Abstract
Birth cohort studies of developmental defects of enamel (DDE) and early childhood caries (ECC) in very low birthweight (VLBW) and normal birthweight (NBW) infants are rare. In this birth cohort of 234 VLBW and 234 NBW infants, we report the incidence of ECC and DDE at 8 and 18-20 mos of corrected age. Infant medical and maternal socio-demographic data were abstracted from medical records at birth. Dental assessments for ECC and DDE (enamel hypoplasia, demarcated and diffuse opacities) were completed at 8 and 18-20 mos. The incidence of hypoplasia was significantly higher in VLBW compared with NBW infants (8 mos, 19% vs. 2%; 18 mos, 31% vs. 8%). The incidence of ECC (International Caries Detection and Assessment System: ICDAS ≥ 2) was 1.4% (8 mos) and 12% (18-20 mos) and was similar between the VLBW and NBW groups. At both ages, using a beta-binomial regression model to control for potential confounders (maternal and infant characteristics), we found increased risk for enamel hypoplasia among the VLBW infants compared with the NBW infants. African Americans had a lower risk for enamel hypoplasia at 18-20 mos. The VLBW infants should be monitored for ECC due to the presence of enamel hypoplasia.
Keywords: children, longitudinal studies, relative risk, dental caries, enamel defects, epidemiology
Introduction
Among the developmental defects of enamel (DDE: enamel hypoplasia, demarcated, diffuse opacity), hypoplasia, in particular, increases the susceptibility for primary dental caries (Oliveira et al., 2006; Caufield et al., 2012). Prevalence estimates for DDE in diverse populations of very low birthweight children (VLBW) range between 52 and 96% (Johnsen et al., 1984; Seow et al., 1989; Lai et al., 1997; Aine et al., 2000; Takaoka et al., 2011). VLBW occurs most frequently with pre-term birth, and disproportionately among African Americans and those of lower socio-economic status (Hamilton et al., 2012).
The central hypothesis for DDE in VLBW infants is through altered calcium homeostasis from systemic causes during the pre- and post-natal periods, and/or through local causes because of endotracheal intubation and mechanical ventilation during the post-natal period (Seow et al., 1989). Consequently, ameloblasts and odontoblasts can be affected in the pre- and post-natal periods due to various maternal and child risk factors (Caufield et al., 2012). The clinical expression of these systemic and/or local insults during the enamel matrix formation, mineralization, and maturation phases results as qualitative (opacity) or quantitative (hypoplasia) defects (Suckling, 1989). In the only cohort study, Lai and colleagues (1997) followed 50 VLBW and normal birthweight (NBW) children from 30 to 52 mos and found that enamel defects were related to increased caries in the VLBW group. More recently, Caufield et al. (2012) suggested an association between enamel hypoplasia and severe ECC.
There are longitudinal studies investigating ECC risk (Oliveira et al., 2006; Thitasomakul et al., 2009; Targino et al., 2011; Warren et al., 2012; Zhou et al., 2012). However, there are no longitudinal birth cohort studies assessing the relationship between VLBW and ECC taking into account multiple putative mediators, moderators, and confounders. The causal relationship between birthweight and ECC is through a complex set of pathways that includes biological and environmental mediators, with DDE being one of the biological factors (Nelson et al., 2010).
In this study, we hypothesized that the incidence of DDE and ECC among VLBW would be higher compared with that in NBW infants as a result of the pre- and post-natal systemic complications of preterm birth, after adjustment for the confounding effects of maternal socio-demographic and infant factors.
Methods
Study Design
The infants and biological mothers are part of a larger cohort study to assess the effect of birth group (exposure: VLBW, NBW) on ECC (outcome). For assessment of the causal relationship between birth group and ECC, a complex set of pathways that includes biological (DDE, salivary Streptococcus mutans) and environmental (medical, socio-behavioral) mediators, together with the influence of potential confounders (socio-demographics) and genetics, is being investigated. This report presents a reduced model of the effects of confounders on ECC and DDE in infants before 2 yrs of age.
Study Setting and Participants
The infants and mothers were recruited at birth from 2 hospitals whose neonatal intensive-care units treat the majority of infants with medical complications in Cleveland and surrounding suburbs. VLBW infants were preterm (< 37 weeks’ gestation) with birthweight ≤ 1,500 g. NBW infants were healthy and full-term (≥ 37 weeks’ gestation), with birthweight ≥ 2,500 g. Infants with major neurological problems and/or congenital malformations or those whose mothers had major psychiatric or physical illness, HIV exposure, were < 18 yrs of age, suffered from drug or alcohol addiction, did not speak English, or who lived greater than a two-hour driving distance were excluded to minimize selection bias due to compliance. One infant out of twins/triplets who met the inclusion/exclusion criteria was randomly selected. The infants were recruited during a 3-year time period (2007-2010). Follow-up visits were conducted at approximately 8 and 18-20 mos of corrected age (i.e., actual weeks since date of birth minus weeks premature) to coincide with the primary tooth eruption patterns. The sample size required for testing the hypothesis of the original study is given in the Appendix. The Institutional Review Boards of the participating hospitals gave approval, and parent/guardian consent was obtained. This manuscript conforms to the STROBE guidelines for observational studies.
Measures
Demographics and Medical Assessments
Socio-demographic and medical data were abstracted from medical records and used for this study and included: mother’s age, race (African American vs. Caucasian/other), ethnicity (Hispanic vs. not Hispanic), education (< 12 yrs, ≥ 12 yrs), number of live births (para), smoking status (yes, no), marital status (single, other), and socio-economic status (SES, low, high; Hollingshead, 1957). Medical data included infant’s birthweight, gestational age, vaginal or C-section delivery, Apgar scores, days of hospitalization, assisted ventilation, oxygen use, and gender. Infants were also classified (Olsen et al., 2010) as small for gestational age (SGA: ≤ 10th percentile) or appropriate for gestational age (AGA: >10th percentile), and by hospital of birth (UH, Metro).
Dental Examination and Outcomes
The infants were visually examined by means of a portable headlight and mirror at 8 and 18-20 mos. The presence of developmental enamel defects was determined according to the modified DDE index (FDI Commission on Oral Health, Research and Epidemiology, 1992). The index identifies and defines the type (hypoplasia, opacity, combination defects), number (single, multiple), and location (on surface) of tooth enamel defects on the buccal and lingual surfaces of all primary teeth. Plaque was removed with gauze before DDE examination, and tooth surfaces were examined wet. The presence of ECC was determined according to ICDAS (Pitts, 2004).
Dental outcomes included DDE and ECC. The DDE index type was utilized to calculate the 3 summary measures for each infant: mean number of teeth affected by hypoplasia, demarcated opacities, and any DDE defect. Based on the summary measures, infants were further categorized (0 = no, ≥ 1 = yes for hypoplasia, demarcated opacity, or any defect) to determine incidence. According to the AAPD (2008) ECC definition, the numbers of decayed (ICDAS lesion code ≥ 2, which includes early non-cavitated and cavitated lesions) and filled (ICDAS filling code ≥ 3) primary teeth (dft) were calculated. Further, infants were categorized according to dft (0 = no, ≥ 1 = yes) to determine incidence of ECC.
Examiners were trained and calibrated against gold standard examiners. For ICDAS examinations, the reliability was good to excellent, with an inter-rater WKappa of 0.69 – 0.92 and intra-rater WKappa of 0.81 – 0.92 (Nelson et al., 2011). For DDE examinations, the reliability was moderate to excellent, with an inter-rater WKappa of 0.56 - 0.90 and intra-rater WKappa of 0.78 - 0.93. The dental examiners were blinded to the birth group.
Data Analysis
Preliminarily, maternal socio-demographic, infant medical, and unadjusted dental outcomes were compared between the VLBW and NBW groups, with t tests applied for unequal variances for sample means and Pearson chi-square tests for categorical variables.
DDE was modeled based on the 3 summary measures (the number of teeth affected by hypoplasia, demarcated opacities, and any type of defect) separately for the 8- and 18- to 20-month visits. ECC was modeled at 18-20 mos only, since very few infants have ECC at 8 mos. Each endpoint was analyzed in either a beta-binomial (BB) regression model (Haseman and Kupper, 1979) or a zero-inflated beta-binomial model (ZIBB, Cheung, 2006), depending on which model provided a better fit according to the Akaike Information Criterion (AIC). These models take into account the number of erupted teeth and allow for extra-binomial variability. The covariates for each model included exposure (VLBW, NBW) and confounding maternal (race, education, age, marital status, SES) and child (gender, birth hospital) socio-demographic variables. Model coefficients were estimated by maximum likelihood and tested via model-based t tests. Only participants with complete data (assumed to be a random subsample) were included in the analysis.
In addition, the adjusted relative risk for each covariate was estimated by a model standardization technique (Albert et al., 2013). Ninety-five percent confidence intervals for each relative risk were computed by the bootstrap percentile method (DiCiccio and Efron, 1996) based on 2,000 bootstrap samples. Bootstrap samples yielding extreme relative risks (> 1,000 or < 0.001) were excluded from the computation of confidence intervals. SAS Version 9.3 (SAS Institute Inc., Cary, NC, USA) was used for all analyses.
Results
Sample Characteristics
Enrollment and participation are outlined in Fig. 1. Specifically, 890 potential participants were approached, with 566 enrolling into the study. Between enrollment and the start of any study visits, 98 were removed from the study, with a resulting sample of 468 (VLBW, 234; NBW, 234) active participants. Maternal characteristics (Table 1) were generally similar, but the VLBW group had more mothers who were Hispanic, lower SES, and with fewer live births (para). Those in the VLBW were significantly different from those in the NBW group in medical characteristics (Table 1), mainly due to the inclusion criteria of our study. Of 468 active participants, 386 (82.5%) and 378 (81%) participated at 8 and 18-20 mos, respectively. Some birth characteristics of maternal participants were significantly different from those of non-participants: At 8 mos, they had fewer live births and were married, and at 18-20 mos, they were older and there were fewer smokers.
Figure 1.
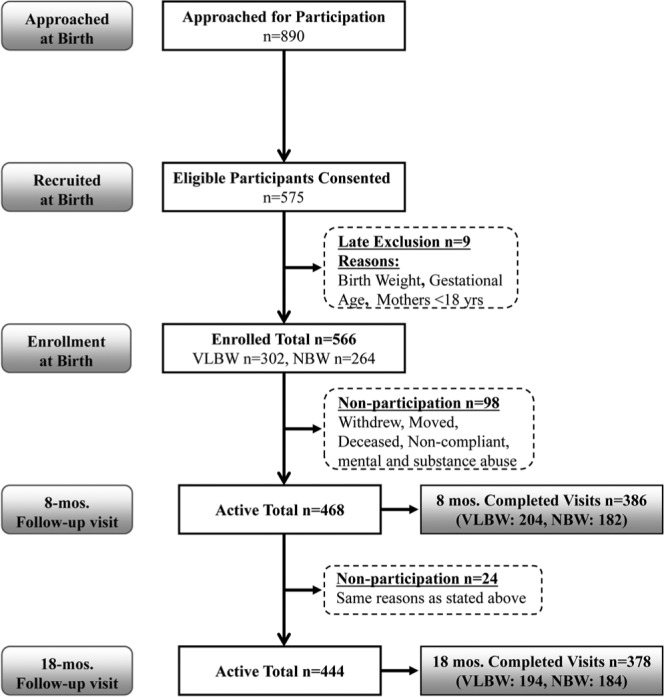
Flow chart of study population recruitment and participation of Very Low Birthweight (VLBW) and Normal Birthweight (NBW) infants.
Table 1.
Birth, Socio-demographic, and Medical Characteristics of 468 Active Infants and Mothers
| Very Low Birthweight (n = 234) | Normal Birthweight (n = 234) | p value | |
|---|---|---|---|
| Maternal | |||
| Age in yrs (mean ± SD) | 26.0 ± 6.0 | 26.4 ± 5.2 | .3 |
| Race | |||
| Black | 158 (71.2%) | 156 (68.7%) | .57 |
| Other | 64 (28.8%) | 71 (31.3%) | |
| Ethnicity | |||
| Hispanic/Latino | 23 (10.9%) | 11 (5.1%) | .03* |
| Other | 189 (89.1%) | 203 (94.9%) | |
| Marital status | |||
| Single | 164 (70.7%) | 164 (70.4%) | .94 |
| Other | 68 (29.3%) | 69 (29.6%) | |
| Education | |||
| < 12 yrs | 40 (17.9%) | 40 (17.4%) | .9 |
| ≥ 12 yrs | 184 (82.1%) | 190 (82.6%) | |
| Socio-economic status (SES) | |||
| Low | 161 (72.9%) | 145 (63.3%) | .03* |
| High | 60 (27.1%) | 84 (36.7%) | |
| Smoking | |||
| Yes | 42 (19.7%) | 43 (18.7%) | .78 |
| No | 171 (80.3%) | 187 (81.3%) | |
| Para (mean ± SD) | 1.4 ± 1.5 | 2.0 ± 1.4 | < .001* |
| Infant | |||
| Gender | |||
| Male | 120 (51.3%) | 128 (54.7%) | .46 |
| Female | 114 (48.7%) | 106 (45.3%) | |
| Birthweight in gms (mean ± SD) | 1,040.4 ± 277.1 | 3,322.0 ± 409.5 | < .001* |
| Gestation age in wks (mean ± SD) | 28.2 ± 2.8 | 39.4 ± 1.1 | < .001* |
| Hospital stay in days (mean ± SD) | 71.7 ± 36.4 | 3.0 ± 1.1 | < .001* |
| Apgar at 5 min (mean ± SD) | 6.9 ± 2.1 | 8.9 ± 0.3 | < .001* |
| Apgar at 1 min (mean ± SD) | 4.9 ± 2.7 | 8.4 ± 1.0 | < .001* |
| Small for gestational age (SGA) | |||
| Yes | 50 (21.4%) | 12 (5.1%) | < .001* |
| No | 184 (78.6%) | 222 (94.9%) | |
| Mode of delivery | |||
| C-section | 140 (60.3%) | 80 (34.8%) | < .001* |
| Vaginal | 92 (39.7%) | 150 (65.2%) | |
| Oxygen use | |||
| No | 51 (22.0%) | 225 (98.7%) | < .001* |
| Yes | 181 (78.0%) | 3 (1.3%) | |
| Assisted ventilation | |||
| No | 29 (12.5%) | 223 (97.8%) | < .001* |
| Yes | 204 (87.5%) | 5 (2.2%) | |
| Hospital of birth | |||
| UH | 140 (59.8%) | 168 (71.8%) | .006* |
| Metro | 94 (40.2%) | 66 (28.2%) | |
Significant at p < .05.
In total, 386 (VLBW, 204; NBW, 182) and 378 (VLBW, 194; NBW, 184) infant-caregiver dyads participated at the 8- and 18- to 20-month visits, respectively. The mean corrected age at visits was significantly (p < .05) lower in the VLBW (8 mos, 9.2 ± 1.6; 18-20 mos, 20.6 ± 3.2) compared with the NBW group (8 mos, 9.7 ± 1.9; 18-20 mos, 21.3 ± 3.3). Since the VLBW dental visits were coordinated with their follow-up medical visits, they had fewer reschedules, no-shows, and lost contact information compared with the NBW infants. Maternal birth characteristics (Appendix Tables 1, 2) were generally similar, but at 8 and 18-20 mos the VLBW had consistently more mothers who were of lower SES, and with fewer live births (para).
At 8 mos, the VLBW group had a significantly higher proportion (28% vs. 18%) with unerupted teeth compared with the NBW infants, but at 18-20 mos, all infants had erupted teeth. Among those infants with erupted teeth (n = 296) at 8 mos, the mean number of teeth was similar between the VLBW and NBW groups, while at 18 mos the VLBW infants (15.0 ± 3.0) had significantly fewer teeth compared with the NBW infants (15.6 ± 2.7).
Hypothesis Testing
The unadjusted incidence of enamel hypoplasia (Table 2) was significantly higher in the VLBW compared with the NBW group (8 mos, 19% vs. 2%; 18-20 mos, 31% vs. 8%). Any DDE defect was also significantly higher among the VLBW infants, but demarcated opacities were similar (Table 2). At both ages, in the VLBW group, enamel hypoplasia was the predominant defect, while in the NBW group it was demarcated opacities (Table 2). At 8 and 18-20 mos, the overall incidence of ECC was 1.4% (4/296) and 12% (46/378), respectively, but it did not differ significantly between the VLBW and NBW groups (Table 2).
Table 2.
Unadjusted Incidence and Means of Dental Outcomes between Very Low Birth Weight Infants and Normal Birth Weight Infants
| 8 mos‡ |
18 mos |
|||||
|---|---|---|---|---|---|---|
| Dental Outcomes | VLBW(n = 149) | NBW(n = 149) | p value | VLBW(n = 194) | NBW(n = 184) | p value |
| Number of teeth (mean ± SD) | 4.1 ± 2.8 | 4.2 ± 2.6 | .75 | 15.0 ± 3.0 | 15.6 ± 2.7 | .05* |
| % with Early Childhood Caries (ECC) | 2 (1.3%) | 2 (1.3%) | 1 | 21 (10.8%) | 25 (13.6%) | .41 |
| No. of teeth with ECC (mean ± SD) | 0.04 ± 0.37 | 0.03 ± 0.23 | .7 | 0.40 ± 1.4 | 0.36 ± 1.2 | .78 |
| % with demarcated opacity | 12 (8.1%) | 8 (5.4%) | .35 | 38 (19.6%) | 29 (15.8%) | .33 |
| Demarcated (mean ± SD) | 0.12 ± 0.46 | 0.11 ± 0.56 | .91 | 0.51 ± 1.75 | 0.27 ± 0.74 | .08 |
| % with hypoplasia | 28 (18.8%) | 3 (2.0%) | < .001* | 60 (30.9%) | 15 (8.2%) | < .001* |
| Hypoplasia (mean ± SD) | 0.29 ± 0.69 | 0.02 ± 0.14 | < .001* | 0.72 ± 1.59 | 0.18 ± 0.68 | < .001* |
| % with any enamel defect | 37 (24.8%) | 12 (8.1%) | < .001* | 85 (43.8) | 45 (24.5%) | .001* |
| Any defect (mean ± SD) | 0.48 ± 1.05 | 0.15 ± 0.60 | .001* | 1.37 ± 2.63 | 0.53 ± 1.18 | < .001* |
VLBW, very low birthweight; NBW, normal birthweight.
Dental outcomes calculated only for 296 infants with erupted teeth
Significant at p < .05.
For the multivariate analysis, the BB model provided a better fit (according to AIC) for DDE, and the ZIBB model provided a better fit for ECC. All models provided an adequate fit according to a 0.05 alpha-level chi-square test. Table 3 indicates that, at 18-20 mos, those in the VLBW had approximately 5 and 2.3 times greater risk for enamel hypoplasia and any defect, even after adjustment for socio-demographic variables (Hypoplasia RR = 4.77, 95% CI 2.8, 9.9; Any defect RR = 2.27, 95% CI 1.6, 3.3). Enamel hypoplasia was 47% less likely in African Americans. At 8 mos, based on 254 complete cases with teeth (Appendix Table 3), only ‘birth group’ (VLBW) in the model showed significantly increased risk (RR = 14.5, 95% CI 5.3, 41.0) for enamel hypoplasia. Neither ‘Birth group’ nor any other socio-demographic variables affected demarcated opacities (8 and 18-20 mos) or ECC at 18-20 mos.
Table 3.
Adjusted Relative Risk (95% confidence interval) for Developmental Defects of Enamel (DDE) and Early Childhood Caries (ECC) at 18-20 mos
| Hypoplasiaa |
Any Defectsa |
ECCb |
||||
|---|---|---|---|---|---|---|
| Variable | RR (95% CI) | p value | RR (95% CIc) | p value | RR (95% CI) | p value |
| Birth group | ||||||
| NBW (reference) | ||||||
| VLBW | 4.77 (2.82, 9.95) | < .001* | 2.27 (1.64, 3.34) | < .001* | 0.98 (0.43, 2.04) | 0.96 |
| Socio-economic status (SES) | ||||||
| High (reference) | ||||||
| Low | 1.08 (0.61, 2.05) | .8 | 1.02 (0.69, 1.59) | .93 | 0.64 (0.17, 2.92) | 0.52 |
| Race | ||||||
| Other (reference) | ||||||
| Black | 0.53 (0.31, 0.92) | .03* | 0.75 (0.50, 1.16) | .18 | 2.21 (0.19, 17.23) | 0.47 |
| Education | ||||||
| ≥ 12 yrs (reference) | ||||||
| < 12 yrs | 0.94 (0.38, 1.94) | .87 | 0.95 (0.53, 1.59) | .85 | 0.85 (0.12, 2.08) | 0.82 |
| Marital status | ||||||
| Married (reference) | ||||||
| Other | 1.51 (0.82, 3.17) | .23 | 1.62 (1.03, 2.82) | .06 | 0.84 (0.16, 6.99) | 0.86 |
| Age (yrs) | ||||||
| 21-35 (reference) | ||||||
| < 21 | 1.15 (0.57, 2.13) | .67 | 1.02 (0.64, 1.66) | .93 | 0.58 (0.11, 2.47) | 0.49 |
| > 35 | 1.14 (0.28, 2.46) | .78 | 1.05 (0.45, 1.81) | .9 | 0.90 (0.11, 3.81) | 0.91 |
| Gender | ||||||
| Male (reference) | ||||||
| Female | 0.80 (0.46, 1.36) | .37 | 0.71 (0.49, 1.01) | .06 | 0.72 (0.13, 1.92) | 0.61 |
| Hospital of birth | ||||||
| UH (reference) | ||||||
| Metro | 1.38 (0.79, 2.36) | .21 | 1.25 (0.82, 1.85) | .24 | 0.38 (0.08, 1.84) | 0.21 |
Beta-binomial (BB) model.
Zero-inflated Beta-binomial (ZIBB) model.
Out of the 27 analyses shown in the Table, 21 used all 2,000 bootstrap samples for the confidence interval, 4 used between 1,997 and 1,999 samples, and 2 used 1,891 and 1,987 samples, respectively.
VLBW, very low birthweight; NBW, normal birthweight.
Significant at p < .05.
Discussion
To our knowledge, this is the first epidemiological study to assess the causal relationship between a large birth cohort of VLBW and NBW infants and ECC, by studying biological and environmental mediator pathways, potential moderators, and confounders for ECC. This first report looks at the risk for DDE and ECC at the time of newly erupting primary teeth after adjustment for confounding effects at 8 and 18-20 mos. The effects of mediator pathways on ECC in this cohort will be studied longitudinally at later infant assessments (i.e., 36 mos), when the entire primary dentition is present. The findings at 8 and 18-20 mos support our hypothesis of increased DDE in VLBW infants, but ECC was similar between the birth groups. Due to pre- and post-natal medical complications in VLBW infants, manifestation of DDE can occur as early as 8 and 18-20 mos. But the delay in tooth eruption among VLBW (i.e., the teeth may not have been present for long) could have influenced ECC results, despite those in the VLBW group having increased enamel hypoplasia. Although non-significant, those in the VLBW group did have increased numbers of teeth affected by ECC (Table 2), similar to prior findings (Lai et al., 1997) in children approximately 41 mos old.
VLBW and Enamel Defects
Our findings suggest that VLBW infants are at a significant risk for enamel hypoplasia, a quantitative defect, at both 8 and 18-20 mos. These results are similar to those in prior reports in the literature for children > 24 mos of age (Seow et al., 1989; Lai et al., 1997; Aine et al., 2000: Rythén et al., 2012). The VLBW group had significantly greater medical needs, suggesting that altered calcium homeostasis combined with local insults during the pre- and/or post-natal period may have had an effect on the enamel matrix formation and mineralization phases. Intra-uterine growth restriction (IUGR), as measured by SGA, was significantly associated with increased DDE (Pinho et al., 2012) in 1- to 5-year-old children. Our descriptive results at 18-20 mos indicate that 19% of VLBW infants (16/85) and 7% of NBW infants (3/45) who had DDE were SGA. So, we speculate that the likely mechanism is not just intra-uterine malnutrition, but also a host of other medical problems that creates an environment for calcium and mineral insufficiency both pre- and post-natally. In particular, low SES associated with VLBW may be a surrogate for inadequate nutrition, high risk behaviors, and medical conditions, thereby having an influence on enamel hypoplasia (Caufield et al., 2012).
An interesting unexpected finding was that, at 18-20 mos, African Americans had a significantly decreased risk for enamel hypoplasia, even after adjustment for birth group and other socio-demographic variables. A lower non-significant risk for African Americans was also observed at 8 mos. Deciduous enamel has been found to be significantly thicker in blacks compared with whites (Harris et al., 2001), and whether this provides a protective effect for hypoplasia needs to be explored further.
A difference in demarcated opacities, a qualitative defect, between the groups was not found, suggesting that post-natal insults may have occurred during later phases of enamel maturation. Due to medical and technological advancements, the post-natal survival and care of VLBW infants have improved vastly over the past decades (Philip, 2005), thus probably reducing potential damage to the maturing enamel.
The incidence of DDE found in our VLBW infants was lower compared with the prevalence rates found in previous literature (Johnsen et al., 1984; Seow et al., 1989; Lai et al., 1997; Aine et al., 2000; Takaoka et al., 2011). First, our study infants were younger (< 2yrs) than in previous studies; thus, the entire primary dentition was not present; Second, our rates may also be underestimated due to cooperation issues (moving, crying) when young infants are being examined. A prior study of this same cohort indicated that photographic examinations detected significantly more DDE compared with clinical examinations (Chen et al., 2013).
VLBW and ECC
Our results indicate that the incidence of ECC at 18-20 mos was 12%—two times higher than a national sample of 2-year-old U.S. children (6.5%; Nunn et al., 2009)—possibly reflecting the lower SES of our sample. While there were no differences in ECC between birth groups, it is possible that VLBW had newly erupted teeth that were thus not present long enough for demineralization activity. The presence of enamel hypoplasia provides ideal retentive surfaces for early bacterial colonization, thus promoting ECC (Caufield et al., 2012). In future reports, the role of S. mutans bacterial activity between the groups will be investigated.
An important strength of this study is that children were examined from infancy, which afforded us the knowledge of their birth history as well as the ability to track the DDE on surfaces/teeth longitudinally, to see subsequent caries development. The limitations include losses to follow-up, as expected with any longitudinal investigation. However, with successful retention strategies, nearly 82% of the cohort was retained. Additionally, we accounted for these losses in the initial recruitment numbers, and thus sufficient power existed to detect differences between groups for this report. The difficulties in examining moving and crying infants may have resulted in underestimated incidence rates, but our examiners were all pediatric dentists/residents trained in examining infants.
VLBW infants are at increased risk for enamel hypoplasia, with the mechanism pointing to in utero insults during tooth formation. Although ECC was similar between the groups, the VLBW group needs continued monitoring for ECC, due to the presence of enamel hypoplasia.
Supplementary Material
Acknowledgments
We thank all study participants, staff, dental examiners, clinic coordinators, and Drs. Marc Collin and Maureen Hack (for access to ‘preemie’ clinics).
Footnotes
The study was supported by the National Institute of Dental and Craniofacial Research (grant R01DE017947) and by the National Center for Research Resources (grants CTSC UL1 RR024989 and CTSC UL1TR000439).
The author(s) declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Aine L, Backstrom MC, Maki R, Kuusela AL, Koivisto AM, Ikonen RS, et al. (2000). Enamel defects in primary and permanent teeth of children born prematurely. J Oral Pathol Med 29:403-409. [DOI] [PubMed] [Google Scholar]
- Albert JM, Wang W, Nelson S. (2013). Estimating overall exposure effects for zero-Inflated regression models with application to dental caries. Stat Methods Med Res [E-pub ahead of print September 8, 2011] (in press). [Google Scholar]
- American Academy of Pediatric Dentistry (AAPD) (2008). Definition of Early Childhood Caries (ECC). Council on Clinical Affairs. Adopted 2003, last revised 2008; found at http://www.aapd.org/assets/1/7/D_ECC.pdf (URL accessed 6/24/2013).
- Caufield PW, Li Y, Bromage TG. (2012). Hypoplasia-associated Severe Early Childhood Caries–a proposed definition. J Dent Res 91:544-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lee W, Ferretti GA, Slayton RL, Nelson S. (2013). Agreement between photographic and clinical examinations in detecting developmental defects of enamel in infants. J Public Health Dent. [Epub ahead of print 4/7/2013] (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung YB. (2006). Growth and cognitive function of Indonesian children: zero-inflated proportion models. Stat Med 25:3011-3022. [DOI] [PubMed] [Google Scholar]
- DiCiccio TJ, Efron B. (1996). Bootstrap confidence intervals. Statist Sci 11:189-228. [Google Scholar]
- FDI Commission on Oral Health, Research and Epidemiology (1992). A review of developmental defects of enamel index (DDE index). Int Dent J 42:411-426. [PubMed] [Google Scholar]
- Hamilton BE, Martin JA, Ventura SJ. (2012). Births: Preliminary data for 2011. National Vital Statistics Reports 61(5). Hyattsville, MD: National Center for Health Statistics; URL accessed at: http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_05.pdf on 6/24/2013. [PubMed] [Google Scholar]
- Harris EF, Hicks JD, Barcroft BD. (2001). Tissue contributions to sex and race: differences in tooth crown size of deciduous molars. Am J Phys Anthropol 115:223-237. [DOI] [PubMed] [Google Scholar]
- Haseman JK, Kupper LL. (1979). Analysis of dichotomous response data from certain toxicological experiments. Biometrics 35:281-293. [PubMed] [Google Scholar]
- Hollingshead AB. (1957). Two factor index of social position. New Haven, CT, USA: Yale University Press. [Google Scholar]
- Johnsen D, Krejci C, Hack M, Fanaroff A. (1984). Distribution of enamel defects and the association with respiratory distress in very low birthweight infants. J Dent Res 63:59-64. [DOI] [PubMed] [Google Scholar]
- Lai PY, Seow WK, Tudehope DI, Rogers Y. (1997). Enamel hypoplasia and dental caries in very-low birthweight children: a case-controlled, longitudinal study. Pediatr Dent 19:42-49. [PubMed] [Google Scholar]
- Nelson S, Albert JM, Lombardi G, Wishnek S, Asaad G, Kirchner HL, et al. (2010). Dental caries and enamel defects in very low birth weight adolescents. Caries Res 44:509-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S, Eggertsson H, Powell B, Mandelaris J, Ntragatakis M, Richardson T, et al. (2011). Dental examiners consistency in applying the ICDAS criteria for a caries prevention community trial. Community Dent Health 28:238-242. [PubMed] [Google Scholar]
- Nunn ME, Dietrich T, Singh HK, Henshaw MM, Kressin NR. (2009). Prevalence of Early Childhood Caries among very young urban Boston children compared with US children. J Public Health Dent 69:156-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AF, Chaves AM, Rosenblatt A. (2006). The influence of enamel defects on the development of early childhood caries in a population with low socioeconomic status: a longitudinal study. Caries Res 40:296-302. [DOI] [PubMed] [Google Scholar]
- Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. (2010). New intrauterine growth curves based on United States data. Pediatrics 125:e214-e224. [DOI] [PubMed] [Google Scholar]
- Philip AG. (2005). The evolution of neonatology. Pediatr Res 58:799-815. [DOI] [PubMed] [Google Scholar]
- Pinho JR, Filho FL, Thomaz EB, Lamy ZC, Libério SA, Ferreira EB. (2012). Are low birth weight, intrauterine growth restriction, and preterm birth associated with enamel developmental defects? Pediatr Dent 34:244-248. [PubMed] [Google Scholar]
- Pitts NB. (2004). “ICDAS”—An international system for caries detection and assessment being developed to facilitate caries epidemiology, research and appropriate clinical management. Community Dent Health 21:193-198. [PubMed] [Google Scholar]
- Rythén M, Niklasson A, Hellström A, Hakeberg M, Robertson A. (2012). Risk indicators for poor oral health in adolescents born extremely preterm. Swed Dent J 36:115-124. [PubMed] [Google Scholar]
- Seow WK, Masel JP, Weir C, Tudehope DI. (1989). Mineral deficiency in the pathogenesis of enamel hypoplasia in prematurely born, very low birthweight children. Pediatr Dent 11:297-302. [PubMed] [Google Scholar]
- Suckling GW. (1989). Development defects of enamel—historical and present-day perspectives of their pathogenesis. Adv Dent Res 3:87-94. [DOI] [PubMed] [Google Scholar]
- Takaoka LA, Goulart AL, Kopelman BI, Weiler RM. (2011). Enamel defects in the complete primary dentition of children born at term and preterm. Pediatric Dentistry 33:171-176. [PubMed] [Google Scholar]
- Targino AG, Rosenblatt A, Oliveira AF, Chaves AM, Santos VE. (2011). The relationship of enamel defects and caries: a cohort study. Oral Dis 17:420-426. [DOI] [PubMed] [Google Scholar]
- Thitasomakul S, Piwat S, Thearmontree A, Chankanka O, Pithpornchaiyakul W, Madyusoh S. (2009). Risks for early childhood caries analyzed by negative binomial models. J Dent Res 88:137-141. [DOI] [PubMed] [Google Scholar]
- Warren JJ, Kramer KW, Phipps K, Starr D, Dawson DV, Marshall T, et al. (2012). Dental caries in a cohort of very young American Indian children. J Public Health Dent 72:265-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yang JY, Lo EC, Lin HC. (2012). The contribution of life course determinants to early childhood caries: a 2-year cohort study. Caries Res 46:87-94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


