Abstract
Objective:
To investigate the effect of teriflunomide on the efficacy and safety of seasonal influenza vaccine.
Methods:
The 2011/2012 seasonal influenza vaccine (containing H1N1, H3N2, and B strains) was administered to patients with relapsing forms of multiple sclerosis (RMS) treated for ≥6 months with teriflunomide 7 mg (n = 41) or 14 mg (n = 41), or interferon-β-1 (IFN-β-1; n = 46). The primary endpoint was the proportion of patients with influenza strain–specific antibody titers ≥40, 28 days postvaccination.
Results:
More than 90% of patients achieved postvaccination antibody titers ≥40 for H1N1 and B in all groups. For H3N2, titers ≥40 were achieved in ≥90% of patients in the 7 mg and IFN-β-1 groups, and in 77% of the 14-mg group, respectively. A high proportion of patients already had detectable antibodies for each influenza strain at baseline. Geometric mean titer ratios (post/prevaccination) were ≥2.5 for all groups and strains, except for H1N1 in the 14-mg group (2.3). The proportion of patients with a prevaccination titer <40 achieving seroprotection was ≥61% across the 3 treatment groups and 3 influenza strains. However, fewer patients in the 14-mg than the 7-mg or IFN-β-1 groups exhibited seroprotection to H3N2 (61% vs 78% and 82%, respectively).
Conclusion:
Teriflunomide-treated patients generally mounted effective immune responses to seasonal influenza vaccination, consistent with preservation of protective immune responses.
Classification of evidence:
This study provides Class II evidence that teriflunomide generally does not adversely impact the ability of patients with RMS to mount immune responses to influenza vaccination.
Teriflunomide is a new once-daily oral disease-modifying therapy (DMT) recently approved in the United States and Australia for treatment of relapsing forms of multiple sclerosis (RMS). Teriflunomide selectively and reversibly inhibits dihydro-orotate dehydrogenase, a key mitochondrial enzyme in de novo pyrimidine synthesis required by rapidly dividing lymphocytes. Through this cytostatic effect, teriflunomide limits expansion of stimulated T and B cells thought to be responsible for the damaging inflammatory process associated with multiple sclerosis (MS). Slowly dividing or resting cells, including lymphocytes and nonlymphoid cells, rely on the pyrimidine salvage pathway to meet their pyrimidine demand. Since this pathway is not affected by teriflunomide, basic homeostatic functions appear to be preserved and lymphocytes remain available for immune surveillance.1
In the phase III Teriflunomide Multiple Sclerosis Oral (TEMSO) trial, teriflunomide significantly reduced annualized relapse rate, 12-week confirmed disability progression, and MRI disease activity markers, with a well-characterized safety profile.2,3 A very low incidence of serious infections and no serious opportunistic infections were reported, demonstrating that teriflunomide is not globally immunosuppressive, but functions as an immunomodulator with normal immune defenses remaining largely intact.3
Immunomodulatory agents may affect patient ability to mount effective immune responses to vaccinations. Evidence on effect of DMTs in MS is scant, although patients treated with interferon-β-1a (IFN-β-1a) have been shown to mount effective immune responses to influenza vaccination.4 This study evaluated antibody responses to seasonal influenza vaccination in patients with RMS treated with teriflunomide, which can largely be considered a recall response as most patients are exposed to circulating virus and/or are vaccinated regularly.
METHODS
Study design.
The Teriflunomide and Vaccination (TERIVA) study (NCT01403376) was a multicenter, multinational, parallel-group study involving 128 patients in 3 treatment groups. Groups 1 and 2 included patients with RMS treated with either teriflunomide 7 mg or 14 mg once daily for at least 6 months over the course of 2 long-term extension studies (NCT00228163: open-label extension of a phase II study, which started in 20015,6; NCT00803049: blinded extension of the phase III TEMSO study, which started in 20043,7,8). Group 3 included patients with RMS who had received a stable dose of IFN-β-1 for at least 6 months, and represents a reference population of patients with RMS who have previously been reported to mount normal immune responses to seasonal influenza vaccination.4
The study comprised a screening period of up to 21 days, followed by administration of seasonal influenza vaccine on day 1 and antibody assessments at day 28 (±2 days) postimmunization (figure e-1 on the Neurology® Web site at www.neurology.org). Patients were immunized with a single IM or intradermal administration of the 2011/2012 inactivated seasonal influenza vaccines, Vaxigrip or Mutagrip (both Sanofi Pasteur, Lyon, France). Both vaccines contained the following influenza strains: A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2), and B/Brisbane/60/2008 (B). These strains were the same as those included in the 2010/2011 seasonal influenza vaccine. Choice of vaccine was performed according to country standards; Mutagrip was administered only in Germany to 20 patients (12 in the IFN-β-1 group, and 3 and 5 in the 7-mg and 14-mg teriflunomide groups, respectively).
Standard protocol approvals, registrations, and patient consents.
The TERIVA study protocol and participation consents were submitted to independent ethics committees or institutional review boards, and subsequently reviewed and approved. The study was performed at sites approved to participate in either of the 2 long-term teriflunomide extension studies. Subjects participating in these extension studies were asked to participate voluntarily in the TERIVA study. Patients gave written informed consent before entering TERIVA.
Study population.
Male or female subjects aged between 18 and 60 years with RMS treated for at least 6 months with once-daily teriflunomide (7 mg or 14 mg) or IFN-β-1, and who were expected to remain on treatment for the duration of the study and who signed a specific informed consent form, were eligible for inclusion in the TERIVA study.
Subjects with concomitant infectious pathology at the time of vaccination, MS relapse within 1 month of vaccination, or administered systemic corticosteroids within 1 month of vaccination were excluded from the study. Additional exclusion criteria included contraindication to influenza vaccine or in receipt of any vaccination within the last 6 months; prior use of investigational drugs or participation in a clinical trial within 1 year before screening (for patients in the IFN-β-1 group only); prior/concomitant use (within 1 year of study entry) of cladribine, mitoxantrone, or other immunosuppressant agents; prior/concomitant use of glatiramer acetate (within 1 year of study entry); or IV immunoglobulins (within 3 months of study entry). Pregnant or breastfeeding women were also excluded from the study.
Study endpoints.
The hemagglutination inhibition assay (HIA) was used to detect strain-specific anti-influenza antibodies 28 days postimmunization. HIAs were performed blinded to sample source and in accordance with standard procedures. HIAs were performed using serial 2-fold dilutions of serum and results were presented as titers (i.e., the highest dilution of serum that achieved complete inhibition of hemagglutination).
Evaluation of vaccine effectiveness in this study was performed in accordance with European guidelines, which apply 3 criteria to evaluate the immune response to influenza vaccine.9 First, the seroprotection rate (i.e., the proportion of subjects achieving a postvaccination titer ≥40 with the HIA) should be achieved in ≥70% of vaccinees postvaccination for individuals aged 18–60 years and in ≥60% for those over 60 years. Second, the mean geometric increase (i.e., the quotient of postvaccination and prevaccination geometric mean titers [GMTs]) should be ≥2.5 in individuals aged 18–60 years and ≥2.0 in individuals over 60 years. Third, the seroresponse rate (i.e., the proportion of previously seronegative subjects exceeding a postvaccination titer of 40, and the proportion of previously seropositive subjects with a ≥4-fold increase in prevaccination and postvaccination sera) should be achieved in ≥40% of vaccinees aged 18–60 years and in ≥30% in those over 60 years. For each virus strain and each age class, at least one of the 3 criteria should be met. In this study, the primary efficacy endpoint was the proportion of patients who achieved seroprotection to the influenza vaccine strains, defined as an influenza antibody titer ≥40 for each strain 28 days postvaccination. The following secondary endpoints were also assessed: the proportion of patients with seroconversion (i.e., a prevaccination antibody titer ≤10 and a postvaccination antibody titer ≥40); the proportion of patients with a prevaccination antibody titer <40 achieving seroprotection; the proportion of patients with either a ≥2- or ≥4-fold increase in strain-specific antibody titers compared with prevaccination titers at 28 days postvaccination; GMTs at baseline and day 28 for each strain, and the corresponding GMT ratio (postvaccination/prevaccination).
Statistical analysis.
Assuming a response rate of around 70% (measured as the proportion of patients with antibody titers ≥40), sample size was calculated as 37 evaluable subjects per group based on the required number of subjects needed to obtain a precision of ±12.5% (corresponding to confidence interval [CI] widths of 25%) around the point estimate of the true response rate under a 90% CI for the true response rate.
Efficacy analyses were conducted in the per-protocol population, which comprised all patients consenting to enter the study who received influenza vaccination and had an antibody titer at day 28. The safety population comprised all patients who consented and were enrolled into the study.
For the primary efficacy analysis, the proportion of patients with influenza antibody titers ≥40 at 28 days postvaccination was summarized together with the corresponding 90% CI using normal approximation. Differences between each teriflunomide treatment group and the IFN-β-1 reference group were estimated along with 90% CIs for each vaccine strain. Although there were 2 teriflunomide treatments and 3 viral strains, no adjustments for multiplicity were made. For secondary efficacy analyses, the evaluation of ≥2- or ≥4-fold increases in influenza antibody titers compared with prevaccination titers, proportions of patients, and corresponding 90% CIs using normal approximation were calculated for each strain and each treatment group. Differences between each teriflunomide treatment group and the IFN-β-1 reference group were estimated. GMTs at baseline and day 28 and the respective ratios (postvaccination/prevaccination) were summarized by strain and treatment group.
For analysis of safety, incidences of treatment-emergent adverse events (TEAEs), treatment-emergent serious adverse events (SAEs), and adverse events leading to permanent treatment discontinuation occurring from time of vaccination to day 28 were documented within each group.
RESULTS
Study disposition and baseline demographics.
The TERIVA study ran from September 2011 to January 2012 and involved 14 different study sites in 5 countries (Austria, Canada, Germany, Russian Federation, and Ukraine). A total of 128 patients were enrolled into the study: 41, 41, and 46 patients in the teriflunomide 7 mg, teriflunomide 14 mg, and IFN-β-1 groups, respectively. The majority of patients in the IFN-β-1 group were receiving Avonex (IFN-β-1a; 30 μg once-weekly IM; 34.8%), Rebif (IFN-β-1a; 44 μg 3 times per week SC; 28.3%), or Betaseron (IFN-β-1b; 0.25 mg every other day SC; 21.7%); the remaining patients received either Genfaxone (IFN-β-1a; 44 μg 3 times per week IM [Russia only]; 8.7%) or Rebif (IFN-β-1a; 22 μg, 3 times per week SC; 6.5%). All included patients were vaccinated and completed the study.
Of the 128 patients enrolled, 122 were included in the per-protocol population (40, 39, and 43 in the 7-mg, 14-mg, and IFN-β-1 groups, respectively). Five patients were excluded from the per-protocol population because of recent or concomitant exposure to a systemic or topical corticosteroid, and one patient in the IFN-β-1 group was excluded because of poor compliance with study treatment.
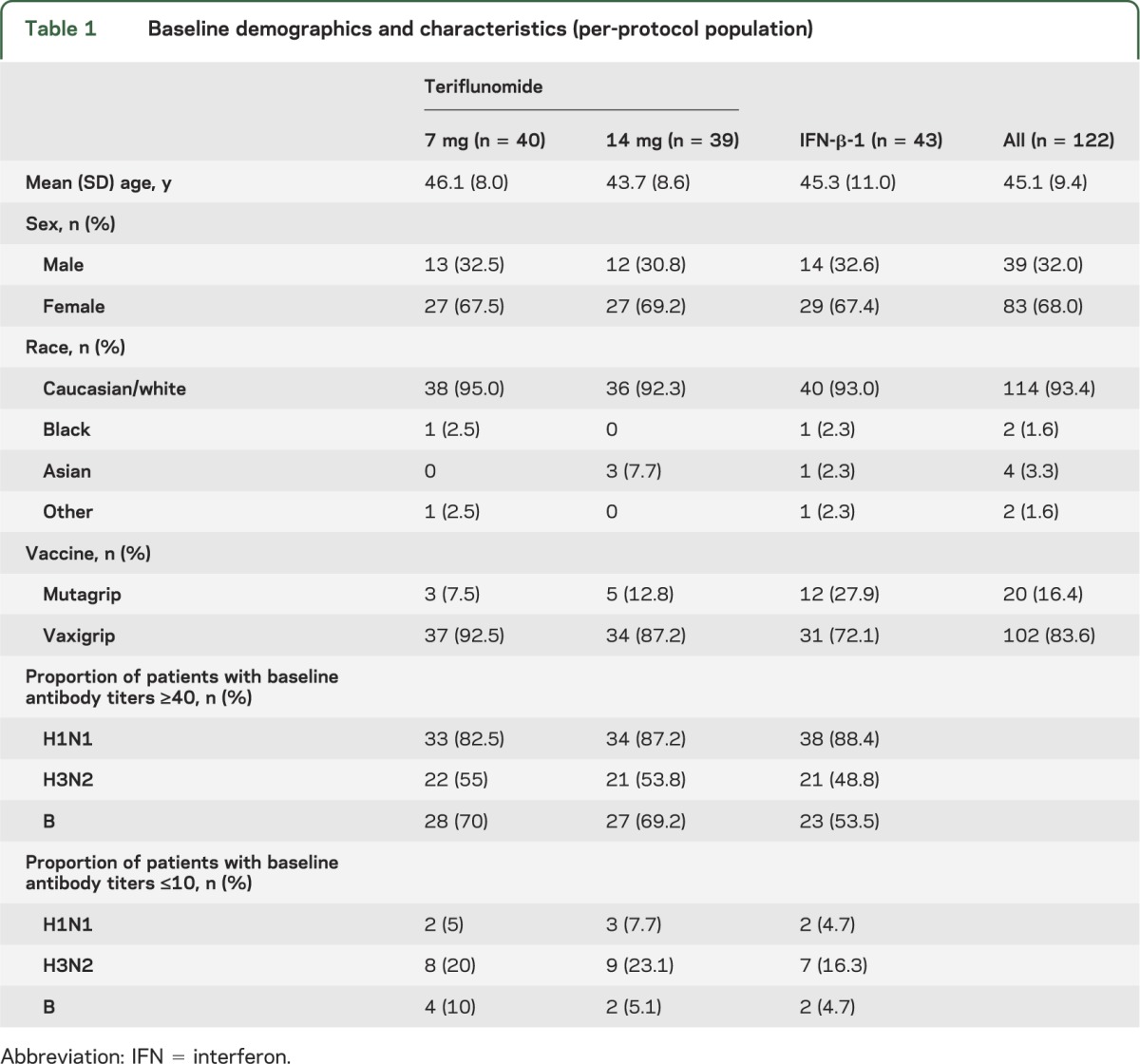
The study population was generally balanced across treatment groups (table 1). Patients had received teriflunomide treatment for a median of 5.7 years (range 1.6–10.4 years) and the reference population received IFN-β-1 for a median duration of 5.7 years (range 1.0–17.9 years; tables e-1 and e-2). More than half of the per-protocol population (57%) had received influenza vaccination previously, more so for patients in the IFN-β-1 group (69.6%) than either of the teriflunomide groups (43.9% and 56.1% for 7 mg and 14 mg, respectively).
Table 1.
Baseline demographics and characteristics (per-protocol population)
Efficacy.
More than 90% of patients achieved postvaccination antibody titers ≥40 for H1N1 and B in all groups. The proportion of patients achieving seroprotection to H3N2 was lower in the teriflunomide 14-mg group (76.9%) compared with the teriflunomide 7-mg (90.0%) and IFN-β-1 (90.7%) groups (table 2). Rates of seroprotection in the teriflunomide groups were consistent regardless of the total duration of treatment exposure prior to vaccination or the nature of the extension study from which patients were enrolled (tables e-3 and e-4). Thus, more than 70% of patients achieved an HI titer of ≥40 for all influenza strains across all treatment groups (table 2 and figure 1).
Table 2.
Primary and secondary efficacy endpoints
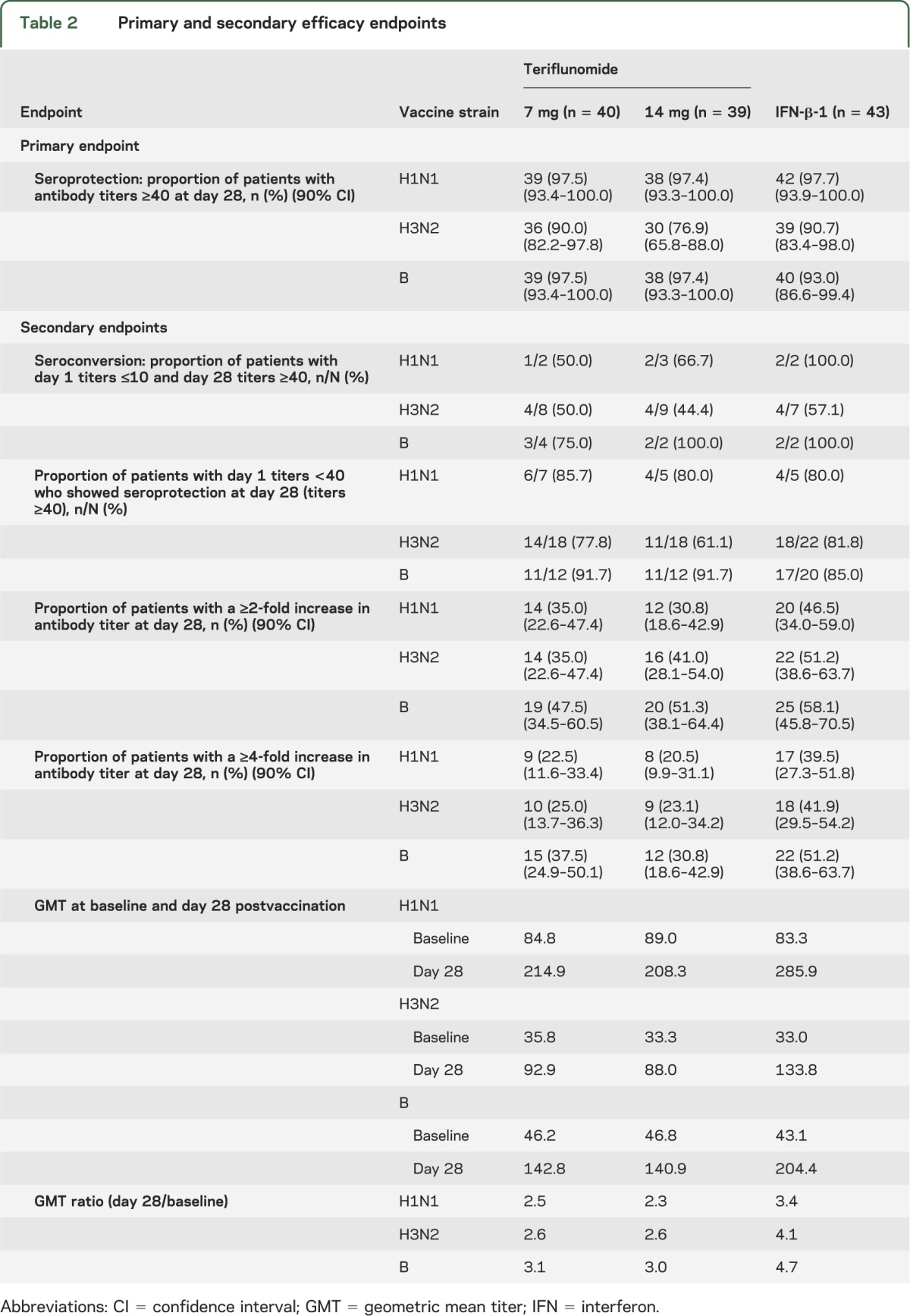
Figure 1. Proportion of patients with influenza antibody titers ≥40 at 28 days postvaccination (per-protocol population).
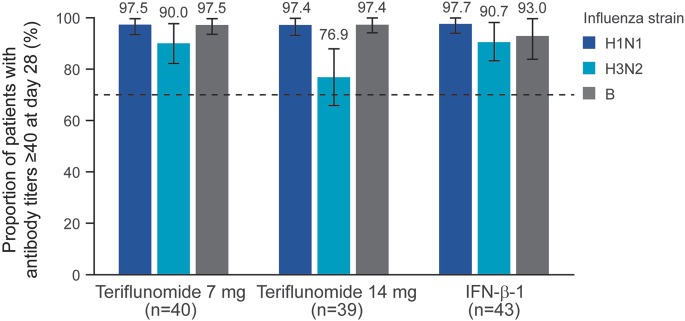
European criteria for efficacy of influenza vaccination in an 18- to 60-year-old population require achievement of a hemagglutination inhibition titer ≥40 by 70% of patients, as indicated by the dashed line. IFN = interferon.
A high proportion of patients across all treatment groups had high HI titers (≥40) for each vaccine strain at baseline (table 1). Correspondingly, few patients had antibody titers ≤10 at baseline (table 1). This reflects the fact that between 44% and 70% of the study population had received influenza vaccination previously and also because the influenza vaccines used in this study included the same strains as those used in the 2010/2011 vaccine. Of the limited number of patients with a prevaccination titer ≤10, approximately 50% or more achieved seroconversion (day 28 titer ≥40), with similar proportions across each of the treatment groups. The lowest seroconversion rates were observed for the H3N2 strain in all 3 groups (table 2).
The proportion of patients with a prevaccination titer <40 achieving seroprotection was ≥61% across the 3 treatment groups and 3 influenza strains. However, fewer patients in the teriflunomide 14-mg group than the teriflunomide 7-mg or IFN-β-1 groups exhibited seroprotection to the H3N2 strain (table 2).
The proportion of patients with a ≥2- or ≥4-fold increase in influenza strain-specific antibody titers from baseline to day 28 was slightly higher in the IFN-β-1 group than either of the teriflunomide groups (table 2). Across all influenza strains, the GMT ratio postvaccination/prevaccination ranged from 2.3 to 3.1 in the teriflunomide groups and from 3.4 to 4.7 in the IFN-β-1 group. The ratio exceeded 2.5 for all treatment groups and for all influenza strains, with the exception of H1N1 in the 14-mg teriflunomide group (table 2).
Safety and tolerability.
The study demonstrated no new safety concerns with teriflunomide administration, and influenza vaccination was generally well tolerated by the entire study population (table e-5). Two cases of infection were reported during the study. The first was a case of thoracic herpes zoster in a female patient in the teriflunomide 14-mg group, which occurred 9 days postvaccination, persisted for 14 days, was mild in severity, and responded to antiviral treatment. The second was a case of infective cholecystitis, also in the teriflunomide 14-mg group, which was associated with an increase in white blood count and facial rash, which had recovered by the end of the study. These 2 cases were not considered as SAEs. Indeed, no SAEs were reported in this study, and the overall incidence of TEAEs was higher in the IFN-β-1 group (45.7%) than the 2 teriflunomide groups (26.8% and 36.6% for 7 mg and 14 mg, respectively). No treatment discontinuation occurred due to a TEAE. None of the participants experienced an MS relapse during the study period.
DISCUSSION
The TERIVA study demonstrates that patients with RMS treated with teriflunomide are generally able to mount effective immune responses to seasonal influenza vaccination. Specifically, the proportion of patients with postvaccination antibody titers ≥40 to all influenza strains was greater than 70% in both teriflunomide treatment groups (range 76.9–97.5%), thereby meeting the European criterion for the efficacy of influenza vaccine in adult subjects aged 18–60 years, which was a priori identified as the primary criterion.9
Neither the duration of teriflunomide treatment prior to vaccination nor the origin of the teriflunomide extension study in which patients were enrolled had a significant impact on overall seroprotection rates. Thus, vaccine responses were preserved in patients treated with teriflunomide across a broad range of treatment exposure, ranging from 1.6 to 10.4 years. Although the response to all vaccine strains in all treatment groups was sufficient for vaccination to be considered protective, it should be noted that there was a slightly diminished response in the teriflunomide 14-mg group with respect to some evaluations for the H3N2 and H1N1 strains. Nevertheless, the development of adequate protective immune responses confirms the ability of teriflunomide to inhibit the activity of pathogenic autoreactive lymphocytes without causing global immunosuppression.
Influenza vaccination was well tolerated in all treatment groups in the TERIVA study. No death, SAE, or TEAE leading to treatment discontinuation was reported in the study, and no new or unexpected safety observations arose in either of the teriflunomide treatment groups. In addition, no MS relapses were reported during the study period. These safety data are in accordance with those from the TEMSO trial, in which teriflunomide was generally well tolerated with a well-characterized safety profile. Furthermore, the low incidence of serious infections and the absence of serious opportunistic infections seen in TEMSO lends further support to the observations made in this study that immune surveillance is preserved under teriflunomide treatment.3
Immune responses to influenza vaccine in patients with MS treated with IFN-β-1a were similar to untreated patients with respect to the proportion of patients achieving HI titers ≥40. No significant safety concerns emerged associated with vaccination in IFN-β-1a-treated patients.4 Our study was consistent with the literature showing that effective immune responses were observed in a reference RMS population treated with IFN-β-1 for at least 6 months, with good safety and tolerability. It should be noted that this study was not designed or powered to make direct comparisons between immune responses in the teriflunomide groups and the IFN-β-1 reference population.
Effective influenza vaccination remains important in patients with MS because influenza can cause serious complications and has been shown to be associated with a higher occurrence of exacerbations in patients with MS.10,11 The observations made in this study are therefore highly reassuring for patients administered teriflunomide treatment.
We conclude that influenza vaccine was found to be safe and effective in patients with MS receiving teriflunomide. Our findings, combined with efficacy and safety data from the TEMSO study, support the view that while teriflunomide appears to effectively limit abnormal activation of pathogenic lymphocyte responses implicated in MS relapses, teriflunomide therapy does not significantly interfere with adaptive activation of immune responses and generally appears to spare the serologic response to influenza antigens.
Supplementary Material
GLOSSARY
- CI
confidence interval
- DMT
disease-modifying therapy
- GMT
geometric mean titer
- HIA
hemagglutination inhibition assay
- IFN-β-1a
interferon-β-1a
- MS
multiple sclerosis
- RMS
relapsing forms of multiple sclerosis
- SAE
serious adverse event
- TEAE
treatment-emergent adverse event
- TEMSO
Teriflunomide Multiple Sclerosis Oral (trial)
- TERIVA
Teriflunomide and Vaccination (study)
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Amit Bar-Or, Françoise Menguy-Vacheron, Deborah Bauer, Stefan Jodl, Philippe Truffinet, and Myriam Benamor contributed to the development of the study design, data analysis, and interpretation. They were all involved in the development, reviewing, and revising of the manuscript. Mark S. Freedman, Marcelo Kremenchutzky, and Paul W. O’Connor all contributed to the development of the study design. They were all involved in reviewing the text of the manuscript. Deborah Bauer was responsible for all statistical analyses of the data, as well as development and revising of the manuscript. Scott Chambers is a medical writer who provided editorial support in the preparation and editing of the manuscript text, figures, and tables, addressing author, reviewer, and editorial comments, and assisting with the submission process. Editorial support was funded by Genzyme, a Sanofi company.
STUDY FUNDING
Supported by Genzyme, a Sanofi company. Editorial support was funded by Genzyme, a Sanofi company.
DISCLOSURE
A. Bar-Or: speaker, consulting fees, and/or research support: Amplimmune, Aventis, Bayhill Therapeutics, Berlex/Bayer, Biogen Idec, BioMS, Diogenix, Eli-Lilly, EMD Serono, Genentech, Genzyme-Sanofi, GSK, Guthy Jackson Greater Good Foundation, Medimmune, Mitsubishi Pharma, Novartis, Ono Pharmacia, Receptos, Roche, Teva Neuroscience, Wyeth. Editorial Board membership: Clinical and Experimental Neurology, Neurology®. M.S. Freedman: research/educational grant support: Genzyme; consulting fees: Actelion, Bayer, Biogen Idec, Celgene, Genzyme, Glycominds, Teva, Merck Serono, Novartis, Opexa, and sanofi-aventis. M. Kremenchutzky: consulting fees and/or research support from Bayer, Biogen Idec, EMD Serono, Genzyme, Novartis, sanofi-aventis, and Teva. F. Menguy-Vacheron: employee of Genzyme, a Sanofi company. D. Bauer: employee of Sanofi. S. Jodl: former employee of Sanofi. P. Truffinet: employee of Genzyme, a Sanofi company. M. Benamor: employee of Sanofi. Scott Chambers: employee of Fishawack Communications Ltd, contracted to provide editorial services to Sanofi, funded by Genzyme, a Sanofi company. P.W. O’Connor: consulting fees and/or research support: Actelion, Bayer, Biogen Idec, BioMS, Cognosci, Daiichi Sankyo, EMD Serono, Genentech, Genmab, Novartis, Roche, sanofi-aventis, Teva, and Warburg Pincus. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Gold R, Wolinsky JS. Pathophysiology of multiple sclerosis and the place of teriflunomide. Acta Neurol Scand 2011;124:75–84 [DOI] [PubMed] [Google Scholar]
- 2.Wolinsky JS, Narayana AP, Nelson F, et al. Magnetic resonance imaging outcomes from a phase III trial of teriflunomide. Mult Scler Epub 2013 Feb 27 [DOI] [PubMed]
- 3.O'Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011;365:1293–1303 [DOI] [PubMed] [Google Scholar]
- 4.Schwid SR, Decker MD, Lopez-Bresnahan M. Immune response to influenza vaccine is maintained in patients with multiple sclerosis receiving interferon beta-1a. Neurology 2005;65:1964–1966 [DOI] [PubMed] [Google Scholar]
- 5.O'Connor PW, Li D, Freedman MS, et al. A phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology 2006;66:894–900 [DOI] [PubMed] [Google Scholar]
- 6.Confavreux C, Li DK, Freedman MS, et al. Long-term follow-up of a phase 2 study of oral teriflunomide in relapsing multiple sclerosis: safety and efficacy results up to 8.5 years. Mult Scler 2012;18:1278–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Connor P, Wolinsky JS, Confavreux C, et al. Extension of a phase III trial (TEMSO) of oral teriflunomide in multiple sclerosis with relapses: clinical and MRI data 5 years after initial randomisation. Mult Scler 2011;17(suppl 17):S414.P924 [Google Scholar]
- 8.Comi G, O'Connor P, Wolinsky J, et al. Extension of a phase III trial (TEMSO) of oral teriflunomide in multiple sclerosis with relapses: safety outcomes with up to 4 years of follow-up. Mult Scler 2011;17(suppl 17):S182.P439 [Google Scholar]
- 9.European Agency for the Evaluation of Medicinal Products Committee for Proprietary Medicinal Products: Note for Guidance on Harmonization of Requirements for Influenza Vaccines. London: European Agency for the Evaluation of Medicinal Products; 1997 [Google Scholar]
- 10.Confavreux C, Suissa S, Saddier P, Bourdès V, Vukusic S. Vaccinations and the risk of relapse in multiple sclerosis. N Engl J Med 2001;344:319–326 [DOI] [PubMed] [Google Scholar]
- 11.De Keyser J, Zwanikken C, Boon M. Effect of influenza vaccination and influenza illness on exacerbations in multiple sclerosis. J Neurol Sci 1998;195:51–53 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


