Abstract
Antimicrobial peptides represent an important aspect of the innate defense system that contributes to the control of bacterial colonization and infection. As studies have progressed it has become clear that antimicrobial peptides manifest other functions in addition to their antimicrobial effects. These functions include chemotaxis of numerous types of host cells involved in both the innate and adaptive response. In this review the antimicrobial activity, regulation, and the contribution to host homeostasis of α-defensins and LL-37 as well as β-defensins are discussed in context of their specific tissue locations in the junctional and oral epithelium respectively.
In the oral cavity, gingivitis and periodontitis are one of the most common infections known to humans. The oral cavity is a unique environment where hard tissues protrude through soft tissue in a moist environment, containing niches for both commensal and pathogenic bacteria. The epithelial lining, which was once thought to be a passive physical barrier, is now being recognized for its active role in innate host defense (16; 19). For example, antimicrobial peptides contribute to the protection of host tissue from dental plaque, a polymicrobial biofilm, residing around the tooth and root surface (16; 36). The original discovery and research of the defensins and LL-37, two different types of antimicrobial peptides; revolved around their anti-bacterial properties yet, recent studies suggest that these antimicrobial peptides also contribute to host defense and homeostasis by recruiting immune cells in times of health and disease.
Indeed, a shift has occurred from the study of the antimicrobial properties of antimicrobial peptides, specifically defensins and LL-37, to a focus on the interaction with host immune functions (20; 49; 75). A common approach to study the effect(s) of antimicrobial peptides involves non-invasive methods to measure activity in fluids such as saliva and gingival crevicular fluid, which contain systemically and locally produced products (18). The gingival crevicular fluid provides an accurate representation of the concentrations of inflammatory mediators in the adjacent serum and tissues (18; 28). Hence much of the data pertaining to the oral cavity are studied by the collection of antimicrobial peptides in oral secretions. These studies have illuminated activities that connect antimicrobial peptides to immune functions in the oral cavity that are in addition to the previously documented antimicrobial functions.
As the focus has shifted from antimicrobial functions to effects on the immune system, new activities of the defensins and LL-37 are now being reported within tissues throughout the body. For example, defensins and LL-37 have the ability to enhance phagocytosis by macrophages (40, 75). They can also serve as chemoattractants for monocytes, macrophages, T lymphocytes, and immature dendritic cells (11, 75, 77). Furthermore, defensins have the ability to suppress the production of pro-inflammatory cytokines of certain microbial antigens (40). The defensins and LL-37 can activate and de-granulate mast cells (40, 75), as well as regulate the complement system (40, 75). Also, defensins are able to enhance antigen specific immune response (40, 77). Although not all the research presented here has focused on the oral cavity, some of these newly reported functions have been confirmed within oral tissues.
The contribution of antimicrobial peptides to periodontal tissue function will be discussed in this review. Both the antimicrobial activity and potential contributions to normal tissue homeostasis will be considered. Since the two major groups of antimicrobial peptides are found in either neutrophils or the gingival epithelium, the respective contributions of these peptides will be put in the context of their location in the periodontium (Figs. 1 and 2). Specifically, neutrophil antimicrobial peptides will be examined in the junctional epithelium where neutrophils are abundant in both health and disease, while β-defensins will be reviewed with a focus on the gingival epithelium, where they interact with both the innate and adaptive immune functions.
Fig. 1.
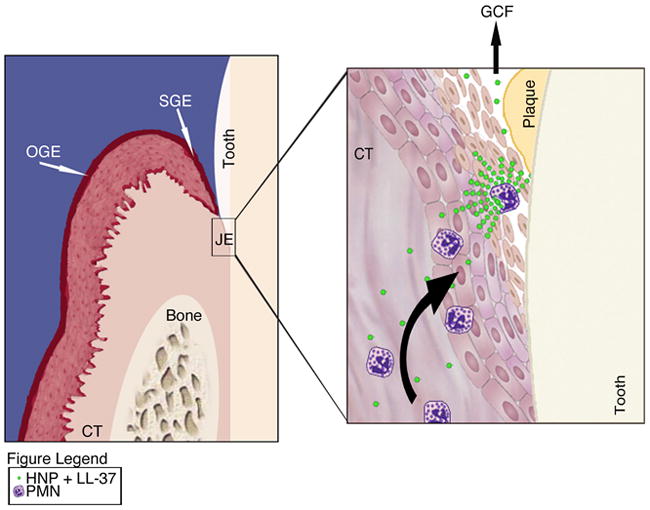
Illustrated is the oral epithelium made up of oral gingival epithelium (OGE), oral sulcular epithelium (OSE), connective tissue (CT) and junctional epithelium (JE). The close up of the junctional epithelium exemplifies a polymorphonuclear neutrophil (PMN) degranulating upon bacterial stimulation. The contents released contain both human neutrophil peptides (HNP) that include α–defensin and cathelicidin LL-37 that act either directly or on epithelial cells to recruit more PMN into areas of bacterial insult.
Fig. 2.
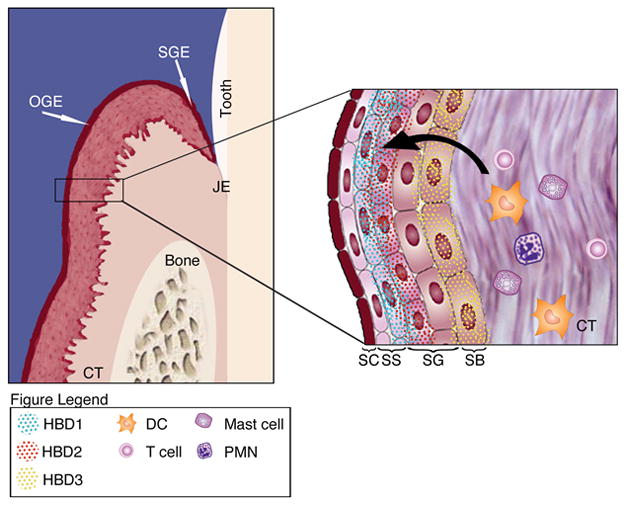
Illustrated is the oral epithelium made up of oral gingival epithelium (OGE), oral sulcular epithelium (OSE), connective tissue (CT) and junctional epithelium (JE). The close up of the OGE displays the stratified sections of the keratinized cells that are comprised of stratified corneum (SC), stratified spinosum (SS), stratified granulosum (SG) and stratified basal (SB) epithelial cells. Human β–defensins (HBD) are expressed in the OSE within specific stratified layers. Expression of HBD leads to infiltration of polymorphonuclear neutrophils (PMN), T-cells, Langerhans dendritic cells (DC) and mast cells into the stratified epithelium.
General overview of defensins and LL37
In general, the mechanism of antimicrobial action of antimicrobial peptides is the formation of pores in the cytoplasmic membrane of bacteria (56; 75). The common structure of the defensins consists of a β-sheet structure and 3 disulphide bonds (56). Human defensins can be classified into two groups based on structure: α and β defensins. This classification is based on distinctions in the connecting patterns of three disulfide bonds and the spacing of cysteine (36). Research has focused primarily on the six human α and four β-defensins (1; 36). The α-defensins 1-4, are also known as human neutrophil peptides and are found in the oral cavity, whereas α defensins 5-6 are found in mucosal Paneth cells associated with the gut (40). Although there have been at least 28β-defensin genes described, in humans β-defensin 1-4 are the most studied (41). The α-helical antimicrobial peptide LL-37, in contrast, is the only cathelicidin produced in humans (56).
α-defensins
As the name indicates human neutrophil peptides are found in the neutrophils; the derivation of the name “human neutrophil peptide” comes from the fact that they were initially isolated from neutrophil primary (azurophilic) granules (75). Human neutrophil peptides are synthesized in neutrophil precursor cells called promyelocytes within the bone marrow (45). Prior to maturation, the peptide is stored in primary granules of neutrophils before eventual release into the tissue. Activation of the human neutrophil peptide molecule happens when the propiece is proteolytically cleaved from the precursor peptide in the Golgi body (34; 44; 71; 75). Human neutrophil peptides 1-3 are numerous in the oronasal cavity, where they reside in neutrophil granules, monocytes and natural killer cells. The tissue and secretions of the oronasal cavity, including the oral and salivary gland tissues, as well as, saliva, gingival crevicular fluid and nasal secretions, are abundant with human neutrophil peptides 1-3 (40). In contrast, human neutrophil peptide-4 is found in primary neutrophil azurophil granules of neutrophils (40). In the azurophilic granules, human neutrophil peptides 1-4 make up approximately 30% to 50% of the total proteins (21; 46; 79). However, in the entire neutrophil, human neutrophil peptides 1-4 comprise approximately 5–7% of the total protein (11; 25). There have not been any studies of human neutrophil peptide-4 in the mouth, as it is present in such small quantities compared to human neutrophil peptides 1-3.
The structures of the various human neutrophil peptides contain 29-35 amino acids and are close in size and composition, with slight changes distinguishing them (40). In human neutrophil peptides 1-4, a distinct 3-D structure with rigid β-sheets is formed when the 1-6 cysteine amino acid residues, the 2-4 cysteine amino acid residues and the 3-5 cysteine amino acid residues are linked by disulfide bonds (40; 75). More specifically, with respect to amino acid content and structure, human neutrophil peptide-1 contains 30 amino acid residues with a net charge of 3+ and has an additional N-terminal alanine residue. Human neutrophil peptide-2 has 29 amino acid residues and net charge of 3+. Human neutrophil peptide-3 has 30 amino acid residues, a net charge of 2+ and an additional N-terminal aspartic acid residue. Human neutrophil peptide-4 is slightly larger with 34 amino acid residues and a net charge of 3+ and is more variable in its amino acid composition (40). Human neutrophil peptides 1-3 is encoded by genes DEFA1 and DEFA3, where human neutrophil peptide-4 is encoded by gene DEFA4 (40; 75). Human neutrophil peptides 1-3 are highly homologous proteins that represent the majority of the antimicrobial peptides within neutrophils.
LL-37
Another important defense peptide, LL-37, also resides in the neutrophils. Although LL-37 can be found in the gingival epithelium, expression appears to be a product of neutrophil migration rather than production in epithelial cells (16). LL-37 belongs to a group of major mammalian antimicrobial proteins called cathelicidin. It is a cationic peptide derived from the 18kDa precursor protien cathelicidin by proteolytic cleavage (15) and is not active until the conserved pro-region of the cathelicidin is cleaved after the protein is secreted (16; 75). Upon activation, this peptide can contribute to many important functions within the gingival epithelium.
The cathelicidin proteins all have the same basic structure containing a pre-region (N-terminal putative signal peptide), a pro-region (a conserved cathelin-like domain), and a C-terminal microbicidal domain (75). The gene that encodes for cathelicidin consists of four exons. The last exon encodes for the cleavage site and the C-terminal antimicrobial domain. As described above, humans only produce one cathelicidin: hCAP18. To produce LL-37, hCPA18 is cleaved by proteinase 3 or elastase, which releases the C-terminal antimicrobial end. This cleavage produces a peptide with two leucine residues and 37 amino acid residues, from which the name LL-37 is derived (75).
Human β-defensins
Human β-defensins are expressed in all human epithelial tissues. In the oronasal cavity, the tissues that contain human β-defensins include: gingiva, tongue, salivary glands, and mucosa (16; 40). Production and storage of human β-defensins occur in the gingival epithelial cells, as well as in dendritic cells, although at a much lower level (58; 78). In epithelium, expression of human β-defensins is varied; human β-defensin-1 is constitutively expressed, and while human β-defensin-2 and human β-defensin-3 levels are more variable depending on stimulation (16; 78). Human β-defensin-4 inducible expression has been identified in epithelial cells and keratinocytes; however, it is found in lower concentrations than human β-defensins 1-3 and therefore its study is not very pervasive in the literature (45). While the human β-defensins are produced in the oronasal epithelial tissues, they are not confined to their locus generis; they are able to migrate into the secretions of the oral tissue such as saliva, gingival crevicular fluid, and nasal secretions (40) (36).
The structure of human β-defensins 1-3 varies between 36-45 amino acid residues along with varying charges. In human β-defensins, a distinct 3-D structure with rigid β-sheets is formed when the 1-5 cysteine amino acid residues, the 2-4 cysteine amino acid residues and the 3-6 cysteine amino acid residues are linked by disulfide bonds (40; 75). More specifically, human β-defensin-1 is made up of 36 amino acids carrying a 5+ charge while human β-defensin-2 has 41 residues with a 7+ charge and human β-defensin-3 contains 45 residues of β-sheet structure in solution that forms dimers with a net +11 charge. Human β-defensin-4 has 49 amino acid residues with a +7 charge (40).
Defensins in the junctional epithelium: α-defensins and LL-37
Overview of junctional epithelium structure
Neutrophils, which contain human neutrophil peptides and LL-37, are found in both healthy and diseased junctional epithelial tissue, making this tissue the main reservoir for neutrophil associated antimicrobial peptide activity contributing to host homeostasis (Fig. 1). The junctional epithelium lies at the bottom of the gingival sulcus and consists of non-keratinized squamous epithelium. The junctional epithelium is highly porous, consisting of a large fluid filled intracellular space where the cells are interconnected by desmosomes and gap junctions (5). To manage the constant microbial challenge presented by the biofilm and planktonic bacteria in the oral cavity, the periodontium expresses select host defense mediators in a highly organized manor.
There are two distinct types of immune responses: innate immune response, which occurs quickly and an adaptive immune response, in which highly specialized cells and processes eliminate or prevent pathogens. In the last 10 years, research focused on innate immune responses in the periodontium has highlighted extensive activities that contribute to healthy tissue, while the disruption of innate inflammatory mediators can actively participate in the destruction of tissue and bone resulting in disease(12; 18; 38; 72). The innate immune response can be induced by both commensal and pathogenic bacteria; however, the response to commensal oral bacteria may contribute to the defensive status of the periodontal tissue (18; 23). The adaptive immune response involves the recruitment of cells such as lymphocytes, killer T-cells, and helper T-cells to the site of inflammation.
In the junctional epithelium, one of the key components to innate host defense is neutrophil migration. Neutrophils are the most abundant immune cell in the periodontium where approximately 30,000 transit per minute (67) (62). Neutrophil migration has been linked to the coordinated expression of E-selectin, intercellular adhesion molecules and interleukin-8, which direct the movement of neutrophils from the gingival tissue, which is highly vascular, to the gingival crest (27; 51; 54; 66–68). Once the neutrophils reach the gingival crest, they form a barrier between the dental plaque biofilm and the host tissue (5). Within the junctional epithelium, the density of neutrophils creates a gradient where there are more neutrophils toward the superficial layers, in close proximity to sub-gingival plaque (69). In early and chronic periodontal disease, neutrophils are the most abundant leukocyte (42) (69).
α-defensins: antimicrobial activity
Subgingival plaque, which contains bacteria and bacterial products, is adjacent to the junctional epithelium where human neutrophil peptides are abundant. The antimicrobial activity of α-defensins (human neutrophil peptides) is not as potent as the β-defensins or LL37. In general, α-defensins induce non-oxidative killing in phagocytes or can act directly as an antimicrobial when discharged into tissue (17). Much of what is known about the antimicrobial activity of human neutrophil peptides in the oral cavity comes from studies of saliva and gingival crevicular fluid. Upon degranulation, neutrophils, release human neutrophil peptides in secretions such as gingival crevicular fluid and saliva. Human neutrophil peptide-1, found in gingival crevicular fluid and saliva, has been identified as being able to target Streptococcus mutans, Pseudomonas aeruginosa, Aggregatibacter actinomycetemcomitans, and Porphyromonas gingivalis. On the other hand, human neutrophil peptide-2 and -3 in gingival crevicular fluid and saliva have been shown to target P. gingivalis and A. actinomycetemcomitans (30). Another study showed that human neutrophil peptides 1-3 activity in Escherichia coli relied on a mechanism that sequentially permeabilized the outer, then and inner membrane. Upon breach of the inner membrane, DNA, RNA and protein synthesis stops and E. coli can no longer form colonies (46). Human neutrophil peptides do not always work alone to provide antimicrobial activity. For example a synergy between human neutrophil peptide-1 and LL-37, has been shown to act against E. coli and Staphylococcus aureus (16). As stated before, α-defensins’ antimicrobial activity against most oral bacteria is not as potent as other antimicrobial peptides, although there is some evidence that they are more active against yeast because such as Candida albicans (16).
α-defensins: regulation in disease
Neutrophils in the junctional epithelium are the major source of human neutrophil peptide regulation. The increase in neutrophil numbers during episodes of gingival disease explain the finding that human neutrophil peptides 1-3 levels in saliva and gingival crevicular fluid can be up-regulated 15-fold in cases of aggressive periodontitis and 60-fold in chronic periodontitis (30). While an increase of human neutrophil peptides is a characteristic response to periodontitis, it is not only in periodontal disease that an increase in the levels of human neutrophil peptides can be seen. Human neutrophil peptide-1 in saliva have been reported to be higher in patients with oral diseases, such as lichen planus, Behçets disease, and recurrent aphthous stomatitis (44). Also in the disease Morbus Kostman, a severe congenital neutropenia, a deficiency in human neutrophil peptides has been shown, with reports of a 30% decrease(30) (16). Human neutrophil peptides are a product of neutrophils and also contribute to neutrophil recruitment, as described in the following section, thereby contributing to the regulation of neutrophil migration into diseased tissues.
α-defensins: immune response
There is a paucity of literature about the role of α-defensins in gingival tissue per se, but there have been studies conducted on other tissues. These studies have revealed that the α-defensins have numerous activities outside the classic antimicrobial, antiviral and anti-parasitic effects initially described (77). They have been shown to induce chemokines for neutrophils, monocytes, macrophages, mast cells, dendritic cells and T lymphocytes (11; 75; 77). They can be directly chemotactic, have selective cytokine/chemokine induction, and serve as adjuvants (77). In addition, α-defensins participate in wound healing and are able to increase collagen production, as well as inhibit complement activity and induce cell death (77). These imunomodulatory activities may have a special significance in the periodontium where human neutrophil peptides may contribute to neutrophil migration (Fig. 1).
In the junctional epithelium, where neutrophils are prolific, there is an interleukin-8 gradient expressed that allows for neutrophil migration. Interleukin-8 is known to improve neutrophil recruitment to effector sites (29). Human neutrophil peptides have been shown to up-regulate interleukin-8 expression (29). Mast cells, which are prolific recruiters of neutrophils, can interact with α-defensins (63). Mast cells reside in close proximity to the basal layer of the epithelium and blood vessels that the neutrophils pass through on their way to the junctional epithelium. α-defensins, from animals such as humans, rabbits, and guinea pigs, can induce mast cell de-granulation and histamine release (4; 75). The products of mast cell granules, in turn, activate the host to cause increased neutrophil influx (48; 75). This may contribute to a positive feedback loop for neutrophil recruitment, where α-defensins help to establish neutrophil migration to the junctional epithelium.
Human neutrophil peptides may also promote neutrophil migration in the periodontium by the induction of inflammatory cytokines. It has been shown that human neutrophil peptides 1-3 stimulation in mice leads to the production of inflammatory mediators: tumor necrosis factor-α, monocyte chemotactic protein-1 and macrophage inflammatory protein-2. Monocytes that have been co-stimulated by S. aureus or phorbol-12-myristate-13 acetate and human neutrophil peptides 1-3 have also been shown to up-regulate the production of tumor necrosis factor-α and interleukin-1β (inflammatory cytokines), while down-regulating the production of interleukin-10 (anti-inflammatory cytokine) (11; 75). Another study showed, that human neutrophil peptide-1 was able to promote the maturation of dendritic cells, which are known to link the innate and adaptive immune response (59). In this study human neutrophil peptide-1 was also shown to enhance the production of the pro-inflammatory cytokines tumor necrosis factor-α, interleukin-6, and interleukin-12p70, while leaving the production of the regulatory cytokine interleukin-10 un-affected (59). This data provide an additional mechanism by which human neutrophil peptides possibly increase local inflammatory mediator concentrations, which in turn induces the recruitment of more neutrophils.
LL-37: antimicrobial activity
Another important antimicrobial peptide located in the neutrophils is LL-37. LL-37 targets different bacteria from those of human neutrophil peptides, allowing the two to work together to defend the junctional epithelium from bacterial invasion. The bactericidal activity of LL-37 involves interacting with negatively charged bacterial molecules and insertion into the membrane; membrane disruption occurs facilitating changes to lipid packing and organization and subsequent pore formation (7). LL-37 has exhibited strong antimicrobial activity against E. coli, P. aeruginosa, Klebsiella pneumoniae, S. aureus, Neisseria gonorrhoeae (7). The examination of antimicrobial activity of LL-37 against oral bacteria has shown specificity to P. gingivalis, A. actinomycetemcomitans, Streptococcus gordonii, Prevotella intermedia, Fusobacterium nucleatum and Streptococcus sanguinis (7; 30), with the greatest activity against A. actinomycetemcomitans and Capnocytopahaga sp. (16). Regulation of LL-37 expression by bacteria is another factor to consider when looking at the overall ability of LL-37 to participate in host defense. Typically, bacterial stimulation of the neutrophil causes degranulation which releases LL-37 into tissues. However, the down-regulation of LL-37 can be induced by byproducts of bacteria such as N. gonorrhoeae, E. coli, and Vibrio cholera in tissues outside the oral cavity (7).
Interestingly, the antimicrobial activity of LL-37 exhibits a dual function in that it kills bacteria and neutralizes the lipopolysaccharide from gram-negative bacteria (30). As common with all antimicrobial agents LL-37 displays varying potency against different bacterial species (30). This suggests that LL-37 is selective in its antimicrobial activities and while it can induce death, it is not effective against all types of bacteria. An example of the effect of LL-37 on lipopolysaccharide is demonstrated by one study that utilized microarray analysis on human monocytes treated with lipopolysaccharide or in combination with LL-37 (50). In the first treatment group, lipopolysaccharide was shown to induce 125 genes while the addition of LL-37 down-regulated 106 of those genes. The numerous genes affected by LL-37 highlights the often complex and vast effects of this small peptide on a variety of host cells. In another study LL-37 was able to protect the host from sepsis by inhibiting the immonostimulatory products induced by bacterial products such as lipopolysaccharide or lipoteichoic acid (7; 52; 77). LL-37 can bind to lipopolysaccharide-binding protein or receptor to block lipopolysaccharide signaling thereby suppressing lipopolysaccharide induction of inflammatory cytokines (61). Consistent with this, examination of LL-37 neutralization of lipopolysaccharide from the oral pathogen P. gingivalis has shown complete inhibition of the lipopolysaccharide-induced inflammatory cytokines using a whole blood cytokine assay (74). The lipopolysaccharide from periodontopathogenic bacteria comprise many variants creating a heterogeneous population of lipopolysaccharide structures in a given plaque sample. A recent study sought to examine these different structures in relation to LL-37 neutralization of lipopolysaccharide (65). Gingival fibroblasts or periodontal ligament fibroblasts were stimulated with lipopolysaccharide isolated from different periodontopathogens alone or in combination with LL-37 and assayed for cytokine production. Although there was cytokine inhibition in all LL-37 treated cells stimulated by periodontopathogenic lipopolysaccharide, the inhibition differed between bacteria. Surprisingly, lipopolysaccharide of a non-periodontal bacterium, E. coli did not show inhibition from LL-37 treatment. These results implicate LL-37 as having lipopolysaccharide-structure specific activity although more work is needed to understand the mechanisms involved. Together these studies show that LL-37 can exert immunomodulatory effects on whole blood, gingival fibroblasts and periodontal ligament fibroblasts. This activity may have broad implications in periodontal disease.
LL-37: Regulation in disease
Despite a difference in molecular structure, the regulatory activities of LL-37 are similar in pattern to those described for human neutrophil peptides. They are both found in the neutrophils that are known to migrate to areas of disease to help the innate host defense and they appear at first to have many redundant activities. For example, similar to the increases of human neutrophil peptides in gingival crevicular fluid and saliva in patients with aggressive or chronic periodontal disease, the overall level of LL-37 is shown to increase in response to the progression of periodontitis (47). LL-37 is shown to be up-regulated by F. nucleatum and P. intermedia and not affected by P. gingivalis, Tannerella forsythia and Treponema denticola in patients with aggressive and chronic periodontitis (30). This observation is supported by the finding that the gingival crevicular fluid levels of LL-37 are significantly elevated in patients with chronic periodontitis when compared to other groups (30). While both human neutrophil peptides and LL-37 have antimicrobial activities, induce cytokines and chemokines that recruit cells; examination of the specificity of their actions show that they are, in fact different. For example, while both target P. gingivalis, A. actinomycetemcomitans, and S. sanguinis; LL-37 targets S. gordonii, P. intermedia, and F. nucleatum, where human neutrophil peptides target S. mutans and P. aeruginosa. Diseases that lead to low production of LL-37 often result in periodontal disease which implicates a very important function for LL-37 that appears to be uniquely different from the activity of human neutrophil peptides. Morbus Kostmann is a disease that is caused by a severe congenital neutropenia. Patients with the disease are often afflicted with periodontal disease and have been found to be deficient in LL-37, with a 30%-50% decrease in α-defensins in the saliva, plasma and neutrophils (16; 30). Deficiencies in LL-37 are also found in Papillon-Lefèvre syndrome and Haim-Munk syndrome, where the precursor cathelicidin is unable to undergo the cleavage of the conserved pro-region of cathelicidin, which causes activation (16; 30). The deficiency found in LL-37 might be important in understanding of the role of LL-37 in periodontal disease. For example, since it is known that LL-37 is active against A. actinomycetemcomitans, which is common in progressive periodontal disease,(16) it is logical to surmise that when LL-37 is lacking and an increase in periodontal disease is observed, the disease could be correlated to the loss of function provided by LL-37.
LL-37: host immunity
The cathelicidins have many of the same functions as α defensins that allow for modulation of both innate and adaptive immune functions (77). While LL-37 can inhibit cytokine expression when coupled with bacterial products, it can also induce expression of cytokines associated with inflammation and chemotaxis. Interleukin-1β, a potent inflammatory cytokine involved in many cellular functions such as proliferation, differentiation and innate immune response shown to modulate cytokine expression in oral epithelial cells (22; 24; 76). LL-37 has been shown to act synergistically with interleukin-1β to induce the production of interleukin-6, interleukin-10 and monocyte chemotactic protein-1/monocyte chemotactic protein-3 in human peripheral blood mononuclear cells (77). Periodontal disease can come from multiple etiologies and it is easy to imagine that interleukin-1β and LL-37 could work together to orchestrate an elaborate immune response depending on type of insult. Another cytokine of importance in the periodontium is interleukin-8. As discussed previously, this functions as a chemoattractant for neutrophils. LL-37 has been shown to induce interleukin-8 production from myeloid and epithelial cells (64) and has been found to be expressed in gingival crevicular fluid and gingival tissues (70) where it likely contributes to the interleukin-8 production required for neutrophil infiltration into the junctional epithelium. In addition to inducing other cells to produce chemokines, LL-37 can also act directly as a chemoattractant by signaling through the G protein coupled formylpeptide receptor like-1 on neutrophils, monocytes, T-cells and mast cells (16; 77). Formylpeptide receptor like-1 is also expressed on endothelial cells where LL-37 induces proliferation and the formation of vessel-structures, thus contributing to the healing response as seen in mice deficient in the LL-37 homologue, CRAMP that have decreased vascularization during wound repair(39). In summary, LL-37 can function to prevent sepsis, induce cytokines, recruit cells toward insult and directly induce angiogenesis providing an extensive repertoire of tools for the overall innate immune response.
Stressing the importance of neutrophil migration
With the forgoing understanding of the connection between human neutrophil peptides and LL-37, it is important to stress the role of neutrophil migration. LL-37 and human neutrophil peptides play a pivotal role in relationship to neutrophil recruitment and yet the presence of activated neutrophils is required for release of these molecules. Patients with severe neutropenia such as in Morbus Kosmann’s disease develop spontaneous periodontal disease, highlighting the importance of neutrophils in disease. This lends support to the idea that a major component of periodontal health is the transport of neutrophils from the highly vascularized connective tissue to the gingival crest. It is well documented that neutrophil migration through the periodontium is a requirement for healthy periodontium in both humans and mice (60). The presence of neutrophils within the periodontal tissue is the main regulatory function for control of the number of commensal bacteria that reside on the tooth and its root surface, (3; 10; 34; 57; 73) and is of particular importance to the junctional epithelium where neutrophils are the primary source of human neutrophil peptides and LL-37.
Oral Epithelium: Home of the β-defensin
Overview of epithelial structure: where do the β-defensins reside?
The oral epithelium is divided into keratinized and non-keratinized tissue. In keratinized tissue there are four layers of epithelium: cornium, granulosum spinousum and basal (Fig. 2). The non-keratinized tissue is lacking the cornium and granulosum layers. The basal layer is the interface with the underlying lamina propria, which contains blood vessels helping to supply the epithelium with nutrients and signals from the body.
In contrast to human neutrophil peptides, whose location is linked to neutrophils, β-defensins are located in different epithelial cells at different times, tied to the relative health and disease of the tissue. For example, in the gingival tissue, human β-defensin-1 and human β-defensin-2 are expressed primarily in the granular and spinous layers of the gingival tissue. The expression is associated with epithelial cell differentiation. For example human β-defensins 1-2 mRNA is found in the spinous layer and the peptide is found in the upper spinous, granular and cornified layers, with the strongest expression directly adjacent to the biofilm on the tooth surface and inflamed sulcular epithelium (16; 17). Interestingly, human β-defensin-2, which is not usually found to be expressed in situations of health in other host tissues, is expressed in health in the oral cavity. This low level of human β-defensin-2 expression could be due to stimulation by commensal bacteria (16; 43). In apparent contradiction, another source stated that the overall expression of human β-defensin-2 was higher in healthy periodontal tissue than in patients with periodontal disease (36). This discrepancy was also noted in the literature by a review article by Gursoy et al. (see review for full discussion) (32).
Human β-defensin-3 is also expressed in different locations depending on state of health or disease. In healthy oral tissue, keratinocytes, which are formed in the basal layer and migrate through the epithelium where they form coneocytes. These differentiated cells produce high levels of human β-defensin-3 proteins and mRNA expression (58). In health, human β-defensin-3 is expressed primarily in the basal layer, whereas in disease, the expression will be found to have extended to the spinous layer in Langerhans and Merkel cells (16; 36; 40; 58). The basal cell expression of human β-defensin-3 may serve as a link between innate and adaptive immunity as a facilitator of cross talk between gingival epithelia and connective tissue, and may even be a component of adaptive immunity in the periodontium (36).
β-defensins: antimicrobial activity
The β-defensins possess antibacterial, anti-fungal and anti-viral properties. The antimicrobial activity of the β-defensins shows greater variation in the oral cavity than in other tissues of the body (20). When comparing the defensins, human β-defensin-3 has potent antimicrobial activity against both gram-negative and gram-positive bacteria when present at low concentrations, and is more active against bacteria and fungi than human β-defensin-2 and human β-defensin-1, with human β-defensin-1 having the lowest activity (16; 36). Human β-defensin-1, has been shown to target P. gingivalis, A. Actinomycetemcomitans, F. nucleatum (30). Human β-defensin-2 it has been shown to be active against gram-negative bacteria and Candida albicans, but is more restricted in its activity against gram-positive bacteria (16; 36). Human β-defensin-3’s targets include P. gingivalis, A. actinomycetemcomintans, S. mutans, T. denticola, F. nucleatum, Burkholderia cepacia, S. sanguinis and P. intermedia (30). It is interesting to note that while the β-defensins target these bacteria, the amount of β-defensin required for minimal inhibitory concentration can be at a level higher than present in the gingival crevicular fluid. This lends support to the idea that the function of β-defensins is not completely antimicrobial, but rather depends on other factors such as the stimulation of the immune system or working in concert with other mechanisms for activity (30). In addition, there are biological factors that can affect the antimicrobial activity of β-defensins. One such example is salt concentration, where at high salt concentrations the anti-microbial activity of human β-defensins 1, 2, 4 is impaired, whereas human β-defensin-3 activity is not altered (1; 26). Therefore, the effective concentrations of β-defensins in vivo required for antimicrobial action are not clear.
The human β-defensin-2 and human β-defensin-3 antimicrobial functions have considerable variability against multiple strains of the same species or oral bacteria, with aerobes being more susceptible than anaerobes to their effects (16; 37). In particular, the effectiveness of human β-defensin-2 and human β-defensin-3 were tested against a collection of oral organisms and the bacteria demonstrated strain rather than species specificity. Human β-defensin-2 and human β-defensin-3 were found to be effective against 100% of some facultative bacteria, which included strains of S. sanguinis, S. mutans, Actinomyces naeslundii and E. coli. For the anaerobes, which included strains of P. gingivalis, F. nucleatum, A. actinomycetemcomitans and Peptostreptococcus micros, only 21.4% and 50% were susceptible to human β-defensin-2 and human β-defensin-3, respectively (37). This difference in activity is very interesting and is consistent with the findings that some periodontal pathogens such as P. gingivalis and T. denticola have developed resistance to β-defensins (20). One study (36) showed that P. gingivalis lipopolysaccharide can affect the mRNA expression of human β-defensins 1-3. P. gingivalis has a unique heterogeneous population of lipid A structures within the lipopolysaccharide molecule that vary depending on environmental stimuli and have been shown to either activate or inhibit immune functions in vitro (2; 13). More specifically, it was discovered that the mRNA of human β-defensins 1-3 is significantly up-regulated by P. gingivalis lipopolysaccharide 1690 (penta-acylated lipid A) but down-regulated by P. gingivalis lipopolysaccharide 1435/1449 (tetra-acylated lipid A). (36) Further study showed P. gingivalis grown at body temperature yields a lipid A structure corresponding to slight immune activation that is susceptible to the antimicrobial activities of human β-defensins 2-3 but when grown at temperatures associated with fever, 41C, the lipid A was more robust in immune activation and very resistant to human β-defensin killing (14). This indicates P. gingivalis can modulate the lipopolysaccharide structure affecting its susceptibility to the antimicrobial assault by β-defensins. The effectiveness of HDBs against commensal bacteria but not periodontopathogens suggests that the overall function of human β-defensin antimicrobial action is to control the accumulation of commensal bacteria.
β-defensins: bacterial induced expression
The levels of human β-defensin expression are modulated by periodontal disease. One study showed that human β-defensin-3 mRNA is present in the gingival crevicular fluid of the majority of healthy subjects, but reductions in human β-defensin-3 mRNA were found in subjects with periodontitis (6). In addition, the levels of human β-defensin are modulated differently by different oral bacteria. For example human β-defensin-1 is up-regulated by P. gingivalis, and P. intermedia but down-regulated by T denticola. In contrast, levels of human β-defensin-1 expression do not change in the presence of T. forsythia and F. nucleatum. Human β-defensin-2 expression has been shown to be up-regulated by A. actinomycetemcomitans, P. gingivalis, F. nucleatum and P. intermedia. Human β-defensin-3 mRNA expression can be regulated by periodontal pathogens such as A. actinomycetemcomitans, P. gingivalis, S. mutans, T. denticola, F. nucleatum, B. cepacia, S. sanguinis, and P. intermedia (6; 30; 58). Specifically, human β-defensin-3 has been shown to be up-regulated by A. actinomycetemcomitans, P. gingivalis, F. nucleatum and P. intermedia but down-regulated by T. forsythia and T. denticola (30). The effects of different oral bacteria on the expression of β-defensins indicates modulatory immune effects of the β-defensins may occur from changes to the oral microbial community composition,
Human β-defensin levels have not only been shown to fluctuate with periodontal disease but with other diseases as well. For example, the expression of human β-defensins 1-2 has been shown to be dysregulated in diabetes (30) possibly resulting in an overgrowth of Candida glabrata and Candida tropicalis (47). Furthermore, human β-defensin-1 is down-regulated in some squamous cell carcinomas (1) possibly participating in in the control of cell growth, consistent with this is its role in tumor suppression (8). The diseased state represents an imbalance in host homeostasis therefore; examination of human β-defensin levels in disease may give insight into the role of human β-defensins in health.
β-defensins: cytokine induced expression
Human β-defensin expression is also modulated by the inflammatory state of the tissue. Inflammatory cytokines can result in increased expression of human β-defensins (1). For example, human β-defensin-2 and -3 levels of expression are induced by several different cytokines, such as tumor necrosis factor-α, interleukin-1β, and interferon-γ (1; 16; 58). Consistent with cytokine induction of β-defensin expression, inflamed epithelia is where the most fluctuation in human β-defensin levels are observed (16). This suggests a more specialized role for human β-defensin-2 in the innate epithelial defense when compared with human β-defensin-1, with is constitutive expression and indicate that immune effects of human β-defensin-2 may be more susceptible to local inflammatory conditions (36).
β-defensins: host immunity
The ability of commensal bacteria to induce expression of human β-defensin-2 in gingival tissues may be to prime the innate immune status as well as keep overall bacterial numbers down (16). For example, the literature has shown that human β-defensins interact with many different facets of the hosts’ immune system. Human β-defensins have been shown to engage the CCR6 receptor on select immune effecter cells, such as dendritic cells and T-cells, evoking a chemokine response, which causes these cells to be recruited to site (31). Human β-defensins 1-3 can also function directly as chemokines for dendritic cells, which, in combination with recruiting T-cells may be a link to adaptive immune activation (1). More specifically, human β-defensin-1 and human β-defensin-2 are ligands for receptor CCRP, which helps with dendritic cell maturation while human β-defensin-2 can stimulate antigen presentation activity in dendritic cells (43). Besides being a chemokine for monocytes, macrophages, and mast cells human β-defensin-3 is also a chemoattractant for CD45 RA+/CD4+ T lymphocytes and immature dendritic cells (58). The human β-defensin recruitment of dendritic cells and maturation induction is one mechanism that could be a link between innate and adaptive immune response in oral tissues. Dendritic cells act as messengers between innate and adaptive immune functions. When an immature dendritic cell is activated, it then migrates to lymphocytes where they coordinate with T-cells and B-cells to produce an adaptive immune response. β-defensins link innate and adaptive immune responses by activating local dendritic cells and recruiting T-cells into nearby epithelial tissues, demonstrating how human β-defensins play a larger role in tissue function than was previously appreciated.
Defensins and LL-37 in Wound Healing
The defensins and LL-37 not only function as an antibacterial and regulate immunity, they may also contribute to wound healing in the periodontium. While there is no evidence of their direct participation in wound healing in oral tissue, their importance in wound healing has been demonstrated in other tissues.
There are four phases to wound healing: inflammation, re-epithelization, granulation tissue formation and tissue remodeling. Both LL-37 and β-defensins have been shown to promote epithelial cell migration and epithelial cell differentiation (9). LL-37 and human β-defensin-3 are also involved with keratinocyte migration and proliferation by the induction of STAT proteins (7; 53), human β-defensin-3 specifically through the activation of the epidermal growth factor receptor (53). Human β-defensin-2 can also increase keratinocyte migration and proliferation. These functions could be important in the re-epithelization phase of repair that requires migration, differentiation and proliferation of epithelial cells (33; 53). Human β-defensin-2 has been demonstrated to promote wound healing, in vitro, in the intestine(55) while LL-37 has been shown to stimulate re-epithelization of wounded skin, and participate in angiogenesis. Consistent with this, wound repair functions of LL-37 can be inhibited by antibodies that neutralize LL-37 (9; 75) which could serve to minimize overgrowth of the epithelium. In fact, LL-37 is currently being tested as a therapeutic in prostate cancer (35). In summary, many functions of the defensins contribute to wound repair however, to date there does not appear to be any study of these activities in the periodontium where wound repair functions are vital for recovery from surgery or disease.
Conclusion
Many of the functions detailed in this report are typically thought of as part of a robust immune response in the periodontium. However, most antimicrobial peptides are not only part of the immune response but are also expressed in healthy tissues where they contribute to host homeostasis. To paint a broad picture of antimicrobial peptides in healthy tissues; the combined antimicrobial activities likely serve to manage and minimize accumulation of commensal bacterial while the cell recruitment of innate and adaptive effectors assist in the maintenance of bacteria as well as prime the adaptive immune cells with continued sampling of the environment. Although there are no current studies that detail antimicrobial peptides in wound healing within the periodontium, it is easy to surmise, with the continual regeneration of gingival tissues from actions such as mastication or the continual transit of neutrophils through junctional epithelium that antimicrobial peptides are required to maintain tissue structure by way of cell differentiation, recruitment and proliferation. Future study into the mechanisms by which the antimicrobial peptides function specifically in the periodontium to maintain healthy tissue functions could provide exciting opportunities in therapeutics for periodontal diseases or possibly even oral cancer.
Acknowledgments
Research funded by: NIH DE018274, “Oral Bacteria Modulate”
References
- 1.Abiko Y, Saitoh M, Nishimura M, Yamazaki M, Sawamura D, Kaku T. Role of beta-defensins in oral epithelial health and disease. Med Mol Morphol. 2007;40:179–184. doi: 10.1007/s00795-007-0381-8. [DOI] [PubMed] [Google Scholar]
- 2.Al-Qutub MN, Braham PH, Karimi-Naser LM, Liu X, Genco CA, Darveau RP. Hemin-dependent modulation of the lipid A structure of Porphyromonas gingivalis lipopolysaccharide. Infect Immun. 2006;74:4474–4485. doi: 10.1128/IAI.01924-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attström R, Schroeder HE. Effect of experimental neutropenia on initial gingivitis in dogs. Scand J Dent Res. 1979;87:7–23. doi: 10.1111/j.1600-0722.1979.tb01935.x. [DOI] [PubMed] [Google Scholar]
- 4.Befus AD, Mowat C, Gilchrist M, Hu J, Solomon S, Bateman A. Neutrophil defensins induce histamine secretion from mast cells: mechanisms of action. J Immunol. 1999;163:947–953. [PubMed] [Google Scholar]
- 5.Bosshardt DD, Lang NP. The junctional epithelium: from health to disease. J Dent Res. 2005;84:9–20. doi: 10.1177/154405910508400102. [DOI] [PubMed] [Google Scholar]
- 6.Brancatisano FL, Maisetta G, Barsotti F, Esin S, Miceli M, et al. Reduced human beta defensin 3 in individuals with periodontal disease. J Dent Res. 2011;90:241–245. doi: 10.1177/0022034510385686. [DOI] [PubMed] [Google Scholar]
- 7.Bucki R, Leszczynska K, Namiot A, Sokolowski W. Cathelicidin LL-37: a multitask antimicrobial peptide. Arch Immunol Ther Exp (Warsz) 2010;58:15–25. doi: 10.1007/s00005-009-0057-2. [DOI] [PubMed] [Google Scholar]
- 8.Bullard RS, Gibson W, Bose SK, Belgrave JK, Eaddy AC, et al. Functional analysis of the host defense peptide Human Beta Defensin-1: new insight into its potential role in cancer. Mol Immunol. 2008;45:839–848. doi: 10.1016/j.molimm.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell EL, Serhan CN, Colgan SP. Antimicrobial aspects of inflammatory resolution in the mucosa: a role for proresolving mediators. J Immunol. 2011;187:3475–3481. doi: 10.4049/jimmunol.1100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrassi A, Abati S, Santarelli G, Vogel G. Periodontitis in a patient with chronic neutropenia. J Periodontol. 1989;60:352–357. doi: 10.1902/jop.1989.60.6.352. [DOI] [PubMed] [Google Scholar]
- 11.Chaly YV, Paleolog EM, Kolesnikova TS, Tikhonov, Petratchenko EV, Voitenok NN. Neutrophil alpha-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur Cytokine Netw. 2000;11:257–266. [PubMed] [Google Scholar]
- 12.Champagne CM, Buchanan W, Reddy MS, Preisser JS, Beck JD, Offenbacher S. Potential for gingival crevice fluid measures as predictors of risk for periodontal diseases. Periodontol 2000. 2003;31:167–180. doi: 10.1034/j.1600-0757.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- 13.Coats SR, Jones JW, Do CT, Braham PH, Bainbridge BW, et al. Human Toll-like receptor 4 responses to P gingivalis are regulated by lipid A 1- and 4′-phosphatase activities. Cell Microbiol. 2009;11:1587–1599. doi: 10.1111/j.1462-5822.2009.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis MA, Percival RS, Devine D, Darveau RP, Coats SR, et al. Temperature-dependent modulation of Porphyromonas gingivalis lipid A structure and interaction with the innate host defenses. Infect Immun. 2011;79:1187–1193. doi: 10.1128/IAI.00900-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.da Silva BR, de Freitas VA, Nascimento-Neto LG, Carneiro VA, Arruda FV, et al. Antimicrobial peptide control of pathogenic microorganisms of the oral cavity: a review of the literature. Peptides. 2012;36:315–321. doi: 10.1016/j.peptides.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Dale BA, Fredericks LP. Antimicrobial peptides in the oral environment: expression and function in health and disease. Curr Issues Mol Biol. 2005;7:119–133. doi: 10.1093/jac/dki103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dale BA, Krisanaprakornkit S. Defensin antimicrobial peptides in the oral cavity. J Oral Pathol Med. 2001;30:321–327. doi: 10.1034/j.1600-0714.2001.300601.x. [DOI] [PubMed] [Google Scholar]
- 18.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 19.Darveau RP, Tanner A, Page RC. The microbial challenge in periodontitis. Periodontol 2000. 1997;14:12–32. doi: 10.1111/j.1600-0757.1997.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 20.Diamond G, Ryan L. Beta-defensins: what are they really doing in the oral cavity? Oral Dis. 2011;17:628–635. doi: 10.1111/j.1601-0825.2011.01799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamond G, Beckloff N, Ryan LK. Host defense peptides in the oral cavity and the lung: similarities and differences. J Dent Res. 2008;87:915–927. doi: 10.1177/154405910808701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 23.Dixon DR, Bainbridge BW, Darveau RP. Modulation of the innate immune response within the periodontium. Periodontol 2000. 2004;35:53–74. doi: 10.1111/j.0906-6713.2004.003556.x. [DOI] [PubMed] [Google Scholar]
- 24.Eskan MA, Benakanakere MR, Rose BG, Zhang P, Zhao J, et al. Interleukin-1beta modulates proinflammatory cytokine production in human epithelial cells. Infect Immun. 2008;76:2080–2089. doi: 10.1128/IAI.01428-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganz T, Lehrer RI. Defensins. Curr Opin Immunol. 1994;6:584–589. doi: 10.1016/0952-7915(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 26.Garcia JR, Krause A, Schulz S, Rodriguez-Jimenez FJ, Kluver E, et al. Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001;15:1819–1821. [PubMed] [Google Scholar]
- 27.Gemmell E, Walsh LJ, Savage NW, Seymour GJ. Adhesion molecule expression in chronic inflammatory periodontal disease tissue. J Periodontal Res. 1994;29:46–53. doi: 10.1111/j.1600-0765.1994.tb01090.x. [DOI] [PubMed] [Google Scholar]
- 28.Giannobile WV. Crevicular fluid biomarkers of oral bone loss. Curr Opin Periodontol. 1997;4:11–20. [PubMed] [Google Scholar]
- 29.de Gomes PS, Fernandes MH. Defensins in the oral cavity: distribution and biological role. J Oral Pathol Med. 2010;39:1–9. doi: 10.1111/j.1600-0714.2009.00832.x. [DOI] [PubMed] [Google Scholar]
- 30.Gorr SU, Abdolhosseini M. Antimicrobial peptides and periodontal disease. J Clin Periodontol. 2011;38 (Suppl 11):126–141. doi: 10.1111/j.1600-051X.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 31.Gupta S, Ghosh SK, Scott ME, Bainbridge B, Jiang B, et al. Fusobacterium nucleatum-associated beta-defensin inducer (FAD-I): identification, isolation, and functional evaluation. J Biol Chem. 2010;285:36523–36531. doi: 10.1074/jbc.M110.133140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gursoy UK, Kononen E. Understanding the roles of gingival beta-defensins. J Oral Microbiol. 2012:4. doi: 10.3402/jom.v4i0.15127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hakkinen L, Uitto VJ, Larjava H. Cell biology of gingival wound healing. Periodontol 2000. 2000;24:127–152. [PubMed] [Google Scholar]
- 34.Hart TC, Shapira L, Van Dyke TE. Neutrophil defects as risk factors for periodontal diseases. J Periodontol. 1994;65:521–529. doi: 10.1902/jop.1994.65.5s.521. [DOI] [PubMed] [Google Scholar]
- 35.Hensel JA, Chanda D, Kumar S, Sawant A, Grizzle WE, et al. LL-37 as a therapeutic target for late stage prostate cancer. Prostate. 2011;71:659–670. doi: 10.1002/pros.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin L. An update on innate defense molecules of human gingiva. Periodontol 2000. 2011;56:125–142. doi: 10.1111/j.1600-0757.2010.00364.x. [DOI] [PubMed] [Google Scholar]
- 37.Joly S, Maze C, McCray PB, Jr, Guthmiller JM. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol. 2004;42:1024–1029. doi: 10.1128/JCM.42.3.1024-1029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamma J, Mombelli A, Tsinidou K, Vasdekis V, Giannopoulou C. Cytokines in gingival crevicular fluid of adolescents and young adults. Oral Microbiol Immunol. 2009;24:7–10. doi: 10.1111/j.1399-302X.2008.00466.x. [DOI] [PubMed] [Google Scholar]
- 39.Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohlgraf KG, Pingel LC, Dietrich DE, Brogden KA. Defensins as anti-inflammatory compounds and mucosal adjuvants. Future Microbiol. 2010;5:99–113. doi: 10.2217/fmb.09.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohlgraf KG, Ackermann A, Lu X, Burnell K, Belanger M, et al. Defensins attenuate cytokine responses yet enhance antibody responses to Porphyromonas gingivalis adhesins in mice. Future Microbiol. 2010;5:115–125. doi: 10.2217/fmb.09.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 43.Krisanaprakornkit S, Kimball JR, Weinberg A, Darveau RP, Bainbridge BW, Dale BA. Inducible expression of human beta-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect Immun. 2000;68:2907–2915. doi: 10.1128/iai.68.5.2907-2915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kucukkolbasi H, Kucukkolbasi S, Dursun R, Ayyildiz F, Kara H. Determination of defensin HNP-1 in human saliva of patients with oral mucosal diseases. J Immunoassay Immunochem. 2011;32:284–295. doi: 10.1080/15321819.2011.569045. [DOI] [PubMed] [Google Scholar]
- 45.Lehrer RI, Lu W. alpha-Defensins in human innate immunity. Immunol Rev. 2012;245:84–112. doi: 10.1111/j.1600-065X.2011.01082.x. [DOI] [PubMed] [Google Scholar]
- 46.Lehrer RI, Barton A, Daher KA, Harwig SS, Ganz T, Selsted ME. Interaction of human defensins with Escherichia coli Mechanism of bactericidal activity. J Clin Invest. 1989;84:553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madhwani T, McBain AJ. Compositional modification of nascent in vitro dental plaques by human host-defence peptides. FEMS Immunol Med Microbiol. 2012;64:374–381. doi: 10.1111/j.1574-695X.2011.00922.x. [DOI] [PubMed] [Google Scholar]
- 48.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 49.McCormick TS, Weinberg A. Epithelial cell-derived antimicrobial peptides are multifunctional agents that bridge innate and adaptive immunity. Periodontol 2000. 2010;54:195–206. doi: 10.1111/j.1600-0757.2010.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mookherjee N, Brown KL, Bowdish DM, Doria S, Falsafi R, et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J Immunol. 2006;176:2455–2464. doi: 10.4049/jimmunol.176.4.2455. [DOI] [PubMed] [Google Scholar]
- 51.Moughal NA, Adonogianaki E, Thornhill MH, Kinane DF. Endothelial cell leukocyte adhesion molecule-1 (ELAM-1) and intercellular adhesion molecule-1 (ICAM-1) expression in gingival tissue during health and experimentally-induced gingivitis. J Periodontal Res. 1992;27:623–630. doi: 10.1111/j.1600-0765.1992.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 52.Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, et al. Augmentation of the lipopolysaccharide-neutralizing activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by replacement with hydrophobic and cationic amino acid residues. Clin Diagn Lab Immunol. 2002;9:972–982. doi: 10.1128/CDLI.9.5.972-982.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, et al. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 2007;127:594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 54.Nylander K, Danielsen B, Fejerskov O, Dabelsteen E. Expression of the endothelial leukocyte adhesion molecule-1 (ELAM-1) on endothelial cells in experimental gingivitis in humans. J Periodontol. 1993;64:355–357. doi: 10.1902/jop.1993.64.5.355. [DOI] [PubMed] [Google Scholar]
- 55.Otte JM, Werner I, Brand S, Chromik AM, Schmitz F, et al. Human beta defensin 2 promotes intestinal wound healing in vitro. J Cell Biochem. 2008;104:2286–2297. doi: 10.1002/jcb.21787. [DOI] [PubMed] [Google Scholar]
- 56.Otto M. Bacterial sensing of antimicrobial peptides. Contrib Microbiol. 2009;16:136–149. doi: 10.1159/000219377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Page RC, Beatty P, Waldrop TC. Molecular basis for the functional abnormality in neutrophils from patients with generalized prepubertal periodontitis. J Periodontal Res. 1987;22:182–183. doi: 10.1111/j.1600-0765.1987.tb01562.x. [DOI] [PubMed] [Google Scholar]
- 58.Pingel LC, Kohlgraf KG, Hansen CJ, Eastman CG, Dietrich DE, et al. Human beta-defensin 3 binds to hemagglutinin B (rHagB), a non-fimbrial adhesin from Porphyromonas gingivalis, and attenuates a pro-inflammatory cytokine response. Immunol Cell Biol. 2008;86:643–649. doi: 10.1038/icb.2008.56. [DOI] [PubMed] [Google Scholar]
- 59.Presicce P, Giannelli S, Taddeo A, Villa ML, Della Bella S. Human defensins activate monocyte-derived dendritic cells, promote the production of proinflammatory cytokines, and up-regulate the surface expression of CD91. J Leukoc Biol. 2009;86:941–948. doi: 10.1189/jlb.0708412. [DOI] [PubMed] [Google Scholar]
- 60.Roberts FA, Darveau RP. Beneficial bacteria of the periodontium. Periodontol 2000. 2002;30:40–50. doi: 10.1034/j.1600-0757.2002.03004.x. [DOI] [PubMed] [Google Scholar]
- 61.Rosenfeld Y, Papo N, Shai Y. Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides. Peptide properties and plausible modes of action. J Biol Chem. 2006;281:1636–1643. doi: 10.1074/jbc.M504327200. [DOI] [PubMed] [Google Scholar]
- 62.Schiott CR, Loe H. The origin and variation in number of leukocytes in the human saliva. J Periodontal Res. 1970;5:36–41. doi: 10.1111/j.1600-0765.1970.tb01835.x. [DOI] [PubMed] [Google Scholar]
- 63.Schramm R, Thorlacius H. Neutrophil recruitment in mast cell-dependent inflammation: inhibitory mechanisms of glucocorticoids. Inflamm Res. 2004;53:644–652. doi: 10.1007/s00011-004-1307-8. [DOI] [PubMed] [Google Scholar]
- 64.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 65.Suphasiriroj W, Mikami M, Shimomura H, Sato S. Specificity of antimicrobial peptide LL-37 to neutralize periodontopathogenic lipopolysaccharide activity in human oral fibroblasts. J Periodontol. 2012 doi: 10.1902/jop.2012.110652. [DOI] [PubMed] [Google Scholar]
- 66.Tonetti MS. Molecular factors associated with compartmentalization of gingival immune responses and transepithelial neutrophil migration. J Periodontal Res. 1997;32:104–109. doi: 10.1111/j.1600-0765.1997.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 67.Tonetti MS, Imboden MA, Lang NP. Neutrophil migration into the gingival sulcus is associated with transepithelial gradients of interleukin-8 and ICAM-1. J Periodontol. 1998;69:1139–1147. doi: 10.1902/jop.1998.69.10.1139. [DOI] [PubMed] [Google Scholar]
- 68.Tonetti MS, Imboden MA, Gerber L, Lang NP, Laisue J, Mueller C. Localized expression of mRNA for phagocyte-specific hemotactic cytokines in human periodontal infections. Infect Immun. 1994;62:4005–4014. doi: 10.1128/iai.62.9.4005-4014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsukamoto Y, Usui M, Yamamoto G, Takagi Y, Tachikawa T, et al. Role of the junctional epithelium in periodontal innate defense and homeostasis. J Periodontal Res. 2012 doi: 10.1111/j.1600-0765.2012.01490.x. [DOI] [PubMed] [Google Scholar]
- 70.Turkoglu O, Kandiloglu G, Berdeli A, Emingil G, Atilla G. Antimicrobial peptide hCAP-18/LL-37 protein and mRNA expressions in different periodontal diseases. Oral Dis. 2011;17:60–67. doi: 10.1111/j.1601-0825.2010.01704.x. [DOI] [PubMed] [Google Scholar]
- 71.Valore EV, Ganz T. Posttranslational processing of defensins in immature human myeloid cells. Blood. 1992;79:1538–1544. [PubMed] [Google Scholar]
- 72.Van Dyke TE. The management of inflammation in periodontal disease. J Periodontol. 2008;79:1601–1608. doi: 10.1902/jop.2008.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waldrop TC, Anderson DC, Hallmon WW, Schmalstieg FC, Jacobs RL. Periodontal manifestations of the heritable Mac-1, LFA-1, deficiency syndrome. Clinical, histopathologic and molecular characteristics. J Periodontol. 1987;58:400–416. doi: 10.1902/jop.1987.58.6.400. [DOI] [PubMed] [Google Scholar]
- 74.Walters SM, Dubey VS, Jeffrey NR, Dixon DR. Antibiotic-induced Porphyromonas gingivalis LPS release and inhibition of LPS-stimulated cytokines by antimicrobial peptides. Peptides. 2010;31:1649–1653. doi: 10.1016/j.peptides.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 75.Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 76.Yang J, Hooper WC, Phillips DJ, Talkington DF. Interleukin-1beta responses to Mycoplasma pneumoniae infection are cell-type specific. Microb Pathog. 2003;34:17–25. doi: 10.1016/s0882-4010(02)00190-0. [DOI] [PubMed] [Google Scholar]
- 77.Yeung AT, Gellatly SL, Hancock RE. Multifunctional cationic host defence peptides and their clinical applications. Cell Mol Life Sci. 2011;68:2161–2176. doi: 10.1007/s00018-011-0710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yin L, Chino T, Horst OV, Hacker BM, Clark EA, et al. Differential and coordinated expression of defensins and cytokines by gingival epithelial cells and dendritic cells in response to oral bacteria. BMC Immunol. 2010;11:37. doi: 10.1186/1471-2172-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang H, Porro G, Orzech N, Mullen B, Liu M, Slutsky AS. Neutrophil defensins mediate acute inflammatory response and lung dysfunction in dose-related fashion. Am J Physiol Lung Cell Mol Physiol. 2001;280:L947–954. doi: 10.1152/ajplung.2001.280.5.L947. [DOI] [PubMed] [Google Scholar]


