. 1967;104:627–633. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nature reviews. Microbiology. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Terkawi MA, Aboge GO, Goo YK, Luo Y, Li Y, Yamagishi J, Nishikawa Y, Igarashi I, Sugimoto C, Fujisaki K, Xuan X. Characterization of a leucine aminopeptidase of Babesia gibsoni. Parasitology. 2009;136:945–952. doi: 10.1017/S0031182009006398. [DOI] [PubMed] [Google Scholar]
- Kantyka T, Shaw LN, Potempa J. Papain-like proteases of Staphylococcus aureus. Adv Exp Med Biol. 2011;712:1–14. doi: 10.1007/978-1-4419-8414-2_1. [DOI] [PubMed] [Google Scholar]
- Kashket ER. Proton motive force in growing Streptococcus lactis and Staphylococcus aureus cells under aerobic and anaerobic conditions. J Bacteriol. 1981;146:369–376. doi: 10.1128/jb.146.1.369-376.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperkiewicz P, Gajda AD, Drag M. Current and prospective applications of non-proteinogenic amino acids in profiling of proteases substrate specificity. Biol Chem. 2012;393:843–851. doi: 10.1515/hsz-2012-0167. [DOI] [PubMed] [Google Scholar]
- Kehl-Fie TE, Chitayat S, Hood MI, Damo S, Restrepo N, Garcia C, Munro KA, Chazin WJ, Skaar EP. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell host & microbe. 2011;10:158–164. doi: 10.1016/j.chom.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lipscomb WN. Differentiation and identification of the two catalytic metal binding sites in bovine lens leucine aminopeptidase by x-ray crystallography. Proc Natl Acad Sci U S A. 1993;90:5006–5010. doi: 10.1073/pnas.90.11.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar SL, Nagarajan V, Oszmiana A, Rivera FE, Miller HK, Davenport JE, Riordan JT, Potempa J, Barber DS, Koziel J, Elasri MO, Shaw LN. NsaRS is a cell-envelope-stress-sensing two-component system of Staphylococcus aureus. Microbiology. 2011;157:2206–2219. doi: 10.1099/mic.0.049692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LL, Hsu WH, Wu CP, Chi MC, Chou WM, Hu HY. A thermostable leucine aminopeptidase from Bacillus kaustophilus CCRC 11223. Extremophiles. 2004;8:79–87. doi: 10.1007/s00792-003-0364-1. [DOI] [PubMed] [Google Scholar]
- Masip L, Veeravalli K, Georgiou G. The many faces of glutathione in bacteria. Antioxidants & redox signaling. 2006;8:753–762. doi: 10.1089/ars.2006.8.753. [DOI] [PubMed] [Google Scholar]
- Matsui M, Fowler JH, Walling LL. Leucine aminopeptidases: diversity in structure and function. Biol Chem. 2006;387:1535–1544. doi: 10.1515/BC.2006.191. [DOI] [PubMed] [Google Scholar]
- Miller HK, Carroll RK, Burda WN, Krute CN, Davenport JE, Shaw LN. The extracytoplasmic function sigma factor sigmaS protects against both intracellular and extracytoplasmic stresses in Staphylococcus aureus. J Bacteriol. 2012;194:4342–4354. doi: 10.1128/JB.00484-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morty RE, Morehead J. Cloning and characterization of a leucyl aminopeptidase from three pathogenic Leishmania species. J Biol Chem. 2002;277:26057–26065. doi: 10.1074/jbc.M202779200. [DOI] [PubMed] [Google Scholar]
- Newton GL, Rawat M, La Clair JJ, Jothivasan VK, Budiarto T, Hamilton CJ, Claiborne A, Helmann JD, Fahey RC. Bacillithiol is an antioxidant thiol produced in Bacilli. Nature chemical biology. 2009;5:625–627. doi: 10.1038/nchembio.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odintsov SG, Sabala I, Bourenkov G, Rybin V, Bochtler M. Staphylococcus aureus aminopeptidase S is a founding member of a new peptidase clan. J Biol Chem. 2005;280:27792–27799. doi: 10.1074/jbc.M502023200. [DOI] [PubMed] [Google Scholar]
- Poreba M, McGowan S, Skinner-Adams TS, Trenholme KR, Gardiner DL, Whisstock JC, To J, Salvesen GS, Dalton JP, Drag M. Fingerprinting the substrate specificity of M1 and M17 aminopeptidases of human malaria, Plasmodium falciparum. PLoS One. 2012;7:e31938. doi: 10.1371/journal.pone.0031938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera FE, Miller HK, Kolar SL, Stevens SM, Jr, Shaw LN. The impact of CodY on virulence determinant production in community-associated methicillin-resistant Staphylococcus aureus. Proteomics. 2012;12:263–268. doi: 10.1002/pmic.201100298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L, Golonka E, Potempa J, Foster SJ. The role and regulation of the extracellular proteases of Staphylococcus aureus. Microbiology. 2004;150:217–228. doi: 10.1099/mic.0.26634-0. [DOI] [PubMed] [Google Scholar]
- Singh AK, Singh R, Tomar D, Pandya CD, Singh R. The leucine aminopeptidase of Staphylococcus aureus is secreted and contributes to biofilm formation. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2012;16:e375–381. doi: 10.1016/j.ijid.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Skinner-Adams TS, Stack CM, Trenholme KR, Brown CL, Grembecka J, Lowther J, Mucha A, Drag M, Kafarski P, McGowan S, Whisstock JC, Gardiner DL, Dalton JP. Plasmodium falciparum neutral aminopeptidases: new targets for anti-malarials. Trends Biochem Sci. 2009;35:53–61. doi: 10.1016/j.tibs.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Soutourina O, Poupel O, Coppee JY, Danchin A, Msadek T, Martin-Verstraete I. CymR, the master regulator of cysteine metabolism in Staphylococcus aureus, controls host sulphur source utilization and plays a role in biofilm formation. Mol Microbiol. 2009;73:194–211. doi: 10.1111/j.1365-2958.2009.06760.x. [DOI] [PubMed] [Google Scholar]
- Stack CM, Lowther J, Cunningham E, Donnelly S, Gardiner DL, Trenholme KR, Skinner-Adams TS, Teuscher F, Grembecka J, Mucha A, Kafarski P, Lua L, Bell A, Dalton JP. Characterization of the Plasmodium falciparum M17 leucyl aminopeptidase. A protease involved in amino acid regulation with potential for antimalarial drug development. J Biol Chem. 2007;282:2069–2080. doi: 10.1074/jbc.M609251200. [DOI] [PubMed] [Google Scholar]
- Sullivan MA, Yasbin RE, Young FE. New shuttle vectors for Bacillus subtilis and Escherichia coli which allow rapid detection of inserted fragments. Gene. 1984;29:21–26. doi: 10.1016/0378-1119(84)90161-6. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Hashimoto W, Kumagai H. Escherichia coli K-12 can utilize an exogenous gamma-glutamyl peptide as an amino acid source, for which gamma-glutamyltranspeptidase is essential. J Bacteriol. 1993;175:6038–6040. doi: 10.1128/jb.175.18.6038-6040.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Kamatani S, Kumagai H. Purification and characterization of aminopeptidase B from Escherichia coli K-12. Bioscience, biotechnology, and biochemistry. 2001;65:1549–1558. doi: 10.1271/bbb.65.1549. [DOI] [PubMed] [Google Scholar]