Abstract
Background
This study was conducted to investigate the effects of Brewer’s yeast supplementation on serum lipoproteins and blood pressure in patients with Type 2 diabetes mellitus.
Methods:
In a randomized double blind clinical trial, 90 adults with type 2 diabetes mellitus were recruited, and divided randomly into 2 groups, trial group received brewer’s yeast (1800 mg/day) and control group received placebo for 12 weeks. Weight, BMI, food consumption (based on 24 hour food recall), fasting serum lipoproteins (Cholesterol, Triglyceride, LDL-c, HDL-c), systolic and diastolic blood pressures were measured before and after the intervention. Data analyses were performed by Statistical Package for Social Sciences ver. 18.0, and the statistical tests included Independent t-test, Paired t-test, Kolmogorov-Smirnov and analysis of covariance. This trial was registered in Iranian Registry of Clinical Trials (IRCT), No.IRCT138807062513N1.
Results:
Eighty-four subjects (21 men and 63 women) aged 46.3±6.1 years completed the study. After 12 weeks supplementation, systolic and diastolic blood pressures were decreased in the group receiving brewer’s yeast (4.1±1.5, P=0.007 and 5.7±0.6, P=0.001 respectively). No-significant changes in LDL-c, HDL-c, Triglyceride and Cholesterol were shown.
Conclusion:
Supplementation with Brewer’s yeast besides the usual treatment of type 2 diabetes mellitus can reduce systolic and diastolic blood pressures in diabetic patients.
Keywords: Diabetes, Brewer’s yeast, Blood pressure
Introduction
Diabetes mellitus is one of the most common metabolic disorders in the world (1, 2). More than 246 million people are diagnosed with diabetes worldwide (3). The number of patients with this disease is predicted to reach over 366 million people in 2030 (4). In recent years, increasing prevalence of diabetes mellitus as a chronic disease has prompted investigators to find ways to control it. Researchers are always looking for non-pharmacologic and nutritional approaches to improve the control of type 2 diabetes with a lower cost. In 1958, Mertz discovered that brewer’s yeast has a potent effect on hypoglycemic role of insulin (5).This discovery led to the isolation of Glucose Tolerance Factor (GTF) from brewer’s yeast. Nowadays, in GTF-related studies, the yeast is used as an applicable matter (6). Brewer’s yeast supplements or nutritional yeasts are dried and inactivated yeast cells called “Saccharomyces cerevisiae” which have lost their fermentation activity (7).
GTF facilitates binding of insulin to the target cells by creating a triple complex between GTF, insulin, and its receptors on target cell membranes. GTF not only potentially increases insulin effects, but also may reduce cholesterol and triglyceride levels (8). Different results have been reported from various studies about effects of brewer’s yeast on lipid profile. A study reported no significant changes in the levels of serum lipoproteins after supplementation with brewer’s yeast (9). Other studies showed beneficial effects of brewer’s yeast on serum total cholesterol, triglyceride and HDL-c levels in comparison with chromium chloride (10, 11). In a clinical trial in Iran, the authors observed significant reductions in triglyceride levels, total cholesterol and LDL-c and significant increased in HDL-c levels after supplementation with brewer’s yeast (12). Moreover, hypertension is one of the complications of type 2 diabetes mellitus, which will accelerate cardiovascular, and nephropathy complications in diabetes mellitus (13, 14). Also high triglycerides and low HDL-c levels are among the complications of diabetes, so monitoring the rate of their changes is of special importance (15).
The current study is different in terms of sample size, research method, number of tablets, duration of intervention, and age of samples comparing with previous research studies. This study aimed to investigate the effect of brewer’s yeast supplementation on blood pressure and serum lipoproteins in patients with type 2 diabetes.
Materials and Methods
Subjects:
Among 1200 cases with type 2 diabetes mellitus referring to Diabetes Clinic of Dezful Ganjavian Hospital, 90 patients with type 2 diabetes were recruited by simple random sampling. Six participants were excluded, 4 of them due to irregular consumption of pills, one patient due to lack of willingness to continue collaboration, and one due to pregnancy. Inclusion criteria were diagnosis of type 2 diabetes by a physician, age range of 35–55 years and diabetes lasted more than two years. Exclusion criteria were cardiovascular diseases, hepatitis, renal diseases, gout, Parkinson’s, depression, consumption of steroidal and non steroidal anti-inflammatory drugs or Monoamine oxidase inhibitors and insulin therapy.
Study design:
This trial was registered in Iranian Registry of Clinical Trials (IRCT), No.IRCT138807062513N1. Medical, drugs & supplements history, anthropometric measurements and demographic data were collected at the first interview. Then, in a double-blind clinical trial, included individuals were randomly divided into 2 groups receiving brewer’s yeast tablets (42 patients) and placebo (42 patients). The brewer’s yeast group received 6 tablets of brewer’s yeast daily manufactured by Health Aid Ltd. Marlborough Hill, United Kingdom. Each brewer’s yeast tablet contained 300 mg brewer’s yeast plus excipient. Placebo group received 6 placebo tablets daily with similar shape, color and size to the brewer’s yeast tablets. Placebo tablets consisted of cellulose microcrystalline compounds, magnesium stearate, caramel, malt and stearic acid, and were manufactured by Health Aid Ltd. Marlborough Hill, United Kingdom. The tablets were from a single lot. Both groups consumed 2 tablets with each meal for 12 weeks.
The subjects were asked not to make any changes in their diet, oral diabetes medications, blood pressure-lowering drugs, lipid lowering agents and their usual physical activity during the intervention. Weight, body mass index, systolic and diastolic blood pressure levels, fasting serum triglyceride, total cholesterol, LDL-c and HDL-c were measured before and after 12 weeks of intervention in both groups. Twenty-four hour dietary recall questionnaires were completed by a trained dietitian at baseline and at the twelfth week for both groups. In addition, the researchers followed up consumption of the pills regularly every 2 weeks by telephone. Furthermore, tablets were delivered to the subjects alternately in three stages (once per month) to better control and to ensure the correct and regular consumption of tablets by patients. Of all patients, informed consent was obtained. This research was approved by Ethics Committee of Tehran University of Medical Sciences.
Clinical and laboratory setting:
Height and weight were measured before and after the treatments by a trained dietitian in the same and standard situations. Weight was measured by accurate bascule (seca762, seca gmbh & co. kg. Hammer Steindamm 9 – 25. 22089 Hamburg. German) while patients were in fasting state with minimal clothing and height was measured by Seca wall height gauge (seca 206, seca gmbh & co. kg. Hammer Steindamm 9 – 25. 22089 Hamburg. German) while subjects were standing without shoes and in standard position.
Systolic and diastolic blood pressures were measured by a trained physician by using a Mercury sphygmomanometer (diplomat-presameter® Desk model by error tolerance of +/− 3 mmHg, Rudolf Riester GmbH · Bruckstr. 31 · D-72417 Jungingen Germany) while the patients were sitting on the chair for at least 5 minutes before the measurements. To be more accurate, an average of three times measured values in each session was reported. To minimize human measurement errors between before and after the intervention, all measurements were done by the same person.
Measurement of total serum cholesterol was done by colorimetric method using commercially available kit (Cat no.10-508 ZiestChem Diagnostics Tehran, Iran) with sensitivity of 1 mg/dl. Serum triglyceride was measured by colorimetric method using biochemistry kit (Cat no.10-525 ZiestChem Diagnostics Tehran, Iran) with sensitivity of 4 mg/dl. Serum HDL-c concentrations were measured by enzymatic methods using biochemistry kit (Cat no.10-507 ZiestChem Diagnostics Tehran, Iran), with sensitivity of 1 mg/dl. LDL-c concentrations in serum were automatically measured using enzymatic methods by Elitech kits (Cat no.LDLD-0230 SEPPIM.FRANCE) with sensitivity of 0.3 mg/dl by autoanalyzer Hitachi 911.
Data analysis:
Data from 24-hour food recall were analyzed by using Food Processor II software (FPII, ver2. ESHA Research. Salem. OR.). For statistical analysis, SPSS 18 software was used. Kolmogorov Smirnov test was used for assessing the normality of data distribution. To compare qualitative data, chi-square test and to compare quantitative data, independent t- test, pair t-test and analysis of covariance were used.
Results
Demographic and anthropometric characteristics
Eighty-four subjects (21 men and 63 women) with mean age of 46.3 ± 6.1 years finished the study. The group taking brewer’s yeast included 42 patients (11 men and 31 women) and the group-taking placebo included 42 patients (10 men and 32 women). There was not any statistically significant differences between two groups regarding gender distribution (P=0.8, Chi-square test). No significant differences observed between recipient of brewer’s yeast and placebo groups in terms of age and anthropometric measurements (Independent sample t-test). Moreover, at the end of the intervention differences of anthropometric variables between two study groups were not statistically significant. There was not any statistically significant differences between two groups regarding anti-hypertensive drug usage (χ2=0.513, P=0.632), anti-dyslipidemia drug usage (χ2=0.431, P=0.662) before intervention (Table 1).
Table 1:
Age and anthropometric variables in study groups at baseline*
| Variable | †Brewer’s yeast group | ‡Placebo group | P-value |
|---|---|---|---|
| Age (yr) | 6.21±46.80 | 6.11±45.70 | NS¶ ** |
| (Height) (m) | 8.61±158.20 | 7.02±158.80 | NS¶ |
| Weight(Kg) | 11.69±75.12 | 13.24±75.53 | NS¶ |
| BMI (Kg/m2) | 4.40±30.03 | 4.74±29.91 | NS¶ |
| Anti-hypertensive drug usage (Yes, No) | (14, 28) | (11, 31) | NS¥ |
| Anti-dyslipidemia drug usage (Yes, No) | (21, 21) | (24, 18) | NS¥ |
Mean ± SD/Independent sample t-test/
NS : Non-significant /
Subjects: 42(11men, 31 women)/
Subjects:42(10men, 32 women)/
Chi-square
Nutrients intake
In terms of energy, macronutrients and micronutrients in the beginning and at the end of the intervention, study groups were not significantly different from each other (Independent t-test, Table 2). There were no significant differences within each group in terms of energy, macro-and micronutrients during the intervention (Paired t-test, Table 2).
Table 2:
Energy, macronutrients and micronutrients in study groups before and after the intervention *
| Variable | †Brewer’s yeast group | ‡Placebo group |
|---|---|---|
| Energy (Kcal) | ||
| B.I | 1431.0±416.7 | 1447.0±454.8 |
| A.I | 1329.0±389.8 | 1354.0±431.3 |
| Carbohydrate (g) | ||
| B.I | 172.1±60.6 | 170.1±59.8 |
| A.I | 168.3±57.7 | 171.0±60.5 |
| Protein (g) | ||
| B.I | 57.4±16.3 | 53.4±20.6 |
| A.I | 50.0±14.3 | 49.2±19.1 |
| Total Fat (g) | ||
| B.I | 60.5±24.2 | 65.1±31.6 |
| A.I | 55.0±21.0 | 59.1±28.7 |
| Cholesterol (g) | ||
| B.I | 201.9±126.0 | 190.0±108.9 |
| A.I | 191.3±120.8 | 178.8±102.6 |
| SFA (g) | ||
| B.I | 17.2±7.1 | 17.3±8.0 |
| A.I | 16.1±6.6 | 16.6±7.6 |
| MUFA (g) | ||
| B.I | 19.5±8.5 | 17.2±6.2 |
| A.I | 18.7±8.3 | 16.3±5.6 |
| PUFA(g) | ||
| B.I | 19.5±11.2 | 22.2±13.5 |
| A.I | 17.7±9.8 | 19.7±11.9 |
| (g) Fibre | ||
| B.I | 15.4±5.5 | 14.9±5.7 |
| A.I | 14.0±5.6 | 13.6±6.1 |
| (mg) Sodium | ||
| B.I | 1492.1±587.0 | 1410±562.2 |
| A.I | 1506.7±584.6 | 1430.0±558.4 |
| (mg) Pottasium | ||
| B.I | 1677.9±511.7 | 1573.1±463.0 |
| A.I | 1568.8±489.5 | 1472.0±443.1 |
| Chromium(μg) | ||
| B.I | 2.5±1.8 | 2.2±1.6 |
| A.I | 2.1±1.7 | 1.9±1.4 |
Mean ± SD/B.I: Before Intervention/A.I: After Intervention/Subjects: 42(11men, 31 women)/
Subjects:42(10men, 32 women)/SFA: Saturated Fatty Acid / MUFA: Mono Unsaturated Fatty Acid/PUFA: Poly Unsaturated Fatty Acid
Blood pressure and Serum lipid profile
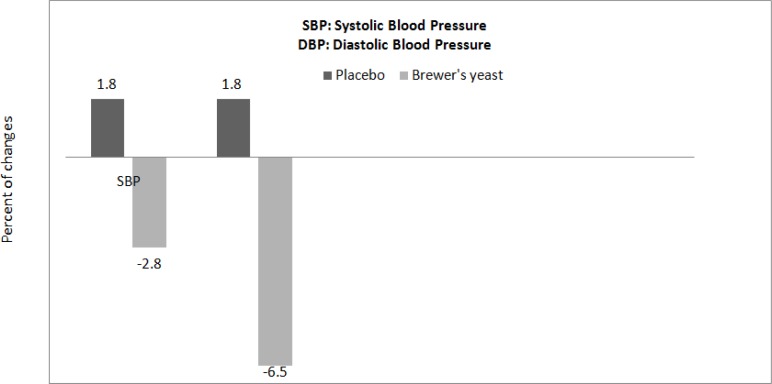
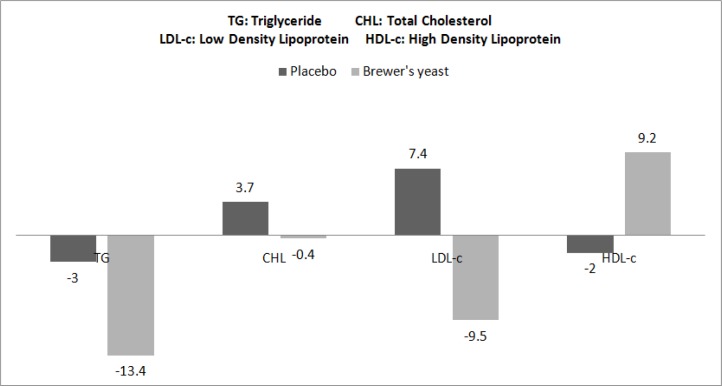
There were no significant differences for blood pressure and serum lipoproteins between two groups at baseline (Independent t-test, Table 3). After the intervention, systolic and diastolic blood pressures significantly decreased in the group taking brewer’s yeast (P=0.007 and P=0.001 respectively, Paired t-test). After adjusting for baseline systolic and diastolic blood pressures, the final systolic and diastolic blood pressures were statistically significant between the groups (P=0.05 and P=0.03 respectively, ANCOVA, Table 3). Percents of changes for systolic and diastolic blood pressures during intervention in brewer’s yeast group were significantly different from placebo group (P=0.02 and P= 0.002 respectively, Independent t-test, Fig. 1). Percent of changes in serum lipoproteins is shown in Fig. 2. After the intervention, triglyceride, LDL-c and HDL-c levels were significantly reduced in-group taking brewer’s yeast (P=0.002, P= 0.001 and P= 0.001 respectively, Paired t-test, Table 3).
Table 3:
Blood pressure and Serum lipoproteins in study groups before and after intervention*
| Variable | † Brewer’s yeast group | ‡ Placebo group |
|---|---|---|
| SBP (mmHg) | ||
| B.I | 16.44b±131.5 | 17.1±125.7 |
| A.I | 14.90 a, b± 127.4 | 127.6±18.4a |
| DBP (mmHg) | ||
| B.I | 84.72±8.4d | 80.7±10.1 |
| A.I | 79.04±9.0 c, d | 81.3±9.9 c |
| Total Cholesterol (mg/dl) | ||
| B.I | 192.5±41.7 | 193.0±54.37 |
| A.I | 191.7±41.2 | 200.2±59.16 |
| Triglyceride(mg/dl) | ||
| B.I | 166.4±74.5e | 191.9±95.4 |
| A.I | 144.1±61.9e | 186.0±93.3 |
| LDL (mg/dl) | ||
| B.I | 114.7±28.7f | 100.3±36.53 |
| A.I | 103.7±26.1f | 107.8±34.00 |
| HDL (mg/dl) | ||
| B.I | 50.6±7.9 g | 11.3±53.5 |
| A.I | 55.3±7.8 g | 10.6±52.4 |
Mean ± SD/B.I: Before Intervention /A.I: After Intervention / Subjects: 42(11men, 31 women)/
Subjects:42(10men, 32 women)/ SBP: Systolic Blood Pressure/a, c 0.05 P< (Covariance) b, d, e, f, g0.05 P< (Paired t-test)DBP: Diastolic Blood Pressure
Fig. 1:
Percent of changes of systolic and diastolic blood pressure in study groups
Fig. 2:
Percent of changes of Serum Lipoproteins in study groups
Discussion
In this study, taking 1800 mg of brewer’s yeast per day reduced systolic and diastolic blood pressures in patients with type 2 diabetes. Dietary sodium, potassium, magnesium and calcium are among factors influencing blood pressure. According to Table 2 no changes can be seen in these micronutrients between the two study groups during the intervention. Thus, significant reduction in systolic and diastolic blood pressures can be due to supplementation with brewer’s yeast.
In a study conducted by Hata and colleagues, fermented milk containing the yeast Saccharomyces cerevisiae was given to 30 non-diabetic elderly subjects with hypertension. After 8 weeks, systolic and diastolic blood pressures were significantly reduced (16). However this study was performed with a shorter time, its results, is confirmed by ours. In addition, Kanauchi and colleagues conducted an in vitro trial on spontaneous hypertensive rats as case and mild hypertensive rats as control group. Significant decrease in systolic blood pressure in spontaneous hypertensive rats was shown in this study (17). Masuda in a study on the SHR rats observed that after feeding the rats with sour milk containing brewer’s yeast and Lactobacillus helveticus, blood pressure decreased significantly (18).
There are different theories on the mechanism of brewer’s yeast effects on blood pressure. Blood pressure lowering effects of brewer’s yeast is attributed to its biological peptides, potassium, magnesium and calcium (17, 19). It seems that the biological peptide named KRF814 is derived from hydrolysis of brewer’s yeast by alkaline phosphatase. This biological peptide can reduce the activity of angiotensin-converting enzyme and thereby may decrease blood pressure (17). We did not observe any adverse effect in patients receiving brewer’s yeast. In the present study, despite significantly decreased triglyceride and LDL-c and significantly increased HDL-c in brewer’s yeast consumers, no significant differences could be detected compared with placebo group at the end of the study. In explorations, which studied the effects of brewer’s yeast on serum triglyceride in people with normal levels of triglyceride, no significant difference has been found (9, 10, 20). Although, in studies on the effects of brewer’s yeast on serum triglyceride in patients with hypertriglyceridemia, significant difference has been found (11,12). It seems that lack of significant changes in serum triglyceride levels may be due to their normal initial levels. In the case of brewer’s yeast effects on serum cholesterol, several studies have been done, but no significant decreases observed in total cholesterol levels and neither did we (10, 12). Related to brewer’s yeast effects on LDL-c, other studies did not show any significant changes between the intervention groups. In present study, despite significant increases in HDL-c levels in-group receiving brewer’s yeast, no significant differences between the two trial groups were observed. In studies conducted by Racek and colleagues and Wang and colleagues also, no significant change in HDL-c level of the subjects was obtained (9, 10). In some studies, HDL-c increased significantly after consumption of brewer’s yeast compared with controls. In comparison with ours, in mentioned studies, the subjects were older and were suffering from dyslipidemia (11, 14). Among factors affecting serum lipoproteins, are intake of macronutrients, micronutrients and their ratios that in the above-mentioned studies, have not been well controlled. So it seems that one of the strengths of current study is controlling nutrient intake by statistical analyses. In a meta-analysis study, on brewer’s yeast effects in patients with type 2 diabetes from 1996 to 2008 revealed that conflicting results in various studies were due to major limitations including: small size (7, 20), short duration of intervention (21), non-randomized design (11) and different doses of supplementation (12, 21). Therefore, it is suggested that these limitations must be eliminated in future clinical trials; also nutrients intake and correlation between biopeptides and blood pressure should take into the account (22).
Conclusion
Supplementation with 1800 mg/day brewer’s yeast in addition to usual treatments can have a modest beneficial effect on systolic and Diastolic blood pressure in patients with Type 2 diabetes.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgments
Hereby, the authors are thankful of the Dezful Ganjavian Hospital staffs for their collaboration. This research was supported financially by the Tehran University of Medical Sciences, license No. 10428. The authors declare that there is no conflict of interest.
References
- 1.Brown WV. Microvascular complications of diabetes mellitus: renal protection accompanies cardiovascular protection. Am J Cardiol. 2008;102(12A):10–13. doi: 10.1016/j.amjcard.2008.09.068. [DOI] [PubMed] [Google Scholar]
- 2.Marcovina SM, Koschinsky ML, Albers JJ, et al. Report of the National Heart, Lung, and Blood Institute Workshop on Lipoprotein (a) and Cardiovascular Disease: recent advances and future directions. Clin Chem. 2003;49:1785–96. doi: 10.1373/clinchem.2003.023689. [DOI] [PubMed] [Google Scholar]
- 3.Williams K, Sniderman AD, Wagenknecht LE, et al. Comparison of the associations of apolipoprotein B and low-density lipoprotein cholesterol with other cardiovascular risk factors in the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2003;108:2312–6. doi: 10.1161/01.CIR.0000097113.11419.9E. [DOI] [PubMed] [Google Scholar]
- 4.Swapan KB. Genetic Epidemiology Of Adult Onset Type 2 Diabetes in Asian-Indian Population : Past, Present and Future. Int J Hum Jenet. 2006;6(1):1–13. [Google Scholar]
- 5.Moyad MA. Brewer’s/baker’s yeast (Saccharomyces cerevisiae) and preventive medicine: part I. Urol Nurs. 2007;27(6):560–1. [PubMed] [Google Scholar]
- 6.Lamson DW, Plaza SW. The safety and efficacy of high-dose chromium. Altern Med Rev. 2002;7(3):218–35. [PubMed] [Google Scholar]
- 7.Li YC. Effects of brewer’s yeast on glucose tolerance and serum lipids in Chinese adults. Biol Trace Elem Res. 1994;41(3):341–7. doi: 10.1007/BF02917434. [DOI] [PubMed] [Google Scholar]
- 8.Moyad MA. Brewer’s/baker’s yeast (Saccharomyces cerevisiae) and preventive medicine: Part II. Urol Nurs. 2008;28(1):73–5. [PubMed] [Google Scholar]
- 9.Racek J, Trefil L, Rajdl D, et al. Influence of chromium-enriched yeast on blood glucose and insulin variables, blood lipids, and markers of oxidative stress in subjects with type 2 diabetes mellitus. Biol Trace Elem Res. 2006;109(3):215. doi: 10.1385/BTER:109:3:215. [DOI] [PubMed] [Google Scholar]
- 10.Wang MM, Fox EA, Stoecker BJ, et al. Serum cholesterol of adults supplemented with brewer’s yeast or chromium chloride. Nutrition Research. 1989;9(9):989–998. [Google Scholar]
- 11.Bahijiri SM, Mira SA, Mufti AM, et al. The effects of inorganic chromium and brewer’s yeast supplementation on glucose tolerance, serum lipids and drug dosage in individuals with type 2 diabetes. Saudi Med J. 2000;21(9):831–7. [PubMed] [Google Scholar]
- 12.Khosravi-Boroujeni H, Rostami A, Ravanshad SH, et al. Favorable effects on metabolic risk factors were observed with a daily intake of brewer’s yeast in type 2 diabetic patients with hypercholesterolemia: a semi-experimental study. J Diabetes. 2011;4(2):153–58. doi: 10.1111/j.1753-0407.2011.00163.x. [DOI] [PubMed] [Google Scholar]
- 13.Mahan lk, Escott-Stump . Medical Nutrition Therapy for Hypertension. In: Saunders, editor. Krauses’s Food & Nutrition Therapy. 12th ed. Elsevier Inc; Philadelphia: 2008. p. 867. [Google Scholar]
- 14.Patel K, Larson C, Hargreaves M, et al. Community screening outcomes for diabetes, hypertension, and cholesterol: Nashville REACH 2010 project. J Ambul Care Manage. 2010;33(2):155–62. doi: 10.1097/JAC.0b013e3181dd4619. [DOI] [PubMed] [Google Scholar]
- 15.Kruit JK, Brunham LR, Verchere CB, et al. HDL and LDL cholesterol significantly influence beta-cell function in type 2 diabetes mellitus. Curr Opin Lipidol. 2010;21(3):178–85. doi: 10.1097/MOL.0b013e328339387b. [DOI] [PubMed] [Google Scholar]
- 16.Hata Y, Yamamoto M, Ohni M, et al. A placebo-controlled study of the effect of sour milk on blood pressure in hypertensive subjects. Am J Clin Nutr. 1996;64:767–71. doi: 10.1093/ajcn/64.5.767. [DOI] [PubMed] [Google Scholar]
- 17.Kanauchi O, Igarashi K, Ogata R, et al. A yeast extract high in bioactive peptides has a blood-pressure lowering effect in hypertensive model. Curr Med Chem. 2005;12(6):3085–90. doi: 10.2174/092986705774933461. [DOI] [PubMed] [Google Scholar]
- 18.Masuda O, Nakamura Y, Takano T. Antihypertensive peptides are present in aorta after oral administration of sour milk containing these peptides to spontaneously hypertensive rats. J Nutr. 1996;126(12):3063–8. doi: 10.1093/jn/126.12.3063. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura Y, Masuda O, Takano T. Decrease of tissue angiotensin I-converting enzyme activity upon feeding sour milk in spontaneously hypertensive rats. Biosci Biotechnol Biochem. 1996;60(3):488–9. doi: 10.1271/bbb.60.488. [DOI] [PubMed] [Google Scholar]
- 20.Offenbacher EG, Pi-Sunyer FX. Beneficial effect of chromium-rich yeast on glucose tolerance and blood lipids in elderly subjects. Diabetes. 1980;29:919–25. doi: 10.2337/diab.29.11.919. [DOI] [PubMed] [Google Scholar]
- 21.Grant AP, McMullen JK. The effect of brewers yeast containing glucose tolerance factor on the response to treatment in Type 2 diabetics, A short controlled study. Ulster Med J. 1982;51(2):110–4. [PMC free article] [PubMed] [Google Scholar]
- 22.Nahas R, Moher M. Complementary and alternative medicine for the treatment of type 2 diabetes. Can Fam Physician. 2009;55(6):591–6. [PMC free article] [PubMed] [Google Scholar]