ABSTRACT
This paper addresses the fourth theme of the Indiana Global Health Research Working Conference, Clinical Effectiveness and Health Systems Research. It explores geographic variation in health care delivery and health outcomes as a source of learning how to achieve better health outcomes at lower cost. It focuses particularly on the relationship between investments made in capacities to deliver different health care services to a population and the value thereby created by that care for individual patients. The framing begins with the dramatic variation in per capita health care expenditures across the nations of the world, which is largely explained by variations in national wealth. The 1978 Declaration of Alma Ata is briefly noted as a response to such inequities with great promise that has not as yet been realized. This failure to realize the promise of Alma Ata grows in significance with the increasing momentum for universal health coverage that is emerging in the current global debate about post-2015 development goals. Drawing upon work done at Dartmouth over more than three decades, the framing then turns to within-country variations in per capita expenditures, utilization of different services, and health outcomes. A case is made for greater attention to the question of value by bringing better information to bear at both the population and individual levels. Specific opportunities to identify and reduce waste in health care, and the harm that is so often associated with it, are identified by learning from outcome variations and practice variations.
KEY WORDS: global health, delivery science, practice variation, shared decision making, patients’ preferences
INTRODUCTION
In recent years, Dartmouth has built on its distinctive contributions linking population and personal health by extending its commitment to expand the science that supports health care delivery. It designed a new interdisciplinary curriculum in health care delivery science, drawing heavily from the management sciences, to bring together researchers and practitioners to foster implementation of innovations that improve value. It formed new research partnerships, including the High Value Health Care Collaborative (HVHC), to apply common methods and measures to improve execution in delivery, by studying variation in processes and impact on both cost and health outcomes. Those same partnerships are also focusing on shared decision making, and measurement of patients’ preferences for outcomes and for treatments, to improve value realized by those who live with the consequences of care that is delivered (or not).
While the decades of research at Dartmouth have focused largely on the United States health care system, the relationships between system capacity and value delivered to patients are universal. The research on outcome and practice variation has been replicated in different countries and contexts. Attention to the lessons learned, and to the replication of this research in developing countries, could serve to guide investment decisions toward better care and better health at lower cost.
Achievement of this “triple aim” in low-resource settings could then inform much needed service innovation in the United States and other developed countries of the world. The opportunity for developed countries to learn about value-driven innovation in design of delivery systems from developing countries has been called “reverse innovation”. The paper closes with a brief description of Dartmouth’s efforts to extend its partnership model globally for mutual cross-border learning that could help all nations realize the promise of Alma Ata, and thereby achieve progress toward universal health coverage with delivery of health services that produce value for individuals and populations.
GEOGRAPHY AS DESTINY IN HEALTH CARE
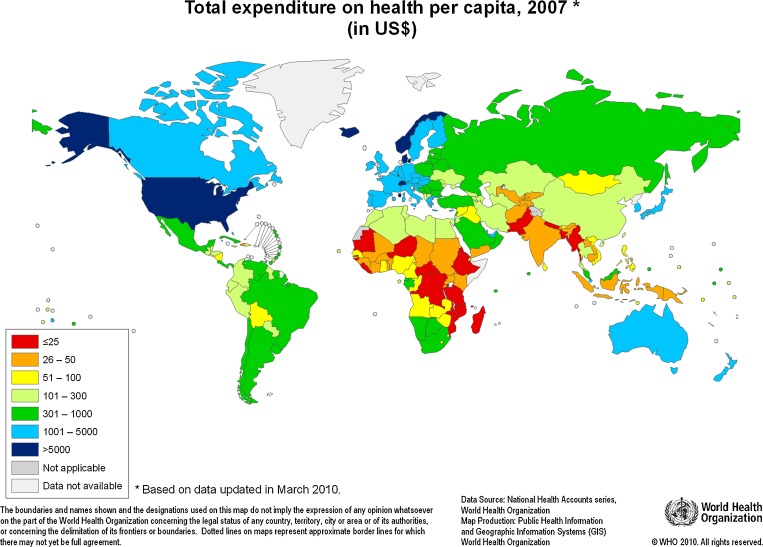
The economic and ethical discourse in global health often begins with the stunning inequities in access to health services across the range of wealth of nations. In 2010, the per capita expenditures on health care varied 500-fold—from a high of $8,437 in the United States to a low of $16 in Eritrea. Five countries had per capita expenditures greater than $5,000, while many more countries had expenditures of less than $50 (Fig. 1).1
Figure 1.
Total expenditure on health per capita, 2007 (in US$) from the World Health Organization. http://www.who.int/nha/use/the_pc_2007.png Published with permission.
Nearly 35 years ago, these gross inequities across nations were deemed “politically, socially and economically unacceptable” by participants gathered at the World Health Organization Conference in Alma Ata.2 The Declaration of Alma Ata reaffirmed that health care was a basic human right. And there was a clear merging of personal and public health agendas: it was declared that the people have both a right and a duty to participate individually and collectively in the planning and implementation of their health care.
To achieve this level of engagement, all countries were urged to redouble their investments in primary health care—building systems to support the first level of contact with national health systems locally, and bringing health care as close as possible to where people live and work. Few countries heeded the recommendations. In many, primary care investments declined as specialists gained ascendency in medical schools and academic health science centers. New diagnostic and therapeutic technologies heightened expectations for return on investments in the hospital-based acute care sector, especially in wealthier countries.
Among these high income countries, per capita expenditures on health care vary widely across regions.3–5 These within-country variations have been most extensively documented in the United States by Wennberg and colleagues at Dartmouth.3 Wennberg began his studies of the epidemiology of health care more than 40 years ago in Vermont. He had traveled there from Johns Hopkins as newly appointed director of the Regional Medical Program. His goal was to document variations in service and the unmet needs of the people who lived in the most rural regions of the state, where the capacity to deliver health care services was limited.6,7
Instead, Wennberg found striking variations in the rates of service delivery throughout the state that could not be explained by characteristics of patients or their clinical needs. Children were more than ten-fold more likely to undergo tonsillectomy in one hospital market area as opposed to another; men were four-fold more likely to have a prostatectomy for benign disease; and women six-fold more likely to undergo hysterectomy. Procedure rates that were high or low varied from one market to another; each geographic region had its own “surgical signature”. There were also striking differences in outcomes, including operative mortality, for seemingly similar patients in different hospitals. For the residents of Vermont, in health care, geography was destiny.7,8
Practice variation was not peculiar to Vermont or to the United States. The same phenomenon had been described for tonsillectomy rates among school districts in England and Wales in 1938.12 In 1982, Wennberg and others documented variation in rates of surgical interventions in small geographic regions of England and Norway, as well as the United States. With adjustments for inter-country variations in rates, the systematic component of variation within countries was procedure-specific: high for tonsillectomy, hysterectomy and prostatectomy; low for appendectomy, cholecystectomy and hernia repair.13
Subsequent research confirmed the hypothesis that the variation was explained by the limits of medical evidence and resulting professional uncertainty. The greater the uncertainty, the more discretion the clinician had in relying on opinion rather than evidence.
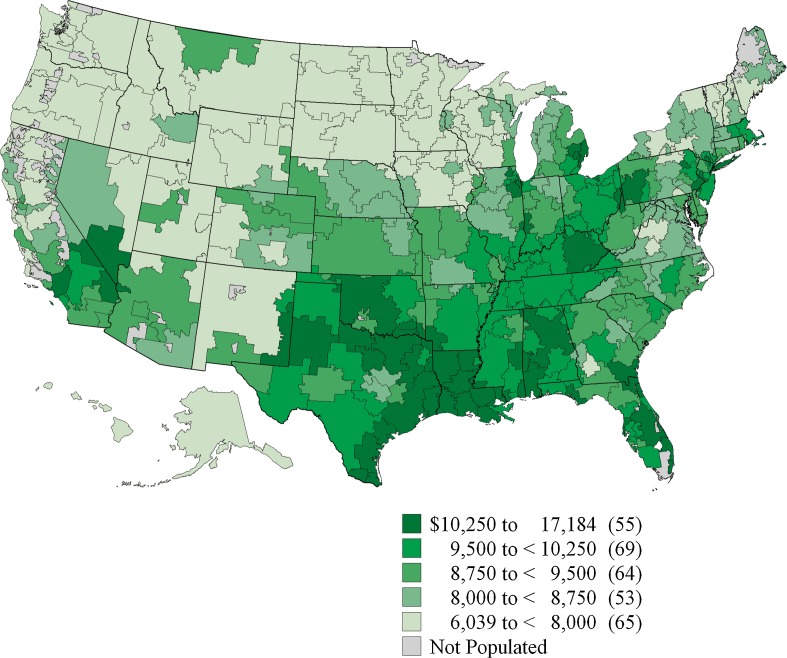
In the ensuing decades, Wennberg and colleagues extended their work to describe per capita resource availability and utilization (e.g., hospital beds and bed-days, intensive care unit beds and bed-days, primary care and specialty physicians and physician-visits, nurses, diagnostic imaging, etc.). There were consistent associations between capacity and utilization, and utilization largely determined per capita expenditures. The Dartmouth Atlas of Health Care was published first in 1996, documenting variations in inputs and costs for Medicare beneficiaries across 306 hospital referral regions within the United States. Per capita expenditures varied three-fold. The Atlas has been regularly updated since; in 2010, the range was $6,039 to $17,184 (Fig. 2).3
Figure 2.
Expenditures on health care per capita for Medicare beneficiaries in 2006 from the Dartmouth Atlas of Health Care3. Published with permission.
It took another seven years to answer the obvious question: Did higher expenditures lead to better outcomes? The definitive answer was “no”.14,15 Outcomes were no better, and they may have been worse with greater intensity of services and the resulting greater costs. Outcomes varied dramatically from one place to another and one provider to another when the same intervention was delivered to similar patients. There was evident waste and harm in poor performance and the resulting delivery of poor quality care.
And there was waste and harm in delivering the wrong care—care that patients would not choose if they were better informed. The right treatment for a particular patient depends on his or her goals when trade-offs among treatment outcomes have to be weighed. Patients’ preferences matter to an extent rarely recognized by either clinicians or patients. And when clinicians do recognize the importance of patients’ preferences, they have great difficulty in diagnosing them accurately.7–11,16,17
Shared decision-making, a process by which clinicians informed patients of treatment options and outcomes and engaged them to elicit their goals and treatment preferences, was introduced. Decision aids were developed to support shared decision-making and randomized trials in the United States, Canada and the United Kingdom showed that they improved patients’ knowledge, led to more realistic expectations, and reduced utilization of many surgical interventions and other treatments.14
The same geographic variations in service delivery, expenditures, and in outcomes have been documented in many developed countries. The NHS Atlas of Health Care, first published in 2010, displayed geographic variation in service delivery comparable to those in the United States. In England, per capita expenditures for care of patients with cancer or musculoskeletal, circulatory or respiratory problems vary two-fold to three-fold among NHS primary care trusts.4,5
Similar variations in service delivery that cannot be explained by differences in patients’ clinical circumstances or preferences have been found wherever it has been studied in Europe, North America, and Asia. The Wennberg International Collaborative, hosted jointly by Dartmouth and the London School of Economics, brings together investigators from around the world in an effort to develop and adopt common methods and measures of health care inputs and outputs so as to accelerate cross-border learning.18
Conversations with health ministers and other leaders in low- and middle-income countries suggest that geographic variation in health care service delivery and expenditures and the outcomes achieved is universal.19 New initiatives in studying such geographic variations are underway in the Peoples’ Republic of China, Rwanda, Kosovo, Peru and other countries.
USING VARIATIONS IN OUTCOMES TO IMPROVE QUALITY
As noted, the early Dartmouth research disclosed surprising variations in both outcomes when the same intervention was delivered to seemingly similar patients, and in rates of interventions delivered to seemingly similar patients in different geographic regions. For example, in the first Dartmouth Atlas, 30-day hospital mortality following coronary artery bypass surgery (CABG) varied from 2% to 10% across the Atlas’s 306 hospital referral regions (HRRs). The rate at which CABG was performed among Medicare beneficiaries in the 306 HRRS varied from 3/100,000 to just under 12/100,000.3,20
The first observation raises questions about process quality and safety in delivering care. The second observation raises questions about whether or not the right care is being delivered. Both questions have major implications for global health development. Wide variations in the quality of care, including surgical mortality rates exist across countries and contexts. Investments in the wrong delivery capacities, as well as failures to engage individual patients in decision-making, can distort the care patients receive.
An early example of learning from outcomes variation was the Northern New England Cardiovascular Study Group comprised of Dartmouth-Hitchcock Medical Center and the four other medical centers in Northern New England performing CABG during the early 1990s.21 When the group discovered widely varying operative mortality rates, they enlisted industrial engineers to apply quality management sciences to document differences in process design that were associated with good or bad outcomes. Over time, this careful documentation of variable delivery processes achieved a 60% relative reduction in postoperative mortality rates, from roughly 5% to roughly 2%.
More recently, Dartmouth has used a similar approach to learning from variation in delivery processes with members of the High Value Healthcare Collaborative (HVHC). The initial comparative study focused on delivery of total knee replacement at the five founding health system members of the HVHC: Cleveland Clinic, Denver Health, Dartmouth-Hitchcock Medical Center, Intermountain Health Care, and Mayo Clinic. There were striking differences in delivery processes that could be associated with significant differences in both lengths-of-stay and post-operative complications during the index hospital admission.22
As follow-up of patients continues, the HVHC members have already identified variations in delivery that were associated with improved clinical outcomes and reduced costs. All members have moved toward adopting these “best practices”, which include: (1) better coordination of management of patients across anesthesia, internal medicine, and orthopedic surgery staff; (2) dedicated operating room teams that were strongly associated with shorter operating times; and (3) better management of patients’ expectations, especially for the recovery and rehabilitation aspects of care.
The HVHC now includes 14 health systems and will be addressing at least eight additional conditions. It has received a major grant from the Center for Medicare and Medicaid Innovation to introduce shared decision-making in member health systems to measurably improve quality of decisions about what to deliver as well as performance in delivering interventions.23
There is just as much to be learned from variations in processes used by different clinicians in delivering care in the ambulatory setting where there is little or no evidence to support the myriad decisions made about when to order a diagnostic test, including imaging and other high-cost interventions. Even low-cost tests can set off a clinical cascade of chasing down the implications of information or misinformation that often leads to more harm than good for patients. The benefit:harm ratio may be shown to tip heavily toward harm when carefully scrutinized. But in the absence of evidence or its deliberate interpretation and application, wishful thinking drives wishful doing.24
And there is little evidence to guide decisions about when to admit to the hospital, refer to a consulting clinician, or have the patient come back for monitoring after a change in medical management. As many as 80 million adult Americans have high blood pressure. When a treatment regimen is changed for one of those patients, there is no evidence to say whether she should be told to return for a check-up in 2 weeks, 2 months, or 2 years. It has been difficult for questions like these to compete with studies of the human genome for the attention of academic health science centers and the funding only they can attract. The resulting ignorance has proved to be very expensive.25
VARIATIONS IN PRACTICES AND THE GLOBAL RULE OF ROEMER’S LAW
Investing in the human and physical capacity to provide health care to a population of patients is the central activity of health care economies throughout the world. In government-funded systems, the investments are generally made based on the evidence that different services can meet the perceived needs and wants of the individuals that comprise the population to be served. In market-based economies, efficiency depends on addressing the information needs of the consumer, which is especially a challenge in health care.14
Investment in capacity to deliver one kind of services—surgery and other forms of acute care in the hospital setting, for instance—always have an opportunity cost; other investments in alternative services such as coordinated primary care teams and other elements of the primary health care systems recommended at Alma Ata may not be made. Wennberg perceived a need for more hospital beds in rural Vermont and developed methods to document those needs. As already noted, he did not find evidence of need for more hospital beds. Rather, he found that clinicians in different geographic regions made widely varying assessment of clinical need for particular clinical services, including surgery and hospitalization.7
Wennberg was not the first to question the wisdom of investments in building hospital beds without data to document need. A decade earlier, in 1959, the economist Milton Roemer was asked about the wisdom of the Hill-Burton Act, passed by the U. S. Congress in 1946. It was the same year that, in the aftermath of the national trauma of WWII, the United Kingdom passed legislation that would lead 2 years later to the launch of the NHS, a tax funded health service that would provide care free to the patient at the point of service. The Hill-Burton Act was a far less ambitious move toward equity in access to care. Grants and loans were made available to communities to build hospitals or add beds to existing hospitals if the per capita rate of beds was less than 4.5 beds per 1,000 in the population. When asked about the wisdom of the law more than a decade after its implementation, Roemer replied that he didn’t know whether it was wise or not. He wondered where the goal of 4.5 beds per 1,000 came from. And then he expressed the concern that in health care “a built bed is a filled bed”.14,25
In these early days of facility planning and capacity investment in developed countries, Roemer’s observations were prophetic. For most of the next 50 years, built hospital beds have been filled. There continues to be much about the decision to admit a patient to the hospital that is discretionary. It is not surprising that those decisions would be influenced by the availability of resources that would otherwise sit idle. Roemer’s law applies equally to investments in human capital. Trained surgeons and specialists as well as generalist physicians manage to keep busy.14,25
Because built capacity is generally used, decisions to invest in capacity to deliver some services but not others can have a profound effect on the care delivered in a region. If these investments are not guided by accurate measures of the needs and wants of the individuals who comprise the population, the uninformed investment decisions will distort the care received by individuals. That distorted care may fall short of meeting people’s needs while exceeding people’s wants for other services. In developed countries, examples of these distortions in the direction of over-diagnosis and over-treatment are legion.26,27
Consider the investments made in imaging, radiation therapy delivered in multiple ways, surgery performed with and without robots, androgen deprivation and chemotherapy—all to treat prostate cancers that never would have been diagnosed were it not for a simple screening test that many men choose not to have when informed of the consequences.28 The investments in these therapeutic modalities would not have been necessary at the level made were the screening decisions informed. The same could be said for the investments made in developed countries over the past 45 years in the surgical or percutaneous treatment of coronary disease.26,27
Academic health science centers everywhere need to embrace a definition of “science” broad enough to include the questions of delivery if we are to reduce the waste of inefficient and poor quality care that falls short of patients’ needs. They must also embrace the intentional elicitation and measurement of patients’ preferences if we are to reduce the waste of giving care to patients that they would not choose if well informed. There are methodological challenges, but we need to be guided by the aggregate of individual patients’ needs and wants as we invest in capacity to deliver care if we are to reach “the triple aim” of better care and better health at lower cost.10,29,30
In the United States, we have invested heavily in building hospital beds and training hospitalists. Despite an aging population, we have invested little in training geriatricians and the community-based palliative care teams that could provide coordinated and compassionate end-of-life care. Is it any wonder then that 55% of Americans who say that they would prefer to die at home die in the hospital? Roemer would not be surprised. Investments in capacity to deliver the kind of end-of-life care that patients and families prefer when well informed and well supported should be a pressing priority in all countries. More hospital beds are undoubtedly needed to increase capacity for curative care in many developing countries. But as they are built, they should not be filled by patients who would prefer to receive end-of-life care at home.31
INNOVATING FOR VALUE THROUGH KNOWLEDGE EXCHANGE AND CROSS-BORDER LEARNING
Much has been written about models of innovation drawn from the management sciences to facilitate value-driven design of health care. It has been argued that health care is highly susceptible to disruptive innovation. One reason is that less-skilled people who are accessible in more convenient, less expensive settings could perform tasks historically restricted to expensive specialists in centralized, inconvenient locations. The use of nurses in pharmacies and retail stores working with guidelines for a constrained set of conditions and related services is an example.32
For the most part, disruption has proved difficult in the health care economies of wealthier countries where interests—financial and otherwise—in the current organization and incentive structure lead to resistance. The use of health coaching and decision aids to support doctors and patients to improve decision quality and self-management of chronic illness has been shown to produce better care at lower cost in many, if not all, settings. Uptake by clinicians has been slow.24
There are stunning examples of innovative design of health care service delivery in low-resource settings. Aravind Eye Institute in India has defined and redistributed tasks across levels of training to achieve highly effective and safe pre-surgical to surgical and post-surgical care for patients with eye disease. They have shared what they’ve learned across regions and international borders.33,34 Aravind is in the process of applying their high-level clinical operations management skills to other clinical areas, including more complex illnesses common in the geriatric population.34
Similar approaches to operations management with training and support to maximize available human resources with a focus on engaging and supporting patients in their communities have produced outcomes equal to or better than those in resource-rich settings. Practices that originated in Rwanda, Haiti and Peru in treatment of patients with HIV and multi-drug resistant tuberculosis have been adapted to and adopted in the urban setting in the United States.35–37
The concept of reverse innovation was introduced to health care with the example of a high performing organization recognizing its need to be intentional about fostering innovation. GE introduced and supported “local growth teams” in India and China where they could better appreciate local context and what would produce value for users.38 Organizational assets were made available to the teams without the organizational red tape that usually accompanies and constrains their use. The result was development of two devices that produced real value in those settings and in resource-rich settings as well.36 As noted in these examples from India, Rwanda, Haiti and Peru and others, the opportunities for reverse innovation applies as well in service innovation.39
At Dartmouth, we believe that reverse innovation can serve as a foundation for cross-border knowledge exchange free of the outmoded assumptions about the asymmetry of contributions made in North–South collaborations. To “turn the world upside down” in this way may be the best means to finally realize the promise of Alma Ata—creating greater value through the efficient delivery of the right services in developed as well as developing countries.40
FROM IDEAS TO ACTION: REALIZING THE PROMISE OF ALMA ATA
As noted in the introduction, it was the more than five-hundred fold variation in per capita expenditures across countries exhibited in Fig. 1 and the associated disparities in access to health care that gave rise to the Declaration of Alma Ata in 1978. When the participants reaffirmed that health was a fundamental human right, they rightly observed that achieving its highest possible level for individuals and communities would require the attention of leadership from other social and economic sectors in addition to the health sector. Social determinants as well as public health measures were often as important as, or more important than, medical or surgical interventions in achieving that goal.41
They were also astute in declaring that the people have both a right and a duty to participate individually and collectively in the planning and implementation of their health care. The primary health care system they envisioned was a means toward an end that included full engagement of patients and populations in assuring that limited resources were used to deliver care that created value in the lives of those who lived with the consequences.
It can be said that no country, regardless of the resources they have invested in health care, has been able to realize the level of engagement of patients and the people envisioned at Alma Ata, letting their informed voice and choice determine the design and delivery of health services. Without that informed voice and choice reporting and revealing the wants and needs of the individuals that comprise the population to be served, health care investments gravitate toward specialized, technology-intensive acute care. The resulting mismatch between capacities to deliver different services and the aggregate wants and needs of the population leads to waste estimated at 20–40% of health care expenditures across all nations, a situation that constitutes a major impediment to achieving universal health coverage.42
Dartmouth has embarked on a program to address such waste and harm through the application of health care delivery science in global partnerships. In September of 2011, Dartmouth sponsored a Salzburg Global Seminar on health care delivery science co-chaired by the Minister of Health of Rwanda and attended by more than 60 faculty members and fellows from 27 countries.43 In November of 2012, Dartmouth co-sponsored at Salzburg with the World Bank Institute the First Global Symposium on the Right to Health and Health Systems.44
In collaboration with the London School of Economics and Political Science, Dartmouth is extending the Wennberg International Collaborative to include low-income and middle-income countries. The goal is to adapt the methods and measures that inform the Dartmouth Atlas of Health Care to study the epidemiology of health care and its relationship to the epidemiology of disease burden across countries and contexts.
We are building collaborations modeled on the HVHC in the Peoples’ Republic of China, where we have entered into an ambitious 5-year agreement with the Ministry of Health, and in the United Kingdom. Reduction of waste and harm through the application of shared decision-making and improved delivery of services are central to the reform agendas of both countries.
Dartmouth is also looking to adapt its new educational offerings in health care delivery science to the global context. The first students in the new Masters in Health Care Delivery Science have included leaders in health care from China, Columbia, France, India, Kosovo, Singapore, Sweden, and Rwanda, as well as the United States. We are learning a great deal from these students about different approaches to the universal problems of health care delivery. Students from low-resource settings have taught those of us from wealthier countries about efficient modes of service delivery that achieve equal or better outcomes at a fraction of the cost. Cross-country teams have examined cross-cultural differences in resistance among patients and professionals to service delivery innovations. This cross-border learning for adaptation and adoption will improve the curriculum and make it more relevant in the United States as well as in their own countries.45
The goal of these and other partnerships is to apply a science of health care delivery that will accelerate the development and implementation of innovative approaches that reduce the waste and harm that is all too prevalent in health care across the globe. As a global commitment to universal health coverage grows, it must be matched by a commitment to assure that the resulting increase in access is to delivery of care that can be trusted to achieve better health at lower cost to people everywhere.
Acknowledgements
This paper is based on a keynote address given at the Indiana Global Health Research Working Conference on October 5, 2012. The author thanks Thomas Inui, William Tierney, and Jeffrey Jackson for their roles in the development of the conference and this special issue of JGIM and for reviewing drafts of my address and this paper.
Conflicts of Interest
AGM is senior clinical adviser to the Informed Medical Decisions Foundation and receives consulting fees for decision aid content and design. He also receives royalties from Health Dialog, which distributes decision aids and other forms of decision support developed in collaboration with the Foundation.
REFERENCES
- 1.World Health Organization. (2010) National Health Accounts Series. Total expenditure on health per capita, 2007.
- 2.World Health Organization. (2008) The World Health Report. Primary Health Care: Now More Than Ever.
- 3.Dartmouth Atlas. www.dartmouthatlas.org. (accessed 5 March, 2013)
- 4.Appleby J, Raleigh V, Frosini F, Bevan G, Gao H, Lyscom T. Variations in Health Care: The good, the bad and the inexplicable. London: King’s Fund; 2011. [Google Scholar]
- 5.Department of Health (2010). ‘NHS Atlas of Variation in Healthcare: Reducing unwarranted variation to increase value and improve quality’. Right Care website. Available at: www.rightcare.nhs.uk/atlas/ (accessed on 5 March 2013) (interactive web version of Atlas: http://www.sepho.org.uk/extras/maps/NHSatlas/atlas.html).
- 6.Wennberg J, Gittelsohn A. Small area variations in health care delivery. Science. 1973;182(117):1102–8. doi: 10.1126/science.182.4117.1102. [DOI] [PubMed] [Google Scholar]
- 7.Wennberg JE. Tracking Medicine: A researcher’s quest to understand health care. Oxford: Oxford University Press; 2010. [Google Scholar]
- 8.Roos NP, Wennberg JE, Malenka DJ, Fisher ES, McPherson K, Andersen TF, Cohen MM, Ramsey E. Mortality and reoperation after open and transurethral resection of the prostate for benign prostatic hyperplasia. N Engl J Med. 1989;320(17):1120–4. doi: 10.1056/NEJM198904273201705. [DOI] [PubMed] [Google Scholar]
- 9.Wennberg JE, Mulley AG, Jr, Hanley D, Timothy RP, Fowler FJ, Roos NP, et al. An assessment of prostatectomy for benign urinary tract obstruction: geographic variations and the evaluation of medical care outcomes. JAMA. 1988;259:3027–30. doi: 10.1001/jama.1988.03720200049032. [DOI] [PubMed] [Google Scholar]
- 10.Mulley AG. Assessing Patients' Utilities: Can the ends justify the means? Med Care. 1989;27:S269–S28l. doi: 10.1097/00005650-198903001-00021. [DOI] [PubMed] [Google Scholar]
- 11.Mulley AG. Medical decision making and practice variation. In: Andersen TF, Mooney G, editors. The Challenges of Medical Practice Variation. London: MacMillan; 1990. [Google Scholar]
- 12.Glover JA. The incidence of tonsillectomy in children. Proc R Soc Med. 1938;31:1219–36. [PMC free article] [PubMed] [Google Scholar]
- 13.McPherson K, Wennberg JE, Hovind E, Clifford P. Small-area variations in the use of common surgical procedures: an international comparison of New England, England, and Norway. N Engl J Med. 1982;307:1310–4. [DOI] [PubMed]
- 14.Fisher ES, Wennberg DE, Stukel TA, et al. The Implications of Regional Variations in Medicare Spending. Part 1: Utilization of services and quality of care. Ann Intern Med. 2003;138(4):273–87. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 15.Fisher ES, Wennberg DE, Stukel TA, et al. The Implications of Regional Variations in Medicare Spending. Part 2: Health Outcomes and Satisfaction with Care. Ann Intern Med. 2003;138(4):288–298. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- 16.Mulley A, Trimble C, Elwyn G. Patients Preferences Matter: Stop the Silent Misdiagnosis. London: The Kings Fund; 2012. [DOI] [PubMed] [Google Scholar]
- 17.Mulley A, Trimble C, Elwyn G. Stop the silent misdiagnosis: Patients preferences matter. BMJ. 2012 [DOI] [PubMed]
- 18.The Wennberg Collaborative. Dartmouth Institute for Health Policy and Clinical Practice and the London School of Economics and Political Science. http://www.wennbergcollaborative.org/. Accessed 5 March 2013.
- 19.Jaime Bayona, Personal Communication. 2012
- 20.Dartmouth Atlas (2005). ‘Cardiac Surgery’. Dartmouth Atlas of Health Care website. Available at: www.dartmouthatlas.org/downloads/reports/Cardiac_report_2005.pdf (accessed on 5 March 2013).
- 21.O’Connor G, Plume S, Olmstead E, et al. A Regional Intervention to Improve the Hospital Mortality Associated with Coronary Artery Bypass Graft Surgery. JAMA. 1996;275:841. [PubMed]
- 22.Tomek IM, Sabel AL, Froimson MI et al. A Collaborative of Leading Health Systems Finds Wide Variations in Total Knee Replacement Delivery And Takes Steps To Improve Value. Health Affairs 2012;31 (6): [DOI] [PubMed]
- 23.The Dartmouth Institute for Health Policy and Clinical Practice. High Value Healthcare Collaborative Receives $26 Million Health Care Innovation Grant. http://tdi.dartmouth.edu/press/press-releases/high-value-healthcare-collaborative-receives-26-million-health-care-innovat Accessed 5 March 2013.
- 24.Mulley AG. Inconvenient truths about supplier induced demand and unwarranted variation in medical practice. BMJ. 2009;339:1007–09. doi: 10.1136/bmj.b4073. [DOI] [PubMed] [Google Scholar]
- 25.Roemer M. Bed supply and hospital utilization: a natural experiment. Hospitals. 1961;35:36–42. [PubMed] [Google Scholar]
- 26.Welch HG. Overdiagnosis: Making People Sick in Pursuit of Health. Boston: Beacon Press; 2012. [Google Scholar]
- 27.Brownlee S. Overtreatment. Why Too Much Medicine is Making Us Sicker. New York: Bloomsbury; 2010. [Google Scholar]
- 28.Frosch D, Kaplan R, Felitti V. The evaluation of two methods to facilitate shared decision making for men considering the prostate-specific antigen test. J Gen Intern Med. 2001;16(6):391–8. [DOI] [PMC free article] [PubMed]
- 29.Mulley AG, Jr, Wennberg JE. Reducing Unwarranted Variation in Clinical Practice by Supporting Clinicians and Patients in Decision Making. In: Gigerenzer G, Gray M, editors. Better Doctors, Better Patients, Better Decisions: Envisioning Health Care in 2020. Cambridge: MIT Press; 2010. [Google Scholar]
- 30.Berwick DM, Nolan TW, et al. The triple aim: care, health, and cost. Health Affairs. 2008;27(3):759–769. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]
- 31.Spence D, Merriman A, Binagwaho A. Palliative care in Africa and the Caribbean. PLoS Med. 2004;1(1):e5. doi: 10.1371/journal.pmed.0010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christensen CM, Bohmer R, et al. Will disruptive innovations cure health care? Harvard Business Review. 2000;78(5):102–112. [PubMed] [Google Scholar]
- 33.Bhandari A, Dratler S, Raube K, Thulasiraj RD. Specialty Care Systems: A Pioneering Vision For Global Health. Health Affairs. 2008, 27, no.4. [DOI] [PubMed]
- 34.Aravind S. Personal Communication, 2011.
- 35.Porter ME, Lee S, Rhatigan J, Kim JY. (2009) Partners in Health: HIV Care in Rwanda. http://hbr.org/casestudies. Accessed 5 March 5, 2013.
- 36.Bohmer, RMJ, Friedman J. (2007) Partners in Health: The P.A.C.T. Project. http://hbr.org/casestudies. Accessed 5 March 2013.
- 37.Talbot J, Rhatigan J. (2011) Multidrug-resistant Tuberculosis Treatment in Peru. HBR http://cb.hbsp.harvard.edu/cb/web/product_detail.seam?E=2783237&R=GHD003-PDF-ENG&conversationId=333270. Accessed 5 March 2013.
- 38.Immelt JR, Govindarajan V, Trimble C. How GE Is Disrupting Itself. Harvard Bus Rev. 2009;87:56–65.
- 39.Govindarajan V, Trimble C. (2012) Reverse Innovation: HBR Press.
- 40.Crisp N. Turning the World Upside Down. New York: Oxford University Press; 2010. [Google Scholar]
- 41.Closing the gap in a generation – health equity through action on the social determinants of health. Geneva, World Health Organization, 2008 [DOI] [PubMed]
- 42.World Health Organization. (2010) The World Health Report. Health systems financing: the path to universal coverage. [DOI] [PMC free article] [PubMed]
- 43.Innovating for Value in Health Care Delivery: Better Cross-Border Learning, Smarter Adaptation and Adoption http://www.salzburgglobal.org/current/sessions-b.cfm?IDSPECIAL_EVENT=2867 (accessed 5 March 2013).
- 44.Realizing the Right to Health: How can a rights-based approach best contribute to the strengthening, sustainability and equity of access to medicines and health systems? http://www.salzburgglobal.org/current/sessions-b.cfm?IDSpecial_Event=3630 (accessed 5 March 2013).
- 45.Master of Health Care Delivery Science at Dartmouth http://mhcds.dartmouth.edu/ (accessed 5 March 2013).