In this series, a clinician extemporaneously discusses the diagnostic approach (regular text) to sequentially presented clinical information (bold). Additional commentary on the diagnostic reasoning process (italics) is integrated throughout the discussion.
Clinical Information
A 49-year-old woman was in her usual state of good health when she noted the sudden onset of weakness in her legs and arms.
Clinician
The term weakness here seems to imply true motor weakness, rather than a general sense of fatigue. Considering involvement of the arms and legs (sparing the face) and the acuity, the lesion could localize to the cervical spine (e.g., herniated disk), peripheral nerves (e.g., acute inflammatory demyelinating polyradiculoneuropathy [AIDP]), or muscles (e.g., toxic myopathy or periodic paralysis).
Diagnostic Reasoning
The problem representation is an abstract one-sentence summary that elaborates the key features of the case. It triggers plausible diagnostic hypotheses and directs exploration of further historical elements, physical examination features, and diagnostic testing. Possible solutions to the brief problem representation—a healthy adult with acute onset quadriparesis—are stratified by location along the neuroaxis.
The patient lived in California and was on an extended vacation in Mexico for 2 months when her symptoms developed. She first noted mild diffuse bilateral thigh pain that resolved with gentle self-massage. The next day, her pain recurred in both thighs after riding an all-terrain vehicle (no trauma or injury occurred). The following day, she developed progressive weakness of both legs. By that evening, the weakness was so severe that she fell to the ground while walking up a short flight of stairs. She did not lose consciousness and was able to rise with assistance and continue to a restaurant for dinner. After dinner, she was unable to rise from a seated position and required wheelchair escort back to her accommodations. The next morning, she awoke with persistent weakness in the legs and progressive weakness of her arms, preventing her from rising from bed. A few hours later she began having trouble holding up her head. She denied facial muscle weakness, shortness of breath, or trouble speaking.
The pattern of proximal extremity weakness and subsequent cervical muscle weakness is consistent with a myopathy, although the rapidity of onset is atypical. Hypokalemic periodic paralysis can cause acute diffuse weakness. Rapidly ascending weakness is compatible with acute inflammatory demyelinating polyneuropathy (AIDP or Guillain-Barré Syndrome); muscle pain can occur, although sensory paresthesias are more typical. An antecedent diarrheal illness, perhaps associated with her travels, could have been the inciting event. Other rapid onset motor neuropathies from toxins, uremia, and porphyria warrant consideration. An acute myasthenic crisis could present in this way, but the absence of bulbar symptoms makes this less likely. Diffuse muscle pain would not be seen with a cervical spine lesion. Although her illness began while in Mexico, there is no fever to suggest an infectious myositis caused by viral or parasitic pathogens. Her respiratory function appears to be intact, but could rapidly deteriorate with many of the aforementioned etiologies, and therefore should be monitored closely.
The discussant has activated multiple illness scripts (mental representations of disease) based on neuroanatomic localization, and is going through a systematic compare and contrast exercise, assessing the degree of match between the illness scripts and the problem representation. For nearly each disorder (myositis, AIDP, myasthenic crisis), the absence of a defining feature (slow onset, sensory prodrome, or bulbar symptoms, respectively) makes that particular diagnosis less likely.
The patient was transported to a hospital in California later that day. She continued to report weakness in the neck, trunk, arms, and legs, but denied shortness of breath, trouble speaking, or visual disturbances. She denied constipation, but had difficulty urinating, without dysuria.
Medical history included thrombotic thrombocytopenia purpura (TTP), treated with plasma exchange followed by splenectomy 10 years prior. Her only medication was an oral contraceptive. She worked in the information technology sector. She was married with two healthy adolescent children. She did not use tobacco or illicit drugs, and did not abuse alcohol. Her parents survived gastric cancer and an unknown brain cancer.
The difficulty with urination rekindles the possibility of cervical cord lesion or compression, although the diffuse muscle pain would be highly unusual. Her TTP was likely refractory or recurrent, which is an indication for splenectomy, but diffuse weakness is not a feature of that disorder. Without a spleen, she is at higher risk for infection with encapsulated organisms, such as pneumococcus or babesiosis, but she has not exhibited fever or other localizing signs of infection beyond myalgia. West Nile virus and tick paralysis can also cause a rapid flaccid paralysis. The strong family history of cancer and an undefined neuromuscular process raises the possibility of a paraneoplastic syndrome.
Candidate diagnoses from the major categories of disease—infection (splenectomy), autoimmunity (TTP), and cancer (family history)—are triggered in the clinician’s mind by predisposing conditions.
She appeared comfortable with normal vital signs, except for respiratory rate of 23 breaths per minute. The oxygen saturation by pulse oximetry was 96% while breathing 2L by nasal cannula. Examination of her cardiovascular, respiratory, and abdominal systems was normal. She was fully alert and oriented and fluent in her speech. She had difficulty articulating the words: “Methodist episcopal”. Memory was intact. Cranial nerves were normal with the exception of both sternocleidomastoids, which were slightly weak. Motor examination showed normal tone and bulk; there was diffuse weakness throughout all four limbs and she could not lift her arms or legs against gravity. The patient had symmetrically diminished biceps (1+), triceps (1+) and brachioradialis (1+) deep tendon reflexes; normal ankle (2+) reflexes; and symmetrically increased patellar (3+) reflexes. There was no Babinski sign. Sensation was normal. Coordination and gait could not be tested due to weakness.
Her dysarthria and sternocleidomastoid weakness may point to a multifocal cranial neuropathy or a neuromuscular disorder. Cervical spinal cord compression or injury warrants consideration with quadraparesis and preserved reflexes, but normal sensation argues against a myelopathy. Reflexes can occasionally be preserved very early in the course of AIDP, although with this degree of weakness the reflexes should be absent. Bulbar and neck involvement are more characteristic of neuromuscular rather than myopathic diseases. Although there is no evidence of ocular involvement, weakness of neck extension, dysarthria, and diffuse limb weakness are compatible with myasthenia gravis or myasthenic crisis, especially given the rapidity of her symptoms. Other neuromuscular disorders that can lead to paralysis include botulism and Lambert–Eaton syndrome. Intact mental status is typical of the aforementioned syndromes, as is autonomic dysfunction, which could explain her urinary difficulties. Her oxygen requirement with simultaneously normal pulmonary exam should be evaluated with an arterial blood gas measurement; a rising pCO2 would signal hypoventilation and impending respiratory failure. Bulbar and limb involvement could signal motor neuron diseases (e.g., amyotrophic lateral sclerosis [ALS]), although the acuity of onset and possible respiratory dysfunction is atypical.
The discussant continues to consider candidate diagnoses (illness scripts in his mind) using neuroanatomic localization as a guide, searching for a strong match with the problem representation. His attention to respiratory status highlights how illness scripts include not only diagnostic features, but also capture the course of disease and allow clinicians to anticipate future events, including those that are potentially life-threatening (e.g., respiratory compromise).
Laboratory evaluation revealed a leukocyte count of 16,600/μL with a neutrophil predominance. Her hemoglobin was normal. Platelet count was 477,000/μL. Her chemistry panel was notable for a sodium of 137 meq/L, chloride 116 meq/L, potassium 1.6 meq/L, bicarbonate 13 meq/L, BUN 15 meq/L, creatinine 1.4 mg/dL, and glucose 108 mg/dL. Creatine kinase was 251 Units/L (normal 30 to 170 Units/L). Coagulation studies and liver function testing were normal.
Severe hypokalemia explains her profound and progressive muscle weakness (although hypokalemia-induced dysarthria is unusual). Hypokalemia results from inadequate intake, gastrointestinal or urinary losses, or transcellular shifting. In this case, there is no suggestion of alcoholism or a restricted diet, she has no vomiting or diarrhea, and there is no known diuretic use or features of hyperaldosteronism (such as hypertension or metabolic alkalosis). Severe hypokalemia coincident with acute myopathy is characteristic of hypokalemic periodic paralysis, which can be associated with hyperthyroidism. Her age is late for an initial presentation, and the duration of weakness is somewhat prolonged, as self-correction over hours is common. The non-anion gap hyperchloremic metabolic acidosis with marked reduction in serum bicarbonate and potassium is characteristic of a distal renal tubular acidosis (RTA).
Distinction between a transcellular shift (which often self-corrects with time) and total body depletion of potassium is critical, because aggressive potassium repletion with the former may invite overcorrection and hyperkalemia.
The leukocytosis with neutrophilia suggests an inflammatory condition that may be infectious or autoimmune, or less likely, malignant. Given her splenectomy and recent travel, infection must be excluded.
The finding of hypokalemia focuses the neuroanatomic approach (the muscle is the most highly susceptible organ in the neuroaxis to low potassium) and shifts the discussant’s attention from “where” to “why”? The low serum bicarbonate that accompanies the hypokalemia favors urinary loss of potassium from a renal tubular acidosis, but the discussant has not abandoned the competing hypothesis of transient shifts in potassium through genetic or thyrotoxic mechanisms leading to periodic paralysis.
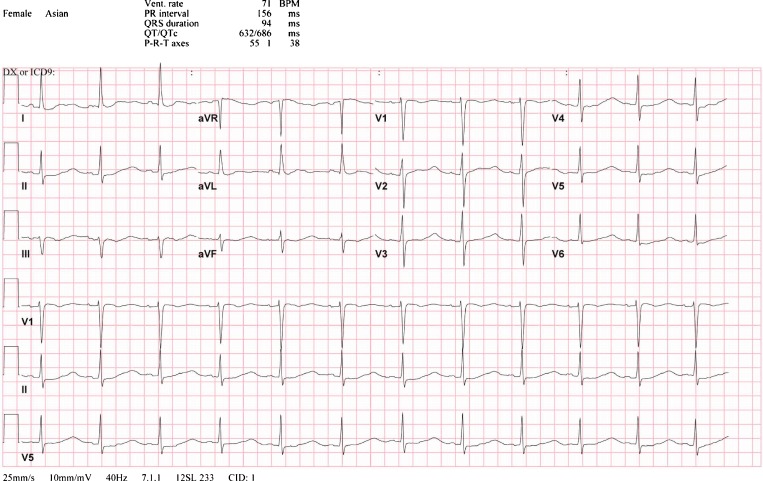
Urinalysis revealed a pH of 7, large hemoglobin, no glucose, positive leukocyte esterase, negative nitrite, 30 mg/dL protein, specific gravity 1.010, < 5 WBC PHPF, 11–20 RBC PHPF. An ECG showed a normal sinus rhythm, mild ST depression in lead II, TU fusion in lead I, and prominent U waves with diminished or absent T wave amplitude in all other limb and precordial leads (Fig.1). A chest x-ray showed low lung volumes, but was otherwise normal. A bedside forced vital capacity (FVC) was 1.6 L (normal 2.0–3.0 L for this patient’s height and weight) with a negative inspiratory pressure (NIP) of −40 cm H2O (normal −60 cm H2O to −80 cm H2O)
Figure 1.
The patient’s admission electrocardiogram demonstrates mild ST depression in lead II, diminished or absent T waves in almost all leads, and prominent U waves in leads I, II, aVF, and V2–V6.
Her reduced FVC and NIP suggest respiratory impairment from hypokalemia-induced myopathy and warrant close monitoring in an intensive care setting. The urinalysis reflects intra-renal inflammation from infection, glomerulonephritis, or interstitial nephritis. The dipstick heme positivity (based on the presence of pigmenturia) is more pronounced than the RBC count detected by microscopy, heralding myoglobinuria from hypokalemia-induced rhabdomyolysis. The alkaline urine is consistent with the impaired urine acidification that characterizes RTA. The abnormal urine sediment warrants exclusion of infection, microscopic examination for dysmorphic RBCs and RBC casts, and consideration of infiltrative interstitial processes such as Sjögren’s syndrome, systemic lupus erythematosus (SLE), or sickle cell disease. This ECG demonstrates changes commonly observed in hypokalemia: ST segment depression, lowering or absence of T wave amplitude and prominent U waves.
The progression of the case illustrates how the key problem to be solved (embodied in the problem representation) can evolve rapidly. In this case the clinicians and the discussant sequentially revised their problem representation from acute onset quadriplegia, to acute muscle weakness (with respiratory failure), to hypokalemia, and finally, to RTA. This continual reexamination of the underlying core process is problem revision, a key component of skillful problem solving.
Additional urine studies demonstrated urine sodium of 69 meq/L, urine potassium of 10.9 meq/L, and urine chloride of 67 meq/L. Microscopic examination did not reveal cellular casts or dysmorphic RBCs. Urine and blood cultures were negative. HIV antibodies were not detected. TSH was 3.68 μUnits/mL (normal 0.40 to 4.00 μUnits/mL). ANA titer was > 1:640. Anti ds-DNA was not detected. SSA was > 5.0 H Units (negative < 0.8, positive > 1.0), SSB was 1.5 H Units (negative < 0.8, positive > 1.0).
The patient’s weakness and muscle pain improved with potassium supplementation, and she was able to walk unassisted on the fourth hospital day. An electromyography study performed after potassium supplementation had commenced was normal.
A non-anion gap metabolic acidosis with a positive urinary anion gap (urinary sodium + urine potassium – urine chloride = +13) confirms reduced urinary acidification (rather than gastrointestinal losses of bicarbonate). The positive value, indicating bicarbonaturia, and elevated urine pH (>5.5) suggests a distal (type I) RTA. The serological profile (elevated ANA, SSA and SSB titers) points to Sjögren’s syndrome, a recognized cause of interstitial nephritis and distal RTA, as the underlying diagnosis.
The resolution of weakness with potassium supplementation and normal EMG imply lack of a persistent underlying inflammatory myopathy, which can complicate many primary autoimmune conditions. Her symptomatic hypokalemia appears to have been the index presentation of Sjögren’s syndrome. The patient should be queried about sicca symptoms. Although SLE can cause interstitial nephritis, she has no other clinical features of that disorder.
The patient subsequently described several years of dry mouth requiring frequent sips of water and development of several dental caries within the last few years. She also reported a several-year history of dry, itchy eyes that required near constant use of lubricating eye drops and an inability to make tears.
A salivary gland biopsy showed chronic sialadenitis, consistent with Sjögren’s syndrome. Potassium and bicarbonate supplementation and lubricating eye drops were prescribed. After 4 years, she has had no recurrences of weakness.
DISCUSSION
Medicine’s most favored aphorisms include: “always take a complete history”, “the answer is in the history 75 % of the time” or “listen to the patient—he’s telling you the diagnosis.” Indeed, the diagnosis of Sjögren’s syndrome was raised and explored in this patient when the consulting nephrologist, after seeing the serum electrolytes, asked about sicca symptoms. Rather than celebrate these triumphs of history taking, it is more instructive to understand how one arrives at the right questions.
Studies suggest that thoroughness is not the key to diagnostic success because it invites the collection of extensive extraneous information.1,2 Instead, the early inclusion of the final diagnosis among early hypotheses is a better predictor of diagnostic accuracy.3,4 This early triggering comes from pattern recognition (the consulting nephrologist) or the orderly framing and reframing of the problem (the discussant).
This case embodies repeated problem revision—going from weakness to myopathy to hypokalemia to RTA—before reaching the solution of Sjögren’s syndrome. Each preliminary conclusion triggered new hypotheses about the underlying disease process. Recognition that a more fundamental question must be asked is critical to clinical reasoning and diagnostic success. This repeated “drilling down” should lead any clinician to seek additional data to explore the differential diagnosis for interstitial nephritis and RTA, which includes Sjogren’s syndrome, SLE, and sickle cell disease.
We should shift our traditional admonition from “be thorough” to “be hypothesis-driven.” The former is not practical or useful, while the latter characterizes an expert problem-solving approach that can be fostered in trainees. Physicians, in fact all human decision makers, seek as much information as they think will be helpful, based on a limited subset of hypotheses (illness scripts) under active consideration. No doctor evaluating an acutely quadriplegic patient reflexively asks “do you have dry eyes?” because a sicca-associated syndrome should never be among the initial hypotheses. But as the discussant repeatedly revised the problem representation and arrived at hypokalemic myopathy due to RTA, a trigger to ask that key historical question was activated. Thus, the history is a dynamic component of the clinical reasoning process—requiring updating each time new hypotheses are triggered—rather than a static entry point into a diagnostic workup.
CLINICAL TEACHING POINTS
The best-documented ECG changes associated with hypokalemia include ST segment depression, diminished T wave amplitude, and prominent U waves, rather than QT prolongation. 5,6
The urinary anion gap (Urine sodium + Urine potassium – Urine chloride) aids in determining whether a non-anion gap metabolic acidosis is being generated by gastrointestinal or renal losses of bicarbonate (a positive value indicates a renal tubular acidosis and a negative value indicates gastrointestinal losses of bicarbonate)7.
The constellation of severe hypokalemia, non-anion gap metabolic acidosis, high urine pH and a positive urinary anion gap is diagnostic of distal renal tubular acidosis.7,8
Distal renal tubular acidosis has been extensively reported in patients with Sjögren’s syndrome, typically associated with renal medullary interstitial nephritis.8–11
The presence of RTA differentiates Sjögren’s syndrome from hypokalemic periodic paralysis.10–15
Acknowledgements
This case was described briefly in: Vickrey BG, Samuels MA, Ropper AH. How neurologists think: A cognitive psychology perspective on missed diagnoses. Ann Neurol. 2010 Apr;67(4):425–33.
Funders
None.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
REFERENCES
- 1.Hodges B, Regehr G, McNaughton N, Tiberius R, Hanson M. OSCE checklists do not capture increasing levels of expertise. Acad Med. 1999;74:1129–1134. doi: 10.1097/00001888-199910000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Eva KW, Link CL, Lutfey KE, McKinlay JB. Swapping horses midstream: factors related to physicians’ changing their minds about a diagnosis. Acad Med. 2010;85:1112–1117. doi: 10.1097/ACM.0b013e3181e16103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrows HS, Norman GR, Neufeld VR, Feightner JW. The clinical reasoning of randomly selected physicians in general medical practice. Clin Invest Med. 1982;5:49–55. [PubMed] [Google Scholar]
- 4.Tsukamoto T, Ohira Y, Noda K, Takada T, Ikusaka M. The contribution of the medical history for the diagnosis of simulated cases by medical students. Int J Med Educ. 2012;3:78–82. doi: 10.5116/ijme.4f8a.e48c. [DOI] [Google Scholar]
- 5.Lepeschkin E, Surawicz B. The measurement of the Q-T Interval of the electrocardiogram. Circulation. 1952;6:378–388. doi: 10.1161/01.CIR.6.3.378. [DOI] [PubMed] [Google Scholar]
- 6.Surawicz B, Lepeschkin E. The electrocardiographic pattern of hypopotassemia with and without hypocalcemia. Circulation. 1953;8:801–828. doi: 10.1161/01.CIR.8.6.801. [DOI] [PubMed] [Google Scholar]
- 7.Batlle DC, Hizon M, Cohen E, Gutterman C, Gupta R. The use of the Urinary Anion Gap in the diagnosis of hyperchloremic metabolic acidosis. N Engl J Med. 1988;318(10):594–599. doi: 10.1056/NEJM198803103181002. [DOI] [PubMed] [Google Scholar]
- 8.Caruana R, Buckalew V. The syndrome of distal (type 1) renal tubular acidosis. Clinical and laboratory findings in 58 cases. Medicine. 1998;67:84–99. doi: 10.1097/00005792-198803000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Ren H, Wang W, Chen X, Zhang W, Pan X, Wang X, Lin Y, Zhang S, Chen N. Renal Involvement and Followup of 130 Patients with Primary Sjögren’s Syndrome. J Rheumatol. 2008;35:278–284. [PubMed] [Google Scholar]
- 10.Soy M, Pamuk ON, Gerenli M, Celik YA. Primary Sjögren’s syndrome patient with distal renal tubular acidosis, who presented with symptoms of hypokalemic periodic paralysis: report of a case study and review of the literature. Rheumatol Int. 2005;26:86–89. doi: 10.1007/s00296-005-0587-9. [DOI] [PubMed] [Google Scholar]
- 11.Yılmaz H, Kaya M, Ozbek M, Ureten K, Safa Yıldırım I. Hypokalemic periodic paralysis in Sjögren’s syndrome secondary to distal renal tubular acidosis. Rheumatol Int. 2012. [DOI] [PubMed]
- 12.Stedwell RE, Allen KM, Binder LS. Hypokalemic paralyses: a review of the etiologies, pathophysiology, presentation, and therapy. Am J Emerg Med. 1992;10:143–148. doi: 10.1016/0735-6757(92)90048-3. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto T, Shiiki H, Takahi Y, Dohi K. Primary Sjögren’s syndrome presenting as hypokalaemic periodic paralysis and respiratory arrest. Clin Rheumatol. 2001;20:365–368. doi: 10.1007/s100670170028. [DOI] [PubMed] [Google Scholar]
- 14.Poux JM, Peyronnet P, Le Meur Y, Favereau JP, Charmes JP, Leroux-Robert C. Hypokalemic quadriplegia and respiratory arrest revealing primary Sjögren’s syndrome. Clin Nephrol. 1992;37:189–191. [PubMed] [Google Scholar]
- 15.Dowd JE, Lipsky PE. Sjögren’s syndrome presenting as hypokalemic periodic paralysis. Arthritis Rheum. 1993;36:1735–1738. doi: 10.1002/art.1780361213. [DOI] [PubMed] [Google Scholar]