Abstract
Recent evidence in humans indicate that defective phagocytic clearance of dying cells is linked to progression of advanced atherosclerotic lesions, the precursor to atherothrombosis, ischemic heart disease, and leading cause of death in the industrialized world. During atherogenesis, apoptotic cell turnover in the vascular wall is counterbalanced by neighboring phagocytes with high clearance efficiency, thereby limiting cellularity and maintaining lesion integrity. However, as lesions mature, phagocytic removal of apoptotic cells (efferocytosis) becomes defective, leading to secondary necrosis, expansion of plaque necrotic cores, and susceptibility to rupture. Recent genetic causation studies in experimental rodents have implicated key molecular regulators of efferocytosis in atherosclerotic progression. These include MER tyrosine kinase (MERTK), milk fat globule-EGF factor 8 (MFGE8), and complement C1q. At the cellular level, atheromata are infiltrated by a heterogenous population of professional phagocytes, comprised of monocytes, differentiated macrophages, and CD11c+ dendritic-like cells. Each cell type is characterized by disparate clearance efficiencies and varying activities of key phagocytic signaling molecules. It is in this context that we outline a working model whereby plaque necrosis and destabilization is jointly promoted by (1) direct inhibition of core phagocytic signaling pathways and (2) expansion of phagocyte subsets with poor clearance capacity. Towards identifying targets for promoting efficient apoptotic cell clearance and resolving inflammation in atherosclerosis and during ischemic heart disease and post myocardial infarction, this review will discuss potential in vivo suppressors of efferocytosis at each stage of clearance and how these putative interventional targets may differentially affect uptake at the level of vascular phagocyte subsets.
Keywords: Cardiovascular disease, Efferocytosis, Phagocytosis, MERTK, Secondary necrosis
Pathophysiological relevance of defective apoptotic cell clearance in atherosclerosis and ischemic heart disease
Vascular diseases such as advanced atherosclerosis leading to ischemic myocardial infarction (MI), are characterized by a blood-borne myeloid infiltration of leukocytes that include monocyte/macrophages, CD11C+ dendritic-like cells, and to a lesser extent various lymphoid subsets [1]. These immune cells extravasate from the circulation past the subendothelial layer of the vascular wall, initially in response to intimal-retained apolipoprotein B-100 lipoproteins [2]. Once embedded within the vascular intima, macrophages phagocytose aggregated cholesterol-rich lipoproteins, giving rise to the characteristic foam cells of early atherogenesis. Underlying the intimal body, smooth muscle cells of the medial vascular wall lining also become activated and transdifferentiate and transmigrate towards the lumen as they act to fortify the endothelial lining by generating a fibrous cap. For not completely understood reasons, these reactive processes begin to degenerate as lesions mature, and one of the earliest tell-tale signs of the conversion from early stable lesions to advanced ruptureprone inflammatory plaque is the accumulation of non-phagocytosed apoptotic cells [3]. Typically, apoptosis is a form of regulated cellular death that occurs billions of times each day without incident or inflammation; about one million cells die every second in our body [4]. In cooperation with neighboring phagocytes, apoptotic death is programmed to lead to compartmentalization and nonphlogistic metabolism of intracellular self-antigens [5]. However, during maturation of atherosclerotic lesions, apoptotic cells, and particularly apoptotic macrophages, lose membrane integrity and become secondarily necrotic, contributing over time to an expanding region of tissue necrosis that destabilizes plaque and is intimately linked to thrombin deposition and acute MI [6]. Post MI and during heart failure, sub-optimal clearance efficiency appears to continue, potentially leading to maladaptive vascular remodeling and tissue repair in the healing heart [7]. Evidence in mice and men suggests that one mechanism behind post-apoptotic necrosis in cardiovascular disease (CVD) is defective phagocytic clearance, or ‘‘efferocytosis,’’ of apoptotic cells [8, 9]. Though several recent studies, as described below, have shed light on a handful of the key regulators of clearance in the vasculature, the natural causes of defective efferocytosis that emerge as lesions mature, remain unknown. Given the strong association between complex, necrotic plaques and acute athero-thrombotic events, it is important to understand the cellular and molecular mechanisms of apoptotic cell clearance in vascular disease, and why/how this is compromised. In the following review, we will highlight candidate negative regulators of clearance during CVD at key stages of clearance, including apoptotic cell recognition and binding, phagocyte recognition and internalization, and finally post-engulfment metabolism and the ensuing inflammatory responses. We will also discuss how these events may differ between phagocyte subsets and the implications of phagocyte heterogeneity on therapeutics that target inflammatory clearance efficiency during coronary vascular disease and post MI.
Nowhere to hide: a surplus of find-me signals in CVD
Defective clearance in advanced plaque is likely not for lack of “getting the message out.” Rather, advanced plaque is a chronic milieu of inflammatory mediators that are in abundant supply and continuously elicit progressive phagocyte recruitment from the nearby circulation [10]. This is in part due to lipoprotein induced up-regulation of vascular cell adhesion molecules such as vascular cell adhesion molecule-1 (VCAM), intercellular adhesion molecule-1 (ICAM), and E-selectin [11]. However, as lesions mature, recent evidence suggests that apoptotic cells themselves may play a role in eliciting phagocytes. Indeed, agents that elicit directional migration of phagocytes, so-called find-me signals, can be harvested and transferred in vitro from the supernatants of disparate cells types that have been stimulated to undergo apoptosis [12]. Consistent with this, acute chemical inducement of lesional apoptosis in vivo can incite blood-borne monocyte recruitment into vascular lesions [13]. Monocyte recruitment also continues post MI. For example, the ischemic myocardium mobilizes phagocytic Ly-6cHI monocytes to help promote digestion of damaged tissue [14]. Unlike microbial infections, monocytic phagocytes, not polymor-phonuclear granulocytes/neutrophils, are the predominant phagocyte to be recruited to atherosclerotic lesions. In this light, it is interesting to note that apoptotic cells have been reported to secrete a keep-out signal such as lactoferrin, and thereby inhibit migration of granulocytes [15]. Though the in vivo mechanisms by which phagocytes transduce recognition of chemoattractant recruitment signals has not been fully elucidated, a number of soluble extracellular find-me or come-get-me signals have been identified (see review article is this issue [16, 17]). These include thrombospondin, which is released by dying fibroblasts during wound healing [18], and other soluble factors released from apoptotic cells, including fractalkine (CX3CL1) [19], tRNA synthetases and ribosome components, all of which have been reported to incite monocyte attraction [20, 21]. Notably, secretion of the most characterized find-me lipid chemoattractant, lysophosphatidyl-choline, has been directly linked to apoptosis, as its production by a phospholipase is caspase-3 dependent [22]. Most recently and in an in vivo setting, nucleotides such as ATP and UTP, which can be recognized by the G-protein-coupled receptor (GPCR) P2Y [23], have also been found to be released from apoptotic cells and can incite directed migration of monocytes and macrophages. It has not yet been elucidated how nucleotide release is coupled to apoptosis and whether this occurs in atheromata, though it can readily be speculated that atherosclerotic necrotic cores, the so called “graveyards of dead macrophages,” likely contain free nucleotide remnants that could function as find-me signals for phagocytic clearance. In this context, it is known that phagocytes have mechanisms to respond to secondarily necrotic cells. For example, LRP/CD91 can recognize extracellular heat shock proteins [24] and dendritic cells can be triggered to mature upon exposure to uric acid from necrotic cells [25]. Of course, find-me signals are only as useful as the accessibility of the path en route to the apoptotic cell. Post infiltration, a key role of professional phagocytes such as the macrophage is to chemotax towards the source of apoptotic elicitation agent and form a phagocytic synapse between effector and target cell membrane [26]. This directed step may be especially problematic in advanced lesions where confounding flux in extracellular matrix production and degradation by metalloproteinases could impede directional chemotaxis [27]. This, in combination with athero-relevant regulation of cytoskeletal signaling pathways may combine to arrest directional phagocyte migration towards find-me signals. For example, Rho-GTPase mediated regulation of cytoskeletal chemotaxis can be impaired by increased phagocyte cholesterol loading [28]. The aforementioned considered, in many cases there nonetheless exists a high density of plaque phagocytes in close proximity to TUNEL positive apoptotic cells. Thus, the need for chemotaxis over relatively long distances may certainly be limited in atherosclerotic lesions. In fact, free apoptotic cells in plaque can readily be detected in close proximity to non-ingesting macrophage phagocytes (Fig. 1), certainly suggesting that other factors besides find-me signals are at play.
Fig. 1.
Efferocytosis in plaque. Accumulation of apoptotic cells accompanies advanced lesional maturation and necrotic core expansion in murine plaque. In the left image, an atherosclerotic lesion is populated by circular nuclei (blue; hoechst) and F4/80 + phagocytes within the intima (green). The circumscribed area is shown at higher magnification to the right where TUNEL positive apoptotic cells can be found in association (A) or free (F) from neighboring phagocytes. Bar = 10 µm (Color figure online)
Negative regulators of uptake and “don’t eat me” signals in plaque
Post secretion of inflammatory find-me cues, apoptotic cells must distinguish themselves from their non-apoptotic/viable neighbors. Thus, an invitation to a meal [29] must be accompanied by down-regulation of intrinsic phagocytosis-suppressor molecules known as don’t eat me signals. Only a handful of such signals/ligands have been characterized and in the case of CVD, it is unknown to what extent such don’t eat me signals regulate clearance. One known example of a don’t eat me ligand is CD31 (or platelet endothelial cell adhesion molecule/PECAM-1). Homophilic CD31 interactions between macrophage phagocytes and viable leukocytes during low fluid shear stress lead to intercellular CD31-mediated detachment [30]. This process is reversed during apoptosis and CD31 may act oppositely as a tethering agent. Interestingly, CD31 gene polymorphism and increases in levels of its circulating soluble form have been associated with ischemic stroke [31], suggesting that extracellular CD31 could affect phagocyte interactions with apoptotic cells. Another example of a don’t eat me molecule is the CD47 integrin-associated protein. In vitro, programmed cell death leads to downregulation of CD47. Also, artificial disruption of interactions between CD47 on non-apoptotic target cells and its cognate ligand, signal regulatory protein alpha (SIRPα or SHPS-1) on phagocytes, can permit uptake of viable cells [32, 33]. Though it is currently unknown as to what happens to CD47 levels on apoptotic cells in plaque, that this pathway is relevant during disease has been shown in cancer, where increased presentation of CD47 provides malignant cells a means of escaping immune surveillance [34]. Not all phagocyte suppressing molecules are found on the cell surface of phagocytes. For example and more recently, genetic studies in Caenorhabditis elegans identified the phosphatidylinositol 3 phosphate (PIP) lipid phosphatase myotubularin (MTM-1) as a negative regulator of cell corpse engulfment [35, 36]. Accumulation of PIPs at the phagocytic cup is required during phagocytic engulfment and as described below, PIP accumulation is suppressed under situations that exacerbate atherosclerosis [37, 38].
Eat-me signals in CVD
After a phagocyte is juxtaposed next to its apoptotic prey, it must recognize apoptotic cell-associated molecular patterns (ACAMPs) to trigger outside-in signaling and initiate actin polymerization. This is necessary to generate the force required to internalize large apoptotic bodies for lysosomal degradation. Besides the overt morphological indications as membrane blebbing and cell-shrinkage, such eat-me signals on the surface of apoptotic cells include Annexin I and phosphatidylserine [38, 39]. Oxidation of phosphatidylserine and other phospholipids may further enhance the “palatability” of eat-me signals [40] and may be especially relevant in the oxidative environment of advanced plaque and post MI. In addition, cell surface glycosylation patterns and changes in cell surface charge can be altered during apoptosis and further be recognized by phagocytes [41]. Of course, exposure of eat-me signals requires down-regulation of don’t eat me ligands as described above. In the case of protease-harboring neutrophils that penetrate damaged myocardial tissue and die rapidly post infiltration, there is an obvious need to control efficient neutrophil clearance and unregulated protease release. Recent evidence suggests that endogenous pentraxin molecules stored within neutrophil granules may serve as eat-me signals during apoptosis [42]. Recent studies also suggest that phosphatidylserine-independent eat-me signals are modifiable by proteases such as Cathepsin [43]. In the context of a protease rich inflammatory milieu, such may be a mechanism of defective clearance. Studies in our own laboratory indicate that macrophages rendered apoptotic by many different atherosclerosis-relevant mechanisms are equally good substrates for healthy phagocytes ([44] and Yankun Li and Ira Tabas, unpublished data). However, it remains possible that various combinations of conditioned medium and athero-relevant stimuli may combinatorially lead to modification of eat-me recognition motifs.
Soluble ligand-receptor bridging molecules (opsonins) and atherosclerosis
Binding of apoptotic cells to phagocytes often occurs through bridging molecules such as Gas6 and lactadherin (milk fat globule-EGF factor 8; MFG-E8), which bind to their cognate receptors, MERTK and αβ integrins, respectively [45, 46]. In the case of the bridging molecules Gas6 and protein S, these MERTK ligands can be produced by various cell types of the vasculature, including vascular smooth muscle cells and endothelial cells [47]. Two causation studies have directly looked at how deficiency of key bridging molecules affects atherosclerotic progression. An in vivo study by the Mallat group used Mfge8−/− mice and found that low-density lipoprotein receptor deficient (Ldlr−/−) mice reconstituted with Mfge8−/− bone marrow had larger numbers of apoptotic cells, increased plaque necrosis, and increased IFNγ compared with control lesions [46]. In vitro studies have separately shown that down-regulation of MFGE8 post TLR4 signaling can inhibit apoptotic cell uptake [48]. Another bridging molecule involved in the recognition of apoptotic cells by phagocytes is the complement factor C1q. C1qa−/− mice on the fat-fed Ldlr−/− background had larger and more complex aortic root lesions and an increase in the number of apoptotic cells in these lesions compared with C1qa+/+; Ldlr−/− mice. Finally, immune B-cells have been identified in plaque and natural IgM antibodies have also been implicated in opsonizing apoptotic cells towards enhanced engulfment [49].
At the level of the phagocyte in CVD
Factors that directly act on the phagocyte in CVD
Two obvious explanations for defective clearance in a setting of accelerated apoptosis are either overwhelming apoptosis or phagocyte depletion. The in vivo data however do not support these notions. For example, billions of cells die each day by apoptosis and are cleared nonpholigistically, evidence of our body’s high capacity for clearance. In instances where there is naturally a high rate of apoptotic cell turnover, such as during selection of T-cells in the thymus, TUNEL positive cells are rarely identified, consistent with a high basal capacity for clearance [50]. In addition, chemically and genetically induced accelerated apoptosis in early lesions does not promote features of plaque instability, strongly suggesting that differential factors present in advanced plaque can negatively regulate clearance efficiency [13, 51]. Thus, at the level of the lesion, in a milieu densely populated by phagocytes, it is unlikely that efferocytosis is overwhelmed by apoptotic targets or phagocyte depletion. Another readily apparent candidate to explain defective efferocytosis in lipid-laded atherosclerotic plaque is subendothelially retained ApoB100 lipoprotein that has been phagocytosed by macrophage foam cells [2, 52]. Besides the effects of cholesterol loading on macrophage chemotaxis as described above, in our own hands and in an assay where apoptotic cells were directly overlaid onto phagocytes, we have found that cholesteryl-ester-laden and non-apoptotic unesterfied (free) cholesterol-loaded macrophage foam cells to be excellent phagocytes in vitro [44]. Furthermore, ingestion of cholesterol-loaded meals can actually promote survival, permitting subsequent secondary clearance events after metabolism of the first [53]. Considering other lipid/ metabolic factors that act directly on the vascular wall, increases in saturated fatty acids have recently been implicated as suppressors of efferocytosis in plaque. Specifically, recent studies indicate that defective efferocytosis in genetically obese mice are related to increased levels of saturated fatty acids, leading to defective phosphatidyl inositol 3-kinase (PI3K) activation and reduced PIP3 in macrophage phagocytic cups [37].
Phagocyte receptors in CVD
Phagocytes express a diverse collection of cell-surface receptors that are capable of tethering and transducing signals that lead to mobilization of phagocyte engulfment machinery (Fig. 2) [54]. Though this assortment of receptors reflects the critical importance of fail-safe clearance mechanisms in maintaining tissue homeostasis, their diversity in both form and function indicate they are not merely redundant. Structurally distinct receptor families are capable of recognizing unrelated ligands on apoptotic cell surfaces, leading to divergent downstream pathways that modulate cytoskeletal signaling, cell survival, pro and anti- inflammatory signaling, and metabolic processing of engulfed cells. Key apoptotic cell receptors include those that recognize phosphatidylserine, such as the newly implicated brain specific angiogenesis inhibitor 1 (BAI1) [55], T-cell immunoglobulin mucin receptors [56, 57], and stabilin receptors. Candidate inhibitory mechanisms within atherosclerotic lesions include oxidized lipoproteins, which in vitro have been shown to directly interfere with efferocytosis by competitively binding apoptotic cell receptors on phagocytes. Specifically, both fully oxidized LDL as well as LDL that is only minimally oxidized, referred to as “minimally modified” LDL, have been shown to inhibit the engulfment of apoptotic cells by altering actin signaling through a pathway involving CD14 and TLR4 [58]. In vivo, still much is left to be learned regarding the contribution of apoptotic cell receptors during the different stages of atherosclerosis and post MI. During advanced atherosclerosis, so far only a handful of receptors have been causally linked to apoptotic clearance and plaque necrosis. For example, cell-surface and protein cross-linking enzyme transglutaminase 2 (TG2), in cooperation with integrins, can engage lactadherin-opsonized apoptotic cells and promote engulfment [59]. In vivo, atherogenic Ldlr−/− mice engrafted with Tg2−/− bone marrow cells exhibit larger aortic root lesions and expanded necrotic cores relative to control [60]. Another interesting candidate is the apoptotic cell receptor MERTK of the TAM receptor tyrosine kinase family. In vitro, macrophages engineered with a kinase dead form of MERTK fail to specifically engulf macrophages that have been rendered apoptotic by atherogenic stimuli [44]. In vivo, mice deficient in MERTK exhibit reduced efferocytosis and increased plaque necrosis and inflammation and features of autoimmunity [61, 62].
Fig. 2.
Key players during efferocytosis in CVD. Efferocytosis requires the interplay between numerous cellular and extracellular ligands. At the level of the apoptotic cells (AC), presentation of “eat-me” signals [such as phosphatidylserine (ps)] and down-regulation of “don’t-eat” me ligands (such as CD47, CD31, and PAI-1) prepare apoptotic cell ligands for engulfment by macrophage (MU) and immature and mature dendritic (IDC and DC) phagocyte subsets. Neutrophils (PMN) may act as phagocytes and/or apoptotic cells. Interactions between apoptotic target and phagocyte effector often require ‘‘bridging’’ molecules such as Gas6/protein S, lactadherin (MFGE8), complement (C1q, C3b), calreticulin (cal), annexin (anxII), and immunoglobulin (IgM) to facilitate target-effector juxtaposition. Numerous apoptotic cell receptors [such as scavenger receptor A (SRA), TAMs, integrins, LRP, and CD36] and signaling (CrkII-Dock180-Rac1, Rho/actin) and metabolic (NR nuclear receptors) molecules participate during engulfment and modulate downstream inflammatory signaling pathways. “Necrotic” indicates secondary necrotic cells. Arrows indicate differentiation pathways from immature monocyte and dendritic cell precursors. ACAMP apoptotic cell-associated molecular pattern, AC apoptotic cell, MO: monocyte, IDC immature dendritic cell, DC dendritic cell, PAI-1 plasminogen activator inhibitor-1, PMN polymorphonuclear leukocyte or neutrophil, PS phosphatidylserine, TAM Tyro3, Axl tyrosine kinase, MerTK
In addition to such engineered tests of receptor causality, it is important to understand how receptor function naturally is regulated or compromised during the course of atherosclerotic lesion maturation. For example, variations in the expression, activity, or function of key apoptotic cell receptors, such as MERTK, may be affected by the heightened inflammatory milieu that is a hallmark of advanced plaque. This may be exacerbated by conditions that are closely tied to CVD, including insulin resistance. Case in point, diabetic lesions exhibit increased inflammation and inflammation-associated proteases in conjunction with reduced levels of natural tissue protease inhibitors [63, 64]. Proteolytic-mediated cleavage of efferocytosis receptors has been implicated in impaired apoptotic cell clearance and exacerbated disease in cystic fibrosis (see the review article in this issue [65]) and bronchiectasis [66] and proteases such as MMPs and ADAMs (A Disintegrin And Metalloprotease) have been found to be up-regulated in advanced atherosclerosis [67]. In vitro, MERTK is proteolytically cleaved as a result of inflammatory stimuli such as LPS and this leads to the generation of a solubilized MER that can act as a competitive inhibitor of uptake [68]. Finally, certain receptors such as CD91/LRP appear to recognize necrotic ligands [24]. Necrotic cell uptake may involve macropinocytosis or fluid-phase internalization [69].
Inhibition of phagocyte signaling in CVD
After engaging cognate ligands, proteinaceous apoptotic cell receptors transduce extracellular signals into the cytosol, culminating in the activation of low molecular mass GTPases and cytoskeletal signaling [70]. These signaling conduits are distinct from Fc-receptor and complement receptor-mediated internalization pathways and much of our knowledge of them has been elucidated in genetically tractable models such as the nematode and as described in the accompanying review article by Kinchen [36]. In mammals, apoptotic cell engulfment leads to mobilization of ELMO and DOCK180 as they cooperate as a guanine nucleotide exchange factor (GEF) to activate Rac and promote actin signaling and membrane ruffling [71]. In parallel, the small GTP-bound protein RhoA (i.e., RhoA-GTP) is inactivated. In general, Rac and Rho follow an inverse relationship during efferocytosis [72]. RhoA can be activated by the athero-relevant lysophosphatidic acid and appears to be involved in a number of cardiovascular related events [73]. Interestingly, statins such as lovastatin have been shown to inhibit the activity of Rho and enhance efferocytosis by blocking farnesylation pathways that lead to Rho membrane translocation [74]. Downstream of RhoA is its effector kinsae ROCK, a target of ROCK-inhibitors such as fasudil, which are being tested in a number of clinical trials; in our own hands fasudil and other ROCK inhibitors enhance efferocytosis of apoptotic cholesterol-loaded macrophages in vitro in unpublished data (Thorp and Tabas).
Numerous factors in atherosclerotic lesions have been identified that can negatively regulate cytoskeletal signaling pathways. These include oxidant stress and superoxides such as 3-morpholinosydnonimine (SIN-1) [9]. In addition, TNFα induces phospholipase 2 and arachidonic acid production, generating ROS production and modulation of Rho in mature macrophages [75]. Oxidant stress and hypoxia have also been reported as in vitro inhibitors of clearance and may play a role in post MI repair [76]. Aberrant calcium signaling has been recently implicated in efferocytosis as well. Both extracellular and intracellular sources of Ca2+ have long been implicated in phagocytic signaling, particularly during Fc-receptor mediated uptake of opsonized particles [77–80]. Changes in extracellular Ca2+ concentration can directly affect the conformation and therefore capacity of phagocytic receptors to bind targets for engulfment. In neutrophils, Ca2+ can enter from extracellular stores where it concentrates in pseudopods during fungal ingestion [81] and promotes phagolysosomefusion [82]. In macrophages, waves of Ca2+ oscillations from intracellular stores also localize to the periphagosomal regions [83]. Interestingly, certain dendritic cell phagocytic signaling pathways appear to be independent of calcium [84, 85]. In dendritic cells, CamKII promotes β3-mediated engulfment of apoptotic tumor cells [86] and also regulates human dendritic cell maturation [87]. Recent genetic studies in nematodes [88] indicate that efferocytosis requires a junctophilin protein called Undertaker, which couples Ca2+ channels at the plasma membrane to endoplasmic reticulum ryanodine receptors. Consistent with these findings, in mammalian cells, Ravichandran and colleagues report that efferocytosis can be blocked by disabling ER calcium homeostasis with the SERCA inhibitor thapsigargin, interestingly implicating ER stress in efferocytosis. Efferocytosis was also inhibited by silencing STIM-1 (Stromal interaction molecule), which localized at ER-plasma membrane junctions. Finally, coupling of nonphlogistic signaling to apoptotic cell clearance may also require calcium. De Lorenzo et al. [89] report that the calcium-binding protein S100A9 suppresses TNFα signaling during efferocytosis and Gronski et al. [90] show that the calcium chelator BAPTA blocks TGF-β production post apoptotic cell uptake.
Inflammatory consequences of defective efferocytosis in plaque
Clearance of apoptotic cells by phagocytes is by effect non-phlogistic. Merely preventing the release of inflammatory intracellular contents such as uric acid, TLR-stimulating nucleic acids, and high mobility group–box chromosomal protein-1 (HMGB-1), in turn prevents bystander cell signaling pathways that promote further phagocyte recruitment [25, 91]. Prevention of secondary necrosis by efferocytosis is also accompanied by phagocyte anti-inflammatory signaling cascades that drive inflammation resolution [92]. Many of these signaling events involve lipid mediators such as lipoxins, resolvins, and protectins [93]. Immediately downstream of apoptotic cell recognition, activation of the apoptotic cell receptor MERTK leads to suppression of NF-κB and suppressor of cytokine signaling (SOCS) downstream of TLRs [94]. Engagement of apoptotic cells can also lead to expression and secretion of anti-inflammatory TGF-β and IL-10. Lastly, uptake of apoptotic cells by antigen presenting cells such as dendritic cells can lead to T-cell tolerance. Interestingly, in work from the Tabas lab, it appears that atherosclerosis-relevant inducers of apoptosis can affect the phagocyte inflammatory response. For example, when apoptotic cells were killed by lipoprotein-derived free cholesterol loading and overlaid onto macrophage phagocytes, this surprisingly triggered a modest proinflammatory response in the phagocytes: TNFα and IL-1β were induced, whereas the levels of transforming growth factor-beta and IL-10 were not increased [44]. Such cytokines as TNFα have been implicated in efferocytosis suppression and therefore this may be an explanation for how efficient efferocytosis in early atherosclerotic lesions later becomes defective in advanced plaque.
Nuclear receptors and transcriptional control of efferocytosis in lesions
The internalization of dying cells exposes phagocytes to large amounts of apoptotic cell derived lipids and cholesterol that threaten phagocyte homeostasis and require activation of compensatory cell survival and lipid metabolism signaling [53]. Phagocytes sense increased lipid and cholesterol loads through nuclear receptors, such as Liver X receptors (LXRα and LXRβ), which are oxysterol activated transcription factors [95] that in turn activate cholesterol efflux pathways [96]. In addition to their role in cholesterol efflux, nuclear receptors including LXRs and peroxisome proliferator-activated receptors (PPARs) also directly regulate transcription of molecules directly required for apoptotic cell internalization. For example, apoptotic cell engulfment activates LXR which in turn leads to increased expression of the phagocyte receptor MERTK [97]. Interestingly, LXRs have also been shown to regulate dendritic cell phenotype and function through regulation of actin signaling proteins and as discussed below, mature dendritic cells characteristically are compromised in phagocytic efficiency [98]. Both nuclear receptors PPARα and PPARδ also modulate efferocytosis. The phagocytic receptor CD36 has a PPAR response element (PPRE) and is induced by thiazolidinediones to promote engulfment [99]. PPARδ activates C1q, a bridging molecule that links apoptotic cells to phagocytes [100]. Finally, Rebe et al. [101] reported that transglutaminase 2 is induced by LXR/RXR during efferocytosis.
Efferocytosis efficiency and phagocyte heterogeneity in plaque
Accumulating evidence highlights the diversity of phagocyte subpopulations in atherosclerosis (Fig. 3) [102]. Plaque phagocyte subsets differentially express potential in vivo modifiers of phagocytic efficiency and phagocyte chemotaxis that include myeloperoxidase, neutrophil elastase, and matrix metalloproteinases (MMPs) [1, 103, 104]. For example, increased MMP activity in conjunction with reduced expression of Tissue Inhibitors of MMPs (TIMPs) has been linked to phagocyte plaque invasiveness and therefore could facilitate phagocyte migration towards distal apoptotic find-me signals [105, 106].In addition,tissue phagocytes can polarize towards a spectrum of activation states in response to the immediate inflammatory milieu [107]. Phagocytes exhibiting such differential expression patterns have been linked spatially to regions of interest, including the necrotic core [102, 108]. In vitro, “alternatively” activated M2 macrophages preferentially clear apoptotic cells and are often characterized by secretion of anti-inflammatory cytokines such as TGFβ and IL-10 [109]. “Classically” activated M1 macrophages are characterized by the secretion of pro-inflammatory cytokines such as TNFα and IL-6. TNFα-induced reactive oxygen generation has been shown to suppress efferocytosis in mature macrophages, while interestingly leaving less differentiated monocytes unaffected [75]. With respect to immature monocyte phagocytes, to what extent phagocyte heterogeneity is determined at the level of peripheral circulating monocyte precursors remains unresolved, however, the inflammatory milieu can direct the recruitment of heterogeneous monocytic subsets from the circulation [107, 110]. For example, Ly6CLO subsets have shown a predilection to differentiation into immature CD11cHI dendritic-like cells [110].
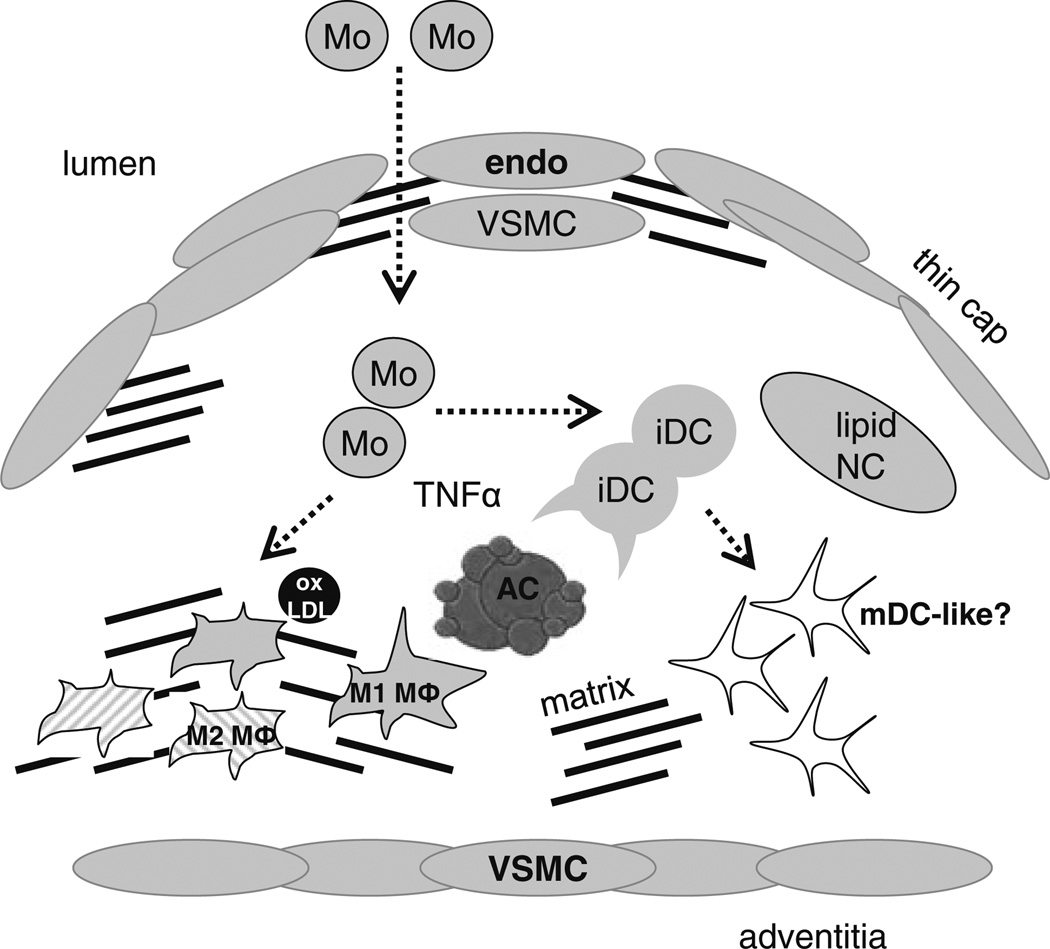
Fig. 3.
Schematic of phagocyte heterogeneity in plaque. Myeloid derived cells, including newly recruited monocyte (Mo) subsets, differentially activated macrophages (M1 and M2 MΦs) (that are symbolized by differentially striped patterns), and immature (iDC) or possible mature CD11C+ dendritic-like cells (mDC) in plaque. Arrows indicate differentiation pathways from immature monocyte and dendritic cell precursors. Endo endothelial cell, VSMC vascular smooth muscle cell, NC necrotic core, AC apoptotic cell, oxLDL oxidized LDL
In the case of dendritic cell phagocytes, the overall impact of these antigen presenting cells with the complex milieu of the plaque is unknown, however recent findings suggest they play a key role in promoting T helper 1 driven plaque immune responses [111]. In vitro, both immature and matured dendritic cells are characterized by reduced efficiency of apoptotic cell clearance [112]. Bone marrow-derived immature DCs express and secrete lactadherin (MFGE8) in exosomes and this and phagocytosis are down regulated after endotoxin-induced maturation or experimental sepsis [113]. Also in vitro, DC-like phenotypes can be induced by lipoprotein-loading of macrophages [114], however in our hands, this does not inhibit efferocytosis capacity [44]. In vivo, several different subsets of DCs can be identified based on cell-surface expression markers. The CD8+ dendritic cell subset selectively endocytose dying cells in culture and in vivo [115], yet this appears to be context dependent, as CD11C+ CD8+ splenic interdigitating dendritic cells are poorly phagocytic [116]. The mechanisms underlying reduced clearance efficiency in antigen presenting cells may be related to changes in metabolic processing of internalized dead cells, with macrophages exhibiting rapid degradation of engulfed targets while DCs do so with slower kinetics so as to sample peptides for MHC cross-presentation [117]. This reduced degradation is partly regulated by tempering of the oxidative burst in a NOX-dependent manner. The effects of other efferocytosis signaling pathways in DC-like cells are less well characterized, though decreases in uptake are linked to downregulation of scavenger receptors such as CD36 and integrins [112]. Also unknown is the extent to which intercellular communication between phagocyte subsets affect clearance efficiency. Interestingly, interstitial macrophages in lung can act to suppress dendritic-cell mediated immunity [118]. Whether phagocyte subset intercommunication affects efferocytosis has not been fully addressed. Finally, non professional phagocyte vascular smooth muscle cells appear to also play a role during clearance in plaque [119].
Future experimental and therapeutic directions
The resolution of inflammation is a key step in controlling infection and disease. Within advanced atherosclerotic lesions, accelerated macrophage death, in the face of defective phagocytic clearance, leads to post-apoptotic necrosis and chronic inflammation. This diseased milieu promotes plaque instability, which can lead to plaque rupture, acute thrombosis, and MI. Macrophage death occurs throughout all stages of atherosclerosis, yet the consequences of this vary in early versus advanced lesions [8]. For example, in early lesions, prompt phagocytic clearance of apoptotic macrophages limits lesion maturation and suppresses inflammation. However, as lesions progress, they become secondarily necrotic and there is an increase in the number of non-cleared apoptotic macrophages, suggesting that the efficiency of efferocytosis is impaired. Therefore, while macrophage death in early lesions may limit athero-progression, macrophage death in advanced lesions, in combination with defective efferocytosis, likely contributes to necrotic, unstable, and rupture-prone plaques. This overall concept presents an opportunity for novel therapeutic strategies directed against the progression of advanced plaques, namely, through restoration and augmentation of defective efferocytosis [8].
Enhancing clearance will likely decrease secondary necrosis and inflammation, leading to reduced macrophage content in advanced lesions and potentially reducing the development of autoimmune T-cells and circulating auto-antibodies. We favor enhancing phagocytic clearance of apoptotic cells over blocking macrophage apoptosis, because living macrophages also contribute to vulnerable plaque formation through production of procoagulant factors [120], and blocking apoptosis may promote early lesion development by contributing to increased cellularity and autocrine and paracrine inflammation. A possible disadvantage of clearance enhancement might be promotion of cholesterol-induced death in the phagocyte through uptake of cholesterol-laden macrophages and cholesterol overload. However, apoptotic cell engulfment is associated with cell-survival signaling [121], and we have shown that after macrophage phagocytes engage cholesterol-loaded apoptotic macrophages, they become protected from cytotoxicity through enhanced cholesterol esterification and efflux and through activation of cell-survival pathways involving Akt and NF-κβ [53].
Another efferocytosis-enhancing strategy is to take advantage of that fact that some phagocyte subpopulations are more efficient at efferocytosis than others. For example, some studies have shown that alternatively activated macrophages (M2) are better phagocytes than classically activated macrophages [122, 123], which is consistent with the role of M2 macrophages in resolution of inflammation. Therefore, molecules involved in alternative macrophage development, such as PPARγ or PPARδ [124, 125], or those that alter the behavior of fully differentiated macrophages into a more anti-inflammatory, pro-resolution state, such as endogenous lipoxins, can be investigated as potential drug targets or drugs. Lipoxins and aspirin-triggered lipoxins have been explored as efferocytosis enhancers in lupus, where defective efferocytosis of granulocytes in joints is thought to promote disease progression [126]. As described above, the hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor lovastatin increases efferocytosis of apoptotic T cells by human monocyte-derived macrophages in vitro and by alveolar macrophages in vivo [74]. Whether statins in humans enhance efferocytosis in atherosclerotic lesions and whether this effect could potentially add to the protective effect of cholesterol lowering by statins in decreasing atherothrombotic vascular disease remains to be investigated. Interestingly, statins have been linked to reduced dendritic cell content in human lesions [127].
Defining the molecular mechanisms of defective efferocytosis in human lesions may also lead to development of therapeutic strategies. For example, the fact that MERTK is rendered inactive through sheddase-mediated cleavage may provide a therapeutic opportunity. Thus, if excess MERTK cleavage were a culprit in human advanced plaques, inhibition of cleavage by drugs might suppress plaque necrosis. To a first approximation, elucidating correlations between alterations or dysfunction of efferocytosis molecules in advanced human lesions and plaque vulnerability and CVD may be helpful in identifying novel drug targets. Nevertheless, the strongest evidence is beginning to emerge from human genetic studies. For example, it will be interesting to determine whether functionally important polymorphisms in specific efferocytosis pathway genes are associated with plaque necrosis and acute coronary syndromes.
The study of the in vivo cellular and molecular regulation of efferocytosis, although still at a very early stage of understanding, may provide the basis for translational therapy in numerous chronic inflammatory disorders. Unlike many drugs that suffer from toxic side effects at higher doses, in theory the targeting and promotion of specific clearance pathways should (1) only lead to the faster removal and processing of already dying cells and (2) would be naturally buffered by cellular don’t-eat me signals and therefore prevent non-specific/toxic viable cell removal. In the case of CVD, phagocytic clearance plays an important role during many stages of disease progression. This includes efficient clearance mechanisms early during lesion atherogenesis followed by unknown mechanisms of defective uptake in advanced lesions [8]. Interesting therapeutic candidates include administration of derivations of recombinant lactadherin, which has already been shown to rescue animals through mechanisms that also lead to enhanced apoptotic cell clearance in a model of sepsis [128]. In addition, administration of immature DC exosomes, containing lactadherin, had similar effects. Thus, liposome based agents or nanoparticles with efferocytosis-enhancing agents, coupled with tissue-targeting motifs, may be an employable strategy. Clearance also plays a key role post plaque rupture during removal of dead cardiomyocytes post MI. Thus, these studies have the potential to elucidate novel therapeutic targets that can be directed against both the accumulation of inflammatory apoptotic cells and the progression of advanced plaques and CVD, namely through restoration and enhancement of defective efferocytosis.
Acknowledgements
I first and foremost thank my postdoctoral mentor Dr. Ira Tabas for support, guidance, and inspiration. I am also grateful to insightful discussions with Drs Alan Tall, Gwendalyn Randolph, Lauren Yvan-Charvet, Molly Ingersoll, and Dorien Schrijvers. Previous support from an American Heart Association National Scientist Development Grant 09SDG2150036 and currently: NIH NHLBI K99 1K99HL097021-01 and an Irving Institute for Clinical and Translational Research Pilot Grant.
References
- 1.Libby P, Nahrendorf M, Pittet MJ, Swirski FK. Diversity of denizens of the atherosclerotic plaque: not all monocytes are created equal. Circulation. 2008;117:3168–3170. doi: 10.1161/CIRCULATIONAHA.108.783068. [DOI] [PubMed] [Google Scholar]
- 2.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabas I. Apoptosis and plaque destabilization in atherosclerosis: the role of macrophage apoptosis induced by cholesterol. Cell Death Differ. 2004;11(1):S12–S16. doi: 10.1038/sj.cdd.4401444. [DOI] [PubMed] [Google Scholar]
- 4.Gregory C. Cell biology: sent by the scent of death. Nature. 2009;461:181–182. doi: 10.1038/461181a. [DOI] [PubMed] [Google Scholar]
- 5.Henson PM. Dampening inflammation. Nat Immunol. 2005;6:1179–1181. doi: 10.1038/ni1205-1179. [DOI] [PubMed] [Google Scholar]
- 6.Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol. 2002;15:439–446. doi: 10.1111/j.1540-8183.2002.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 7.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 9.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 10.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci USA. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frostegard J, Haegerstrand A, Gidlund M, Nilsson J. Biologically modified LDL increases the adhesive properties of endothelial cells. Atherosclerosis. 1991;90:119–126. doi: 10.1016/0021-9150(91)90106-d. [DOI] [PubMed] [Google Scholar]
- 12.Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth IB, Schulze-Osthoff K, Belka C, Stuhler G, Wesselborg S. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 13.Gautier EL, Huby T, Witztum JL, Ouzilleau B, Miller ER, Saint-Charles F, Aucouturier P, Chapman MJ, Lesnik P. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009;119:1795–1804. doi: 10.1161/CIRCULATIONAHA.108.806158. [DOI] [PubMed] [Google Scholar]
- 14.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bournazou I, Pound JD, Duffin R, Bournazos S, Melville LA, Brown SB, Rossi AG, Gregory CD. Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J Clin Invest. 2009;119:20–32. doi: 10.1172/JCI36226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peter C, Wesselborg S, Herrmann M, Lauber K. Dangerous attraction: phagocyte recruitment and danger signals of apoptotic and necrotic cells. Apoptosis. 2010 doi: 10.1007/s10495-010-0472-1. [DOI] [PubMed] [Google Scholar]
- 17.Gregory CD, Jean-Charles L. Microenvironemental influences of apoptosis in vivo and in vitro. Apoptosis. 2010 doi: 10.1007/s10495-010-0485-9. [DOI] [PubMed] [Google Scholar]
- 18.Moodley Y, Rigby P, Bundell C, Bunt S, Hayashi H, Misso N, McAnulty R, Laurent G, Scaffidi A, Thompson P, Knight D. Macrophage recognition and phagocytosis of apoptotic fibroblasts is critically dependent on fibroblast-derived throm-bospondin 1 and CD36. Am J Pathol. 2003;162:771–779. doi: 10.1016/S0002-9440(10)63874-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Truman LA, Ford CA, Pasikowska M, Pound JD, Wilkinson SJ, Dumitriu IE, Melville L, Melrose LA, Ogden CA, Nibbs R, Graham G, Combadiere C, Gregory CD. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112:5026–5036. doi: 10.1182/blood-2008-06-162404. [DOI] [PubMed] [Google Scholar]
- 20.Wakasugi K, Schimmel P. Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science. 1999;284:147–151. doi: 10.1126/science.284.5411.147. [DOI] [PubMed] [Google Scholar]
- 21.Horino K, Nishiura H, Ohsako T, Shibuya Y, Hiraoka T, Kitamura N, Yamamoto T. A monocyte chemotactic factor, S19 ribosomal protein dimer, in phagocytic clearance of apoptotic cells. Lab Invest. 1998;78:603–617. [PubMed] [Google Scholar]
- 22.Peter C, Waibel M, Radu CG, Yang LV, Witte ON, Schulze-Osthoff K, Wesselborg S, Lauber K. Migration to apoptotic “find-me” signals is mediated via the phagocyte receptor G2A. J Biol Chem. 2008;283:5296–5305. doi: 10.1074/jbc.M706586200. [DOI] [PubMed] [Google Scholar]
- 23.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 26.Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell. 2004;14:277–287. doi: 10.1016/s1097-2765(04)00237-0. [DOI] [PubMed] [Google Scholar]
- 27.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagao T, Qin C, Grosheva I, Maxfield FR, Pierini LM. Elevated cholesterol levels in the plasma membranes of macrophages inhibit migration by disrupting RhoA regulation. Arterioscler Thromb Vasc Biol. 2007;27:1596–1602. doi: 10.1161/ATVBAHA.107.145086. [DOI] [PubMed] [Google Scholar]
- 29.Ravichandran KS. “Recruitment signals” from apoptotic cells: invitation to a quiet meal. Cell. 2003;113:817–820. doi: 10.1016/s0092-8674(03)00471-9. [DOI] [PubMed] [Google Scholar]
- 30.Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418:200–203. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 31.Wei YS, Lan Y, Liu YG, Meng LQ, Xu QQ, Xie HY. Platelet-endothelial cell adhesion molecule-1 gene polymorphism and its soluble level are associated with ischemic stroke. DNA Cell Biol. 2009;28:151–158. doi: 10.1089/dna.2008.0817. [DOI] [PubMed] [Google Scholar]
- 32.Okazawa H, Motegi S, Ohyama N, Ohnishi H, Tomizawa T, Kaneko Y, Oldenborg PA, Ishikawa O, Matozaki T. Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J Immunol. 2005;174:2004–2011. doi: 10.4049/jimmunol.174.4.2004. [DOI] [PubMed] [Google Scholar]
- 33.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 34.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou W, Lu Q, Zhao D, Li W, Mapes J, Xie Y, Wang X. Caenorhabditis elegans myotubularin MTM-1 negatively regulates the engulfment of apoptotic cells. PLoS Genet. 2009;5:e1000679. doi: 10.1371/journal.pgen.1000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinchen JM. A model to die for: signaling to apoptotic cell removal in worm, fly and mouse. Apoptosis. 2010 doi: 10.1007/s10495-010-0509-5. [DOI] [PubMed] [Google Scholar]
- 37.Li S, Sun Y, Liang CP, Thorp EB, Han S, Jehle AW, Saraswathi V, Pridgen B, Kanter JE, Li R, Welch CL, Hasty AH, Bornfeldt KE, Breslow JL, Tabas I, Tall AR. Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of ob/ob mice and reversal by a fish oil diet. Circ Res. 2009;105:1072–1082. doi: 10.1161/CIRCRESAHA.109.199570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schutters K, Reutelingsperger C. Phosphatidylserine targeting for diagnosis and treatment of human diseases. Apoptosis. 2010 doi: 10.1007/s10495-010-0503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arur S, Uche UE, Rezaul K, Fong M, Scranton V, Cowan AE, Mohler W, Han DK. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell. 2003;4:587–598. doi: 10.1016/s1534-5807(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 40.Sambrano GR, Steinberg D. Recognition of oxidatively damaged and apoptotic cells by an oxidized low density lipoprotein receptor on mouse peritoneal macrophages: role of membrane phosphatidylserine. Proc Natl Acad Sci USA. 1995;92:1396–1400. doi: 10.1073/pnas.92.5.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savill JS, Henson PM, Haslett C. Phagocytosis of aged human neutrophils by macrophages is mediated by a novel “charge-sensitive” recognition mechanism. J Clin Invest. 1989;84:1518–1527. doi: 10.1172/JCI114328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaillon S, Jeannin P, Hamon Y, Fremaux I, Doni A, Bottazzi B, Blanchard S, Subra JF, Chevailler A, Mantovani A, Delneste Y. Endogenous PTX3 translocates at the membrane of late apoptotic human neutrophils and is involved in their engulfment by macrophages. Cell Death Differ. 2009;16:465–474. doi: 10.1038/cdd.2008.173. [DOI] [PubMed] [Google Scholar]
- 43.Guzik K, Bzowska M, Smagur J, Krupa O, Sieprawska M, Travis J, Potempa J. A new insight into phagocytosis of apoptotic cells: proteolytic enzymes divert the recognition and clearance of polymorphonuclear leukocytes by macrophages. Cell Death Differ. 2007;14:171–182. doi: 10.1038/sj.cdd.4401927. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Gerbod-Giannone MC, Seitz H, Cui D, Thorp E, Tall AR, Matsushima GK, Tabas I. Cholesterol-induced apoptotic macrophages elicit an inflammatory response in phagocytes, which is partially attenuated by the Mer receptor. J Biol Chem. 2006;281:6707–6717. doi: 10.1074/jbc.M510579200. [DOI] [PubMed] [Google Scholar]
- 45.Ishimoto Y, Ohashi K, Mizuno K, Nakano T. Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J Biochem. 2000;127:411–417. doi: 10.1093/oxfordjournals.jbchem.a022622. [DOI] [PubMed] [Google Scholar]
- 46.Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, Blanc-Brude O, Barateau V, Potteaux S, Merval R, Esposito B, Teissier E, Daemen MJ, Leseche G, Boulanger C, Tdgui A, Mallat Z. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 2007;115:2168–2177. doi: 10.1161/CIRCULATIONAHA.106.662080. [DOI] [PubMed] [Google Scholar]
- 47.Benzakour O, Kanthou C. The anticoagulant factor, protein S, is produced by cultured human vascular smooth muscle cells and its expression is up-regulated by thrombin. Blood. 2000;95:2008–2014. [PubMed] [Google Scholar]
- 48.Komura H, Miksa M, Wu R, Goyert SM, Wang P. Milk fat globule epidermal growth factor-factor VIII is down-regulated in sepsis via the lipopolysaccharide-CD14 pathway. J Immunol. 2009;182:581–587. doi: 10.4049/jimmunol.182.1.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y, Khanna S, Goodyear CS, Park YB, Raz E, Thiel S, Gronwall C, Vas J, Boyle DL, Corr M, Kono DH, Silverman GJ. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. J Immunol. 2009;183:1346–1359. doi: 10.4049/jimmunol.0900948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 51.Liu J, Thewke DP, Su YR, Linton MF, Fazio S, Sinensky MS. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptornull mice. Arterioscler Thromb Vasc Biol. 2005;25:174–179. doi: 10.1161/01.ATV.0000148548.47755.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 53.Cui D, Thorp E, Li Y, Wang N, Yvan-Charvet L, Tall AR, Tabas I. Pivotal advance: macrophages become resistant to cholesterol-induced death after phagocytosis of apoptotic cells. J Leukoc Biol. 2007;82:1040–1050. doi: 10.1189/jlb.0307192. [DOI] [PubMed] [Google Scholar]
- 54.Platt N, da Silva RP, Gordon S. Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol. 1998;8:365–372. doi: 10.1016/s0962-8924(98)01329-4. [DOI] [PubMed] [Google Scholar]
- 55.Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 56.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 57.Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, Ito S, Dranoff G, Kaplan GG, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem. 2003;278:1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- 59.Toth B, Garabuczi E, Sarang Z, Vereb G, Vamosi G, Aeschlimann D, Blasko B, Becsi B, Erdodi F, Lacy-Hulbert A, Zhang A, Falasca L, Birge RB, Balajthy Z, Melino G, Fesus L, Szondy Z. Transglutaminase 2 is needed for the formation of an efficient phagocyte portal in macrophages engulfing apoptotic cells. J Immunol. 2009;182:2084–2092. doi: 10.4049/jimmunol.0803444. [DOI] [PubMed] [Google Scholar]
- 60.Boisvert WA, Rose DM, Boullier A, Quehenberger O, Sydlaske A, Johnson KA, Curtiss LK, Terkeltaub R. Leukocyte transglutaminase 2 expression limits atherosclerotic lesion size. Arterioscler Thromb Vasc Biol. 2006;26:563–569. doi: 10.1161/01.ATV.0000203503.82693.c1. [DOI] [PubMed] [Google Scholar]
- 61.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wallet MA, Sen P, Flores RR, Wang Y, Yi Z, Huang Y, Mathews CE, Earp HS, Matsushima G, Wang B, Tisch R. MerTK is required for apoptotic cell-induced T cell tolerance. J Exp Med. 2008;205:219–232. doi: 10.1084/jem.20062293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garcia-Touchard A, Henry TD, Sangiorgi G, Spagnoli LG, Mauriello A, Conover C, Schwartz RS. Extracellular proteases in atherosclerosis and restenosis. Arterioscler Thromb Vasc Biol. 2005;25:1119–1127. doi: 10.1161/01.ATV.0000164311.48592.da. [DOI] [PubMed] [Google Scholar]
- 64.Cardellini M, Menghini R, Martelli E, Casagrande V, Marino A, Rizza S, Porzio O, Mauriello A, Solini A, Ippoliti A, Lauro R, Folli F, Federici M. TIMP3 is reduced in atherosclerotic plaques from subjects with type 2 diabetes and increased by SirT1. Diabetes. 2009;58:2396–2401. doi: 10.2337/db09-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krysko O, Vandenabeele P, Krysko DV, Bachert C. impairment of phagocytosis of apoptotic cells and its role in chronic airway diseases. Apoptosis. 2010 doi: 10.1007/s10495-010-0504-x. [DOI] [PubMed] [Google Scholar]
- 66.Vandivier RW, Fadok VA, Hoffmann PR, Bratton DL, Penvari C, Brown KK, Brain JD, Accurso FJ, Henson PM. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest. 2002;109:661–670. doi: 10.1172/JCI13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Canault M, Peiretti F, Kopp F, Bonardo B, Bonzi MF, Coudeyre JC, Alessi MC, Juhan-Vague I, Nalbone G. The TNF alpha converting enzyme (TACE/ADAM17) is expressed in the atherosclerotic lesions of apolipoprotein E-deficient mice: possible contribution to elevated plasma levels of soluble TNF alpha receptors. Atherosclerosis. 2006;187:82–91. doi: 10.1016/j.atherosclerosis.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 68.Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM, Graham DK. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109:1026–1033. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krysko DV, Denecker G, Festjens N, Gabriels S, Parthoens E, D’Herde K, Vandenabeele P. Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell Death Differ. 2006;13:2011–2022. doi: 10.1038/sj.cdd.4401900. [DOI] [PubMed] [Google Scholar]
- 70.May RC, Machesky LM. Phagocytosis and the actin cytoskeleton. J Cell Sci. 2001;114:1061–1077. doi: 10.1242/jcs.114.6.1061. [DOI] [PubMed] [Google Scholar]
- 71.Lu M, Ravichandran KS. Dock180-ELMO cooperation in Rac activation. Methods Enzymol. 2006;406:388–402. doi: 10.1016/S0076-6879(06)06028-9. [DOI] [PubMed] [Google Scholar]
- 72.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 73.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 74.Morimoto K, Janssen WJ, Fessler MB, McPhillips KA, Borges VM, Bowler RP, Xiao YQ, Kench JA, Henson PM, Vandivier RW. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J Immunol. 2006;176:7657–7665. doi: 10.4049/jimmunol.176.12.7657. [DOI] [PubMed] [Google Scholar]
- 75.McPhillips K, Janssen WJ, Ghosh M, Byrne A, Gardai S, Remigio L, Bratton DL, Kang JL, Henson P. TNF-alpha inhibits macrophage clearance of apoptotic cells via cytosolic phospholipase A2 and oxidant-dependent mechanisms. J Immunol. 2007;178:8117–8126. doi: 10.4049/jimmunol.178.12.8117. [DOI] [PubMed] [Google Scholar]
- 76.Anderson HA, Englert R, Gursel I, Shacter E. Oxidative stress inhibits the phagocytosis of apoptotic cells that have externalized phosphatidylserine. Cell Death Differ. 2002;9:616–625. doi: 10.1038/sj.cdd.4401013. [DOI] [PubMed] [Google Scholar]
- 77.Young JD, Ko SS, Cohn ZA. The increase in intracellular free calcium associated with IgG gamma 2b/gamma 1 Fc receptor-ligand interactions: role in phagocytosis. Proc Natl Acad Sci USA. 1984;81:5430–5434. doi: 10.1073/pnas.81.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hackam DJ, Rotstein OD, Schreiber A, Zhang W, Grinstein S. Rho is required for the initiation of calcium signaling and phagocytosis by Fcgamma receptors in macrophages. J Exp Med. 1997;186:955–966. doi: 10.1084/jem.186.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosales C, Brown EJ. Signal transduction by neutrophil immunoglobulin G Fc receptors. Dissociation of intracytoplasmic calcium concentration rise from inositol 1,4,5-trisphosphate. J Biol Chem. 1992;267:5265–5271. [PubMed] [Google Scholar]
- 80.Zhang J, Guo J, Dzhagalov I, He YW. An essential function for the calcium-promoted Ras inactivator in Fcgamma receptor-mediated phagocytosis. Nat Immunol. 2005;6:911–919. doi: 10.1038/ni1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sawyer DW, Sullivan JA, Mandell GL. Intracellular free calcium localization in neutrophils during phagocytosis. Science. 1985;230:663–666. doi: 10.1126/science.4048951. [DOI] [PubMed] [Google Scholar]
- 82.Jaconi ME, Lew DP, Carpentier JL, Magnusson KE, Sjogren M, Stendahl O. Cytosolic free calcium elevation mediates the phagosome-lysosome fusion during phagocytosis in human neutrophils. J Cell Biol. 1990;110:1555–1564. doi: 10.1083/jcb.110.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marks PW, Maxfield FR. Local and global changes in cytosolic free calcium in neutrophils during chemotaxis and phagocytosis. Cell Calcium. 1990;11:181–190. doi: 10.1016/0143-4160(90)90069-7. [DOI] [PubMed] [Google Scholar]
- 84.Canetti C, Aronoff DM, Choe M, Flamand N, Wettlaufer S, Toews GB, Chen GH, Peters-Golden M. Differential regulation by leukotrienes and calcium of Fc gamma receptor-induced phagocytosis and Syk activation in dendritic cells versus macrophages. J Leukoc Biol. 2006;79:1234–1241. doi: 10.1189/jlb.0705374. [DOI] [PubMed] [Google Scholar]
- 85.Becker SM, Delamarre L, Mellman I, Andrews NW. Differential role of the Ca(2+) sensor synaptotagmin VII in macrophages and dendritic cells. Immunobiology. 2009;214(7):495–505. doi: 10.1016/j.imbio.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Poggi A, Carosio R, Rubartelli A, Zocchi MR. Beta(3)-mediated engulfment of apoptotic tumor cells by dendritic cells is dependent on CAMKII: inhibition by HIV-1 Tat. J Leukoc Biol. 2002;71:531–537. [PubMed] [Google Scholar]
- 87.Herrmann TL, Morita CT, Lee K, Kusner DJ. Calmodulin kinase II regulates the maturation and antigen presentation of human dendritic cells. J Leukoc Biol. 2005;78:1397–1407. doi: 10.1189/jlb.0205105. [DOI] [PubMed] [Google Scholar]
- 88.Cuttell L, Vaughan A, Silva E, Escaron CJ, Lavine M, Van GE, Eid JP, Quirin M, Franc NC. Undertaker, a Drosophila Junctophilin, links Draper-mediated phagocytosis and calcium homeostasis. Cell. 2008;135:524–534. doi: 10.1016/j.cell.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 89.De Lorenzo BH, Godoy LC, Novaes e Brito RR, Pagano RL, Amorim-Dias MA, Grosso DM, Lopes JD, Mariano M. Macrophage suppression following phagocytosis of apoptotic neutrophils is mediated by the S100A9 calcium-binding protein. Immunobiology. 2009;215(5):341–347. doi: 10.1016/j.imbio.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 90.Gronski MA, Kinchen JM, Juncadella IJ, Franc NC, Ravichandran KS. An essential role for calcium flux in phagocytes for apoptotic cell engulfment and the anti-inflammatory response. Cell Death Differ. 2009;16:1323–1331. doi: 10.1038/cdd.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 92.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 95.Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, Mangelsdorf DJ. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 96.Castrillo A, Tontonoz P. Nuclear receptors in macrophage biology: at the crossroads of lipid metabolism and inflammation. Annu Rev Cell Dev Biol. 2004;20:455–480. doi: 10.1146/annurev.cellbio.20.012103.134432. [DOI] [PubMed] [Google Scholar]
- 97.Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Diaz M, Gallardo G, de Galarreta CR, Salazar J, Lopez F, Edwards P, Parks J, Andujar M, Tontonoz P, Castrillo A. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Geyeregger R, Zeyda M, Bauer W, Kriehuber E, Saemann MD, Zlabinger GJ, Maurer D, Stulnig TM. Liver X receptors regulate dendritic cell phenotype and function through blocked induction of the actin-bundling protein fascin. Blood. 2007;109:4288–4295. doi: 10.1182/blood-2006-08-043422. [DOI] [PubMed] [Google Scholar]
- 99.Majai G, Sarang Z, Csomos K, Zahuczky G, Fesus L. PPARgamma-dependent regulation of human macrophages in phagocytosis of apoptotic cells. Eur J Immunol. 2007;37:1343–1354. doi: 10.1002/eji.200636398. [DOI] [PubMed] [Google Scholar]
- 100.Mukundan L, Odegaard JI, Morel CR, Heredia JE, Mwangi JW, Ricardo-Gonzalez RR, Goh YP, Eagle AR, Dunn SE, Awakuni JU, Nguyen KD, Steinman L, Michie SA, Chawla A. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med. 2009;15(11):1266–1272. doi: 10.1038/nm.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rebe C, Raveneau M, Chevriaux A, Lakomy D, Sberna AL, Costa A, Bessede G, Athias A, Steinmetz E, Lobaccaro JM, Alves G, Menicacci A, Vachenc S, Solary E, Gambert P, Masson D. Induction of transglutaminase 2 by a liver X receptor/retinoic acid receptor alpha pathway increases the clearance of apoptotic cells by human macrophages. Circ Res. 2009;105:393–401. doi: 10.1161/CIRCRESAHA.109.201855. [DOI] [PubMed] [Google Scholar]
- 102.Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 103.Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dollery CM, Owen CA, Sukhova GK, Krettek A, Shapiro SD, Libby P. Neutrophil elastase in human atherosclerotic plaques: production by macrophages. Circulation. 2003;107:2829–2836. doi: 10.1161/01.CIR.0000072792.65250.4A. [DOI] [PubMed] [Google Scholar]
- 105.Johnson JL, Sala-Newby GB, Ismail Y, Aguilera CM, Newby AC. Low tissue inhibitor of metalloproteinases 3 and high matrix metalloproteinase 14 levels defines a subpopulation of highly invasive foam-cell macrophages. Arterioscler Thromb Vasc Biol. 2008;28:1647–1653. doi: 10.1161/ATVBAHA.108.170548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Salomon RN, Underwood R, Doyle MV, Wang A, Libby P. Increased apolipoprotein E and c-fms gene expression without elevated interleukin 1 or 6 mRNA levels indicates selective activation of macrophage functions in advanced human atheroma. Proc Natl Acad Sci USA. 1992;89:2814–2818. doi: 10.1073/pnas.89.7.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 108.Wilcox JN, Smith KM, Schwartz SM, Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci USA. 1989;86:2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu W, Roos A, Schlagwein N, Woltman AM, Daha MR, van Kooten C. IL-10-producing macrophages preferentially clear early apoptotic cells. Blood. 2006;107:4930–4937. doi: 10.1182/blood-2005-10-4144. [DOI] [PubMed] [Google Scholar]
- 110.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gautier EL, Huby T, Saint-Charles F, Ouzilleau B, Pirault J, Deswaerte V, Ginhoux F, Miller ER, Witztum JL, Chapman MJ, Lesnik P. Conventional dendritic cells at the crossroads between immunity and cholesterol homeostasis in atherosclerosis. Circulation. 2009;119:2367–2375. doi: 10.1161/CIRCULATIONAHA.108.807537. [DOI] [PubMed] [Google Scholar]
- 112.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miyasaka K, Hanayama R, Tanaka M, Nagata S. Expression of milk fat globule epidermal growth factor 8 in immature dendritic cells for engulfment of apoptotic cells. Eur J Immunol. 2004;34:1414–1422. doi: 10.1002/eji.200424930. [DOI] [PubMed] [Google Scholar]
- 114.Cho HJ, Shashkin P, Gleissner CA, Dunson D, Jain N, Lee JK, Miller Y, Ley K. Induction of dendritic cell-like phenotype in macrophages during foam cell formation. Physiol Genomics. 2007;29:149–160. doi: 10.1152/physiolgenomics.00051.2006. [DOI] [PubMed] [Google Scholar]
- 115.Iyoda T, Shimoyama S, Liu K, Omatsu Y, Akiyama Y, Maeda Y, Takahara K, Steinman RM, Inaba K. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leenen PJ, Radosevic K, Voerman JS, Salomon B, van Rooijen N, Klatzmann D, van Ewijk W. Heterogeneity of mouse spleen dendritic cells: in vivo phagocytic activity, expression of macrophage markers, and subpopulation turnover. J Immunol. 1998;160:2166–2173. [PubMed] [Google Scholar]
- 117.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299:1400–1403. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 118.Bedoret D, Wallemacq H, Marichal T, Desmet C, Quesada CF, Henry E, Closset R, Dewals B, Thielen C, Gustin P, de Leval L, van Rooijen N, Le Moine A, Vanderplasschen A, Cataldo D, Drion PV, Moser M, Lekeux P, Bureau F. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J Clin Invest. 2009;119(12):3723–3738. doi: 10.1172/JCI39717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Clarke MC, Talib S, Figg NL, Bennett MR. Vascular smooth muscle cell apoptosis induces interleukin-1-directed inflammation. Effects of hyperlipidemia-mediated inhibition of phagocytosis. Circ Res. 2009;106(2):363–372. doi: 10.1161/CIRCRESAHA.109.208389. [DOI] [PubMed] [Google Scholar]
- 120.Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GK, Lee RT. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol. 1996;7:330–335. doi: 10.1097/00041433-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 121.Weigert A, Johann AM, von Knethen A, Schmidt H, Geisslinger G, Brune B. Apoptotic cells promote macrophage survival by releasing the anti-apoptotic mediator sphingosine-1-phosphate. Blood. 2006;108:1635–1642. doi: 10.1182/blood-2006-04-014852. [DOI] [PubMed] [Google Scholar]
- 122.Loke P, Gallagher I, Nair MG, Zang X, Brombacher F, Mohrs M, Allison JP, Allen JE. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179:3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- 123.Peng Y, Latchman Y, Elkon KB. Ly6C(low) monocytes differentiate into dendritic cells and cross-tolerize T cells through PDL-1. J Immunol. 2009;182:2777–2785. doi: 10.4049/jimmunol.0803172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Eagle AR, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Odegaard JI, Ricardo-Gonzalez RR, Red EA, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mitchell S, Thomas G, Harvey K, Cottell D, Reville K, Berlasconi G, Petasis NA, Erwig L, Rees AJ, Savill J, Brady HR, Godson C. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol. 2002;13:2497–2507. doi: 10.1097/01.asn.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- 127.Yilmaz A, Reiss C, Tantawi O, Weng A, Stumpf C, Raaz D, Ludwig J, Berger T, Steinkasserer A, Daniel WG, Garlichs CD. HMG-CoA reductase inhibitors suppress maturation of human dendritic cells: new implications for atherosclerosis. Atherosclerosis. 2004;172:85–93. doi: 10.1016/j.atherosclerosis.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 128.Miksa M, Wu R, Dong W, Das P, Yang D, Wang P. Dendritic cell-derived exosomes containing milk fat globule epidermal growth factor-factor VIII attenuate proinflammatory responses in sepsis. Shock. 2006;25:586–593. doi: 10.1097/01.shk.0000209533.22941.d0. [DOI] [PubMed] [Google Scholar]