Abstract
OBJECTIVES
Systematic review of preventive pharmacologic treatments for community-dwelling adults with episodic migraine.
DATA SOURCES
Electronic databases through May 20, 2012.
ELIGIBILITY CRITERIA
English-language randomized controlled trials (RCTs) of preventive drugs compared to placebo or active treatments examining rates of ≥50 % reduction in monthly migraine frequency or improvement in quality of life.
STUDY APPRAISAL AND SYNTHESIS METHODS
We assessed risk of bias and strength of evidence and conducted random effects meta-analyses of absolute risk differences and Bayesian network meta-analysis.
RESULTS
Of 5,244 retrieved references, 215 publications of RCTs provided mostly low-strength evidence because of the risk of bias and imprecision. RCTs examined 59 drugs from 14 drug classes. All approved drugs, including topiramate (9 RCTs), divalproex (3 RCTs), timolol (3 RCTs), and propranolol (4 RCTs); off-label beta blockers metoprolol (4 RCTs), atenolol (1 RCT), nadolol (1 RCT), and acebutolol (1 RCT); angiotensin-converting enzyme inhibitors captopril (1 RCT) and lisinopril (1 RCT); and angiotensin II receptor blocker candesartan (1 RCT), outperformed placebo in reducing monthly migraine frequency by ≥50 % in 200–400 patients per 1,000 treated. Adverse effects leading to treatment discontinuation (68 RCTs) were greater with topiramate, off-label antiepileptics, and antidepressants than with placebo. Limited direct evidence as well as frequentist and exploratory network Bayesian meta-analysis showed no statistically significant differences in benefits between approved drugs. Off-label angiotensin-inhibiting drugs and beta-blockers were most effective and tolerable for episodic migraine prevention.
LIMITATIONS
We did not quantify reporting bias or contact principal investigators regarding unpublished trials.
CONCLUSIONS
Approved drugs prevented episodic migraine frequency by ≥50 % with no statistically significant difference between them. Exploratory network meta-analysis suggested that off-label angiotensin-inhibiting drugs and beta-blockers had favorable benefit-to-harm ratios. Evidence is lacking for long-term effects of drug treatments (i.e., trials of more than 3 months duration), especially for quality of life.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-013-2433-1) contains supplementary material, which is available to authorized users.
KEY WORDS: migraine, evidence based medicine, adverse drug effects
INTRODUCTION
Migraine headaches ranging from moderate to very severe1–3 affect 17 % of women and 6 % of men.4–7 The National Headache Foundation defined migraine as episodic (<15) or chronic (≥15 days per month for at least 3 months).8–10 Many adults with episodic migraine experience serious lifestyle restrictions11–13 and need preventive medications.5,14,15 The US Food and Drug Administration (FDA) has approved four drugs for episodic migraine prevention in adults: two beta blockers (propranolol and timolol) and two antiepileptic drugs (topiramate and divalproex sodium).16 Doctors also prescribe off-label drugs from other classes.16,17
Preventive treatments aim to reduce headache frequency by at least 50 %18–20 without intolerable harms.21,22 In clinical practice, physicians and patients choose preventive treatments based primarily on FDA approval and drug tolerability.9,18,19,23–25 Systematic reviews and meta-analyses with consistent and transparent appraisal of study quality and strength of evidence are essential for arriving at evidence-based migraine preventive treatment and policy decisions.26
Previously published systematic reviews focused on the efficacy of specific drugs rather than comparative effectiveness and tolerability of all pharmacologic options.27,28 In addition, the Institute of Medicine recommends basing treatment decisions on post-marketing studies tracking drug benefits and harms after FDA approval.29–31 Thus, we conducted a systematic literature review of the comparative effectiveness and tolerability of the available preventive medications for episodic migraine in adults in outpatient settings to inform treatment and policy decisions (CRD42012001918).32,33 The topic, research questions, and eligible interventions were nominated and posted for public comments on the Effective Healthcare website. We chose not to synthesize studies of the drug flunarizine (commonly used for adults in Europe) because the FDA has not approved it. Efficacy of nonpharmacologic preventive treatments and prevention of chronic migraine are beyond the scope of this paper.
METHODS
Data Sources and Searches
We searched databases including MEDLINE®, the Cochrane Library, the FDA website, and the World Health Organization International Clinical Trials Registry portal to find English publications through May 20, 2012 (online Appendix Table 1).
Study Selection
Three investigators determined study eligibility. Each title and abstract was reviewed by at least two investigators, and disagreements were resolved through discussion. We determined eligibility according to the PICOTS (Population, Intervention, Comparator, Outcomes, Timing, and Settings) framework. We defined the target population as community-dwelling adults with episodic migraine (online Appendix Table 2).9 We formulated a list of eligible pharmacologic classes available in the US.34 We defined eligible patient-centered outcomes (≥50 % reduction in frequency of migraine attack from baseline, complete cessation of migraine attacks, migraine-related disability, and quality of life).
We excluded studies of treatments for acute attacks, prevention of menstrual migraines or migraine variants, and studies in inpatient settings.8,35,36
We analyzed the effectiveness of drugs from RCTs, adverse effects, and treatment discontinuation due to adverse effects from RCTs and nonrandomized studies.37 We defined harms as the totality of all possible adverse consequences of an intervention regardless of how authors perceived causality of treatments.38
Data Extraction
For each trial, one reviewer extracted the data and a second reviewer checked the abstracted data for accuracy using standardized forms (available at https://netfiles.umn.edu/xythoswfs/webui/_xy-21041343_1-t_zdhvSpvy).
We abstracted the information relevant to the PICOTS framework and minimum data sets to reproduce the results presented by the authors.
We abstracted the number randomized to each treatment group as the denominator to calculate estimates by applying intention-to-treat principles assuming that the same proportions apply in the missing data.39
Risk of Bias Assessment
We evaluated the risk of bias in individual studies of benefits and harms according to: (1) random allocation of subjects to the treatment groups; (2) masking the treatment status to the participants and investigators; (3) adequacy of allocation concealment; (4) adequacy of randomization as estimated based on similarity of the subjects in treatment groups by demographics and by frequency and severity of migraine; (5) planned and executed intention-to-treat principles; and 6) selective outcome reporting when compared with the articles’ protocols (when available) and methods sections.40 Since all outcomes in the review were self-reported, masking of outcome assessment was not essential.
We assumed a low risk of bias when RCTs met all risk-of-bias criteria, a medium risk of bias if one criterion was not met, and a high risk of bias if two or more criteria were not met. We concluded an unknown risk of bias for studies with poorly reported risk-of-bias criteria. We examined risk of bias in nonrandomized studies according to: (1) adjustment for confounding factors to address selection biases and (2) exclusion of subjects from the analyses to address attrition biases. We evaluated disclosure of conflict of interest by the authors of individual studies and funding sources but did not use this information to downgrade quality of individual studies. Incorporating risk of bias of individual studies into the synthesis of evidence, we used individual risk of bias criteria rather than a global score.41,42
Data Synthesis and Analysis
Using Meta-Analyst43 and STATA®44 software at a 95 % confidence level, we calculated the relative risk and absolute risk difference from the abstracted events using default software continuity correction coefficients for 0 events.39 We hypothesized superiority of drugs versus placebo and versus each other.45
We pooled results only from studies that used the same active drug treatments and comparators and the same definitions of outcomes.
Many FDA-regulated and post-marketing trials were not powered to detect statistically significant increases in harms with migraine preventive drugs. We used meta-analysis of RCTs for evaluating drug safety based on all available trials.46 We analyzed sparse adverse effects data with various statistical methods43,47–51 for robustness by comparing statistical significance and magnitude of the harms. In cases of multi-arm trials, we created a single pair-wise comparison.40 To avoid the spurious increase in precision in multiarm trials, we divided placebo arms approximately evenly among the comparisons according to the randomization ratio.39,52
We tested consistency of the results by comparing the direction and strength of the association,53 assessed heterogeneity in results with the chi-squared and I-squared tests,54,55 and explored it with meta-regression and sensitivity analysis, reporting only the results from random effects models,56 which incorporate inevitable differences between trials in patient populations, baseline rates of the outcomes, dosages of drugs, and other factors.47 We examined whether the definition of migraine could contribute to differences in trial results. The FDA had approved four drugs for prevention of episodic migraine based on trials conducted prior to the recent implementation of the migraine definition proposed by the International Headache Society.9 Eligible studies published earlier defined classic or common migraine as per the Ad Hoc Committee on Classification of Headache.57
We calculated the number needed to treat to achieve one event as the reciprocal of absolute risk differences (ARD) in rates of outcome events in active and control groups.44,58,59 The number of avoided or excessive events per population of 1,000 is the difference between the two event rates multiplied by 1,000.
In cases where very few studies provided evidence from head-to-head comparisons, we conducted indirect comparisons using statistical techniques to estimate the treatment effects from studies of each given treatment against controls under an assumption of consistency.60–64 First, we used adjusted indirect frequentist comparisons for individual drugs compared with placebo.62 This analysis provided pairwise triangular comparisons for drugs compared with placebo rather than network meta-analysis. Second, to address the problems with inevitable differences across studies, we used mixed (or multiple) treatment comparison (MTCs) Bayesian network meta-analysis.62–64 We calculated Bayesian odds ratios43,51 with 2.5 to 97.5 % credible intervals and Bayesian network random effects meta-analysis assuming heterogeneous variances across treatments (online Appendix Table 3).65 We synthesized evidence from drug classes in network meta-analysis when individual drugs from the same class demonstrated no significant differences in outcomes. We compared odds ratios from network meta-analyses with odds ratios from direct head-to-head RCTs to examine the consistency of the estimates.66 We concluded no differences in drug effect (hereafter called similar effects) if confidence or credible intervals included one (no effect or no difference).67 All Bayesian results were obtained from the WinBUGS software68 using Markov chain Monte Carlo (MCMC) samples after a 50,000-sample algorithm burn-in.
Grading the Evidence for Each Key Question
We assessed strength of evidence according to risk of bias, consistency, directness, and precision for clinical response and treatment discontinuation due to harms.53 We based our criteria on published guidelines acknowledging inevitable subjectivity of the assessment.40,69 We assigned a medium or high risk of bias in the body of evidence when at least one individual RCT had a medium or high risk of bias, respectively. We defined treatment effect estimates as precise when pooled estimates had narrow 95 % CIs or the pooled sample had >300 events (using 25 % relative effect difference for calculation of optimal information size).70 We did not quantify publication biases or selective outcome reporting biases because of the questionable statistical validity of the available tests.71
We defined a high level of evidence on the basis of consistent findings from low risk-of-bias RCTs. We downgraded strength of evidence to moderate if at least one of the four strength-of-evidence criteria was not met and to low if two or more criteria were not met. We defined evidence as insufficient if treatment effects or associations were examined by no studies or by a single study with unclear or high risk of bias.53 We applied this approach regardless of the statistical significance of the results.
Assessing Applicability
We estimated applicability of the population by evaluating baseline subject characteristics in observational studies and clinical trials.67 We reviewed the drugs applicable to practice in the US and patient-centered outcomes most valued by patients.
RESULTS
Of 5,244 identified references, we included 215 publications of RCTs (Figure 1) and 76 publications of nonrandomized studies. Randomized trials examined 59 drugs from 14 classes (online Appendix Table 4). Most trials were funded by industry but did not disclose conflict of interest by study investigators. More than half of the RCTs had a medium risk of bias (online Appendix Table 5). Most RCTs (86 %) were double blind with unclear adequacy of allocation concealment or randomization.
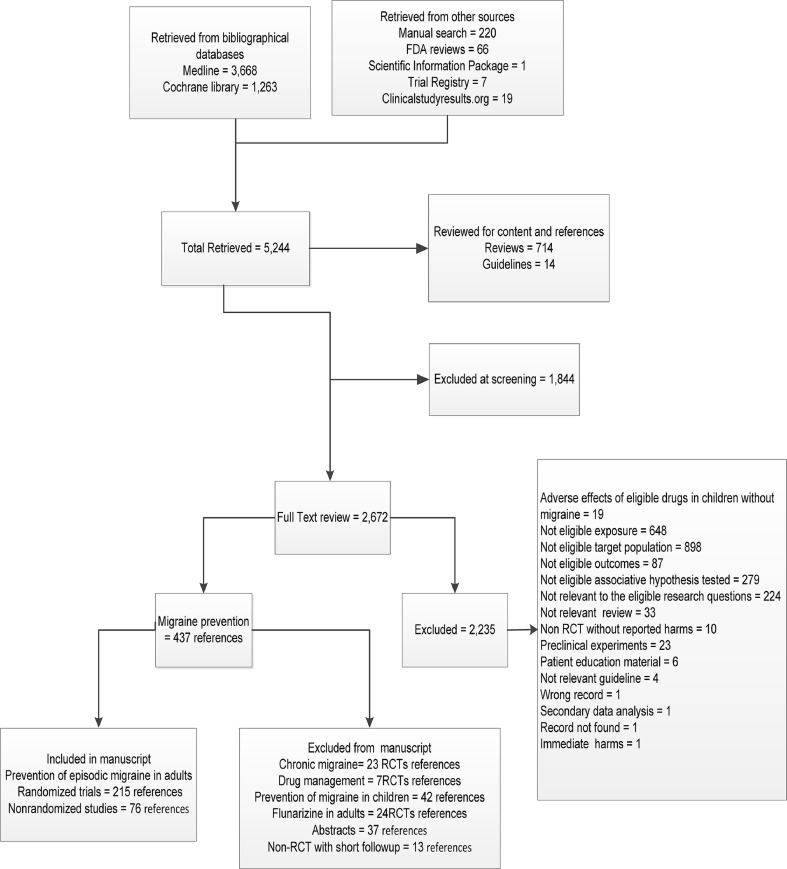
Figure 1.
Study flow.
The results were applicable to the target population. Most RCTs were conducted in the US and Western countries, used the International Headache Society’s definition, and enrolled mostly middle-age women with episodic migraine (online Appendix Table 6). RCTs enrolled on average 210 adults, measured outcomes at 2 to 3 months follow-up, and reported about 15 % attrition.
Enrolled patients were mostly overweight and had an average of five monthly migraine attacks with or without aura. Almost half of enrolled subjects were naïve to migraine-preventive drugs. Patient age and baseline migraine characteristics did not statistically differ in most trials. Substantial variability in reporting comorbidities prevented us from using this information in quantitative synthesis of evidence. Most trials excluded patients with severe medical comorbidities or psychiatric illnesses, stroke, and vascular migraine. RCTs rarely reported patient characteristics that could modify drug effects (e.g. family history of migraine, ,socioeconomic status, or response to prior preventive treatments).
Most trials compared one active agent with placebo or another drug. RCTs rarely reported concomitant treatment details such as exact drugs and doses. However, most trials disallowed concomitant drugs during the run-in period and after randomization (implying no concomitant treatments were used in the RCTs). Strength of evidence was low because of medium or high risk of bias and imprecise estimates from individual or meta-analyzed RCTs.
Efficacy for Prevention of Episodic Migraine
All approved drugs were better than placebo in reducing monthly migraine frequency by ≥50 % in individual patients (clinical response) (Table 1 and online Appendix Table 7). Drugs would achieve a clinical response in 200 to 400 patients per 1,000 treated. We analyzed dose–response associations and found that an increase in target topiramate dose from 50 to 100 mg/day but not from 100 to 200 mg/day resulted in a higher response rate (≥50 % reduction in monthly migraine frequency).72–74
Table 1.
Migraine Prevention with Approved Pharmacologic Preventive Treatments vs. Placebo in Adults, Results from Randomized Controlled Clinical Trials (Random Effects Models)
| Active drug | References | Sample | % with outcome with active drug [placebo] | Relative risk (95 % CI) | Absolute risk difference (95 % CI) | Number needed to treat (95 % CI) | Attributable events per 1,000 treated (95 % CI) | Risk of bias | Direct | Consistency | Precision | Strength of evidence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antiepileptics | ||||||||||||
| Divalproex >50 % reduction on migraine frequency | Pooled79,115,116 | 405 | 43.0 [23.3] | 2.2 (1.1 to 4.2) | 0.24 (0.10 to 0.38) | 4 (3 to 10) | 241 (97 to 384) | Medium | Yes | Yes | Imprecise | Low |
| P value | 0.12 | 0.10 | ||||||||||
| I squared | 0.52 | 0.57 | ||||||||||
| Topiramate on >50 % reduction on migraine frequency | Pooled72,82,95,117–120 | 1422 | 49.6 [25.1] | 2.0 (1.5 to 2.7) | 0.29 (0.18 to 0.40) | 3 (3 to 6) | 288 (176 to 400) | Medium | Yes | Yes | Precise | Moderate |
| P value | 0.04 | <0.01 | ||||||||||
| I squared | 0.56 | 0.74 | ||||||||||
| Topiramate on >50 % reduction on migraine days | Pooled77,82,121 | 1145 | 42.2 [23.3] | 1.7 (1.0 to 2.9) | 0.18 (0.08 to 0.28) | 6 (4 to 13) | 179 (75 to 284) | Low | Yes | Yes | Imprecise | Moderate |
| P value | 0.01 | 0.04 | ||||||||||
| I squared | 0.77 | 0.68 | ||||||||||
| Topiramate on ≥75 % reduction in migraine days | Pooled82,121 | 1086 | 22.3 [11.0] | 1.9 (1.1 to 3.1) | 0.10 (−0.01 to 0.20) | Low | Yes | Yes | Imprecise | Moderate | ||
| P value | 0.12 | 0.03 | ||||||||||
| I squared | 0.58 | 0.80 | ||||||||||
| Beta-blockers | ||||||||||||
| Propranolol >50 % reduction on migraine frequency | Pooled122–125 | 541 | 45.1 [22.3] | 2.0 (1.5 to 2.7) | 0.22 (0.14 to 0.30) | 4 (3 to 7) | 223 (142 to 304) | Medium | Yes | Yes | Imprecise | Low |
| P value | 1.00 | 0.94 | ||||||||||
| I squared | 0 | 0 | ||||||||||
| Timolol ≥50 % reduction in migraine frequency | Pooled122,125,126 | 276 | 49.4 [23.3] | 2.1 (1.5 to 3.1) | 0.27 (0.15 to 0.38) | 4 (3 to 6) | 265 (154 to 377) | Medium | Yes | Yes | Imprecise | Low |
| P value | 0.73 | 0.61 | ||||||||||
| I squared | 0 | 0 | ||||||||||
Bold Significant differences when 95% CIs of absolute risk difference do not include 0
Approved drugs improved other patient-centered outcomes in addition to monthly migraine frequency. Topiramate improved quality of life measured by scores on the Headache Impact Test,75 Migraine-Specific Questionnaire,76 and Migraine Disability Assessment.77 Topiramate improved general health status in a previously published pooled analysis of individual patient data from RCTs.78 Divalproex in a larger dose of 1,500 mg/day increased the likelihood of a >50 % improvement in whether migraine attacks impaired usual activities or necessitated symptomatic medication and in reducing migraine attacks with nausea, vomiting, phonophobia, or photophobia.79 Topiramate73,74,80–82 and propranolol decreased use of drugs for acute migraine attacks.83
Among off-label drugs, pooled analyses offered low-strength evidence that the beta-blocker metoprolol (approved for migraine prevention in Europe) and calcium channel blocker nimodipine were better than placebo in reducing monthly migraine attacks by ≥50 % (Table 2). Antiepileptic gabapentin demonstrated some benefits; however, the validity of the results from one trial was questioned because of exclusion of patients from the analyses and biased tolerability conclusions.84
Table 2.
Migraine Prevention with Off-label Pharmacologic Preventive Treatments vs. Placebo in Adults (the Results from Individual or Pooled with Random Effects Model Randomized Controlled Clinical Trials)
| Active drug | References | Sample | % with outcome with active drug [placebo] | Relative risk (95 % CI) | Absolute risk difference (95 % CI) | Number needed to treat (95 % CI) | Attributable events per 1,000 treated (95 % CI) | Risk of bias | Direct | Consistency | Precision | Strength of evidence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACE inhibitors | ||||||||||||
| Lisinopril | Individual RCT89 | 120 | 23.3 [0.0] | 29.0 (1.8 to 475.4) | 0.23 (0.12 to 0.34) | 4 (3 to 8) | 233 (124 to 343) | Low | Yes | NA | Imprecise | Low |
| Angiotensin II receptor blockers | ||||||||||||
| Candesartan | Individual RCT90 | 120 | 38.3 [3.3] | 11.5 (2.8 to 46.6) | 0.35 (0.22 to 0.48) | 3 (2 to 5) | 350 (219 to 481) | Low | Yes | NA | Imprecise | Low |
| Telmisartan | Individual RCT | 95 | 33[23] | 1.4 (0.7 to 2.7) | 0.1 (−0.1 to 0.3) | High | Yes | NA | Imprecise | Low | ||
| Antiepileptics | ||||||||||||
| Gabapentin | Pooled127–129 | 270 | 45.9 [31.0] | 1.5 (1.1 to 2.0) | 0.17 (0.06 to 0.27) | 6 (4 to 16) | 165 (61 to 269) | Medium | Yes | Yes | Imprecise | Low |
| P value | 0.49 | 0.85 | ||||||||||
| I squared | 0 | 0 | ||||||||||
| Beta-blockers | ||||||||||||
| Metoprolol | Pooled130–133 | 225 | 39.9 [19.4] | 2.0 (1.3 to 3.2) | 0.20 (0.09 to 0.3) | 5 (3 to 11) | 204 (88 to 321) | Medium | Yes | Yes | Imprecise | Low |
| P value | 0.42 | 0.39 | ||||||||||
| I squared | 0 | 0 | ||||||||||
| Magnesium | ||||||||||||
| Magnesium | Pooled134,135 | 137 | 33.8 [25.8] | 1.3 (0.7 to 2.3) | 0.08 (-0.09 to 0.26) | Low | Yes | No | Imprecise | Low | ||
| P value | 0.27 | 0.25 | ||||||||||
| I squared | 0.19 | 0.25 | ||||||||||
| Selective calcium channel blockers | ||||||||||||
| Nimodipine | Pooled130,131 | 126 | 28.6 [6.3] | 4.5 (0.5 to 40.1) | 0.23 (0.06 to 0.39) | 4 (3 to 16) | 229 (64 to 394) | Medium | Yes | No | Imprecise | Low |
| P value | 0.13 | 0.19 | ||||||||||
| I squared | 0.58 | 0.41 | ||||||||||
Bold Significant differences when 95% CIs of absolute risk difference do not include 0
Individual RCTs demonstrated that three off-label beta blockers—acebutolol85 (256 attributable events per 1,000 treated, 95 % CI, 105 to 407), atenolol86 (333 attributable events per 1,000 treated, 95 % CI, 140 to 527), and nadolol87 (250 attributable events per 1,000 treated, 95 % CI, 22 to 478)—were better than placebo in reducing monthly migraine attacks by ≥50 %.
Results from individual RCTs of angiotensin inhibiting drugs (angiotensin-converting enzyme [ACE] inhibitors and angiotensin II receptor blockers [ARB]) demonstrated effective migraine prevention. The ACE inhibitor captopril resulted in complete cessation of migraine (online Appendix Table 8), improved headache index scores by ≥50 %, and reduced depression symptoms in adults with comorbid hypertension and depressive symptoms for whom previous preventive antimigraine drugs had been ineffective.88 The ACE inhibitor lisinopril89 (233 attributable events per 1,000 treated, 95 % CI, 124 to 343) and the ARB candesartan90 (350 attributable events per 1,000 treated, 95 % CI, 219 to 481) were better than placebo in reducing monthly migraine attacks by ≥50 %. Lisinopril was better than placebo in reducing pain measured with the Short Form 36 (SF-36) questionnaire, but did not decrease use of drugs for acute migraine attacks.89 Candesartan decreased migraine-related disability, but had no effect on use of drugs for acute migraine attacks.90 In contrast, the ARB telmisartan was not better than placebo in reducing monthly migraine attacks by ≥50 %.91
Comparative Effectiveness of Drugs for Prevention of Episodic Migraine
Pooled direct analyses demonstrated better effectiveness of propranolol over nifedipine and no differences between propranolol versus timolol or versus metoprolol and metoprolol versus aspirin (online Appendix Table 9). Indirect adjusted frequentist analyses demonstrated no differences among approved drugs in reducing monthly headache frequency by ≥50 % (online Appendix Table 10). Indirect adjusted frequentist analyses offered low-strength evidence that off-label ARB candesartan90 resulted in greater odds of clinical response than approved drugs (online Appendix Table 10). Exploratory network Bayesian meta-analyses demonstrated effectiveness of all approved drugs with no differences between them (Figure 2 and online Appendix Table 11). Among off-label drug classes, angiotensin-inhibiting drugs (ACE inhibitors and ARBs) were more effective in reducing monthly migraine by ≥50 % when compared with antidepressants (OR, 2.8; 95 % CI, 1–7.5), off-label antiepileptics (OR, 2.7 95 % CI, 1–7.5), and ergot alkaloids (OR, 3.9; 95 % CI, 1.2 -14) (online Appendix Table 11).
Figure 2.
Bayesian network meta-analysis of clinical response to drugs vs. placebo (66 RCTs of 14,774 adults) in randomized controlled clinical trials that aimed to prevent migraine in adults. CrI Credible intervals. Clinical response was defined as 50 % or more reduction in monthly migraine attacks or perceived clinically important treatment success. We used a heterogeneous random effects model that assumes correlation within a study (rho = 0.5) and heterogeneity between studies. NSAID Nonsteroidal antiinflammatory drugs.
Adverse Effects with Drugs for Prevention of Episodic Migraine
We identified 159 RCTs reporting adverse effects in 18,134 adults and focused on treatment discontinuation because of any adverse effects reported in 68 RCTs.
Topiramate in target doses of 100 and 200 mg/day (but not 50 mg/day) resulted in treatment discontinuation because of adverse effects more often than placebo (Table 3 and online Appendix Table 12). Compared with placebo, topiramate more often resulted in bothersome taste perversion, paresthesia, and fatigue leading to withdrawal (online Appendix Table 13). Taste perversion, weight loss, and paresthesia were the most common adverse effects (online Appendix Table 14). Larger target doses of topiramate caused higher risk of dry mouth, paresthesia or fatigue, mood problems, nausea, and weight loss92 and led to treatment withdrawal due to higher risk of anorexia, depression, paresthesia, and impaired memory.92
Table 3.
Treatment Discontinuation due to Adverse Effects with Migraine Preventive Drugs in Adults, Evidence from Meta-Analyzed Randomized Controlled Clinical Trials
| Active preventive treatment | Sample | Rate, percent with drug [placebo] | Relative risk (95 % CI) | Absolute risk difference (95 % CI) | Number needed to treat (95 % CI) | Attributable events per 1,000 treated (95 % CI) | Strength of evidence Reasons for lowering strength of evidence |
|---|---|---|---|---|---|---|---|
| Compared with placebo | |||||||
| Approved antiepileptics | |||||||
| Divalproex115,116 | 346 | 9.8 [7.8] | 1.2 (0.5 to 2.7) | 0.02 (−0.05 to 0.10) | Low (medium ROB, imprecise, inconsistent) | ||
| Topiramate21,80,81,95,118, 120,136,137 | 2055 | 16.6 [8.5] | 1.8 (1.3 to 2.4) | 0.06 (0.02 to 0.11) | 16 (9 to 53) | 63 (19 to 107) | Low (medium ROB, imprecise) |
| Approved beta-blockers | |||||||
| Propranolol124,138 | 221 | 13.2 [5.6] | 2.1 (0.6 to 7.7) | 0.06 (0.00 to 0.12) | 16 (8 to 333) | 62 (3 to 120) | Low (medium ROB, imprecise, inconsistent) |
| Off-label ACE inhibitors | |||||||
| Lisinopril89 | 120 | 3.3 [1.7] | 2.0 (0.2 to 21.5) | 0.02(−0.04 to 0.07) | Low (imprecise, individual RCT) | ||
| Off-label angiotensin II receptor blockers | |||||||
| Telmisartan91 | 95 | 2.1 [2.1] | 1.0 (0.1 to 15.2) | 0.00(−0.06 to 0.06) | Low (imprecision, individual RCT) | ||
| Off-label antiadrenergics | |||||||
| Clonidine139,140 | 334 | 2.4 [0.6] | 2.8 (0.4 to 18.5) | 0.02 (−0.01 to 0.05) | Low (medium ROB, imprecise) | ||
| Off-label antidepressants | |||||||
| Amitriptyline93,141 | 507 | 11.2 [5.8] | 1.9 (1.0 to 3.5) | 0.05 (0.01 to 0.10) | 19 (10 to 167) | 54 (6 to 102) | Low (medium ROB, imprecise) |
| Femoxetine142,143 | 124 | 11.7 [6.3] | 1.9 (0.6 to 6.1) | 0.05 (−0.05 to 0.15) | Low (medium ROB, imprecise) | ||
| Off-label antiepileptics | |||||||
| Gabapentin127–129 | 270 | 17.0 [7.7] | 1.9 (0.9 to 4.2) | 0.07 (−0.01 to 0.15) | Low (medium ROB, imprecise) | ||
| Lamotrigine120,144 | 178 | 12.8 [6.0] | 2.4 (0.5 to 12.2) | 0.14 (−0.17 to 0.44) | Low (imprecise, inconsistent) | ||
| Valproate145,146 | 150 | 6.7 [5.3] | 1.3 (0.3 to 4.9) | 0.01 (−0.07 to 0.08) | Low (medium ROB, imprecise) | ||
| Off-label magnesium | |||||||
| Magnesium134,135 | 150 | 7.7 [1.4] | 3.8 (0.7 to 22.4) | 0.06 (0.00 to 0.13) | Low (Imprecise, inconsistent) | ||
| Off-label nonsteroidal antiinflammatory drugs | |||||||
| Naproxen147,148 | 172 | 3.5 [1.2] | 2.3 (0.3 to 15.4) | 0.02 (−0.03 to 0.07) | Low (high ROB, imprecise, inconsistent) | ||
| Off-label selective calcium channel blockers | |||||||
| Nimodipine130,149 | 155 | 3.9 [6.3] | 0.7 (0.2 to 2.6) | −0.03 (−0.09 to 0.04) | Low (medium ROB, imprecise, inconsistent) | ||
| Compared with active treatment: approved antiepileptic vs. off-label antidepressant | |||||||
| Topiramate vs. amitriptyline150,151 | 399 | 18.3 [21.3] | 0.9 (0.6 to 1.3) | −0.04 (−0.11 to 0.04) | Low (medium ROB, imprecise) | ||
SOE Strength of evidence, ROB risk of bias. Bold Significant effects of drugs on treatment response and discontinuation due to adverse effects when 95 % CI of attributable events per 1,000 treated do not include 0
Propranolol caused bothersome adverse effects leading to treatment discontinuation more often than placebo (Table 3). Among specific adverse effects, propranolol increased risk of diarrhea and nausea (online Appendix Table 15). Timolol increased risk of any adverse effects but not harms leading to treatment discontinuation.
Among off-label drugs, pooled direct analyses demonstrated that the antidepressant amitriptyline caused bothersome adverse effects leading to treatment discontinuation more often than placebo (Table 3).
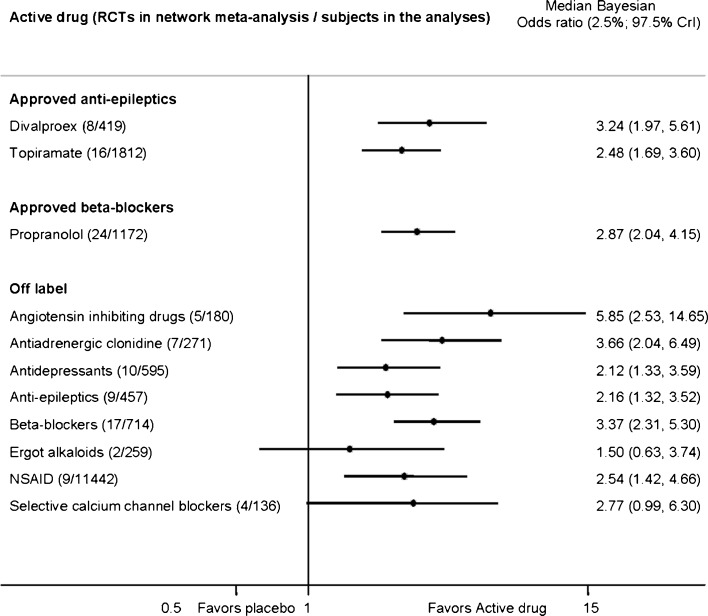
Indirect adjusted frequentist analyses demonstrated no differences in treatment discontinuation due to adverse effects with approved drugs or approved versus off-label drugs. Exploratory Bayesian network meta-analyses demonstrated that topiramate and off-label antiepileptics and antidepressants resulted in bothersome adverse effects leading to treatment discontinuation more often than placebo (Figure 3). According to network meta-analysis, off-label beta-blockers were the least likely to result in adverse effects leading to treatment discontinuation in adults with episodic migraine. Subjects did not experience increased risk of adverse effects that would lead to treatment discontinuation with off-label angiotensin-inhibiting drugs (online Appendix Table 16).
Figure 3.
Bayesian network meta-analysis of treatment discontinuation due to intolerable adverse effects with drugs vs. placebo (47 RCTs of 3,054 adults) in randomized controlled clinical trials that aimed to prevent migraine in adults. CrI Credible intervals. We used a heterogeneous random effects model that assumes correlation within a study (rho = 0.5) and heterogeneity between studies. RCTs of angiotensin-inhibiting drugs do not report intolerable adverse effects. NSAID Nonsteroidal antiinflammatory drugs.
Nonrandomized studies with high risk of bias suggested that 10 to 20 % of patients discontinued antiepileptic drug treatments at 1 year or longer of follow-up.
Drug Effect Modification by Select Patient Characteristics
Evidence was limited to individual RCTs examining drug effect modification by select patient characteristics. Amitriptyline was better than placebo in reducing monthly migraine, but only in patients with depression or baseline frequent and severe migraine93 (OR, 2.4; 95 % CI, 1.45-3.8 for every additional day of migraine at baseline).94 No trials directly compared drug effects in patients with and without aura. Several post hoc subgroup analyses of topiramate versus placebo provided inconsistent efficacy evidence regarding aura.95,96
DISCUSSION
All approved drugs, some off-label beta blockers, and the angiotensin-inhibiting drugs were better than placebo in reducing monthly migraine frequency by ≥50 % percent (clinical response). Drugs demonstrated similarly moderate relative effect size: drugs prevented half or more migraine attacks in 200 to 400 patients per 1,000 treated.
Critical assessment of the available evidence suggested that strength of evidence was moderate only for topiramate and low for other drugs because of risk of bias and imprecise estimates. We found it difficult to evaluate the role of financial conflict of interest and industry participation in data analyses because many studies were conducted prior to mandatory requirements for financial disclosure, leading to inconsistent reporting and insufficient detail from individual studies.97 Studies inconsistently reported subjects’ baseline severity, comorbidities, and concomitant treatments2,98 as well as family history of migraine, socioeconomic status, or response to prior preventive treatments.99,100 Few studies provided evidence for individualized treatment decisions with clear descriptions of planned stratified randomization and subgroup analyses.101 No trials examined the role of genetic polymorphism in drug metabolism and effects.102–104 Migraine prevention trials did not address teratogenic effects,105 anorgasmia,106 impotence,107 and other harms of antiepileptic drugs that can deter long-term adherence to preventive drugs.
Informed clinical decisions should balance the benefits and harms attributable to specific drugs.108 The most recent guidelines from the American Academy of Neurology and the American Headache Society recommend the four FDA-approved drugs—topiramate, divalproex, propranolol, and timolol—for adult migraine prevention.109 These guidelines focused on published evidence only and differed in recommending off-label drugs, including beta-blockers and angiotensin-inhibiting drugs. Further, current guidelines do not include consideration of the balance between benefits and harms of drugs as a basis for clinical decision-making.110
Our report has limitations. We did not contact authors for details about unreported benefits and harms or about methodological quality in cases of poor reporting of risk of bias criteria; the cost-effectiveness of this pursuit is still being debated.111,112 We justify using indirect network meta-analyses since trials did not differ by reported baseline subject characteristics. However, indirect comparisons did not address unreported baseline differences in comorbidities or socioeconomic status. We did not use tests with questionable statistical validity to quantify publication bias; instead, we identified low publication rates of NIH-funded grants and registered studies of migraine prevention. Available data did not allow us to determine exact reasons for low availability of results. Complete information about all conducted studies may change our low strength of evidence conclusions in the future. Although judgments in ranking strength of evidence were subjective,40 our transparent appraisal provides useful information for informed decision-making in practice and future research needs.
Future well-designed RCTs should examine the comparative effectiveness of the approved and the most effective off-label drugs by patient demographics, migraine family history, comorbidities, and response to prior treatments. Analyses of administrative databases should examine emergency room visits for treatment of migraines among adults taking approved and off-label preventive drugs.17 Prospective pharmacovigilance methods113,114 should be used for routine monitoring of off-label drug use and associated adverse effects with migraine preventive drugs.
Based on our comprehensive network analysis of comparative effectiveness and harms with migraine preventive drugs in adults, we conclude that approved drugs and off-label angiotensin-inhibiting drugs (lisinopril, captopril, and candesartan) or off-label beta-blockers (metoprolol, acebutolol, atenolol, and nadolol) were effective in preventing episodic migraine in adults. Exploratory network meta-analyses of all available evidence suggested that off-label angiotensin-inhibiting drugs demonstrated the most favorable benefit to harm ratio.
Electronic supplementary material
(DOC 1534 kb)
Acknowledgments
Contributors
We would like to thank the librarians, Judy Stanke, MA, and Delbert Reed, PhD, for their contributions to the literature search; Jeannine Ouellette for her help in writing the report; Marilyn Eells for editing and formatting the report; and Christa Prodzinski, RN, and Kirsten Johnson, BS, for assistance with data entry, quality control, and formatting tables. We would like to thank Hwanhee Hong, PhD candidate, for her help in conducting Bayesian network meta-analyses.
Funders
Prepared by the University of Minnesota Evidence-based Practice Center under contract no. 290-2007-10064 I with the AHRQ
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Systematic review registration no.: CRD42012001918
REFERENCES
- 1.Goadsby PJ, Raskin NH, et al. Chapter 15. Headache. In: Fauci AS, Braunwald E, Kasper DL, et al., editors. Harrison’s principles of internal medicine. 17. New York: The McGraw-Hill Companies; 2008. [Google Scholar]
- 2.Silberstein SD. Preventive migraine treatment. Neurol Clin. 2009;27:429–443. doi: 10.1016/j.ncl.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Solomon GD, Santanello N. Impact of migraine and migraine therapy on productivity and quality of life. Neurology. 2000;55:S29–S35. doi: 10.1212/WNL.55.4.606. [DOI] [PubMed] [Google Scholar]
- 4.Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache. 2007;47:355–363. doi: 10.1111/j.1526-4610.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- 5.Lipton RB, Scher AI, Kolodner K, Liberman J, Steiner TJ, Stewart WF. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885–894. doi: 10.1212/WNL.58.6.885. [DOI] [PubMed] [Google Scholar]
- 6.Bigal ME, Lipton RB, Winner P, et al. Migraine in adolescents: association with socioeconomic status and family history. Neurology. 2007;69:16–25. doi: 10.1212/01.wnl.0000265212.90735.64. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez-Latorre MA, Roig M. Natural history of migraine in childhood. Cephalalgia. 2000;20:573–579. doi: 10.1046/j.1468-2982.2000.00083.x. [DOI] [PubMed] [Google Scholar]
- 8.Headache Classification Subcommittee of the International Headache Society The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 9.Olesen J, Bousser MG, Diener HC, et al. New appendix criteria open for a broader concept of chronic migraine. Cephalalgia. 2006;26:742–746. doi: 10.1111/j.1468-2982.2006.01172.x. [DOI] [PubMed] [Google Scholar]
- 10.Solomon S. New appendix criteria open for a broader concept of chronic migraine (Comment on: Cephalagia 2006 Jun:26(6):742–6) Cephalalgia. 2007;27:469. doi: 10.1111/j.1468-2982.2007.01292_1.x. [DOI] [PubMed] [Google Scholar]
- 11.Stewart WF, Ricci JA, Chee E, Morganstein D. Lost productive work time costs from health conditions in the United States: results from the American Productivity Audit. J Occup Environ Med. 2003;45:1234–1246. doi: 10.1097/01.jom.0000099999.27348.78. [DOI] [PubMed] [Google Scholar]
- 12.Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML. Burden of migraine in the United States: disability and economic costs. Arch Intern Med. 1999;159:813–818. doi: 10.1001/archinte.159.8.813. [DOI] [PubMed] [Google Scholar]
- 13.Burton WN, Landy SH, Downs KE, Runken MC. The impact of migraine and the effect of migraine treatment on workplace productivity in the United States and suggestions for future research. Mayo Clin Proc. 2009;84:436–445. doi: 10.1016/S0025-6196(11)60562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipton RB, Scher AI, Steiner TJ, et al. Patterns of health care utilization for migraine in England and in the United States. Neurology. 2003;60:441–448. doi: 10.1212/WNL.60.3.441. [DOI] [PubMed] [Google Scholar]
- 15.Lipton RB, Stewart WF. The epidemiology of migraine. Eur Neurol. 1994;34(Suppl 2):6–11. doi: 10.1159/000119525. [DOI] [PubMed] [Google Scholar]
- 16.Rapoport AM. Acute and prophylactic treatments for migraine: present and future. Neurol Sci. 2008;29(Suppl 1):S110–S122. doi: 10.1007/s10072-008-0901-x. [DOI] [PubMed] [Google Scholar]
- 17.Stafford RS. Regulating off-label drug use–rethinking the role of the FDA. N Engl J Med. 2008;358:1427–1429. doi: 10.1056/NEJMp0802107. [DOI] [PubMed] [Google Scholar]
- 18.Silberstein S, Tfelt-Hansen P, Dodick DW, et al. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia. 2008;28:484–495. doi: 10.1111/j.1468-2982.2008.01555.x. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder BM. AAFP/ACP-ASIM release guidelines on the management and prevention of migraines. Am Fam Physician. 2003;67(1392):5–7. [PubMed] [Google Scholar]
- 20.Morey SS. Guidelines on migraine: part 4. General principles of preventive therapy. Am Fam Physician. 2000;62:2359–2360. [PubMed] [Google Scholar]
- 21.Lainez MJ, Freitag FG, Pfeil J, Ascher S, Olson WH, Schwalen S. Time course of adverse events most commonly associated with topiramate for migraine prevention. Eur J Neurol. 2007;14:900–906. doi: 10.1111/j.1468-1331.2007.01869.x. [DOI] [PubMed] [Google Scholar]
- 22.Luykx J, Mason M, Ferrari MD, Carpay J. Are migraineurs at increased risk of adverse drug responses? A meta-analytic comparison of topiramate-related adverse drug reactions in epilepsy and migraine. Clin Pharmacol Ther. 2009;85:283–288. doi: 10.1038/clpt.2008.203. [DOI] [PubMed] [Google Scholar]
- 23.Geraud G, Lanteri-Minet M, Lucas C, Valade D. French guidelines for the diagnosis and management of migraine in adults and children. Clin Ther. 2004;26:1305–1318. doi: 10.1016/S0149-2918(04)80161-9. [DOI] [PubMed] [Google Scholar]
- 24.Evers S, Afra J, Frese A, et al. EFNS guideline on the drug treatment of migraine–revised report of an EFNS task force. Eur J Neurol. 2009;16:968–981. doi: 10.1111/j.1468-1331.2009.02748.x. [DOI] [PubMed] [Google Scholar]
- 25.Dowson AJ, Lipscombe S, Sender J, Rees T, Watson D. New guidelines for the management of migraine in primary care. Curr Med Res Opin. 2002;18:414–439. doi: 10.1185/030079902125001164. [DOI] [PubMed] [Google Scholar]
- 26.Epstein RM, Alper BS, Quill TE. Communicating evidence for participatory decision making. JAMA. 2004;291:2359–2366. doi: 10.1001/jama.291.19.2359. [DOI] [PubMed] [Google Scholar]
- 27.Amanzio M, Corazzini LL, Vase L, Benedetti F. A systematic review of adverse events in placebo groups of anti-migraine clinical trials. Pain. 2009;146:261–269. doi: 10.1016/j.pain.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Whyte CA, Tepper SJ. Adverse effects of medications commonly used in the treatment of migraine. Expert Rev Neurother. 2009;9:1379–1391. doi: 10.1586/ern.09.47. [DOI] [PubMed] [Google Scholar]
- 29.Sung NS, Crowley WF, Jr, Genel M, et al. Central challenges facing the national clinical research enterprise. JAMA. 2003;289:1278–1287. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- 30.Finding what works in health care: Standards for systematic reviews. Heidelberg: National Academies Press; 2011. [PubMed] [Google Scholar]
- 31.Committee on Ethical and Scientific Issues in Studying the Safety of Approved Drugs. Ethical and scientific issues in studying the safety of approved drugs. Washington: National Academies Press; 2012. [PubMed] [Google Scholar]
- 32.Slutsky J, Atkins D, Chang S, Collins Sharp BA. Comparing medical interventions: AHRQ and the Effective Health Care Program. Methods Guide for Effectiveness and Comparative Effectiveness Reviews AHRQ Publication No 10(11)-EHC063-EF. 2011/03/25 ed. Rockville, MD: Agency for Healthcare Research and Quality; 2008:1–4. Available at http://effectivehealthcare.ahrq.gov/ehc/products/118/324/MethodsGuide_Slutsky_Comparing%20Medical%20Interventions.pdf; accessed on February 19, 2013. [PubMed]
- 33.Helfand M, Balshem H. Principles in Developing and Applying Guidance. 2008. [PubMed]
- 34.Whitlock EP, Lopez SA, Chang S, Helfand M, Eder M, Floyd N. AHRQ series paper 3: identifying, selecting, and refining topics for comparative effectiveness systematic reviews: AHRQ and the effective health-care program. J Clin Epidemiol. 2010;63:491–501. doi: 10.1016/j.jclinepi.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Rothner AD. Complicated migraine and migraine variants. Curr Pain Headache Rep. 2002;6:233–239. doi: 10.1007/s11916-002-0040-7. [DOI] [PubMed] [Google Scholar]
- 36.Hansen JM, Thomsen LL, Olesen J, Ashina M. Calcitonin gene-related peptide does not cause the familial hemiplegic migraine phenotype. Neurology. 2008;71:841–847. doi: 10.1212/01.wnl.0000325482.64106.3f. [DOI] [PubMed] [Google Scholar]
- 37.Norris S, Atkins D, Bruening W, et al. Chapter 4. Selecting observational studies for comparing medical interventions. Methods Guide for Effectiveness and Comparative Reviews AHRQ Publication No 10(11)-EHC063-EF. Rockville, MD: Agency for Healthcare Research and Quality. March 2011:56–68. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/196/454/MethodsGuideNorris_06042010.pdf; accessed on February 19, 2013. [PubMed]
- 38.Chou R, Aronson N, Atkins D, et al. AHRQ series paper 4: assessing harms when comparing medical interventions: AHRQ and the Effective Health-Care Program. J Clin Epidemiol. 2008;63:502–512. doi: 10.1016/j.jclinepi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. London: The Cochrane Collaboration; 2011. [Google Scholar]
- 40.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Velde G, van Tulder M, Cote P, et al. The sensitivity of review results to methods used to appraise and incorporate trial quality into data synthesis. Spine. 2007;32:796–806. doi: 10.1097/01.brs.0000258903.67718.d5. [DOI] [PubMed] [Google Scholar]
- 42.Herbison P, Hay-Smith J, Gillespie WJ. Adjustment of meta-analyses on the basis of quality scores should be abandoned. J Clin Epidemiol. 2006;59:1249–1256. doi: 10.1016/j.jclinepi.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9:80. doi: 10.1186/1471-2288-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Egger M, Smith GD, Altman DG. Systematic reviews in health care: meta-analysis in context. 2. London: BMJ Books; 2001. [Google Scholar]
- 45.Treadwell JR, Uhl S, Tipton K, et al. Assessing equivalence and noninferiority. J Clin Epidemiol. 2012;65:1144–1149. doi: 10.1016/j.jclinepi.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Lievre M, Cucherat M, Leizorovicz A. Pooling, meta-analysis, and the evaluation of drug safety. Current controlled trials in cardiovascular medicine. 2002;3:6. doi: 10.1186/1468-6708-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fu R, Gartlehner G, Grant M, et al. Chapter 9. Conducting quantitative synthesis when comparing medical interventions. Methods Guide for Effectiveness and Comparative Effectiveness Reviews AHRQ Publication No 10(11)-EHC063-EF. Rockville, MD: Agency for Healthcare Research and Quality. March 2011:104–19. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/243/554/MethodsGuide--ConductingQuantitativeSynthesis.pdf; accessed on February 20, 2013.
- 48.Rucker G, Schwarzer G, Carpenter J, Olkin I. Why add anything to nothing? The arcsine difference as a measure of treatment effect in meta-analysis with zero cells. Stat Med. 2009;28:721–738. doi: 10.1002/sim.3511. [DOI] [PubMed] [Google Scholar]
- 49.Bradburn MJ, Deeks JJ, Berlin JA, Russell LA. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26:53–77. doi: 10.1002/sim.2528. [DOI] [PubMed] [Google Scholar]
- 50.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 51.Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29:3046–3067. doi: 10.1002/sim.4040. [DOI] [PubMed] [Google Scholar]
- 52.White IR. Multivariate random-effects meta-regression: Updates to mvmeta. The Stata Journal 2011;11.
- 53.Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions-Agency for Healthcare Research and Quality and the Effective Health-Care Program. J Clin Epidemiol. 2010;63:513–523. doi: 10.1016/j.jclinepi.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 54.Viechtbauer W. Confidence intervals for the amount of heterogeneity in meta-analysis. Stat Med. 2007;26:37–52. doi: 10.1002/sim.2514. [DOI] [PubMed] [Google Scholar]
- 55.Knapp G, Biggerstaff BJ, Hartung J. Assessing the amount of heterogeneity in random-effects meta-analysis. Biom J. 2006;48:271–285. doi: 10.1002/bimj.200510175. [DOI] [PubMed] [Google Scholar]
- 56.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 57.Ad Hoc Committee on Classification of Headache Classification of headache. JAMA. 1962;179:717–718. doi: 10.1001/jama.1962.03050090045008. [DOI] [Google Scholar]
- 58.Ebrahim S. The use of numbers needed to treat derived from systematic reviews and meta-analysis. Caveats and pitfalls. Eval Health Prof. 2001;24:152–164. doi: 10.1177/01632780122034858. [DOI] [PubMed] [Google Scholar]
- 59.Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317:1309–1312. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coory M, Jordan S. Frequency of treatment-effect modification affecting indirect comparisons: a systematic review. Pharmcoeconomics. 2010;28:723–732. doi: 10.2165/11535670-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 61.Wells G, Sultan S, Chen L, Khan M, Coyle D. Indirect evidence: Indirect treatment comparisons in meta-analysis. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2009. [Google Scholar]
- 62.Glenny AM, Altman DG, Song F, et al. Indirect comparisons of competing interventions. Health Technol Assess. 2005;9:1–134. doi: 10.3310/hta9260. [DOI] [PubMed] [Google Scholar]
- 63.Song F, Loke YK, Walsh T, Glenny AM, Eastwood AJ, Altman DG. Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ. 2009;338:b1147. doi: 10.1136/bmj.b1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Donegan S, Williamson P, Gamble C, Tudur-Smith C. Indirect comparisons: a review of reporting and methodological quality. PLoS One. 2010;5:e11054. doi: 10.1371/journal.pone.0011054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carlin BP, Louis TA. Bayesian methods for data analysis. Boca Raton: Chapman & Hall/CRC; 2009. [Google Scholar]
- 66.Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Research Synthesis Methods. 2012;3:98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aschengrau A, Seage GR. Essentials of epidemiology in public health. Sudbury: Jones and Bartlett; 2003. [Google Scholar]
- 68.Lunn D, Thomas A, Best N, Spiegelhalter D. WinBUGS- a Bayesian modelling framework: concepts, structure, and extensibility. Statistics and Computing. 2000;10:325–337. doi: 10.1023/A:1008929526011. [DOI] [Google Scholar]
- 69.Berkman ND, Lohr KN, Morgan LC, et al. Reliability Testing of the AHRQ EPC Approach to Grading the Strength of Evidence in Comparative Effectiveness Reviews. Rockville (MD) 2012. [PubMed]
- 70.Guyatt G, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence-imprecision. J Clin Epidemiol 2011. [DOI] [PubMed]
- 71.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 72.Silberstein S. Efficacy and safety of topiramate in migraine prevention: a dose-ranging, placebo-controlled, double-blind, multicenter study. Advanced Studies in Medicine. 2003;3:S565–S568. [Google Scholar]
- 73.Brandes JL, Saper JR, Diamond M, et al. Topiramate for migraine prevention: a randomized controlled trial. JAMA. 2004;291:965–973. doi: 10.1001/jama.291.8.965. [DOI] [PubMed] [Google Scholar]
- 74.Silberstein SD, Neto W, Schmitt J, Jacobs D. Topiramate in migraine prevention: results of a large controlled trial. Arch Neurol. 2004;61:490–495. doi: 10.1001/archneur.61.4.490. [DOI] [PubMed] [Google Scholar]
- 75.Diener HC, Agosti R, Allais G, et al. Cessation versus continuation of 6-month migraine preventive therapy with topiramate (PROMPT): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2007;6:1054–1062. doi: 10.1016/S1474-4422(07)70272-7. [DOI] [PubMed] [Google Scholar]
- 76.Brandes JL, Kudrow DB, Rothrock JF, Rupnow MF, Fairclough DL, Greenberg SJ. Assessing the ability of topiramate to improve the daily activities of patients with migraine. Mayo Clin Proc. 2006;81:1311–1319. doi: 10.4065/81.10.1311. [DOI] [PubMed] [Google Scholar]
- 77.Diener HC, Bussone G, Van Oene JC, Lahaye M, Schwalen S, Goadsby PJ. Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia. 2007;27:814–823. doi: 10.1111/j.1468-2982.2007.01326.x. [DOI] [PubMed] [Google Scholar]
- 78.Dahlof C, Loder E, Diamond M, Rupnow M, Papadopoulos G, Mao L. The impact of migraine prevention on daily activities: a longitudinal and responder analysis from three topiramate placebo-controlled clinical trials. Health Qual Life Outcomes. 2007;5:56. doi: 10.1186/1477-7525-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klapper J. Divalproex sodium in migraine prophylaxis: a dose-controlled study. Cephalalgia. 1997;17:103–108. doi: 10.1046/j.1468-2982.1997.1702103.x. [DOI] [PubMed] [Google Scholar]
- 80.Mei D, Ferraro D, Zelano G, et al. Topiramate and triptans revert chronic migraine with medication overuse to episodic migraine. Clin Neuropharmacol. 2006;29:269–275. doi: 10.1097/01.WNF.000022888.49044.99. [DOI] [PubMed] [Google Scholar]
- 81.Lipton RB, Silberstein S, Dodick D, et al. Topiramate intervention to prevent transformation of episodic migraine: the topiramate INTREPID study. Cephalalgia. 2011;31:18–30. doi: 10.1177/0333102410372427. [DOI] [PubMed] [Google Scholar]
- 82.Bussone G, Diener HC, Pfeil J, Schwalen S. Topiramate 100 mg/day in migraine prevention: a pooled analysis of double-blind randomised controlled trials. Int J Clin Pract. 2005;59:961–968. doi: 10.1111/j.1368-5031.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 83.Forssman B, Henriksson KG, Johannsson V, et al. Propranolol for migraine prophylaxis. Headache. 1976;16(5):238–245. doi: 10.1111/j.1526-4610.1976.hed1605238.x. [DOI] [PubMed] [Google Scholar]
- 84.Schoonman GG, Wiendels NJ, Ferrari MD. Gabapentin in migraine prophylaxis: is it effective and well tolerated? Headache. 2002;42:235. doi: 10.1046/j.1526-4610.2002.02060.x. [DOI] [PubMed] [Google Scholar]
- 85.Nanda RN, Johnson RH, Gray J, Keogh HJ, Melville ID. A double blind trial of acebutolol for migraine prophylaxis. Headache. 1978;18(1):20–22. doi: 10.1111/j.1526-4610.1978.hed1801020.x. [DOI] [PubMed] [Google Scholar]
- 86.Forssman B, Lindblad CJ, Zbornikova V. Atenolol for migraine prophylaxis. Headache. 1983;23(4):188–190. doi: 10.1111/j.1526-4610.1983.hed2304188.x. [DOI] [PubMed] [Google Scholar]
- 87.Freitag FG, Diamond S. Nadolol and placebo comparison study in the prophylactic treatment of migraine. The Journal of the American Osteopathic Association. 1984;84(4):343–347. [PubMed] [Google Scholar]
- 88.Minervini MG, Pinto K. Captopril relieves pain and improves mood depression in depressed patients with classical migraine. Cephalalgia. 1987;7(Suppl 6):485–486. [Google Scholar]
- 89.Schrader H, Stovner LJ, Helde G, Sand T, Bovim G. Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor (lisinopril): randomised, placebo controlled, crossover study. BMJ. 2001;322:19–22. doi: 10.1136/bmj.322.7277.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tronvik E, Stovner LJ, Helde G, Sand T, Bovim G. Prophylactic treatment of migraine with an angiotensin II receptor blocker: a randomized controlled trial. JAMA. 2003;289:65–69. doi: 10.1001/jama.289.1.65. [DOI] [PubMed] [Google Scholar]
- 91.Diener HC, Gendolla A, Feuersenger A, et al. Telmisartan in migraine prophylaxis: a randomized, placebo-controlled trial. Cephalalgia. 2009;29:921–927. doi: 10.1111/j.1468-2982.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 92.Adelman J, Freitag FG, Lainez M, et al. Analysis of safety and tolerability data obtained from over 1,500 patients receiving topiramate for migraine prevention in controlled trials. Pain Med. 2008;9:175–185. doi: 10.1111/j.1526-4637.2007.00389.x. [DOI] [PubMed] [Google Scholar]
- 93.Couch JR, Hassanein RS. Amitriptyline in migraine prophylaxis. Arch Neurol. 1979;36:695–699. doi: 10.1001/archneur.1979.00500470065013. [DOI] [PubMed] [Google Scholar]
- 94.Lampl C, Huber G, Adl J, et al. Two different doses of amitriptyline ER in the prophylaxis of migraine: long-term results and predictive factors. Eur J Neurol. 2009;16:943–948. doi: 10.1111/j.1468-1331.2009.02631.x. [DOI] [PubMed] [Google Scholar]
- 95.Silberstein SD, Hulihan J, Karim MR, et al. Efficacy and tolerability of topiramate 200 mg/d in the prevention of migraine with/without aura in adults: a randomized, placebo-controlled, double-blind, 12-week pilot study. Clin Ther. 2006;28:1002–1011. doi: 10.1016/j.clinthera.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 96.Reuter U, Del Rio MS, Diener HC, et al. Migraines with and without aura and their response to preventive therapy with topiramate. Cephalalgia: an International Journal of Headache. 2010;30(5):543–551. doi: 10.1111/j.1468-2982.2009.01999.x. [DOI] [PubMed] [Google Scholar]
- 97.Barden J, Derry S, McQuay HJ, Moore RA. Bias from industry trial funding? A framework, a suggested approach, and a negative result. Pain. 2006;121:207–218. doi: 10.1016/j.pain.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 98.Fox AW. Disease modification in migraine: study design and sample size implications. Headache. 2008;48:1169–1175. doi: 10.1111/j.1526-4610.2007.01057.x. [DOI] [PubMed] [Google Scholar]
- 99.Hazard E, Munakata J, Bigal ME, Rupnow MFT, Lipton RB. The burden of migraine in the United States: current and emerging perspectives on disease management and economic analysis. Value in Health. 2009;12:55–64. doi: 10.1111/j.1524-4733.2008.00404.x. [DOI] [PubMed] [Google Scholar]
- 100.Russell MB, Hilden J, Sorensen SA, Olesen J. Familial occurrence of migraine without aura and migraine with aura. Neurology. 1993;43:1369–1373. doi: 10.1212/WNL.43.7.1369. [DOI] [PubMed] [Google Scholar]
- 101.Arends LR, Hoes AW, Lubsen J, Grobbee DE, Stijnen T. Baseline risk as predictor of treatment benefit: three clinical = meta-re-analyses. Stat Med. 2000;19:3497–3518. doi: 10.1002/1097-0258(20001230)19:24<3497::AID-SIM830>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 102.Gentile G, Missori S, Borro M, Sebastianelli A, Simmaco M, Martelletti P. Frequencies of genetic polymorphisms related to triptans metabolism in chronic migraine. Journal of Headache & Pain. 2010;11:151–156. doi: 10.1007/s10194-010-0202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schurks M, Zee RY, Buring JE, Kurth T. ACE D/I polymorphism, migraine, and cardiovascular disease in women. Neurology. 2009;72:650–656. doi: 10.1212/01.wnl.0000342517.97178.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feldman HL. Pushing drugs: genomics and genetics, the pharmaceutical industry, and the law of negligence. Washburn Law J. 2003;42:575–599. [PubMed] [Google Scholar]
- 105.Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol. 2012;11:803–813. doi: 10.1016/S1474-4422(12)70103-5. [DOI] [PubMed] [Google Scholar]
- 106.Coebergh JA, Waldinger MD. Reversible anorgasmia with topiramate for migraine prophylaxis. The Journal of Neuropsychiatry and Clinical Neurosciences. 2012;24:E30–E31. doi: 10.1176/appi.neuropsych.11040081. [DOI] [PubMed] [Google Scholar]
- 107.Huang CY, Keller JJ, Sheu JJ, Lin HC. Migraine and erectile dysfunction: evidence from a population-based case–control study. Cephalalgia. 2012;32:366–372. doi: 10.1177/0333102412439801. [DOI] [PubMed] [Google Scholar]
- 108.Bingham MF, Johnson FR, Miller D. Modeling choice behavior for new pharmaceutical products. Value in Health. 2001;4:32–44. doi: 10.1046/j.1524-4733.2001.004001032.x. [DOI] [PubMed] [Google Scholar]
- 109.Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337–1345. doi: 10.1212/WNL.0b013e3182535d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. J Clin Epidemiol. 2010;63:1308–1311. doi: 10.1016/j.jclinepi.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 111.Mullan RJ, Flynn DN, Carlberg B, et al. Systematic reviewers commonly contact study authors but do so with limited rigor. J Clin Epidemiol. 2009;62:138–142. doi: 10.1016/j.jclinepi.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 112.Finding Evidence and Assessing for Reporting Biases when Comparing Medical Interventions: AHRQ and the Effective Health Care Program. In press 2012. The draft is available at: http://effectivehealthcare.ahrq.gov/ehc/products/486/1305/Reporting-Bias_DraftReport_20121023.pdf; accessed on February 19, 2013
- 113.Edwards IR. Off-label pharmacovigilance. Drug Saf. 2011;34:795–797. doi: 10.2165/11596140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 114.Layton D, Hazell L, Shakir SA. Modified prescription-event monitoring studies: a tool for pharmacovigilance and risk management. Drug Saf. 2011;34:e1–e9. doi: 10.2165/11593830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 115.Mathew NT, Saper JR, Silberstein SD, et al. Migraine prophylaxis with divalproex. Arch Neurol. 1995;52:281–286. doi: 10.1001/archneur.1995.00540270077022. [DOI] [PubMed] [Google Scholar]
- 116.Freitag FG, Collins SD, Carlson HA, et al. A randomized trial of divalproex sodium extended-release tablets in migraine prophylaxis. Neurology. 2002;58:1652–1659. doi: 10.1212/WNL.58.11.1652. [DOI] [PubMed] [Google Scholar]
- 117.Storey JR, Calder CS, Hart DE, Potter DL. Topiramate in migraine prevention: a double-blind, placebo-controlled study. Headache. 2001;41:968–975. doi: 10.1046/j.1526-4610.2001.01190.x. [DOI] [PubMed] [Google Scholar]
- 118.Mei D, Capuano A, Vollono C, et al. Topiramate in migraine prophylaxis: a randomised double-blind versus placebo study. Neurol Sci. 2004;25:245–250. doi: 10.1007/s10072-004-0350-0. [DOI] [PubMed] [Google Scholar]
- 119.Silvestrini M, Bartolini M, Coccia M, Baruffaldi R, Taffi R, Provinciali L. Topiramate in the treatment of chronic migraine. Cephalalgia. 2003;23:820–824. doi: 10.1046/j.1468-2982.2003.00592.x. [DOI] [PubMed] [Google Scholar]
- 120.Gupta P, Singh S, Goyal V, Shukla G, Behari M. Low-dose topiramate versus lamotrigine in migraine prophylaxis (the Lotolamp study) Headache. 2007;47:402–412. doi: 10.1111/j.1526-4610.2006.00599.x. [DOI] [PubMed] [Google Scholar]
- 121.Silberstein S, Lipton R, Dodick D, et al. Topiramate treatment of chronic migraine: a randomized, placebo-controlled trial of quality of life and other efficacy measures. Headache. 2009;49:1153–1162. doi: 10.1111/j.1526-4610.2009.01508.x. [DOI] [PubMed] [Google Scholar]
- 122.Tfelt-Hansen P, Standnes B, Kangasneimi P, Hakkarainen H, Olesen J. Timolol vs propranolol vs placebo in common migraine prophylaxis: a double-blind multicenter trial. Acta Neurol Scand. 1984;69:1–8. doi: 10.1111/j.1600-0404.1984.tb07772.x. [DOI] [PubMed] [Google Scholar]
- 123.Diener HC, Tfelt-Hansen P, Dahlof C, et al. Topiramate in migraine prophylaxis–results from a placebo-controlled trial with propranolol as an active control. J Neurol. 2004;251:943–950. doi: 10.1007/s00415-004-0464-6. [DOI] [PubMed] [Google Scholar]
- 124.Diamond S, Medina JL. Double blind study of propranolol for migraine prophylaxis. Headache. 1976;16:24–27. doi: 10.1111/j.1526-4610.1976.hed1601024.x. [DOI] [PubMed] [Google Scholar]
- 125.Standnes B. The prophylactic effect of timolol versus propranolol and placebo in common migraine: beta-blockers in migraine. Cephalalgia. 1982;2:165–170. doi: 10.1046/j.1468-2982.1982.0203165.x. [DOI] [PubMed] [Google Scholar]
- 126.Stellar S, Ahrens SP, Meibohm AR, Reines SA. Migraine prevention with timolol. A double-blind crossover study. JAMA. 1984;252:2576–2580. doi: 10.1001/jama.1984.03350180030025. [DOI] [PubMed] [Google Scholar]
- 127.Wessely P, Baumgartner C, Klingler D, et al. Preliminary results of a double-blind study with the new migraine prophylactic drug Gabapentin. Cephalalgia. 1987;7:477–478. [Google Scholar]
- 128.Mathew NT, Rapoport A, Saper J, et al. Efficacy of gabapentin in migraine prophylaxis. Headache. 2001;41:119–128. doi: 10.1046/j.1526-4610.2001.111006119.x. [DOI] [PubMed] [Google Scholar]
- 129.NCT00742209. Prevention study in adult patients suffering from migraine headaches. 2010.
- 130.Havanka-Kanniainen H, Hokkanen E, Myllylä VV. Efficacy of nimodipine in the prophylaxis of migraine. Cephalalgia: an International Journal of Headache. 1985;5(1):39–43. doi: 10.1046/j.1468-2982.1985.0501039.x. [DOI] [PubMed] [Google Scholar]
- 131.Gelmers HJ. Nimodipine, a new calcium antagonist, in the prophylactic treatment of migraine. Headache. 1983;23(3):106–109. doi: 10.1111/j.1526-4610.1983.hed2303106.x. [DOI] [PubMed] [Google Scholar]
- 132.Kangasniemi P, Andersen AR, Andersson PG, et al. Classic migraine: effective prophylaxis with metoprolol. Cephalalgia. 1987;7:231–238. doi: 10.1046/j.1468-2982.1987.0704231.x. [DOI] [PubMed] [Google Scholar]
- 133.Andersson PG, Dahl S, Hansen JH, et al. Prophylactic treatment of classical and non-classical migraine with metoprolol–a comparison with placebo. Cephalalgia. 1983;3:207–212. doi: 10.1046/j.1468-2982.1983.0304207.x. [DOI] [PubMed] [Google Scholar]
- 134.Peikert A, Wilimzig C, Kohne-Volland R. Prophylaxis of migraine with oral magnesium: results from a prospective, multi-center, placebo-controlled and double-blind randomized study. Cephalalgia. 1996;16:257–263. doi: 10.1046/j.1468-2982.1996.1604257.x. [DOI] [PubMed] [Google Scholar]
- 135.Pfaffenrath V, Wessely P, Meyer C, et al. Magnesium in the prophylaxis of migraine–a double-blind placebo-controlled study. Cephalalgia. 1996;16:436–440. doi: 10.1046/j.1468-2982.1996.1606436.x. [DOI] [PubMed] [Google Scholar]
- 136.Silberstein SD, Lipton RB, Dodick DW, et al. Efficacy and safety of topiramate for the treatment of chronic migraine: a randomized, double-blind, placebo-controlled trial. Headache. 2007;47:170–180. doi: 10.1111/j.1526-4610.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 137.Edwards KR, Potter DL, Wu SC, Kamin M, Hulihan J. Topiramate in the preventive treatment of episodic migraine: a combined analysis from pilot, double-blind, placebo-controlled trials. CNS Spectr. 2003;8:428–432. doi: 10.1017/s1092852900018733. [DOI] [PubMed] [Google Scholar]
- 138.Pradalier A, Serratrice G, Collard M, et al. Long-acting propranolol in migraine prophylaxis: results of a double-blind, placebo-controlled study. Cephalalgia. 1989;9:247–253. doi: 10.1046/j.1468-2982.1989.0904247.x. [DOI] [PubMed] [Google Scholar]
- 139.Boisen E, Deth S, Hübbe P, Jansen J, Klee A, Leunbach G. Clonidine in the prophylaxis of migraine. Acta Neurologica Scandinavica. 1978;58(5):288–295. doi: 10.1111/j.1600-0404.1978.tb02889.x. [DOI] [PubMed] [Google Scholar]
- 140.Adam EI, Gore SM, Price WH. Double blind trial of clonidine in the treatment of migraine in a general practice. J R Coll Gen Pract. 1978;28:587–590. [PMC free article] [PubMed] [Google Scholar]
- 141.Couch JR. Amitriptyline in the prophylactic treatment of migraine and chronic daily headache. Headache. 2011;51:33–51. doi: 10.1111/j.1526-4610.2010.01800.x. [DOI] [PubMed] [Google Scholar]
- 142.Orholm M, Honore PF, Zeeberg I. A randomized general practice group-comparative study of femoxetine and placebo in the prophylaxis of migraine. Acta Neurol Scand. 1986;74:235–239. doi: 10.1111/j.1600-0404.1986.tb07861.x. [DOI] [PubMed] [Google Scholar]
- 143.Orholm M, Le Fevre P. Prophylactic treatment of migraine with femoxetine—a randomized comparison with placebo. Cephalalgia 1985:516–7.
- 144.Steiner TJ, Findley LJ, Yuen AW. Lamotrigine versus placebo in the prophylaxis of migraine with and without aura. Cephalalgia. 1997;17:109–112. doi: 10.1046/j.1468-2982.1997.1702109.x. [DOI] [PubMed] [Google Scholar]
- 145.Hering R, Kuritzky A. Sodium valproate in the prophylactic treatment of migraine: a double-blind study versus placebo. Cephalalgia. 1992;12:81–84. doi: 10.1046/j.1468-2982.1992.1202081.x. [DOI] [PubMed] [Google Scholar]
- 146.Jensen R, Brinck T, Olesen J. Sodium valproate has a prophylactic effect in migraine without aura: a triple-blind, placebo-controlled crossover study. Neurology. 1994;44:647–651. doi: 10.1212/WNL.44.4.647. [DOI] [PubMed] [Google Scholar]
- 147.Welch KMA, Ellis DJ, Keenan PA. Successful migraine prohpylaxis with naproxen sodium. Neurology1985. [DOI] [PubMed]
- 148.Ziegler DK, Ellis DJ. Naproxen in prophylaxis of migraine. Archives of neurology. 1985;42(6):582–584. doi: 10.1001/archneur.1985.04060060084014. [DOI] [PubMed] [Google Scholar]
- 149.(MINES) M-NESG European multicenter trial of nimodipine in the prophylaxis of classic migraine (migraine with aura) Headache. 1989;29:639–642. doi: 10.1111/j.1526-4610.1989.hed2910639.x. [DOI] [PubMed] [Google Scholar]
- 150.Dodick DW, Freitag F, Banks J, et al. Topiramate versus amitriptyline in migraine prevention: a 26-week, multicenter, randomized, double-blind, double-dummy, parallel-group noninferiority trial in adult migraineurs. Clin Ther. 2009;31:542–559. doi: 10.1016/j.clinthera.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 151.Keskinbora K, Aydinli I. A double-blind randomized controlled trial of topiramate and amitriptyline either alone or in combination for the prevention of migraine. Clin Neurol Neurosurg. 2008;110:979–984. doi: 10.1016/j.clineuro.2008.05.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 1534 kb)