Abstract
BACKGROUND
Delivery of comprehensive care for persons with human immunodeficiency virus (HIV) infection in rural and low prevalence settings presents many challenges. We developed and evaluated a telehealth collaborative care (TCC) program for persons with HIV in a rural area.
OBJECTIVE
To determine the feasibility of TCC, and identify factors influencing implementation in rural settings.
DESIGN
Mixed methods evaluation of a quality improvement program with pre-measures and post-measures.
PATIENTS
Veterans with HIV infection in Iowa and Illinois.
INTERVENTION
TCC integrated HIV specialty care delivered by clinical video telehealth, with primary care delivered by generalist providers, in seven Community Based Outpatient Clinics (CBOCs) serving rural areas. Principles guiding TCC design were: 1) clear delineation of specialty and primary care clinic roles in co-managed care; 2) creation of processes to improve care coordination between specialty and primary care teams; and 3) use of a patient registry for population management across sites.
MEASURES
Veterans Affairs (VA) healthcare system performance measures for care for HIV infection and common comorbidities, patient travel time to obtain care, and patient satisfaction. Qualitative evaluation involved semi-structured telephone interviews with patients.
KEY RESULTS
Thirty of 32 eligible patients chose TCC over traveling to the HIV clinic for all care. Among 24 patients in TCC during the June 2011–May 2012 evaluation period, median age was 54 (range, 40–79), most (96 %) were men, and median CD4 count was 707 cells/cm3 (range, 233–1307). VA performance measures were met for > 90 % of TCC patients. Median yearly travel time decreased from 320 min per patient prior to TCC to 170 min during TCC (p < 0.001). Interview themes included: 1) overcoming privacy concerns during care in local primary care clinics; 2) tradeoffs between access, continuity, and care coordination; and 3) the role of specialist involvement in collaborative care.
DISCUSSION
Telehealth Collaborative Care is a feasible approach to providing accessible and comprehensive care for persons with HIV in rural settings.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-013-2385-5) contains supplementary material, which is available to authorized users.
KEY WORDS: HIV, rural health, telehealth, veterans
INTRODUCTION
In large cities in the United States (US), persons living with human immunodeficiency virus (HIV) infection often receive comprehensive healthcare in high-volume HIV specialty clinics that employ co-located, multidisciplinary care teams.1,2 In recent years, these clinics have increasingly developed systems and expertise necessary to deliver comprehensive primary care for an aging population of persons with HIV infection. However, this model does not adapt to rural and low HIV prevalence settings, where there are few healthcare providers with expertise in HIV medicine, and distances between patients and HIV specialty clinics are often great.3 Historically, the majority of rural-dwelling persons with HIV in the US have traveled long distances to HIV specialty clinics in urban areas, often foregoing care due to travel burdens.3
This was the case in the Iowa City Veterans Affairs (ICVA) healthcare system. In 2009, ICVA cared for approximately thirty veterans with HIV infection who traveled more than 1 hour each way from outlying, primarily rural areas to the HIV specialty clinic. These patients bypassed VA primary care clinics closer to their homes in order to receive all care in the HIV specialty clinic. Although the quality of HIV care in the specialty clinic was high—more than 90 % of patients were on antiretroviral therapy and had an undetectable HIV viral load—this created two problems: 1) travel burdens made it difficult for patients to obtain care; and 2) the small HIV specialty clinic lacked the expertise, resources, and systems necessary to provide comprehensive primary care for an aging population.
As part of a quality improvement initiative to address these issues, ICVA developed a telehealth collaborative care (TCC) program for patients with HIV. The goal was to improve the accessibility and comprehensiveness of care for the small, geographically dispersed, and aging population of veterans with HIV in rural Iowa and Illinois. TCC integrated HIV specialty care delivered by clinical video telehealth (CVT) with primary care delivered by generalist providers in VA Community Based Outpatient Clinics (CBOCs) serving rural areas.
The objective of this work is to describe our experience implementing TCC and results of a mixed-methods evaluation of the program, with a focus on qualitative findings from patient interviews that are relevant to other healthcare systems considering similar programs in rural settings.
METHODS
Overview
The quality improvement initiative that created the TCC program at ICVA occurred between September 2009 and May 2012. Quantitative evaluation of the program used a pre-test and post-test design; outcomes included VA performance measures relevant to care for persons with HIV (see below); care satisfaction, and time spent traveling to obtain care. Qualitative evaluation involved semi-structured telephone interviews with patients to elicit their experiences with TCC. We describe this work according to the Standard for Quality Improvement Reporting Excellence (SQUIRE) guidelines for reporting.4
Setting
ICVA includes a main facility in Iowa City and nine CBOCs across Eastern Iowa and Western Illinois. The HIV specialty clinic in the main facility operates one half-day per week with a team composed of a physician specializing in HIV medicine, a clinical pharmacist, and a nurse care manager. In 2009, VA began to redesign primary care delivery in CBOCs according to tenets of the patient-centered medical home model. CBOC personnel were formed into “teamlets” including a primary care provider (internal medicine or family medicine physician, nurse practitioner, or physician’s assistant), a registered nurse care manager, a licensed practical nurse associate, and a clerical associate.
The TCC Intervention
Planning for the TCC intervention began in 2009 during informal conversations with patients who were travelling long distances to the HIV clinic for all of their care. Patients reported that travel burdens often made it difficult to obtain care and expressed interest in care closer to their homes, but had several reasons for bypassing CBOCs. Some were concerned that their local CBOC may not be equipped to handle their care needs, while others worried about loss of privacy and HIV stigma related to care in clinics nearer their homes. Despite these concerns, most patients expressed interest in receiving care in CBOCs, provided they felt secure that their privacy would be protected and that they maintain a direct connection to the HIV specialty clinic team through telehealth.
To follow up on this patient input, members of the HIV clinic team visited each CBOC to discuss the potential for TCC and to request input on program design. After considering several models for collaboration between the HIV clinic and CBOCs, discussion focused on the TCC model to integrate primary care delivered by CBOC teams with HIV specialty care delivered by telehealth. To accomplish this, each scheduled patient visit to the CBOCs would include a face-to-face visit with their local primary care provider, followed by a telehealth visit with the HIV specialty clinic team.
Process maps were completed for patient visits in both CBOCs and the HIV specialty clinic, to determine how face-to-face encounters with CBOC teams and HIV telehealth sessions could be integrated during a single patient visit to the CBOC (integrated process map in online appendix). Due to concerns expressed by patients, each step in the CBOC encounter (e.g. check-in, provider encounters, and laboratory and pharmacy visits) was reviewed with a focus on maintaining privacy. A secure internet-based scheduling tool allowed sequential scheduling of HIV telehealth visits and face-to-face visits with CBOC providers.
Additional discussions with CBOC care teams focused on establishing necessary elements of TCC, including: 1) clear definition of roles for the primary care and HIV specialty teams in co-managed care; 2) processes to coordinate care across sites; and 3) systems to manage care for the population of patients with HIV infection across multiple care sites in the ICVA system (Table 1).
Table 1.
Program Elements
| Program Element | Description |
|---|---|
| Defined roles in co-managed care | |
| - Interactive video conferences involving HIV Specialty and Primary Care Teams | - Review clinical issues in co-management of patients with HIV infection and define specific tasks and team roles in co-management |
| - Personalized patient handout on navigating co-managed care | - Lists members of patient’s HIV specialty clinic and primary care teams and who to contact for various care needs (available in online appendix) |
| Care coordination across sites | |
| - Telehealth care coordination huddles | - Primary care nurse care manager joins patient at end of HIV telehealth visit to discuss issues from that day’s HIV telehealth and primary care visits, and assign tasks for follow up to patient, HIV care team, or primary care team |
| Population management across sites | |
| - Registry | - Electronic registry of patients in care for HIV infection at seven primary care sites automatically populates data relevant to care for HIV infection, common comorbidities, and cardiovascular risk factor management. |
| - Structured telehealth collaborative care notes | - Every 3 months, same member of HIV care team queries registry to identify care tasks and generates note for each patient, assigning tasks to HIV or primary care team. Note is shared across sites in electronic health record |
Defining Clear Roles
CBOC providers requested information about aspects of primary care relevant to co-management of persons with HIV infection, such as frequency of and care for specific comorbidities, and drug interactions involving antiretroviral agents. These topics were reviewed during two follow-up video teleconferences in the initial 3 months of the TCC program. During these conferences, CBOC and HIV clinic staff negotiated roles and responsibilities in co-managed care. For example, the HIV care team would supervise antiretroviral therapy, prophylaxis for opportunistic infections, and discussions about preventing HIV transmission. The CBOC team would supervise other primary and preventive care, such as screening and care for common coexisting conditions (e.g. hypertension, hyperlipidemia, diabetes, smoking cessation, depression, and osteopenia). The nurse care manager in the HIV clinic would triage undifferentiated care issues to the appropriate teams.
In addition, each patient entering TCC received a personalized, two-page brochure on navigating co-managed care, with names and contact information for HIV clinic and CBOC team members, as well as suggestions on who to contact with a list of potential concerns related to HIV therapy, treatment for specific comorbid conditions, or undifferentiated care needs (included in online appendix).
Care Coordination Processes
It was not practical for primary care providers to attend HIV telehealth sessions to ensure care coordination. Instead, we created “telehealth care coordination huddles” during the final 10 min of each HIV telehealth session. Specifically, the nurse care manager from the CBOC primary care team sat with the patient and reviewed, together with the HIV specialty team on videoconference, the specific care plans and medication changes made during that day’s CBOC primary care provider and HIV telehealth visits. In discussion with the patient and HIV team, the CBOC nurse care manager assigned tasks for follow-up after the visit to the HIV care team, CBOC primary care team, and patient.
Population Management Across Sites
Using VA’s Computerized Patient Record System (CPRS) and Corporate Data Warehouse (CDW), we created a registry of all patients in care for HIV in the ICVA system to facilitate population management across sites. For each patient, the registry automatically pulled data relevant to care for HIV infection, common comorbidities, and cardiovascular risk factors. Data included vital signs (i.e. blood pressure and body mass index), selected laboratory values (CD4 count, HIV viral load, lipids, blood glucose, and glycosylated hemoglobin), vaccinations received, and results from screenings routinely performed during CBOC visits [alcohol use by Alcohol Use Disorder Identification Test—Consumption (AUDIT-C) questions,5 depression screening using the Patient Health Questionnaire,6 and tobacco screening and cessation counseling].
Every 3 months, the same member of the HIV clinic team queried this electronic registry to identify care issues in need of action for each patient. Specific care tasks in need of attention were assigned to HIV or CBOC team members, according to previously negotiated care roles, and appropriate HIV clinic or CBOC team personnel were alerted to specific tasks using a structured “TCC tasks note” entered in each patient’s electronic health record prior to their next scheduled visit. This note was available across sites in the shared medical record. Aggregate results for care measures were also examined to identify systematic gaps in care for the TCC population and to inform system redesign.
Patient Population
Patients with HIV infection who lived closer to a CBOC than to the HIV clinic and who had life expectancy greater than 6 months were offered participation in TCC. Following a description of the TCC program, patients chose whether to participate or to continue to travel to the HIV clinic for all care. It was emphasized that they may choose to receive all care in HIV clinic at any point.
Evaluation Methods—Quantitative
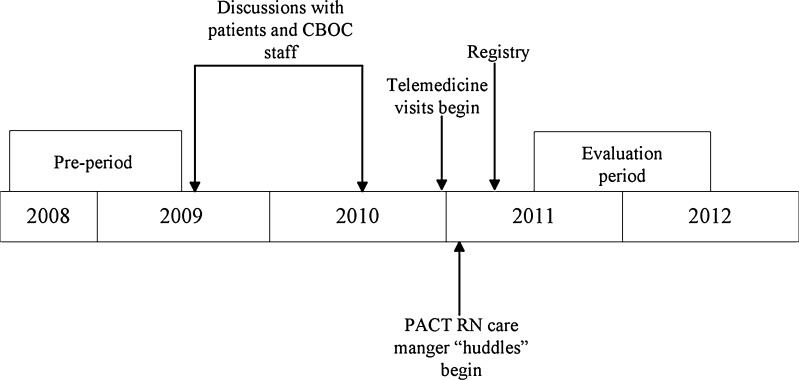
Evaluation occurred between June 1, 2011 and May 31, 2012, following complete implementation of TCC elements (Fig. 1). Measures included: 1) a set of HIV care quality measures tracked in VA;7 2) VA performance measures related to management of cardiovascular risk factors (i.e. hypertension, diabetes, hyperlipidemia, and smoking); 3) frequency of routine screening for alcohol use disorders and depression; 4) satisfaction with TCC visits as determined by VA’s Survey of Healthcare Experiences of Patients (SHEP); and 5) the total time each patient spent traveling to clinic appointments (Table 2).
Figure 1.
Timeline.
Table 2.
Care Measure Description
| Measure | Eligibility | Definition | Responsibility |
|---|---|---|---|
| HIV quality measures | |||
| 1. Retention in care | All | Seen at least twice annually, at least 60 days apart | HIV clinic |
| 2. CD4 measurement | All | CD4 measured at least twice annually | HIV clinic |
| 3. HIV viremia control | ART > 6 months | Last HIV viral load < 50 copies/ml | HIV clinic |
| 4. Syphilis screening | All | Syphilis serology annually | HIV clinic |
| 5. Hepatitis C (HCV) screening | All | HCV serology at least once | PC clinic |
| 6. Hepatitis B (HBV) screening | All | HBV serology panel (sAb, sAg, cAb) at least once | PC clinic |
| 7. Influenza vaccination | All | Vaccine received annually | PC clinic |
| 8. Pneumococcal vaccination | All | Vaccine received once | PC clinic |
| 9. Hepatitis B vaccination | HBV sAb-/sAg- | HBV vaccine first dose received | PC clinic |
| Cardiovascular risk factor measures | |||
| 10. Hypertension control | Hypertension Dx | Last blood pressure < 140/90 | PC clinic |
| 11. Glycemia control | Diabetes Dx | A1C measured in last 6 months and < 9 | PC clinic |
| 12. Lipid monitoring | All | Lipid panel annually | PC clinic |
| 13. Tobacco cessation | All | Annual tobacco screening documented and cessation counseling/pharmacotherapy offered to users | PC clinic |
| Other measures | |||
| 14. Alcohol screening | All | AUDIT-C* performed annually | PC clinic |
| 15. Depression screening | All | Patient health questionnaire (PHQ), or other validated depression screen, performed annually | PC clinic |
| 16 Patient satisfaction | All | Very or completely satisfied with last TCC visit | Both |
| 17. Travel time | All | Estimated yearly total travel time to visits | Both |
ART antiretroviral therapy; sAbs urface antibodyy; sAg surface antigen; PC Primary care
*Alcohol use disorder identification test—consumption questions
We focused on management of cardiovascular risk factors, because of their recognized role in accelerated coronary artery disease among persons with HIV.8 We sought to improve screening for depression and alcohol use disorders, because: 1) these are prevalent, under-recognized, and treatable conditions that influence outcomes among persons with HIV;9,10 2) screening rates in the HIV clinic were low at baseline and few resources existed in the specialty clinic to address this; and 3) there was opportunity to improve screening rates using systems already implemented in CBOCs.
For each patient, total travel time from home to all clinic appointments during the evaluation year was determined using GoogleMap® software and data on the number and site of clinic appointments. The SHEP care satisfaction survey was mailed to patients in September 2011 and April 2012; non-responders received a single follow-up mailing. Patients were asked to consider their most recent TCC visit, and satisfaction was determined using the question “All things considered, how satisfied were you with your visit?—Very satisfied, somewhat satisfied, neither satisfied nor dissatisfied, somewhat dissatisfied, or very dissatisfied."
With exception of the care satisfaction survey, data for all measures were available in the CDW and through chart review during both the 1-year evaluation period and the year before TCC implementation (June 1, 2008–May 31, 2009), the pre-TCC and post-TCC periods (Fig. 1). For patients in care in the ICVA system during both periods, we compared pre-TCC and post-TCC measures using paired sign tests for continuous measures, or McNemar tests for dichotomous measures. Analyses were performed using SAS v. 9.1 (Cary, NC).
Evaluation Methods—Qualitative
All patients participating in TCC in September 2011 were invited to participate in a semi-structured telephone interview to evaluate TCC. The University of Iowa institutional review board (IRB) and Iowa City VA Research and Development Committee approved the study. A research team member contacted patients by phone to explain the interview and obtain informed consent to participate. Interviews were conducted by a qualitatively trained researcher between September and November, 2011.
The interview guide (online appendix) was developed to assess the feasibility of TCC and factors influencing implementation in rural settings. The guide elicited participants’ experience with, perceptions of, and expectations for: shared care; care coordination and role clarity among clinical team(s); use of CVT for HIV specialty visits; stigma and privacy concerns; and the TCC program’s perceived impact on access to HIV specialty care and primary care. These topics were chosen based on the experience of the team implementing TCC and their discussions with patients and primary care providers while designing the program. Additional topics, including questions about cultural shifts associated with HIV and its treatment, were also included in the interview guide. All questions were open-ended, with the exception of questions related to appointment logistics.
Interviews were audio recorded, transcribed verbatim, and audited by the interviewer for accuracy. To create the codebook, a subset of transcripts was independently reviewed by two qualitative researchers, with additional input on clinical issues from the research team’s pharmacist and physician to develop a preliminary list of reoccurring ideas.11 Segments of text representing reoccurring ideas were discussed and compared during in-person coder meetings, then grouped into themes, or codes, representing implicit topics around which reoccurring ideas were organized. Samples of coded text were reviewed regularly among the research team to further delimit themes’ definitions and check for coder reliability; differences in coding text were discussed until consensus was achieved. The codebook was iteratively revised as new themes emerged, either by creating new codes to capture themes at the same level, or to develop sub-codes to further group text in finer-grained categories. The final set of themes was deductively derived based on evaluation domains (e.g., care coordination, stigma, privacy), and inductively derived based on emergent patterns in patient interviews. All changes were tracked in an audit trail. Themes were analyzed for convergent and divergent perspectives and associations. Associations between themes were also identified using MAXQDA software and associated analytical tools (VERBI software; Berlin, Germany). Analysis was concurrent with interviews, which continued until thematic saturation was achieved.
RESULTS
Sample
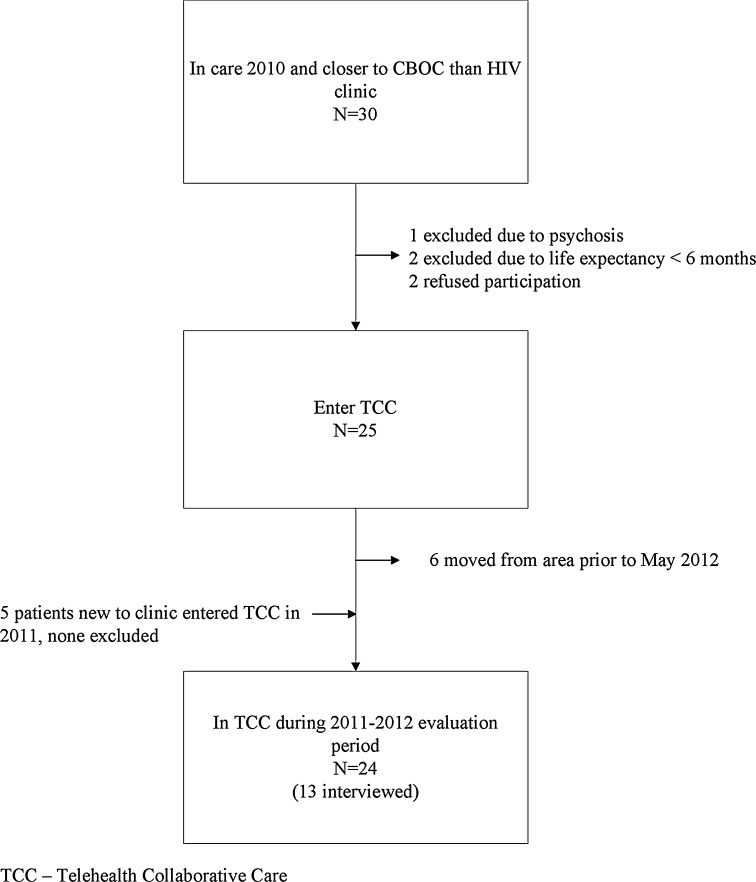
Among 30 patients who lived closer to a CBOC than the HIV clinic at initiation of TCC in 2010, two were excluded due to life expectancy less than 6 months and one due to psychosis and delusions involving television sets that made telehealth inappropriate (Fig. 2). Two were eligible but preferred to travel to the HIV clinic for all care. Six TCC patients moved out of the ICVA service area and five into the area prior to the evaluation period. Overall, 30 patients participated in TCC between 2010 and 2012. None elected to leave TCC and return to traveling to HIV clinic for all care—though this option was offered at all appointments. Twenty-four were in care throughout the 2011–2012 evaluation period, 17 of whom were also in care in ICVA throughout the pre-TCC period.
Figure 2.
Patient participation and evaluation flow chart.
Quantitative Results
Among the 24 patients in care throughout the post-TCC evaluation period, the median age was 54 (range 40–79), and 23 (96 %) were men. Median CD4 count was 707 cells/cm3 (range, 233–1307), and all received antiretroviral therapy throughout the year. Fourteen had a diagnosis of hypertension and five had diabetes. These patients saw 14 primary care providers (eight physicians, four nurse practitioners, and two physician assistants) during 58 TCC visits at seven CBOCs .
Performance on care measures during the post TCC evaluation period exceeded 90 % in all cases (Table 3). More than 90 % of patients were on antiretroviral therapy and had undetectable levels of HIV viremia (< 50 copies/ml) in both pre-TCC and post-TCC periods. Among the 17 patients in care in the ICVA system during both the pre-TCC and post-TCC periods, there was a statistically significant improvement (p < 0.05) in syphilis screening, influenza vaccination, tobacco screening and cessation counseling, and screening for alcohol disorders and depression. Median yearly travel time decreased by 150 min, from 320 min per patient pre-TCC to 170 min post-TCC (p < 0.001). Satisfaction with TCC was high: 14 of 18 patients who returned the satisfaction survey reported that they were very or completely satisfied with their most recent TCC visit.
Table 3.
Care Measure Results
| Pre-TCC (N = 17) | Post-TCC (N = 24) | |||||
|---|---|---|---|---|---|---|
| Measure | N eligible | N met (%) | N eligible | N met (%) | p | |
| HIV Quality Measures | 1. Retention in care | 17 | 13 (76) | 24 | 24 (100) | 0.13 |
| 2. CD4 Measurement | 17 | 14 (82) | 24 | 24 (100) | 0.25 | |
| 3. HIV viremia control | 15 | 15 (100) | 24 | 23 (96) | 0.99 | |
| 4. Syphilis screening | 17 | 6 (35) | 24 | 24 (100) | 0.001 | |
| 5. HCV screening | 17 | 17 (100) | 24 | 24 (100) | — | |
| 6. HBV screening | 17 | 13 (76) | 24 | 22 (92) | 0.5 | |
| 7. Influenza vaccination | 17 | 8 (47) | 24 | 23 (96) | 0.008 | |
| 8. Pneumococcal vaccination | 17 | 15 (88) | 24 | 23 (96) | 0.99 | |
| 9. HBV vaccination | 5 | 2 (40) | 10 | 9 (90) | 0.25 | |
| Cardiovascular Risk Factor Measures | 10. Hypertension control | 10 | 10 (100) | 14 | 14 (100) | — |
| 11. Glycemia control | 4 | 3 (75) | 5 | 5 (100) | 0.99 | |
| 12. Lipid monitoring | 17 | 16 (94) | 24 | 24 (100) | 0.95 | |
| 13. Tobacco cessation | 17 | 5 (29) | 24 | 24 (100) | 0.001 | |
| Other | 14. Alcohol screening | 17 | 3(18) | 24 | 24(100) | < 0.001 |
| 15. Depression screening | 17 | 0(0) | 24 | 24(100) | < 0.001 | |
| 16. Very/completely satisfied with care | — | — | 18 | 16(88) | — | |
| 17. Travel time, minutes, median (IQR) | 17 | 320 (180–594) | 24 | 170 (39–221) | < 0.001 | |
TCC Telehealth Collaborative Care
Qualitative Findings
Among the 24 patients in TCC in September 2011, 13 completed interviews, three declined, three could not schedule interviews during the study period, and five could not be contacted by the study team. Average interview duration was 35 min. Qualitative findings encompassed the full range of topics explored in the interview guide. We present three topics from interviews that are particularly relevant to patients, and inform efforts to implement TCC in other rural settings: 1) role of stigma and privacy concerns in implementation; 2) impact on access, continuity, and care coordination; and 3) role of HIV specialists in collaborative care.
Stigma and Privacy Were not Barriers to TCC Implementation
Concerns regarding HIV stigma and privacy were prominent in patients’ daily lives, but were not significant barriers to participation in TCC. Many patients withheld their HIV status from their family, social networks, or places of employment. Although some patients initially had concerns about expanding the number and locations of care teams aware of their HIV infection, these concerns mostly resolved once in TCC. Patients frequently used the term “professional” to describe VA staff, albeit with different connotations: clinicians were described as professionals who protect privacy and are nonjudgmental on the one hand, and legally bound to uphold patient privacy on the other. Many also reported that the TCC appointment process was structured in a way to protect privacy.
Prior to participating in TCC, many patients reported seeing non-VA primary care providers closer to their homes. In some cases, these providers were unaware of their HIV infection. The integration of a primary care provider in TCC allowed these patients to openly discuss all health issues with a single, local provider for the first time.
Access, Continuity, and Care Coordination
Patients described a trade-off with TCC: improved access to care at the expense of decreased continuity of care and occasional difficulties with care coordination related to working with healthcare teams at two sites. With the exception of one patient, the convenience of traveling to a local CBOC for appointments clearly outweighed issues related to continuity and coordination of collaborative care.
Patients indicated a number of ways access improved with TCC: time spent traveling and away from work decreased; travel was less stressful and costly; and the wait time before visits at CBOCs was typically shorter than at the specialty clinic.
Despite efforts to define and communicate roles of all providers in TCC, patients experienced occasional ambiguity about the roles of HIV clinic and CBOC primary care teams. Patients’ attributed most problems with care coordination to perceived role confusion among providers regarding who should respond to urgent health issues. In rare cases, care for urgent health issues was delayed. The way patients conceptualized HIV infection relative to other chronic conditions also contributed to unclear role boundaries; HIV was described as intertwined with, not distinct from, other health issues.
Many recounted instances where they were aware that their primary and specialty care providers communicated about their care. An important benefit observed by these patients was that TCC provided a sense of a coordinated team of providers delivering more personalized care, compared with patients navigating disparate providers on their own. One participant explicitly noted that the transparency of care afforded by the telehealth care coordination huddles increased his trust in his providers. Patients also reported relying heavily on telephone contact with the HIV clinic nurse care manager when they were uncertain about how to obtain needed care or when there were problems in care coordination.
Specialty Care Access Remains Important
Despite the inconvenience of seeing multiple providers, most patients reported that ongoing, direct access to their HIV specialist via telehealth was important. Most were not comfortable seeing the CBOC primary care provider for all care, even if this provider was in constant communication with the HIV specialist. Although many patients were on well tolerated, once-daily antiretroviral regimens and maintained high CD4 counts, they perceived their HIV care as complex and rapidly evolving. Most did not believe it was reasonable to expect CBOC providers to maintain expertise in the complexities of HIV therapy and recent advances in research. Patients also expressed an interest in periodically discussing advances in HIV research directly with their specialist.
DISCUSSION
This mixed-methods evaluation of a quality improvement program demonstrates that telehealth collaborative care (TCC) is a feasible approach for delivering comprehensive and accessible healthcare for persons with HIV in rural and low prevalence settings. TCC was well accepted, with 30 of 32 eligible patients choosing to participate over 2 years. TCC maintained the high quality of HIV care that previously existed in the specialty clinic, as evidenced by high rates of HIV viremia control, while reducing the amount of time patients spent traveling for care.
Three findings from patient interviews are particularly relevant for other healthcare systems considering TCC and suggest potential best practices when implementing TCC programs. First, when patient concerns regarding HIV stigma and privacy in rural primary care clinics are openly discussed with patients and explicitly addressed in program design, they do not impede patient participation in TCC. Second, the nurse care manager in the HIV specialty clinic provided essential assistance to patients in navigating the TCC system and coordinating care between sites. Patients also valued their participation in the telehealth care coordination huddles, which included the HIV care team and the nurse care manager from the primary care site. Other systems establishing TCC should prioritize roles for such nurse care managers and consider incorporating telehealth care coordination huddles.
Third, patients perceived a need for routine, direct communication with the HIV specialty care team by telehealth. We initially considered several models for collaboration between the HIV specialty clinic and primary care providers, including one that asked primary care teams to provide all care, with access to structured consultation with the HIV specialist outside of patient visits. This resembles the Enhancing Community Health Outcomes (ECHO) model for persons with hepatitis C infection in rural New Mexico.12 We did not pursue this model because our patients stated during early, informal discussions that they preferred to maintain a routine, direct relationship with the HIV specialty clinic via telehealth. This message was repeated in formal interviews with patients in TCC.
In the roughly 15 years since effective treatment became available for HIV infection, patients have received a strong message that HIV care is technically complex and that ongoing specialist involvement is essential.13 As HIV therapy becomes progressively less complex, this perceived need for routine, direct specialist contact may decrease.14 In the meantime, systems establishing collaborative care models for persons with HIV in rural settings should consider that some patients will likely desire ongoing visits with HIV specialists via telehealth.
The TCC model relied heavily on resources available in VA, but which may not exist in other systems serving rural areas. These included a single electronic health record for specialty and primary care sites across a large region, extensive telehealth infrastructure, and universal access for all patients regardless of insurance status. This limits the generalizability of the TCC model outside VA. On the other hand, ongoing reforms in US healthcare, such as creation of accountable care organizations (ACOs) and interoperable electronic health records with regional data exchanges, may make TCC more broadly achievable in rural and low prevalence settings.15
This study has limitations. The sample size was relatively small, which is an inherent aspect of studies of care for rare conditions in rural settings, and there was no “usual care” control group. We mostly examined process measures of care (e.g. rates of tobacco screening and cessation counseling) as opposed to outcome measures (e.g. tobacco quit rates), as this small study lacked power to demonstrate changes in outcomes. Multisite, controlled trials including much larger numbers of patients and more patient-oriented outcomes are necessary to determine the relative cost and effectiveness of alternate strategies for delivering HIV care in rural settings. Finally, TCC patients had generally well-preserved immune function as reflected by high CD4 counts; the TCC model may be less appropriate for patients with advanced Acquired Immune Deficiency Syndrome (AIDS).
CONCLUSION
TCC is a feasible approach to delivering accessible and comprehensive care for persons with HIV infection in rural settings.
Electronic Supplementary Materials
(DOCX 410 kb)
Acknowledgments
The work reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Rural Health, Veterans Rural Health Resource Center—Central Region. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the United States government.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
REFERENCES
- 1.Handford CD, Tynan AM, Rackal JM, Glazier RH. Setting and organization of care for persons living with HIV/AIDS. Cochrane Database Syst Rev. 2006;3:CD004348. doi: 10.1002/14651858.CD004348.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McInnes K, Landon BE, Malitz FE, et al. Differences in patient and clinic characteristics at CARE Act funded versus non-CARE Act funded HIV clinics. AIDS Care. 2004;16:851–857. doi: 10.1080/09540120412331290202. [DOI] [PubMed] [Google Scholar]
- 3.Schur CL, Berk ML, Dunbar JR, Shapiro MF, Cohn SE, Bozzette SA. Where to seek care: An examination of people in rural areas with HIV/AIDS. J Rural Health. 2002;18:337–347. doi: 10.1111/j.1748-0361.2002.tb00895.x. [DOI] [PubMed] [Google Scholar]
- 4.Ogrinc G, Mooney SE, Estrada C, et al. The SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines for quality improvement reporting: Explanation and elaboration. Qual Saf Health Care. 2008;17(Suppl 1):i13–i32. doi: 10.1136/qshc.2008.029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinert DF, Allen JP. The alcohol use disorders identification test: An update of research findings. Alcohol Clin Exp Res. 2007;31:185–199. doi: 10.1111/j.1530-0277.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- 6.Meader N, Mitchell AJ, Chew-Graham C, et al. Case identification of depression in patients with chronic physical health problems: A diagnostic accuracy meta-analysis of 113 studies. Br J Gen Pract. 2011;61:e808–e820. doi: 10.3399/bjgp11X613151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Backus LI, Boothroyd DB, Phillips BR, et al. National quality forum performance measures for HIV/AIDS care: The Department of Veterans Affairs’ experience. Arch Intern Med. 2010;170:1239–1246. doi: 10.1001/archinternmed.2010.234. [DOI] [PubMed] [Google Scholar]
- 8.Triant VA. HIV infection and coronary heart disease: an intersection of epidemics. J Infect Dis. 2012;205(Suppl 3):S355–S361. doi: 10.1093/infdis/jis195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asch SM, Kilbourne AM, Gifford AL, et al. Underdiagnosis of depression in HIV: Who are we missing? J Gen Intern Med. 2003;18:450–460. doi: 10.1046/j.1525-1497.2003.20938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Justice AC, Lasky E, McGinnis KA, et al. Medical disease and alcohol use among veterans with human immunodeficiency infection: A comparison of disease measurement strategies. Med Care. 2006;44:S52–S60. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- 11.Bernard H, Ryan G. Analyzing qualitative data: Systematic approaches. Thousand Oaks: Sage Publications; 2010. [Google Scholar]
- 12.Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199–2207. doi: 10.1056/NEJMoa1009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz MH. Human immunodeficiency virus is (once again) a primary care disease. Arch Intern Med. 2011;171:719–720. doi: 10.1001/archinternmed.2011.130. [DOI] [PubMed] [Google Scholar]
- 14.McKinnell JA, Willig JH, Westfall AO, et al. Antiretroviral prescribing patterns in treatment-naive patients in the United States. AIDS Patient Care STDS. 2010;24:79–85. doi: 10.1089/apc.2009.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emanuel EJ. Why accountable care organizations are not 1990s managed care redux. JAMA. 2012;307:2263–2264. doi: 10.1001/jama.2012.4313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 410 kb)