Abstract
Clinical trial data on selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs) have demonstrated reduced breast cancer incidence in the prevention setting among high-risk women. We conducted an extensive review of clinical trials and recent published reports of barriers to uptake of breast cancer chemoprevention, to provide health care professionals with information to improve decision-making regarding chemoprevention. Despite the positive results of these trials, uptake of chemoprevention has been low due to barriers in identifying high-risk women, lack of understanding of risks and benefits, as well as concerns about side effects. Interventions designed to increase uptake have met with limited success. Clinicians can support women in informed decision-making about SERMs and AIs by effectively communicating breast cancer risk and enhancing knowledge about the risks and benefits of chemoprevention. Promoting uptake and adherence to chemoprevention holds promise for reducing the public health burden of this disease.
Keywords: Breast cancer, Chemoprevention, Selective estrogen receptor modulator, SERM, Aromatase inhibitor, AI, Prevention, Risk
Introduction
Breast cancer confers significant morbidity and mortality on women in the U.S., and the primary prevention of this disease is a major public health issue. Breast cancer risk assessment and available interventions for prevention, such as chemoprevention, are underutilized in the U.S. Selective estrogen receptor modulators (SERMs), tamoxifen and raloxifene, have been shown to reduce breast cancer incidence by up to 50% among high-risk women [1, 2]. Approximately 15% of women age 35–79 years are at increased risk for breast cancer development and are potentially eligible for a SERM [3], but uptake has been poor in the prevention setting [4]. Reasons for lack of SERM uptake for chemoprevention include inadequate time for counseling, an insufficient level of knowledge and information about risk-reduction strategies, and public misconceptions about the risks of SERMs [5]. A recent clinical trial of the aromatase inhibitor (AI), exemestane, among high-risk postmenopausal women reported promising results after short-term follow-up with a favorable safety profile [6]. We conducted an extensive review of chemoprevention trials of SERMs and AIs, as well as recent published reports of barriers to uptake of breast cancer chemoprevention, to provide up-to-date information for health care professionals to improve clinical decision-making.
Breast Cancer Chemopreventive Agents
Selective Estrogen Receptor Modulators (SERMs)
Table 1 summarizes the results of randomized controlled trials of SERMs and AIs for the primary prevention of breast cancer. In 1998, the Breast Cancer Prevention Trial or NSABP-P1 trial demonstrated that the SERM, tamoxifen, given for 5 years reduced breast cancer incidence in high-risk women by 49% (absolute risk of 2.48% vs. 4.25% for placebo, p<.001) [1, 7]. The updated results from 3 additional randomized placebo-controlled trials of tamoxifen (International Breast cancer Intervention Study [IBIS-1], Royal Marsden and Italian trials) demonstrated a 30–40% reduction in estrogen receptor (ER)-positive invasive breast cancer [8–10]. Conversely, tamoxifen had no effect on the incidence of ER-negative tumors. Of note, none of these chemoprevention trials were adequately powered to assess the effects of tamoxifen on breast cancer mortality. Also, limited data exist on the efficacy of tamoxifen for breast cancer risk reduction in BRCA1 and BRCA2 mutation carriers.
Table 1.
Breast cancer chemoprevention trials of selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs)
| No. of Participants |
Eligibility | Duration of Intervention (years) |
Median Follow-up (months) |
Invasive BC rate/1000 person-years |
Invasive BC RR (95% CI) |
ER-positive BC RR (95% CI) |
ER-negative BC RR (95% CI) |
|
|---|---|---|---|---|---|---|---|---|
| Tamoxifen (20mg/d) vs. Placebo | ||||||||
| NSABP P-1 (2005) [7] | 13,388 | Age ≥60 yrs or 35–59 yrs and 5-yr Gail risk >1.66% or LCIS | 5 | 84 | 3.59 vs. 6.29 | 0.57 (0.46–0.70) | 0.38 (0.28–0.50) | 1.31 (0.86–2.01) |
| IBIS-1 (2007) [10] | 7145 | Age 45–70 yrs with 2-fold risk or 40–44 yrs with 4-fold risk or 35–39 yrs with 10-fold risk | 5 | 96 | 4.34 vs. 5.88 | 0.74 (0.58–0.94) | 0.66 (0.50–0.87) | 1.00 (0.61–1.65) |
| Royal Marsden Trial (2007) [8] | 2494 | Age 30–70 yrs, 1° relative with BC at age<50 or 1° bilateral BC or 1° BC plus another 1° or 2° or BBD with 1° relative | 8 | 158 | 4.8 vs. 6.1 | 0.78 (0.58–1.04) | 0.61 (0.43–0.86) | 1.4 (0.7–2.6) |
| Italian Study (2007) [9] | 5408 | Age 35–70 yrs, avg BC risk, hysterectomy | 5 | 132 | 1.77 vs. 2.21 | 0.80 (0.56–1.15) | 0.77 (0.51–1.16) | 1.10 (0.59–2.05) |
| Raloxifene (60mg or 120mg/d) vs. Placebo | ||||||||
| MORE (1999) [11] | 7705 | Age <80 yrs, at least 2 yrs postmenopausal, osteoporosis | 3 | 40 | 0.87 vs. 3.61 | 0.24 (0.13–0.44) | 0.10 (0.04–0.24) | 0.88 (0.26–3.00) |
| CORE (2004) [12] | 5213 | Subset of MORE cohort | 4 | 95 | 2.1 vs. 5.2 | 0.41 (0.24–0.71) | 0.34 (0.18–0.66) | 1.13 (0.29–4.35) |
| RUTH (2006) [13] | 10,101 | Age ≥55 yrs, at least 1 yr postmenopausal, CHD or at increased risk for CHD | 5 | 67 | 0.15 vs. 0.27* | 0.56 (0.38–0.83) | 0.45 (0.28–0.72) | 1.44 (0.61–3.36) |
| Lasofoxifene (0.25mg or 0.5mg/d) vs. Placebo | ||||||||
| PEARL (2010) [15] | 8556 | Age 59–80 yrs, postmenopausal, osteoporosis | 5 | 60 | 0.41 vs. 1.97 | 0.21 (0.08–0.55) | 0.17 (0.05–0.57) | 0.35 (0.04–3.34) |
| Arzoxifene (20mg/d) vs. Placebo | ||||||||
| GENERATIONS (2011) [16] | 9354 | Age 60–85 yrs, postmenopausal, osteoporosis | 4 | 48 | 0.25 vs. 0.58* | 0.44 (0.26–0.76) | NS | NS |
| Raloxifene (60mg/d) vs. Tamoxifen (20mg/d) | ||||||||
| STAR (2010) [18] | 19,747 | Age ≥35 yrs, postmenopausal, 5-yr Gail risk ≥1.66% | 5 | 81 | 5.02 vs. 4.04 | 1.24 (1.05–1.47) | 0.93 (0.72–1.24) | 1.15 (0.75–1.77) |
| Exemestane (25mg/d) vs. Placebo | ||||||||
| ExCel/MAP3 (2011) [6] | 4560 | Age ≥35 yrs, at least 1 yr postmenopausal, Age >60 yrs or 5-yr Gail risk ≥1.66% or prior AH, LCIS, DCIS with mastectomy | 5 | 35 | 0.19 vs. 0.55* | 0.35 (0.18–0.70) | 0.27 (0.12–0.60) | 0.80 (0.21–2.98) |
Abbreviations: AH, atypical hyperplasia; BC, breast cancer; BBD, benign breast disease; CI, confidence interval; CHD, coronary heart disease; CORE, Continuing Outcomes Relevant to Evista; DCIS, ductal carcinomain situ; ER, estrogen receptor; GENERATIONS, Global Investigation to Determine Efficacy of Arzoxifene on At-risk Postmenopausal Patients; IBIS, International Breast cancer Intervention Study; LCIS, lobular carcinoma in situ; MORE, Multiple Outcomes of Raloxifene; NS, not stated; NSABP, National Surgical Adjuvant Breast and Bowel Project; PEARL, Postmenopausal Evaluation And Risk-reduction with Lasofoxifene; RR, relative risk; RUTH, Raloxifene Use for The Heart; STAR, Study of Tamoxifen and Raloxifene.
Annual Incidence (%)
Another SERM, raloxifene, has been shown to reduce the incidence of breast cancer in clinical trials for the treatment and prevention of osteoporosis [11, 12]. Among postmenopausal women with osteoporosis, raloxifene given for up to 7 years reduced the incidence of invasive breast cancer by 76%, with the majority of the benefit restricted to ER-positive tumors [11]. More recently, the Raloxifene Use for The Heart (RUTH) trial among postmenopausal with coronary heart disease (CHD) risk factors demonstrated that raloxifene reduced the risk of invasive breast cancer and vertebral fracture, but had no affect on the risk of CHD [13]. Two additional SERMs, lasofoxifene and arzoxifene, tested in postmenopausal women with osteoporosis demonstrated similar 70–80% reductions in ER-positive breast cancer incidence compared to placebo [14–16]. However, the results of these trials suggest that these agents do not offer major clinical benefits for breast, bone, or cardiovascular health over currently available SERMs [17].
The Study of Tamoxifen and Raloxifene (STAR) trial is the largest breast cancer chemoprevention trial to date with 19,747 women randomized and a median of 81 months of follow-up [2, 18]. Updated analyses from the STAR trial showed that raloxifene retained 76% of the long-term efficacy of tamoxifen in preventing invasive breast cancer among high-risk postmenopausal women with a more favorable side effect profile [18]. The difference in endometrial cancer risk between the two SERMs while not significant in the initial report was significantly higher for the tamoxifen group with longer follow-up. Based upon the results of these trials, tamoxifen was approved by the U.S. Food and Drug Administration (FDA) for breast cancer risk reduction among high-risk women in 1998 and raloxifene in 2007.
Aromatase Inhibitors (AIs)
The risk of developing contralateral tumors is 50% lower in postmenopausal breast cancer patients receiving an AI compared to tamoxifen in the adjuvant setting [19, 20]. Data from adjuvant trials have proven to be a useful model for testing the chemopreventive effects of hormonal therapies, since the results of the prevention trials closely mirrored those of adjuvant studies [21]. The MAP.3 trial, a randomized double-blind placebo-controlled trial of exemestane 25mg daily for 5 years in 4560 high-risk postmenopausal women, is the first chemoprevention trial of an AI [6]. The primary outcome was incidence of invasive breast cancer, with combined invasive and noninvasive breast cancer as well as ER-positive tumors as secondary endpoints. Postmenopausal women with at least one of the following risk factors were enrolled: age 60 years or older, a 5-year breast cancer risk greater than 1.66% according to the Gail model, prior atypical hyperplasia (AH) or lobular carcinoma in situ (LCIS), or ductal carcinoma in situ (DCIS) with mastectomy [6, 22]. The majority of participants were white (93%), and the main high-risk criteria were age 60 years or older (49%), Gail risk >1.66% (40%), AH or LCIS (8%), and DCIS (3%) [6]. After a median follow-up of 35 months, 11 invasive breast cancer events occurred in the exemestane arm compared to 32 in the placebo group. Exemestane reduced invasive breast cancer incidence by 65% (absolute risk of 0.48% vs. 1.41% for placebo, p=0.002) [6]. In the exemestane group, more grade 2 or higher arthritis (6.5% vs. 4.0% for placebo) and hot flashes (18.3% vs. 11.9% for placebo) were seen. Approximately 85% of women were compliant with study treatments and early discontinuation for toxic effects was 15.4% in the exemestane group vs. 10.8% for placebo. No significant differences in new-onset osteoporosis, clinical skeletal fractures, cardiovascular events, or other malignancies were detected, and overall health-related quality of life did not differ between the two groups. The main limitations of the trial were the relatively short-term follow-up of 3 years, the small number of breast cancer events, and the lack of a direct comparison to SERMs.
Another third-generation AI, anastrozole, is being actively investigated in the prevention setting in the IBIS-II trial, which randomizes high-risk postmenopausal women ages 40 to 70 years to either anastrozole (1mg/d) or placebo for 5 years [23]. The primary endpoint is invasive breast cancer incidence. To date, there are no head-to-head trials comparing SERMs to AIs in the primary prevention setting. The Study to Evaluate Letrozole and Raloxifene (STELLAR) or NSABP-P4 was designed to address this question [24]. However, a National Cancer Institute review panel halted initiation of the STELLAR trial due to financial constraints and concerns about low uptake of chemoprevention.
Barriers to Uptake of Breast Cancer Chemoprevention
Based on age and breast cancer risk, an estimated 10 million women in the U.S. may be eligible for chemoprevention [3], but less than 5% of high-risk women agree to take a SERM [4]. Data from the National Health Interview Survey (NHIS) indicated that the prevalence of tamoxifen use among women without a personal history of breast cancer was merely 0.2% in 2000 and decreased to 0.08% by 2005 [25]. Similarly, raloxifene use decreased after FDA approval of this drug for breast cancer risk reduction [5]. It remains to be seen whether there will be greater acceptance of AIs in the prevention setting.
Identification of high-risk women
Low SERM uptake is partly due to the lack of effective strategies to identify high-risk women. The Gail model (www.cancer.gov/bcrisktool) or Breast Cancer Risk Assessment Tool (BCRAT), which takes into account a woman’s age, race, reproductive history, family history, and benign breast disease, is the most commonly used model in the U.S. and has been well-validated at the population level [26]. It can be used in women age 35 years or older and provides an individual’s absolute 5-year and lifetime risk of invasive breast cancer compared to the general population. High-risk criteria used to determine eligibility in chemoprevention trials are at least a 1.67% 5-year risk or 20% or greater lifetime risk of breast cancer. The Gail model recently incorporated the Women’s Contraceptive and Reproductive Experiences (CARE) [27] model and Asian American Breast Cancer Study (AABCS) [28] model to provide more sensitive estimates for black and Asian American women, respectively. Few studies have used this model in Hispanic populations [29, 30]. Eligibility for chemoprevention among U.S. women varies dramatically by race/ethnicity: 18.7% of whites, 5.7% of blacks, 2.9% of Hispanics [3]. Not meeting high-risk criteria may be an initial barrier to chemoprevention uptake among minority groups.
In women with a strong family history of breast cancer (i.e., two or more affected family members, particularly those with an early age of onset), the IBIS or Tyrer-Cuzick model (www.ems-trials.org/riskevaluator), is useful, because it incorporates a detailed family history including second- and third-degree family history of breast and ovarian cancer and their age at diagnosis [31]. The IBIS model estimates the risk of developing invasive breast cancer and DCIS and the probability of carrying a mutation in the BRCA1 and BRCA2 genes [31]. This model may be particularly relevant for calculating risk in women with affected family members. Despite the availability of both the Gail and IBIS breast cancer risk assessment tools on-line, only 18% of primary care physicians report use of software to calculate breast cancer risk [32]. Barriers to assessment of risk in the primary care setting include time constraints during clinic visits and lack of confidence in knowledge about risk assessment [33].
To address this barrier of poor identification of high-risk women, the “Ready, Set, GO GAIL!” project screened for high-risk women with routine use of the Gail model in women age 35 to 70 years who presented for a comprehensive physical examination at the Aurora Health Care women’s center in Wisconsin [34]. During the first year, 5,718 Gail model scores were calculated and 15.2% of women were deemed high risk. At our institution, we are embarking on a project entitled the Breast cancer Family-based Intervention Trial (BFIT) which will target women with a first-degree family history of breast cancer in the clinic and community-based settings [35]. These women may be at high risk for breast cancer owing to shared hereditary and lifestyle factors and may be unaware of their elevated risk. Providing information and education about personal risk at a time when a close relative is diagnosed with breast cancer represents a “teachable moment” when women may want to take personal action for disease prevention.
Risks and Benefits of Chemoprevention
Another reason for low SERM uptake is the perception of patients and physicians that chemoprevention does not offer a favorable risk-benefit ratio [36–39]. Whereas the side effects diminish after stopping 5 years of tamoxifen, the protective effect on breast cancer risk persists for up to 10 years. In the IBIS-1 trial in which participants remained blinded after the primary results were published, no diminution of benefit was observed for up to 10 years after randomization [10]. Although the short-term absolute benefits of tamoxifen may seem small, if the risk-reducing effects of chemoprevention persist for a woman’s lifetime, then the absolute benefit in terms of breast cancer risk reduction would exceed 10% (among women with a lifetime risk >20%). Unlike preventive therapies for other chronic diseases which often require life-long treatment, breast cancer chemoprevention for 5 years can confer long-term benefits with side effects limited to the period of active treatment.
Concern about potential toxicities, such as endometrial cancer, thromboembolic events and menopausal symptoms, is the main contributor to a woman’s unwillingness to try a SERM and a physician’s reluctance to prescribe it [36–38, 40–43]. In a meta-analysis of chemoprevention trials, Cuzick et al. [21] reported a 2.4-fold increase in endometrial cancer (absolute risk of 0.37% vs. 0.15% for placebo) and a 1.9-fold increase in thromboembolic events (absolute risk of 0.83% vs. 0.44% for placebo) with tamoxifen use. In the NSABP-P1 trial, the elevated risk of endometrial cancer was only observed in women over age 50 [1]. In other trials, the risk of endometrial cancer and thromboembolic events was not observed after stopping tamoxifen [8, 10]. Increased vaginal discharge (55% vs. 34% in controls) and hot flashes (78% vs. 65% in controls) and a 14% increase in the incidence of cataracts were reported with tamoxifen during treatment [44]. In the STAR trial, raloxifene was associated with a lower risk of thromboembolic disease, benign uterine complaints, and cataracts compared to tamoxifen [2, 45]. Women on tamoxifen reported more gynecologic and vasomotor symptoms [2], however, overall quality of life was similar in both arms of the STAR trial [45]. AIs do not have the serious side effects of tamoxifen, but are associated with an increased risk of fracture, musculoskeletal side effects, and elevated cholesterol [46–48]. Tamoxifen and raloxifene have a similar favorable effect on bone density with about a 32% reduction in fracture incidence [1, 11, 12].
Low SERM uptake is partly due to fear of side effects, but also due to the lack of effective strategies to inform high-risk women and their health care providers about the risks and benefits of chemoprevention. Physicians who felt insufficiently informed about risk reduction options were less than half as likely to prescribe a SERM than physicians who felt sufficiently trained [49]. Physician recommendation and health care provider communication are among the most influential factors to impact SERM use [36, 38, 50]. Freedman et al. recently published models to predict the risks and benefits of SERMs for women over age 50, based upon age, race/ethnicity, 5-year Gail risk score, and presence of a uterus [51]. Tools such as this can be incorporated into the clinical setting as an aid for health care providers and high-risk women in chemoprevention decision-making.
Adherence to Endocrine Therapy
The efficacy of chemoprevention depends not only on initiation of therapy, but also long-term adherence and persistence of therapy for the planned duration. Poor adherence to tamoxifen tends to attenuate the drug’s therapeutic effects [52]. In a study with 12 years of follow-up, breast cancer patients with 80% or higher adherence had a ≥26% lower risk for recurrence compared with patients with <80% adherence [53]. In the adjuvant setting by year 4 to 5 of treatment, the full adherence rate drops to 50% [54, 55]. In the SERM chemoprevention trials, adherence at 5 years ranged from 64% to 76.3% [1, 10, 45], with the caveat that clinical trial participants are often more compliant than the general population. Veronesi et al. reported that women in a chemoprevention trial were less likely to adhere to tamoxifen than breast cancer patients treated in the adjuvant setting, because higher perceived health risk increases adherence [56]. In the NSABP-P1 trial, low income and Hispanic ethnicity were associated with low adherence [57]. A substudy of the MAP.3 trial also found worse adherence for ethnic minorities vs. whites [58]. Recognizing predictors of adherence to endocrine therapies will assist in the development of targeted interventions that promote adherence to chemoprevention.
Interventions to Increase Uptake of Breast Cancer Chemoprevention
A systematic review published in 2010 addressed patient decisions about breast cancer chemoprevention and found that perceived vulnerability to breast cancer correlated with increased uptake, whereas concern for adverse effects was associated with reduced uptake [4]. This meta-analysis included 13 studies, which were limited by the use of descriptive study designs and the lack of validated survey instruments or reproducible sampling strategies that would enhance generalizability of the results. Table 2 summarizes more recent intervention studies designed to increase SERM uptake for chemoprevention. Interventions involving reading materials or decision aids met with limited success, ranging from 0.5% to 5.6% [38, 39, 42, 59, 60]. In a recent randomized controlled trial of a web-based decision aid which informed women about the risks and benefits of SERMs [59], only 0.5% of 712 eligible participants started raloxifene and none started tamoxifen.
Table 2.
Summary of intervention studies of uptake of breast cancer chemoprevention
| Author (Year) | Intervention | N | Population | Age Range, years (Mean) |
Chemoprevention Uptake |
|---|---|---|---|---|---|
| Fagerlin et al. (2010) [39] | Tailored on-line decision aid "Guide to Decide" | 632 | 5-yr Gail risk ≥1.66% Healthcare Organizations (Henry Ford Health System in Michigan; Group Health in Washington State) |
40–74 (59) | 0.9% TAM |
| Loehberg et al. (2010) [60] | None; screened for participation in IBIS-II | 2524 | Age 50–69 yrs Mammography screening programs in Germany |
50–69 (59.5) | 0.7% participation rate in AI trial |
| Fagerlin et al. (2011) [59] | Tailored on-line decision aid "Guide to Decide" | 1197 | Age 40–74 yrs, postmenopausal, 5-yr Gail risk ≥1.66% Healthcare Organizations (Henry Ford Health System in Michigan; Group Health in Washington State) |
46–74 (62) | 0% TAM 0.5% RAL |
| Owens et al. (2011) [34] | “Ready, Set, GO GAIL!” project High-risk consultation by primary care provider or referral to breast center |
868 | Age 35–70 yrs, 5-yr BC risk ≥1.7% or lifetime risk ≥20% Aurora Health Care in Wisconsin |
35–70 | 2% |
Abbreviations: AI, aromatase inhibitor; BC, breast cancer; IBIS, International Breast cancer Intervention Study; RAL, raloxifene; TAM, tamoxifen.
Since discussions about chemopreventive agents, which are prescription medications, are traditionally initiated by physicians, clinic-based interventions may be more effective. In the “Ready, Set, GO GAIL!” project [34], women deemed high risk according to the Gail model (5-year risk ≥1.7% or lifetime risk ≥20%) either had a high-risk consultation with their primary care provider or were referred to a comprehensive breast cancer center. However, only 14.7% of high-risk women were referred to the breast center, and only 6.4% completed the consultation. Overall SERM uptake for eligible women was 2% with this clinic-based intervention. Therefore, most intervention studies designed to increase uptake of breast cancer chemoprevention have yielded disappointing results.
However, studies that involved consultation at a specialized breast center reported SERM use ranging from 11% to 58% [36, 40, 41, 50, 61, 62]. Variation in SERM uptake may have been due to the strength of the physician’s recommendation [63]. In our experience at an urban academic breast center, we have a high-risk population comprised of women from diverse racial and ethnic backgrounds (55% white, 32% Hispanic, 7% black, and 6% Asian) who mainly have benign breast disease (AH and LCIS). Our SERM uptake rate among eligible women was 30% in the primary prevention setting [64]. Therefore, higher chemoprevention uptake may be achieved with health professionals that have sufficient knowledge and training about breast cancer risk and risk reduction strategies.
Chemoprevention Guidelines
In 2009, the American Society of Clinical Oncology (ASCO) published consensus guidelines on breast cancer chemoprevention [65]. High-risk premenopausal and postmenopausal women, defined as a 5-year Gail risk ≥1.67% or LCIS, may take tamoxifen for 5 years to reduce the risk of ER-positive breast cancer for up to 10 years. Tamoxifen is favored in high-risk premenopausal women and postmenopausal women with a hysterectomy, whereas raloxifene is preferred for use among high-risk postmenopausal women with an intact uterus and those at risk for osteoporosis. Due to the increased risk of uterine cancer, follow-up for women on tamoxifen should include a baseline gynecologic examination before the initiation of treatment and annually thereafter, with a timely work-up for abnormal vaginal bleeding. Routine endometrial biopsies are not recommended in the absence of abnormal vaginal bleeding. SERMs are contraindicated in women with a history of deep vein thrombosis, pulmonary embolus, stoke, or transient ischemic attack. In addition, the STAR trial excluded women on hormone replacement therapy, those with uncontrolled diabetes mellitus or hypertension, and atrial fibrillation [2]. In 2010, the National Comprehensive Cancer Network (NCCN) published similar clinical practice guidelines for breast cancer risk reduction [66]. However, these guidelines do not incorporate AIs for breast cancer chemoprevention among high-risk postmenopausal women.
Figure 1 represents a potential algorithm for decision-making about SERMs and AIs for breast cancer chemoprevention based upon menopausal status, history of blood clots, risk of osteoporosis, and prior hysterectomy. For high-risk premenopausal women without a personal history of thromboembolic events, the only proven chemopreventive agent is tamoxifen. In the chemoprevention trials, the greatest clinical benefit and fewest side effects were derived from tamoxifen use in young high-risk women, age 35–50 years. If tamoxifen is contraindicated due to a history of blood clots, then participation in a clinical trial or consideration of an AI after menopause is a potential option. Similarly, among high-risk postmenopausal women with a history of thromboembolism all SERMs are contraindicated, therefore, exemestane is currently the only proven agent for breast cancer prevention. If the woman is at highrisk for osteoporosis, then SERMs may be preferred over AIs do to the beneficial effects on reducing fracture risk, although presence of osteoporosis is not an absolute contraindication to taking an AI. For postmenopausal women with a prior hysterectomy, tamoxifen may be favored over raloxifene due to its greater efficacy in lowering invasive breast cancer risk [18]. Overall, all three drugs are effective chemopreventive agents for high-risk postmenopausal women, therefore, the choice will depend upon personal preferences and acceptable toxicities.
Fig. 1.
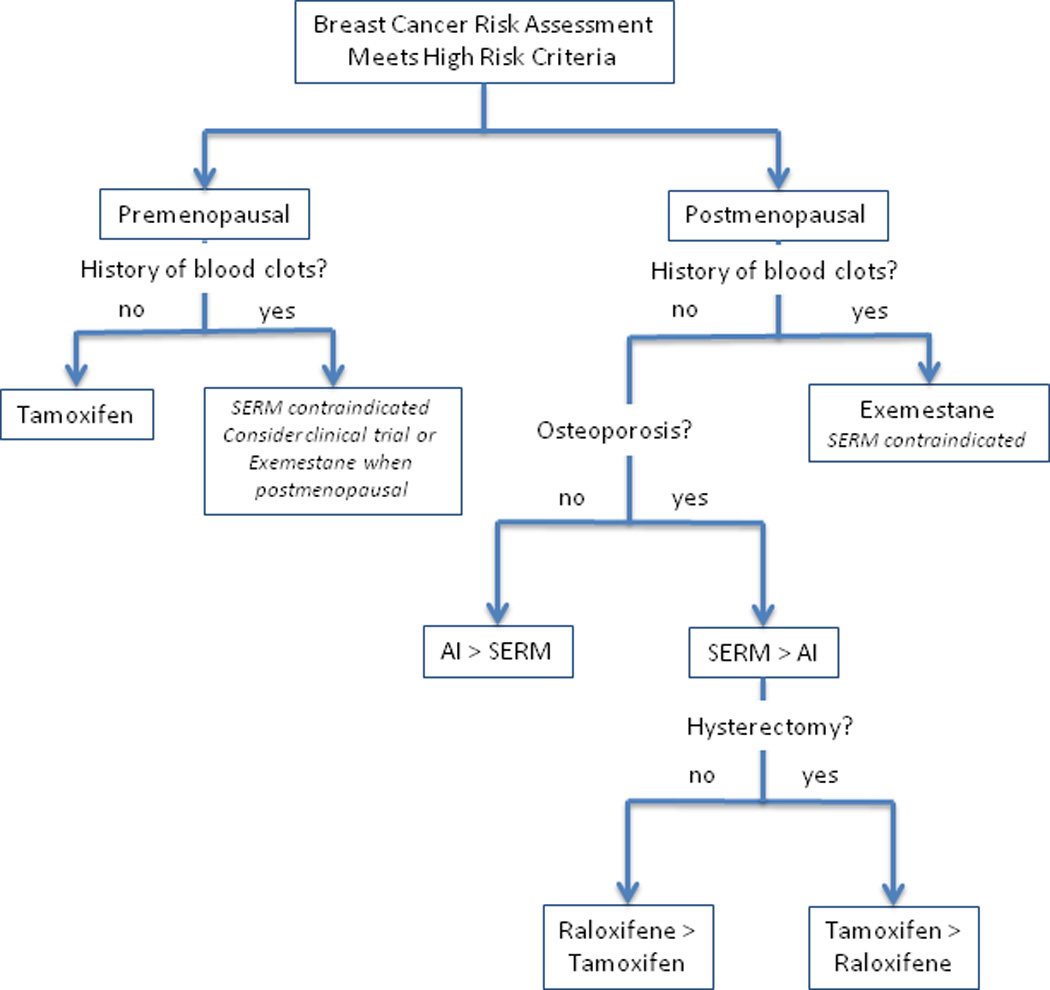
Algorithm to determine appropriate chemopreventive agent in high risk women based upon menopausal status, history of blood clots, risk of osteoporosis, and prior hysterectomy. AI, aromatase inhibitor; SERM, selective estrogen receptor modulator.
Conclusions
Breast cancer chemoprevention has proven efficacy in high-risk populations [1, 2, 18], but these benefits will only be translated to the general population if women adopt and adhere to these chemopreventive agents. Preventive therapy for cancer is currently less well established than in other chronic conditions, such as CHD, and could benefit from lessons learned [67]. Health care providers can do more in the area of cancer prevention by targeting high-risk populations. Chemoprevention needs to be integrated into wider strategies of preventive care that also include non-pharmacologic approaches such as lifestyle modification. Breast cancer screening represents an opportunity to provide advice about breast cancer risk and options for prevention. Therefore, individual risk assessment and risk modification should become an integral part of breast cancer screening programs.
Further research is needed to determine how knowledge about breast cancer, actual and perceived risk, and strategies for prevention are best communicated to high-risk women. Another important research area is the development of novel chemopreventive agents targeting ER-negative breast cancer. Promising agents that are actively being investigated include pharmacologic drugs, such as bisphosphonates, metformin, statins, aspirin, and other non-steroidal anti-inflammatory drugs, as well as dietary supplements, such as omega-3 fish oils, vitamin D, and green tea extract [68]. The high costs of large-scale chemoprevention studies have prompted the search for intermediate markers of breast cancer development. Validation of intermediate biomarkers which correlate with breast cancer risk, such as mammographic density [69], will reduce the time and expense associated with new prevention drug development and will allow breast cancer prevention research to accelerate and expand. Improving short-term breast cancer risk assessment will also allow us to identify high-risk women who are likely to derive benefits from chemoprevention.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
References
Recently published papers of particular interest have been highlighted as:
• Of importance
- 1.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 2.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 3.Freedman AN, Graubard BI, Rao SR, et al. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95:526–532. doi: 10.1093/jnci/95.7.526. [DOI] [PubMed] [Google Scholar]
- 4.Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28:3090–3095. doi: 10.1200/JCO.2009.27.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravdin PM. The lack, need, and opportunities for decision-making and informational tools to educate primary-care physicians and women about breast cancer chemoprevention. Cancer Prev Res (Phila) 2010;3:686–688. doi: 10.1158/1940-6207.CAPR-10-0100. [DOI] [PubMed] [Google Scholar]
- 6. Goss PE, Ingle JN, Ales-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. This is the first chemoprevention trial of an aromatase inhibitor in high-risk postmenopausal women. The results showed a 65% relative risk reduction in the incidence of invasive breast cancer with exemestane for 5 years compared to placebo.
- 7.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 8.Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–290. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 9.Veronesi U, Maisonneuve P, Rotmensz N, et al. Tamoxifen for the prevention of breast cancer: late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J Natl Cancer Inst. 2007;99:727–737. doi: 10.1093/jnci/djk154. [DOI] [PubMed] [Google Scholar]
- 10.Cuzick J, Forbes JF, Sestak I, et al. Long-term results of tamoxifen prophylaxis for breast cancer--96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 11.Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 12.Martino S, Cauley JA, Barrett-Connor E, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]
- 13.Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 14.Cummings SR, Ensrud K, Delmas PD, et al. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med. 2010;362:686–696. doi: 10.1056/NEJMoa0808692. [DOI] [PubMed] [Google Scholar]
- 15.LaCroix AZ, Powles T, Osborne CK, et al. Breast cancer incidence in the randomized PEARL trial of lasofoxifene in postmenopausal osteoporotic women. J Natl Cancer Inst. 2010;102:1706–1715. doi: 10.1093/jnci/djq415. [DOI] [PubMed] [Google Scholar]
- 16.Cummings SR, McClung M, Reginster JY, et al. Arzoxifene for prevention of fractures and invasive breast cancer in postmenopausal women. J Bone Miner Res. 2011;26:397–404. doi: 10.1002/jbmr.191. [DOI] [PubMed] [Google Scholar]
- 17.Becker C. Another selective estrogen-receptor modulator for osteoporosis. N Engl J Med. 2010;362:752–754. doi: 10.1056/NEJMe0912847. [DOI] [PubMed] [Google Scholar]
- 18. Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila) 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. Updated results from the STAR trial demonstrated that raloxifene retained 76% of the efficacy of tamoxifen in preventing invasive breast cancer with a more favorable side effect profile.
- 19.Cuzick J. Aromatase inhibitors for breast cancer prevention. J Clin Oncol. 2005;23:1636–1643. doi: 10.1200/JCO.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 20.Cuzick J. Chemoprevention of breast cancer. Breast Cancer. 2008;15:10–16. doi: 10.1007/s12282-007-0006-z. [DOI] [PubMed] [Google Scholar]
- 21.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 22.Richardson H, Johnston D, Pater J, Goss P. The National Cancer Institute of Canada Clinical Trials Group MAP.3 trial: an international breast cancer prevention trial. Curr Oncol. 2007;14:89–96. doi: 10.3747/co.2007.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuzick J. IBIS II: a breast cancer prevention trial in postmenopausal women using the aromatase inhibitor anastrozole. Expert Rev Anticancer Ther. 2008;8:1377–1385. doi: 10.1586/14737140.8.9.1377. [DOI] [PubMed] [Google Scholar]
- 24.NCI and the STELLAR trial. Lancet. 2007;369(9580):2134. doi: 10.1016/S0140-6736(07)60987-8. [DOI] [PubMed] [Google Scholar]
- 25. Waters EA, Cronin KA, Graubard BI, Han PK, Freedman AN. Prevalence of tamoxifen use for breast cancer chemoprevention among U.S. women. Cancer Epidemiol Biomarkers Prev. 2010;19:443–446. doi: 10.1158/1055-9965.EPI-09-0930. Using data from the National Health Interview Survery, this study reported the low prevalence of tamoxifen use among women without breast cancer since its approval by the FDA for breast cancer chemoprevention.
- 26.Costantino JP, Gail MH, Pee D, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91:1541–1548. doi: 10.1093/jnci/91.18.1541. [DOI] [PubMed] [Google Scholar]
- 27.Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007;99:1782–1792. doi: 10.1093/jnci/djm223. [DOI] [PubMed] [Google Scholar]
- 28.Matsuno RK, Costantino JP, Ziegler RG, et al. Projecting individualized absolute invasive breast cancer risk in Asian and Pacific Islander American women. J Natl Cancer Inst. 2011;103:951–961. doi: 10.1093/jnci/djr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu-Rustum NR, Herbolsheimer H. Breast cancer risk assessment in indigent women at a public hospital. Gynecol Oncol. 2001;81:287–290. doi: 10.1006/gyno.2001.6160. [DOI] [PubMed] [Google Scholar]
- 30.Grann VR, Jacobson JS, Troxel AB, et al. Barriers to minority participation in breast carcinoma prevention trials. Cancer. 2005;104:374–379. doi: 10.1002/cncr.21164. [DOI] [PubMed] [Google Scholar]
- 31.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111–1130. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- 32.Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272–279. doi: 10.3122/jabfm.2009.03.080153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabatino SA, McCarthy EP, Phillips RS, Burns RB, et al. Breast cancer risk assessment and management in primary care: provider attitudes, practices, and barriers. Cancer Detect Prev. 2007;31:375–383. doi: 10.1016/j.cdp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 34. Owens WL, Gallagher TJ, Kincheloe MJ, Ruetten VL. Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract. 2011;7:85–88. doi: 10.1200/JOP.2010.000107. This study implemented routine screening with the Gail model for high-risk women seen in a women's center. Over 15% of women met high-risk criteria, but only 2% of eligible women agreed to a SERM for chemoprevention.
- 35.Dubin-Rhodin A, Greenlee H, Terry MB, et al. Development of the Breast Cancer Family-Based Intervention Trial (BFIT) Database. Washington, D.C.: American Society of Preventive Oncology; 2012. [Google Scholar]
- 36.Bober SL, Hoke LA, Duda RB, Regan MM, Tung NM. Decision-making about tamoxifen in women at high risk for breast cancer: clinical and psychological factors. J Clin Oncol. 2004;22:4951–4957. doi: 10.1200/JCO.2004.05.192. [DOI] [PubMed] [Google Scholar]
- 37.Melnikow J, Paterniti D, Azari R, et al. Preferences of Women Evaluating Risks of Tamoxifen (POWER) study of preferences for tamoxifen for breast cancer risk reduction. Cancer. 2005;103:1996–2005. doi: 10.1002/cncr.20981. [DOI] [PubMed] [Google Scholar]
- 38.Taylor R, Taguchi K. Tamoxifen for breast cancer chemoprevention: low uptake by high-risk women after evaluation of a breast lump. Ann Fam Med. 2005;3:242–247. doi: 10.1370/afm.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fagerlin A, Zikmund-Fisher BJ, Nair V, et al. Women's decisions regarding tamoxifen for breast cancer prevention: responses to a tailored decision aid. Breast Cancer Res Treat. 2010;119:613–620. doi: 10.1007/s10549-009-0618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metcalfe KA, Snyder C, Seidel J, et al. The use of preventive measures among healthy women who carry a BRCA1 or BRCA2 mutation. Fam Cancer. 2005;4:97–103. doi: 10.1007/s10689-005-4215-3. [DOI] [PubMed] [Google Scholar]
- 41.Salant T, Ganschow PS, Olopade OI, Lauderdale DS. "Why take it if you don't have anything?" breast cancer risk perceptions and prevention choices at a public hospital. J Gen Intern Med. 2006;21:779–785. doi: 10.1111/j.1525-1497.2006.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Port ER, Montgomery LL, Heerdt AS, Borgen PI. Patient reluctance toward tamoxifen use for breast cancer primary prevention. Ann Surg Oncol. 2001;8:580–585. doi: 10.1007/s10434-001-0580-9. [DOI] [PubMed] [Google Scholar]
- 43.Stacey D, O'Connor AM, DeGrasse C, Verma S. Development and evaluation of a breast cancer prevention decision aid for higher-risk women. Health Expect. 2003;6:3–18. doi: 10.1046/j.1369-6513.2003.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Day R, Ganz PA, Costantino JP, et al. Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol. 1999;17:2659–2669. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- 45.Land SR, Wickerham DL, Costantino JP, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2742–2751. doi: 10.1001/jama.295.23.joc60075. [DOI] [PubMed] [Google Scholar]
- 46.Buzdar A, Howell A, Cuzick J, et al. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol. 2006;7:633–643. doi: 10.1016/S1470-2045(06)70767-7. [DOI] [PubMed] [Google Scholar]
- 47.Coombes RC, Kilburn LS, Snowdon CF, et al. Survival and safety of exemestane versus tamoxifen after 2–3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet. 2007;369:559–570. doi: 10.1016/S0140-6736(07)60200-1. [DOI] [PubMed] [Google Scholar]
- 48.Thurlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 49.Kaplan CP, Haas JS, Perez-Stable EJ, Des Jarlais G, Gregorich SE. Factors affecting breast cancer risk reduction practices among California physicians. Prev Med. 2005;41:7–15. doi: 10.1016/j.ypmed.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 50.Rondanina G, Puntoni M, Severi G, et al. Psychological and clinical factors implicated in decision making about a trial of low-dose tamoxifen in hormone replacement therapy users. J Clin Oncol. 2008;26:1537–1543. doi: 10.1200/JCO.2007.13.6739. [DOI] [PubMed] [Google Scholar]
- 51.Freedman AN, Yu B, Gail MH, Costantino JP, et al. Benefit/Risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327–2333. doi: 10.1200/JCO.2010.33.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin JH, Zhang SM, Manson JE. Predicting adherence to tamoxifen for breast cancer adjuvant therapy and prevention. Cancer Prev Res (Phila) 2011;4:1360–1365. doi: 10.1158/1940-6207.CAPR-11-0380. [DOI] [PubMed] [Google Scholar]
- 53.Dezentje VO, van Blijderveen NJ, Gelderblom H, et al. Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early-stage breast cancer. J Clin Oncol. 2010;28:2423–2429. doi: 10.1200/JCO.2009.25.0894. [DOI] [PubMed] [Google Scholar]
- 54.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 55.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Veronesi A, Pizzichetta MA, Ferlante MA, et al. Tamoxifen as adjuvant after surgery for breast cancer and tamoxifen or placebo as chemoprevention in healthy women: different compliance with treatment. Tumori. 1998;84:372–375. doi: 10.1177/030089169808400312. [DOI] [PubMed] [Google Scholar]
- 57.Land SR, Cronin WM, Wickerham DL, et al. Cigarette smoking, obesity, physical activity, and alcohol use as predictors of chemoprevention adherence in the national surgical adjuvant breast and bowel project p-1 breast cancer prevention trial. Cancer Prev Res (Phila) 2011;4:1393–1400. doi: 10.1158/1940-6207.CAPR-11-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moy B, Richardson H, Johnston D, et al. NCIC CTG MAP.3: Enrollment and study drug adherence of ethnic minority women in a breast cancer prevention trial. Breast Cancer Res Treat. 2007;106:S141–S142. [Google Scholar]
- 59. Fagerlin A, Dillard AJ, Smith DM, et al. Women's interest in taking tamoxifen and raloxifene for breast cancer prevention: response to a tailored decision aid. Breast Cancer Res Treat. 2011;127:681–688. doi: 10.1007/s10549-011-1450-1. This is a randomized controlled trial of a tailored on-line decision aid to inform women about breast cancer chemoprevention, which resulted in low SERM uptake.
- 60.Loehberg CR, Jud SM, Haeberle L, et al. Breast cancer risk assessment in a mammography screening program and participation in the IBIS-II chemoprevention trial. Breast Cancer Res Treat. 2010;121:101–110. doi: 10.1007/s10549-010-0845-8. [DOI] [PubMed] [Google Scholar]
- 61.Tchou J, Hou N, Rademaker A, Jordan VC, Morrow M. Acceptance of tamoxifen chemoprevention by physicians and women at risk. Cancer. 2004;100:1800–1806. doi: 10.1002/cncr.20205. [DOI] [PubMed] [Google Scholar]
- 62.Goldenberg VK, Seewaldt VL, Scott V, et al. Atypia in random periareolar fine-needle aspiration affects the decision of women at high risk to take tamoxifen for breast cancer chemoprevention. Cancer Epidemiol Biomarkers Prev. 2007;16:1032–1034. doi: 10.1158/1055-9965.EPI-06-0910. [DOI] [PubMed] [Google Scholar]
- 63.McKay A, Latosinsky S, Martin W. Acceptance of tamoxifen chemoprevention by physicians and women at risk. Cancer. 2005;103:209–210. doi: 10.1002/cncr.20744. [DOI] [PubMed] [Google Scholar]
- 64.Reimers LL, Campbell J, Hershman DL, et al. Uptake of selective estrogen receptor modulators and other breast cancer prevention strategies among high-risk women seen in a breast center; San Antonio Breast Cancer Symposium; 2011. [Google Scholar]
- 65.Visvanathan K, Chlebowski RT, Hurley P, et al. American society of clinical oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235–3258. doi: 10.1200/JCO.2008.20.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bevers TB, Armstrong DK, Arun B, et al. Breast cancer risk reduction. J Natl Compr Canc Netw. 2010;8:1112–1146. doi: 10.6004/jnccn.2010.0083. [DOI] [PubMed] [Google Scholar]
- 67.Meyskens FL, Jr, Curt GA, Brenner DE, et al. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: moving what we have learned into the clinic. Cancer Prev Res (Phila) 2011;4:311–323. doi: 10.1158/1940-6207.CAPR-09-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cuzick J, DeCensi A, Arun B, et al. Preventive therapy for breast cancer: a consensus statement. Lancet Oncol. 2011;12:496–503. doi: 10.1016/S1470-2045(11)70030-4. [DOI] [PubMed] [Google Scholar]
- 69.Cuzick J, Warwick J, Pinney E, Duffy SW, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103:744–752. doi: 10.1093/jnci/djr079. [DOI] [PubMed] [Google Scholar]


