Abstract
BACKGROUND
Few longitudinal studies have examined associations between body mass index (BMI) changes in adults with diabetes and the development of disability.
OBJECTIVE
To investigate association patterns between BMI and disability in middle-aged adults with diabetes.
DESIGN AND SETTING
Retrospective cohort design with data from the 1992–2006 Health and Retirement Study (HRS). A group-based joint trajectory method identified distinct BMI change trajectories and their link to subsequent disability trajectories.
PARTICIPANTS
U.S. nationally representative adults aged 51–61 who reported a diagnosis of diabetes in the 1992 HRS (N = 1,064).
MEASUREMENTS
BMI and self-reported disability score were the main variables. Sociodemographic, clinical, behavioral, and diabetes-related factors were also examined.
RESULTS
Four distinct weight trajectories (stable normal weight, 28.7 %; stable overweight, 46.2 %; loss and regain obese, 18.0 %; weight cumulating morbidly obese, 7.1 %) and three disability trajectories (little or low increase, 34.4 %; moderate increase, 45.4 %; chronic high increase, 20.2 %) best characterized the long-term patterns of BMI and disability change in middle-aged adults with diabetes. Adults in stable normal weight had the highest probability of being in the little/low increase disability group; however, one in five adults in that group progressed into chronic high disability, a higher proportion compared to the stable overweight group.
CONCLUSIONS
Although there were various ways in which the two trajectories were linked, the beneficial impacts of optimizing weight in adults with diabetes were supported. In addition, the complexity of diabetes control in those with relatively normal weight was highlighted from this study.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-013-2399-z) contains supplementary material, which is available to authorized users.
KEY WORDS: weight, physical function, diabetes, group-based modeling
Overweight and obesity, defined by body mass index (BMI, kg/m2) of 25.0–29.9 and 30.0 and above, respectively,1 is common in adults with diabetes. Despite acknowledged difficulties with losing weight and maintaining weight loss, current clinical practice guidelines for diabetes and diabetes researchers continue to emphasize the importance of weight management in adults with diabetes.
Research in clinical and community settings has examined general weight change patterns in adults living with diabetes over time, yielding inconsistent findings2–7 and suggesting that the longitudinal course of body weight in middle-aged and older adults with diabetes exhibits not only intra-individual but also inter-individual variation.
Only recently have researchers begun to more explicitly examine distinct weight trajectories in adults with diabetes.8,9 However, these studies are limited by relatively short periods of follow-up (1 and 3 years, respectively); thus, weight fluctuations10 may not be well-detected. In addition, use of medical records rather than population-based data may not capture variations in demographically and geographically heterogeneous adults with diabetes. Further, few studies have investigated how weight changes longitudinally in relation to the development of disability, a key determinant of quality of life in adults with diabetes. In one study of adults (not limited to those with diabetes), Kahng and colleagues11 found that although obesity was associated with more functional disability in cross-sectional analyses, change in BMI was not related to change in physical function over time. We argue that the simultaneous measure of BMI and disability in their study may have obscured the real (or lagged) association between change in BMI and change in disability. In another study that examined the lagged effect of BMI on disability, Ferraro and colleagues12 found that disability risk was higher for obese persons, but that overweight was not consistently associated with higher disability. Whether this pattern exists in adults living with diabetes is not yet known.
The current study aimed to fill these gaps by examining longitudinal data on BMI and disability in a representative sample of U.S. adults aged 51–61 diagnosed with diabetes. We use a dual trajectory model within a group-based trajectory modeling approach (a.k.a., latent class growth model [LCGM])13–15 to evaluate 10-year weight trajectory patterns from 1992 to 2002 and the patterns’ associations with disability trajectories in years 10 to 14 (2002–2006). Three research questions were posed: (1) What are the main patterns of weight and disability trajectories experienced by middle-aged and older adults living with diabetes?; (2) What is the proportion of each trajectory in the population?; and (3) How are weight trajectories associated with disability trajectories later in life, as well as baseline sociodemographic, clinical, behavioral, and diabetes-related factors?
METHODS
Data and Sample
This is a retrospective cohort study, with data drawn from the Health and Retirement Study (HRS), a large contemporary nationally representative samples of middle-aged and older adults for nearly two decades, first interviewing 9,760 adults age 51–61 in 1992 (born 1931–1941), with oversamplings of African Americans, Hispanics, and Florida residents, and biennial follow-up interviews. Individuals who moved to institutions were also tracked. Further details of the HRS mission and administration are described elsewhere.16
For this study, we included the full sample of participants who self-reported that they had been told by a doctor they had diabetes or high blood sugar in the 1992 baseline interview (N = 1,064). These adults were on average 56.0 years of age (SD = 3.2) and nearly half (46.2 %) men; 66.4 % self-identified as non-Hispanic White, 28.0 % as non-Hispanic African American, and 5.6 % as Hispanic or other. The majority (73.2 %) reported their level of schooling as high school or less, and 26.8 % some college or above. Mean BMI at baseline was 30.2 (SD = 6.5). Mean years since diagnosis of diabetes was 8.7 (Median = 6.0, SD = 8,.9) with nearly one in ten (9.2 %) reporting they had been diagnosed in 1992. Treatment types were 34.2 % diet alone, 39.7 % oral therapy, and 26.1 % insulin only or in combination with other regimens. More than two in three adults (68.5 %) were diagnosed with diabetes at age 45 or above.
Because our analyses were conducted using maximum likelihood estimation, all participants were included in the present analyses, regardless of their missing data patterns. From 1994 to 2006, the retention rates of the original 1,064 participants in each wave were 92 %, 82 %, 76 %, 66 %, 63 %, 58 %, and 53 %, respectively; 559 had complete data on all study variables at the last measurement point in 2006. Those who completed the 2006 interview did not differ statistically from those who did not on race/ethnicity, education level, years after diagnosis of diabetes, and late or early onset diabetes. However, they were significantly younger (55.7 vs. 56.4 years of age), more female (56.3 % vs. 49.9 %), had higher mean BMI (30.7 vs. 29.7), reported being treated with diet alone (39.9 % vs. 27.9 %), and lower disability (2.43 vs. 3.50) at baseline, than did adults who did not complete the 2006 interview. To confirm that missing data did not bias trajectory parameter estimates, the dual trajectories were also estimated with a reduced sample (N = 559), in which participants with full data points from 1992 to 2006 on each variable were included. The results of the reduced sample analysis were equivalent to those with the full sample analyses; thus, all analyses used the full sample (N = 1,064), with 6,172 data points analyzed.
Measures
Six measures of body mass index (BMI, kg/m2), calculated by dividing a participant’s self-reported weight in kilograms and the square of their height in meters from 1992 to 2002, were obtained for this study.
HRS participants were asked at each wave if they had any difficulty performing a certain task, avoided it, or needed help or equipment for its performance in Activities of Daily Living (ADLs: bathing, dressing, eating, walking across a room, getting in/out of bed, and using a toilet independently), Instrumental Activities of Daily Living (IADLs: preparing meals, shopping, managing money, using the telephone, and using a map), and strength and mobility activities (walking several blocks, climbing several flights of stairs, stooping/kneeling/or crouching, reaching above the head, and lifting or carrying weights over 10 lb like a heavy bag of groceries) (1 = yes, 0 = no). The sixteen items were summed to obtain a disability score, with higher scores indicating more disability (range 0–16). This composite measure, which captures a broad range of disability from early or “preclinical” disability to later personal care disability, has the advantage of capturing finer graduations in limitations and reducing ceiling or flooring effects.17 Cronbach’s alpha of the 16 items across all waves averaged 0.90.
Several sociodemographic, clinical, behavioral, and diabetes-related variables associated with both weight and disability were examined as covariates at each wave. Sociodemographic variables included age (centered at the grand mean), sex (1 = male, 0 = female), race/ethnicity (0 = non-Hispanic White, 1 = non-Hispanic Black, 2 = Hispanic and others), and educational level (1 = at least some college, 0 = high school or less). Clinical conditions included dichotomous measures (1 = yes, 0 = never) of self-reported physician diagnosed cancer, lung disease, heart attack, arthritis, kidney/bladder problem, psychiatric disorder (including emotional, nervous, or psychiatric problems), high cholesterol, stroke, and fracture/break bone after 45. Behavioral factors included exercise (5 = 3 or more times a week, 4 = 1–2 times a week, 3 = 1–3 times a month, 2 = less than once a month, 1 = never), being a current smoker (1 = yes, 0 = no), and drinker (1 = yes, 0 = never). Diabetes-related factors included diabetes duration (i.e., years after diagnosis of diabetes), diabetes onset type (1 = late onset diabetes, 0 = age at diagnosis of diabetes earlier than 45), and diabetes treatment type (1 = diet alone, 2 = oral medication, 3 = insulin or combination of others).
Statistical Analyses
Given our interest in exploring the nature and extent of heterogeneity in developmental patterns of weight and disability experienced by adults with diabetes, we used a group-based semi-parametric mixture modeling approach performed with SAS TRAJ in SAS Version 9.2 (SAS Institute, Cary, NC).13 This technique allows for the analysis of unbalanced and nested data and assigns each individual to a latent class with a developmental profile that best fits their trajectory.
First, distinct classes (i.e., groups) of trajectories and the shape of each trajectory were identified separately for BMI and disability. We followed Jones and Nagin13,18 by considering both the index of maximum Bayesian Information Criterion (BIC) and the substantive importance of the groups (e.g., parsimony, group size, and standard errors). The distinctiveness of each of the trajectory classes were also ensured by Wald tests13. Detailed model selection procedure in this step can be found in Table 1 of the online appendix.
Dual trajectory modeling13 linking conditional probabilities of membership across trajectory groups was then employed. We estimated the probability of membership in each class of disability, conditional on membership in a given trajectory for body weight, and adjusted individual trajectories for empirically evident covariates, such as age and covariates suggested by univariate tests of Chi-square and ANOVA.
RESULTS
Longitudinal Trajectories of BMI and Disability in Adults with Diabetes
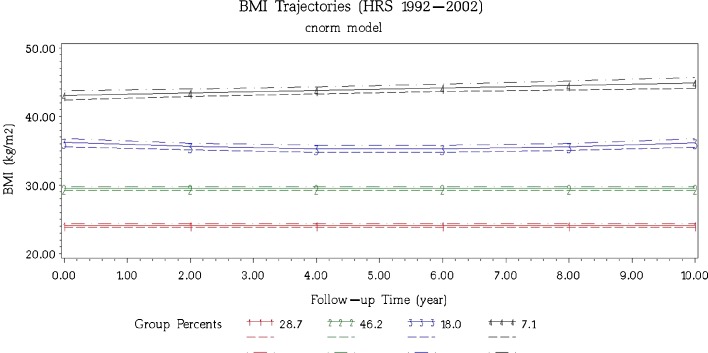
Our 10-year BMI data on a nationally representative sample of 51–61 year-old adults with diabetes detected four distinct weight change trajectories (Fig. 1): the “stable normal weight” adults (28.7 %) were characterized by no significant weight change during the entire follow-up period (BMI = 24.1 kg/m2); the “Stable overweight” adults (46.2 %) who were consistently overweight across the 10 years and without significant weight loss or weight gain (mean BMI = 29.5 kg/m2); the “loss and regain obese” group (18.0 %) lost weight during the initial follow-ups, regain weight in the second half, and ended with a mean weight near that at baseline (mean BMI = 36.2 kg/m2); and the “weight cumulating morbidly obese” group (7.1 %) experienced a linear increase in BMI over time, starting off with a BMI = 43.1 kg/m2 and gaining weight at a rate of about 0.2 kg/m2 per year over the 10-year period.
Figure 1.
The four dominant BMI trajectories during a 10-year follow-up in U.S. middle-aged adults with diabetes from the 1992 Health and Retirement Study. Participants were 51–61 years old at baseline. The solid lines are the mean values of BMI for members in that class. Dashed lines represent 95 % confidence intervals. Group 1: stable normal weight (28.7 %; BMI = 24.098 + 0 year + 0 year2); Group 2: stable overweight (46.2 %; BMI = 29.516 + 0 year + 0 year2); Group 3: loss and regain obese (18.0 %; BMI = 36.210–0.369 year + 0.036 year2); Group 4: weight cumulating morbidly obese (7.1 %; BMI = 43.106 + 0.179 year + 0 year2).
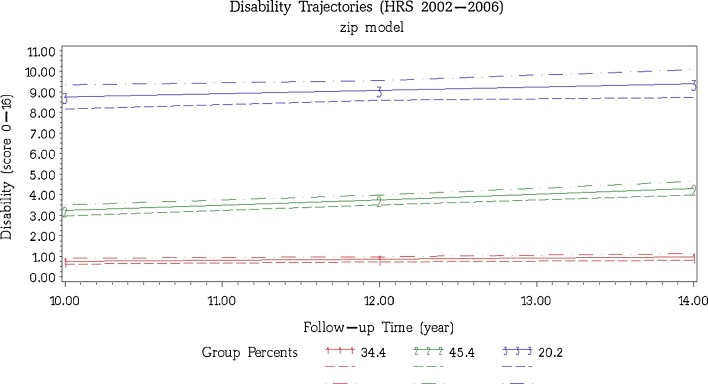
Participants’ physical disability during the 10th to 14th year follow-up was best characterized by three trajectories (Fig. 2). The two largest trajectory groups, accounting for nearly 80 % of the sample, were characterized by low levels of and slightly increasing rates of disability (“little/low increase,” 34.4 %) and modest levels of disability following a pattern of rising disability over time (“moderate increase,” 45.4 %). The “chronic high” group (20.2 % of the sample) represented adults with high levels of disability over the entire period. Parameters of the trajectories of BMI and disability are provided in Table 1.
Figure 2.
The three dominant disability trajectories during the 10th to 14th year follow-up in U.S. middle-aged adults with diabetes from the 1992 Health and Retirement Study. Participants were 51–61 years old at baseline. The solid lines are the mean amount of disability for members in that class. Dashed lines represent 95 % confidence intervals. Group 1: little/low increase (34.4 %; Disability = exp [−0.882 + 0.060*year]); Group 2: moderate increase (45.4 %; Disability = exp [0.462 + 0.071*year]); Group 3: chronoc high (20.2 %; Disability = exp [1.989 + 0.018*year]).
Table 1.
Estimated Parameters in Group-Based Dual Trajectory Model (Estimated Group Percentage in Parenthesis)
| Parameter | Coefficients | Standard error | |
|---|---|---|---|
| BMI (kg/m2) | |||
| Stable normal weight (28.7 %) | Intercept | 24.098*** | 0.132 |
| Stable overweight (46.2 %) | Intercept | 29.516*** | 0.130 |
| Loss and regain obese (18.0 %) | Intercept | 36.210*** | 0.310 |
| Slope | −0.369** | 0.114 | |
| Quadratic | 0.036** | 0.011 | |
| Weight cumulating morbidly obese (7.1 %) | Intercept | 43.106*** | 0.336 |
| Slope | 0.179*** | 0.054 | |
| Disability | |||
| Little or low increaser (34.4 %) | Intercept | −0.882* | 0.374 |
| Slope | 0.060* | 0.030 | |
| Moderate increaser (45.4 %) | Intercept | 0.460** | 0.146 |
| Slope | 0.071*** | 0.012 | |
| Chronic high (20.2 %) | intercept | 1.989*** | 0.142 |
| Slope | 0.018 | 0.012 | |
*p < 0.05, **p < 0.01, ***p < 0.001. The raw mean BMI and disability score for each trajectory at each time point was provided in Table 2 of the supplemental appendix on line. Because the BMI measure is continuous with approximately normal distribution and the disability scores are close to zero-inflated, we used censored normal model for BMI and ZIP model for disability score
Demographic, Clinical, Behavioral and Diabetes-Related Variables in Association with BMI Trajectories
As shown in Table 2, adults in the four weight trajectories were significantly different on sex, race/ethnicity, health conditions (e.g., heart attack, arthritis, kidney/bladder problem, psychiatric disorder), exercise, diabetes duration, diabetes onset type, and anti-diabetic treatment type. For example, compared to the other groups, the weight cumulating morbidly obese group had significantly higher proportions of women, adults who were Black, and reported heart attack, arthritis, kidney/bladder problems, and psychiatric disorders, as well as use of insulin at baseline and lower level of exercise. The stable normal weight group was characterized by the lowest proportion of adults with late onset diabetes, longer duration of diabetes, and more exercise.
Table 2.
Baseline Characteristics of Individuals in Four Dominant BMI Trajectories
| Stable normal weight | Stable overweight | Loss & regain obese | Weight cumulating morbidly obese | p | |
|---|---|---|---|---|---|
| n (%) | 305 (28.7) | 491 (46.2) | 192 (18.0) | 76 (7.1) | |
| Age (years) | 56.1 ± 3.2 | 56.1 ± 3.2 | 55.7 ± 3.2 | 55.6 ± 3.4 | 0.21 |
| Female | 50.2 | 48.8 | 58.8 | 81.6 | < 0.001 |
| Race/ethnicity | |||||
| White | 66.6 | 70.9 | 59.4 | 52.6 | 0.002 |
| Black | 25.1 | 23.9 | 36.9 | 44.7 | < 0.001 |
| Hispanic/others | 8.4 | 5.2 | 3.7 | 2.6 | 0.075 |
| Education (at least some college) | 27.8 | 27.9 | 23.0 | 25.0 | 0.58 |
| Cancer | 8.4 | 6.6 | 4.8 | 9.2 | 0.40 |
| Lung disease (exclude asthma) | 12.0 | 11.2 | 13.9 | 15.8 | 0.59 |
| Heart attack | 25.4 | 21.9 | 27.8 | 39.5 | 0.008 |
| Arthritis | 39.8 | 49.4 | 63.1 | 73.7 | < 0.001 |
| Kidney/bladder problem | 21.1 | 15.7 | 25.1 | 29.0 | 0.005 |
| High cholesterol | 29.4 | 29.3 | 26.7 | 38.2 | 0.33 |
| Ever had Stroke | 9.7 | 6.8 | 4.8 | 7.9 | 0.21 |
| Fracture/break bone after 45 | 16.4 | 12.2 | 15.0 | 11.8 | 0.35 |
| Psychiatric disorder | 13.7 | 14.7 | 9.6 | 28.9 | < 0.001 |
| Exercise | 4.0 ± 1.4 | 3.7 ± 1.5 | 3.3 ± 1.7 | 3.3 ± 1.7 | < 0.001 |
| Diabetes duration | 10.7 ± 10.4 | 7.8 ± 7.5 | 7.8 ± 7.6 | 8.8 ± 7.8 | < 0.001 |
| Late onset diabetes | 59.9 | 73.5 | 69.3 | 67.6 | 0.001 |
| Treatment type | < 0.001 | ||||
| Diet alone | 41.1 | 35.1 | 25.7 | 22.4 | < 0.001 |
| Oral therapy | 31.8 | 44.4 | 40.1 | 38.2 | 0.006 |
| Insulin therapy | 27.1 | 20.5 | 34.2 | 39.5 | < 0.001 |
Data reported as % or means ± SD
Linking BMI Trajectories to Disability Trajectories
As presented in Table 3, nearly half (48 %) of the stable normal weight group were linked to the little or low increase disability group, whereas one in three (32 %) belonged to the moderate increase disability group, and one in five (20 %) to the chronic high disability group, net of age, sex, race/ethnicity, diabetes duration, diabetes onset type, baseline disability score, time-varying comorbidities, exercise, and diabetes treatment type. For the stable overweight group, 40 % of the adults were linked to the little or low increase disability group, nearly half (46 %) were linked to the moderate increase disability group, and the remaining 13 % experienced chronic high disability. A substantial majority (64 %) in the loss and regain obese group belonged to the moderate increase disability group, 26 % in the chronic high disability group, and 10 % in the little or low increase group. Finally, nearly all of those in the weight cumulating morbidly obese group belonged to either the chronic high disability group (51 %) or the moderate increase disability group (47 %). Detailed effects of covariates in the dual trajectory modeling can be found in Table 3 of the online appendix.
Table 3.
Probability of Disability Group Membership Conditional on BMI Group Membership
| BMI (kg/m2) Group 1992–2002 | Disability Group 2002–2006 | ||
|---|---|---|---|
| Little or low increaser | Moderate increaser | Chronic high | |
| Stable normal weight | 0.48*** | 0.32*** | 0.20*** |
| Stable overweight | 0.40*** | 0.46*** | 0.13*** |
| Loss and regain obese | 0.10*** | 0.64*** | 0.26*** |
| Weight cumulating morbidly obese | 0.01 | 0.47*** | 0.51*** |
***p < 0.001. BMI groups were determined by six biennial data collection from1992–2002, and disability groups were determined by three biennial data collection from 2002 to 2006. Probabilities were adjusted for the following covariates: age, sex, race/ethnicity, baseline disability, diabetes duration, diabetes onset type, and time dependent comorbidities, exercise, and diabetes treatment type
DISCUSSION
This is the first work examining a 10-year BMI record in a U.S. nationally representative sample of 51–61 year-old adults with diabetes and their disability in the 10th to14th year follow-up. By employing group-based dual trajectory modeling that sorts various developmental experiences into different types, our findings may shed new light on typical trajectories of weight in adults with diabetes, as well as the predictors and consequences of those trajectories.
Our 10-year BMI results that the majority (74.9 %) of adults with diabetes from age 51–61 to age 61–71 did not change their BMI are consistent with past studies, indicating that 76.1 % of adults who received a new diagnosis of type 2 diabetes maintained a stable weight over a 3-year follow-up.9 The current study also provides new evidence that the probability of stable weight maintenance in adults with diabetes over 10 years is highest in those who were initially of normal weight (28.7 %) or overweight (46.2 %).
Study results highlight the challenges of losing and regaining weight in obese adults. Prior studies of weight management programs for adults with diabetes10,19 show that weight regain is common during a 1-year follow-up period; we found that more than one in six middle-aged adults with diabetes experienced weight loss and regain over 10 years.
In the UK Prospective Diabetes Study (UKPDS), weight gain was higher in patients who were already more than 120 % of their ideal body weight.2 We found that adults with BMIs above 40 at baseline tended to gain weight rather than lose weight over 10 years. In addition, we found that higher proportion of insulin users in this group than in other weight trajectory groups, supporting previous research linking insulin use and weight accumulation.3,20 Since obesity and weight gain are risk factors for diabetes prognosis, more focus should be placed on constructing coping strategies to counteract insulin-related weight gain, especially in obese adults with diabetes.
Prior studies have pointed out that anti-hyperglycemic therapies,6 duration of diabetes,5 and sex7 may affect weight change in diabetes patients; results from the present study support and extend previous findings. The weight cumulating morbidly obese group was disproportionately female, members of minority groups, as well as reporting greater comorbid health conditions and being treated with insulin, compared to other groups. In contrast, the stable normal weight group comprised a relatively high percentage of adults with a longer duration of diabetes.
Adults in the stable normal weight group were more likely to be in the group with little or low increasing disability. Conversely, individuals in the weight cumulating morbidly obese group were most likely to be in the chronic high disability group. Although this study did not test the causal relationships between BMI change and disability, the reverse relationship of probabilities of BMI groups conditional on disability groups were tested (see Tables 4 and 5 in the online Appendix). Unlike the clear information that emerged from linking BMI and subsequent disability, disability trajectories do not give distinct patterns of future BMI trajectories. These results suggest that optimizing weight in a normal BMI range may serve a protective function and guard against progressive disability.
It should be noted, however, that although members in the stable normal weight group had the highest probability of being in the little or low increase disability group, more than one in five adults in the stable normal weight group were linked to chronic high disability, a higher proportion than for stable overweight adults. It is possible that this finding may be confounded with adherence to medication. For example, people who do not gain weight may be those who are not adhering to diabetic medications (e.g., insulin use),20 thus leading to greater disability. However, since there is increasing evidence that in older persons with longer diabetes duration, increases rather than decreases in weight may be in better diabetes control21–23 and normal or low BMI in older adults may be associated with greater disease burden24 or mortality25, it may be more likely that stable normal weight actually masks disease burden.
Despite their longitudinal overweight pattern, 40 % of stable overweight members enjoyed little or no disability, only 8 % lower than for those in the normal weight group. This finding echoes a previous study in a sample of adults of all ages and not limited by health conditions, which found that being overweight was not consistently associated with higher disability12. The reasons may be analogous to the “effects of life-threatening disease on behavioral change,” which has been found in the link of heart attack and smoking cessation behavior26. For instance, overweight individuals may be more conscious about their health outcomes, and thus may pay more attention to health information and be willing to adopt a healthy lifestyle, resulting in maintenance of good physical function.
Our study has limitations. First, as in all longitudinal studies, there was attrition in the sample over time. Although comparison of individuals who did and did not participate in all eight waves of the study revealed no significant differences in race/ethnicity, education level, years after diagnosis of diabetes, and late or early onset diabetes, those who did complete all eight waves were younger in age, more women, had higher mean BMI, used diet alone to manage their diabetes, and reported lower disability. Thus, the results may not generalize to older men, those with lower mean BMI, users of more intensive diabetic therapies, or those who presented with higher disability at baseline.
Our results also share limitations for research based on secondary and self-reported data. Although the validity of self-reported diabetes was proven to be highly accurate in the U.S.27, and the validity of self-reported weight and height for calculating BMI was acceptable as an epidemiologic tool,28,29 there is increasing evidence that the accuracy of self-reported weight and height tends to vary by self-reporter characteristics.30,31 For example, obese persons tend to under-report their weight while underweight persons tend to overestimate it; thus, our BMI change trajectories in adults with diabetes may be afflicted with “regression to the mean.” In addition, the study participants were identified based on HRS question “Have you ever been told by a doctor you have diabetes or high blood sugar?” Since high blood sugar does not necessarily indicate diabetes, our sample may be contaminated with individuals who only have pre-diabetes. Further, although self-reported disability identifies a broad range of disability in older age32, previous research has shown that performance-based and self-reported measures of disability may not measure the same construct33. It may be preferable for future research to adopt objective measures in assessing diabetes, BMI, and disability.
Our study linking BMI and subsequent disability controlled for baseline sociodemographics, and time dependent clinical, behavioral, and diabetes-related variables. The lack of variables such as amputation, menopause status, type of diabetes, or HbA1c level in the current analysis, due to data availability, may fail to control bias due to these life events or to identify possible differential patterns between type 1 and type 2 patients. We suggest that future research depicting the relationship between BMI changes and disability include a more comprehensive set of confounding/moderating variables in data collection and analysis.
In summary, our data suggest that neither BMI nor disability in middle-aged and older adults with diabetes follows fixed patterns of change over time. Four distinct weight trajectories (stable normal weight, 28.7 %; stable overweight, 46.2 %; loss and regain obese, 18.0 %; and weight cumulating morbidly obese, 7.1 %) and three disability trajectories (little or low increase, 34.4 %; moderate increase, 45.4 %; and chronic high, 20.2 %) might best characterize long-term patterns of change in BMI and disability. Although the beneficial impacts of optimizing weight in adults with diabetes on disability later in life were supported in the present study, the evidence that there were various ways in which the two variables were linked supports the value of tailoring treatments and interventions at the individual level for adults with diabetes to achieve long-term health. In addition, more research is needed to unravel the mechanisms that account for poor physical functioning outcomes in adults with relatively normal weight.
Electronic supplementary material
(DOCX 35 kb)
Acknowledgements
This work was supported by Office of Research and Development at National Cheng Kung University (D100-35B19). We acknowledge the valuable editorial help provided by Kaileen Yeh and Siao-Ling Lee. The earlier version of this paper was presented at the 5th Annual Research Retreat of the Penn State Institute for Diabetes and Obesity.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
REFERENCES
- 1.Clinical guidelines on the identification. Evaluation, and treatment of overweight and obesity in adults. Bethesda, MD: National Heart Lung, and Blood Institute; 1998. [PubMed] [Google Scholar]
- 2.U.K. Prospective Diabetes Study Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhry ZW, Gannon MC, Nuttall FQ. Stability of body weight in type 2 diabetes. Diabetes Care. 2006;29(3):493–497. doi: 10.2337/diacare.29.03.06.dc05-1703. [DOI] [PubMed] [Google Scholar]
- 4.Wray LA, Blaum C, Ofstedal MB, Herzog R. Diabetes dianosis and weight loss in middle-aged adults. Res Aging. 2004;26(1):62–81. doi: 10.1177/0164027503258741. [DOI] [Google Scholar]
- 5.Looker HC, Knowler WC, Hanson RL. Changes in BMI and weight before and after the development of type 2 diabetes. Diabetes Care. 2001;24(11):1917–1922. doi: 10.2337/diacare.24.11.1917. [DOI] [PubMed] [Google Scholar]
- 6.de Fine Olivarius N, Andreasen AH, Siersma V, Richelsen B, Beck-Nielsen H. Changes in patient weight and the impact of antidiabetic therapy during the first 5 years after diagnosis of diabetes mellitus. Diabetologia. 2006;49(9):2058–2067. doi: 10.1007/s00125-006-0328-y. [DOI] [PubMed] [Google Scholar]
- 7.Tuthill A, McKenna MJ, O'Shea D, McKenna TJ. Weight changes in type 2 diabetes and the impact of gender. Diabetes Obes Metabol. 2008;10(9):726–732. doi: 10.1111/j.1463-1326.2007.00800.x. [DOI] [PubMed] [Google Scholar]
- 8.Feldstein AC, Nichols GA, Smith DH, Rosales AG, Perrin N. Weight change and glycemic control after diagnosis of type 2 diabetes. J Gen Intern Med. 2008;23(9):1339–1345. doi: 10.1007/s11606-008-0681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldstein AC, Nichols GA, Smith DH, et al. Weight change in diabetes and glycemic and blood pressure control. Diabetes Care. 2008;31(10):1960–1965. doi: 10.2337/dc08-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guare JC, Wing RR, Grant A. Comparison of obese NIDDM and nondiabetic women: short- and long-term weight loss. Obes Res. 1995;3(4):329–335. doi: 10.1002/j.1550-8528.1995.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 11.Kahng SK, Dunkle RE, Jackson JS. The relationship between the trajectory of body mass index and health trajectory among older adults. Res Aging. 2004;26(1):31–61. doi: 10.1177/0164027503258734. [DOI] [Google Scholar]
- 12.Ferraro KF, Su YP, Gretebeck RJ, Black DR, Badylak SF. Body mass index and disability in adulthood: a 20-year panel study. Am J Public Health. 2002;92(5):834–840. doi: 10.2105/AJPH.92.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Socio Meth Res. 2007;35(4):542–571. doi: 10.1177/0049124106292364. [DOI] [Google Scholar]
- 14.Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: a group-based method. Psychol Meth. 2001;6(1):18–34. doi: 10.1037/1082-989X.6.1.18. [DOI] [PubMed] [Google Scholar]
- 15.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Socio Meth Res. 2001;29(3):374–393. doi: 10.1177/0049124101029003005. [DOI] [Google Scholar]
- 16.Juster FT, Suzman R. An overview of the health and retirement study. J Hum Resour. 1995;30:S7–S56. doi: 10.2307/146277. [DOI] [Google Scholar]
- 17.Wang CY, Sheu CF, Protas E. Construct validity and physical performance of older adults in different hierarchical physical-disability level. J Aging Phys Act. 2007;15(1):75–89. doi: 10.1123/japa.15.1.75. [DOI] [PubMed] [Google Scholar]
- 18.D'Unger AV, Land KC, McCall PL, Nagin DS. How many latent classes of delinquent/criminal careers? Results from mixed poisson regression analyses. Am J Sociol. 1998;103(6):1593–1630. doi: 10.1086/231402. [DOI] [Google Scholar]
- 19.Hensrud DD. Dietary treatment and long-term weight loss and maintenance in type 2 diabetes. Obes Res. 2001;9(Suppl 4):348S–353S. doi: 10.1038/oby.2001.141. [DOI] [PubMed] [Google Scholar]
- 20.Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes—causes, effects and coping strategies. Diabetes Obes Metabol. 2007;9(6):799–812. doi: 10.1111/j.1463-1326.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- 21.Jacob AN, Salinas K, Adams-Huet B, Raskin P. Weight gain in type 2 diabetes mellitus. Diabetes Obes Metabol. 2007;9(3):386–393. doi: 10.1111/j.1463-1326.2006.00622.x. [DOI] [PubMed] [Google Scholar]
- 22.Shoff SM, Klein R, Moss SE, Klein BE, Cruickshanks KJ. Weight change and glycemic control in a population-based sample of adults with older-onset diabetes. J Gerontol. 1998;53(1):M27–M32. doi: 10.1093/gerona/53a.1.m27. [DOI] [PubMed] [Google Scholar]
- 23.Zamboni M, Mazzali G, Zoico E, et al. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes (Lond) 2005;29(9):1011–1029. doi: 10.1038/sj.ijo.0803005. [DOI] [PubMed] [Google Scholar]
- 24.Sairenchi T, Iso H, Irie F, Fukasawa N, Ota H, Muto T. Underweight as a predictor of diabetes in older adults: a large cohort study. Diabetes Care. 2008;31(3):583–584. doi: 10.2337/dc07-1390. [DOI] [PubMed] [Google Scholar]
- 25.Carnethon MR, De Chavez PJ, Biggs ML, et al. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012;308(6):581–590. doi: 10.1001/jama.2012.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wray LA, Herzog AR, Willis RJ, Wallace RB. The impact of education and heart attack on smoking cessation among middle-aged adults. J Health Soc Behav. 1998;39(4):271–294. doi: 10.2307/2676339. [DOI] [PubMed] [Google Scholar]
- 27.Goldman N, Lin IF, Weinstein M, Lin YH. Evaluating the quality of self-reports of hypertension and diabetes. J Clin Epidemiol. 2003;56(2):148–154. doi: 10.1016/S0895-4356(02)00580-2. [DOI] [PubMed] [Google Scholar]
- 28.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123(5):894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 29.Bowman RL, DeLucia JL. Accuracy of self-reported weight: a meta-analysis. Behav Ther. 1992;23:637–655. doi: 10.1016/S0005-7894(05)80226-6. [DOI] [Google Scholar]
- 30.Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8(4):307–326. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 31.McAdams MA, Van Dam RM, Hu FB. Comparison of self-reported and measured BMI as correlates of disease markers in US adults. Obesity (Silver Spring) 2007;15(1):188–196. doi: 10.1038/oby.2007.504. [DOI] [PubMed] [Google Scholar]
- 32.Langlois JA, Maggi S, Harris T, et al. Self-report of difficulty in performing functional activities identifies a broad range of disability in old age. J Am Geriatr Soc. 1996;44(12):1421–1428. doi: 10.1111/j.1532-5415.1996.tb04065.x. [DOI] [PubMed] [Google Scholar]
- 33.Hoeymans N, Feskens EJ, van den Bos GA, Kromhout D. Measuring functional status: cross-sectional and longitudinal associations between performance and self-report (Zutphen Elderly Study 1990–1993) J Clin Epidemiol. 1996;49(10):1103–1110. doi: 10.1016/0895-4356(96)00210-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 35 kb)