Abstract
Sulfadoxine-pyrimethamine (SP) is currently the drug of choice for intermittent preventive treatment of Plasmodium falciparum both in pregnancy and infancy. A prolonged parasite clearance time conferred by dhfr and dhps mutations is believed to be responsible for increased gametocyte prevalence in SP treated individuals. However, using a direct feeding assay in Mali, we showed that gametocytes present in peripheral venous blood post-SP treatment had reduced infectivity for Anopheles gambiae sensu stricto (ss) mosquitoes. We investigated the potential mechanisms involved in the dhfr and dhps quintuple mutant NF135 and the single dhps 437 mutant NF54. Concentrations of Sulfadoxine (S) and Pyrimethamine (P) equivalent to the serum levels of the respective drugs on day 3 (S = 61 ug/ml, P = 154.7 ng/ml) day 7 (S = 33.8 ug/ml, P = 66.6 ng/ml) and day 14 (S = 14.2 ug/ml, P = 15.7 ng/ml) post-SP treatment were used to study the effect on gametocytogenesis, gametocyte maturation and infectivity to Anopheles stephensi mosquitoes fed through an artificial membrane. The drugs readily induced gametocytogenesis in the mutant NF-135 strain but effectively killed the wild-type NF54. However, both drugs impaired gametocyte maturation yielding odd-shaped non-exflagelating mature gametocytes. The concomitant ingestion of both S and P together with gametocytemic blood-meal significantly reduced the prevalence of oocyst positivity as well as oocyst density when compared to controls (P < 0.001). In addition, day 3 concentrations of SP decreased mosquito survival by up to 65% (P < 0.001). This study demonstrates that SP is deleterious in vitro for gametocyte infectivity as well as mosquito survival.
Keywords: Plasmodium falciparum, Sulfadoxine-pyrimethamine, Gametocyte, Impaired gametocytogenesis, Mosquito, Infectivity
1. Introduction
In most tropical and many sub-tropical developing countries, malaria is a major cause of morbidity, especially among young children and pregnant women, and mortality, principally among infants and toddlers. Increasing resistance of Anopheles mosquitoes to insecticides has crippled national and regional Malaria Control Programs based on vector control interventions. Furthermore, the spread of chloroquine and Sulfadoxine-Pyrimethamine (SP)-resistant strains of Plasmodium falciparum in Asia, Africa and South America has created a public health concern in the clinical treatment and prevention of malaria in many countries.
Although artemisinin-based combination therapies are now the World Health Organization’s (WHO) recommended first line drugs for the management of uncomplicated malaria, there are very few options for preventing malaria during pregnancy in endemic countries. Intermittent preventive treatment of malaria (IPT), which is aimed at protecting at-risk populations from both clinical malaria and the sequelae of asymptomatic parasitemia (Kayentao et al., 2005; Manzi et al., 2008) continues to heavily rely on SP-containing regimens (Aponte et al., 2009). Widely used in pregnant women in malaria endemic zones (Briand et al., 2007; ter Kuile et al., 2007), IPT is now being contemplated for deployment in infants (IPTi) (Greenwood, 2007), in children (IPTc) (Buffet et al., 2008) and school children (IPTsc) (Clarke et al., 2008; Temperley et al., 2008; Barger et al., 2009). However, the possibility that this strategy may cause an increase of P. falciparum resistance to SP is a subject of debate (Sokhna et al., 2008; Dicko et al., 2010).
Point mutations in the genes encoding dihydrofolate reductase (DHFR) and dihydropteroate synthetase (DHPS), the respective targets of pyrimethamine (P) and sulfadoxine (S), have been associated with in vitro resistance to these drugs (Peterson et al., 1990; Wang et al., 1997). Increasing resistance to P is seen with the DHFR mutations S108N, N51 I and/or C59R, and I164L. A quintuple mutation, DHFR 108, 51, 59, and DHPS 437 and 540, was found to be predictive of clinical failure in Malawi (Kublin et al., 2002).
During the life cycle of Plasmodia a small percentage of the merozoites differentiate into sexual forms (gametocytes). Early stage I and II gametocytes appear after a few cycles of asexual multiplication, by the eighth day in in vitro cultures. Their maturation process, which includes stages III – VI, follows and peaks at around day 12 of culture. Stage V mature gametocytes are reached by day 14 of culture (Ponnudurai et al., 1982). When ingested by a mosquito, male and female gametocytes can unite, forming a zygote, which matures into the ookinete. The ookinete further differentiates into an oocyst, which will eventually release sporozoites. These sporozoites migrate in the mosquito’s salivary glands, thus completing the lifecycle. The ability of the host to infect the mosquito vector is determined by the presence of infectious gametocytes and the gametocytes’ sporogonic capacity.
Several anti-malarial drugs are known to induce P. falciparum gametocytes both in vivo and in vitro, (Buckling et al., 1999) which are suggestive of a response to drug-related stress. SP treatments increase the rate of gametocyte carriage in treated patients compared with untreated controls (Robert et al., 2000a,b; von Seidlein et al., 2001a).
Drug-resistant parasites are believed to be selected by drug pressure. Studies in South America found that in an area with rare SP failure, the presence of resistance-conferring mutations at the time of treatment was associated with prolonged parasite clearance times and with higher rates, levels and duration of gametocytemia (Mendez et al., 2002). Although some studies found a high infectivity of post-SP gametocytes (Targett et al., 2001), most published data and our own preliminary findings suggest that post-SP gametocytes present a decreased infectivity (Robert et al., 2000a). The mechanisms involved in the low infectiousness of post-SP gametocyte are poorly understood.
Deterring the spread of anti-malarial drug resistance will require an understanding of the specific mechanisms involved. The objective of this study was to investigate the impact of SP on the biology of P. falciparum gametocytogenesis, gametocyte maturation, oocyst formation in Anopheles mosquitoes, and on mosquito viability. We confirm that S, P and SP induced gametocytogenesis, notably in mutant parasites. However, both drugs inhibit gametocyte maturation in both mutant and wild-type parasites. Furthermore, a high dose of S is toxic for Anopheles stephensi.
2. Materials and methods
2.1. Standard membrane feeding assay
Gametocyte culture (7.5 ml) was harvested and added to 10 Eppendorf centrifuge tubes, each with 380 µl of packed cells, and centrifuged (20 s, 20,000 g). The supernatant was removed, pellets were resuspended with 75 µl non-immune serum and collected in a 15 ml tube. The compounds to be tested were diluted in 90 µl non-immune human serum which were mixed with 180 µl of gametocyte suspension.
The suspension was fed to 3–5 days old mosquitoes. Six days after the feed, 10–20 mosquitoes per feeder were dissected and checked for oocysts after staining the stomach with 2% Mercurochrome (Ponnudurai et al., 1989a).
2.2. Determination of the dhfr and dhps genotypes
Laboratory adapted P. falciparum strains (NF-54 (Ponnudurai et al., 1981), NF-114, NF-135, NF-151 , 3D7 (Rosario, 1981), 7G8 (Burkot et al., 1984) and Dd2 (Wellems et al. ,1990)) were screened for inclusion in the study. These strains were selected because they were routinely grown and used for gametocyte production in the laboratory at Nijmegen, The Netherlands.
Parasitemic blood from cultures of each strain was spotted onto 3MM Whatman filter papers in the Nijmegen laboratory and sent to the Malaria Research and Training Center (MRTC) in Bamako, Mali for genotyping. DNA was extracted from these filter paper blots as previously described (Djimde et al., 2001). Nested mutation-specific PCR and nested PCR followed with restriction endonuclease digestion were used to determine the alleles of dhfr at codons 51, 59 and 108 and dhps at 437 and 540 as described (Djimde et al., 2008).
2.3. Gametocyte production
Frozen P. falciparum samples were thawed and grown in vitro to steady state in an automated tipper system in red blood cells (RBCs) of blood group O and non-immune A serum, and harvested after 13 or 14 days (Ponnudurai et al., 1982a). Subcultures at 0.5%, 1% or 3% parasitemia were grown in a shaker (Ponnudurai et al., 1983), a tipper (Ponnudurai et al., 1982a) or a candle jar (Ifediba and Vanderberg, 1981) at 5% hematocrit, respectively. The culture medium (RPMI, HEPES, hypoxanthine, human serum and bicarbonate) was changed twice each day without adding fresh RBCs. The cultures were maintained at 37° C to avoid any exflagellation. Thin smears were made regularly, Giemsa-stained and examined to monitor gametocyte presence and age. The infectivity of gametocytes was determined by a pre-feed experiment (van der Kolk et al., 2005).
2.4. Capacity to exflagellate
The capacity to exflagellate was tested for each culture containing mature stage V gametocytes prior to mosquitoes feeding (Ponnudurai et al., 1982b). The quality of mature gametocytes was assessed by testing the ability of the males to exflagellate. All materials and reagents were at 37 ° C before starting the experiment. Approximately 20 ul of culture were centrifuged and 2 ul of pellet were mixed with a drop of FCS on a slide. The slide was then put in a humidified box at room temperature and covered for 10 min. The preparation was covered with a cover-slide, sealed with Vaseline and observed under a light microscope. The exflagellated male gametocytes app eared as small moving RBCs but at higher resolution flagella could be observed.
2.5. Drug treatment of parasite culture
The parasite cultures were treated with different concentrations of S and P at different stages of gametocyte development. These concentrations were chosen to correspond with physiological serum levels of S or P on days 3, 7, 14, 21 and 28 following a single oral dose of SP. We hereafter refer to these concentrations as SD3 (sulfadoxine day 3 = 61 ug/ml), SD7 (sulfadoxine day 7 = 33.8 ug/ml), SD14 (sulfadoxine day 14 = 14.2 ug/ml), SD21 (sulfadoxine day 21 = 7.6 ug/ml) and SD28 (sulfadoxine day 28 = 3.8 ug/ml); PD3 (pyrimethamine day 3 = 154.7 ng/ml), PD7 (pyrimethamine day 7 = 66.6 ng/ml), PD14 (pyrimethamine day 14 = 15.7 ng/ml), PD21 (pyrimethamine day 21 = 19.7 ng/ml) and PD28 (pyrimethamine day 28 = 9.9 ng/ml); and SPD3 (sulfadoxine-pyrimethamine day 3), SPD7, SPD14, SPD21 and SPD28 (our unpublished data).
The following experimental design was based on the biology and timing of the different steps in gametocytogenesis.
2.5.1. Effect of SP on the induction of gametocytes
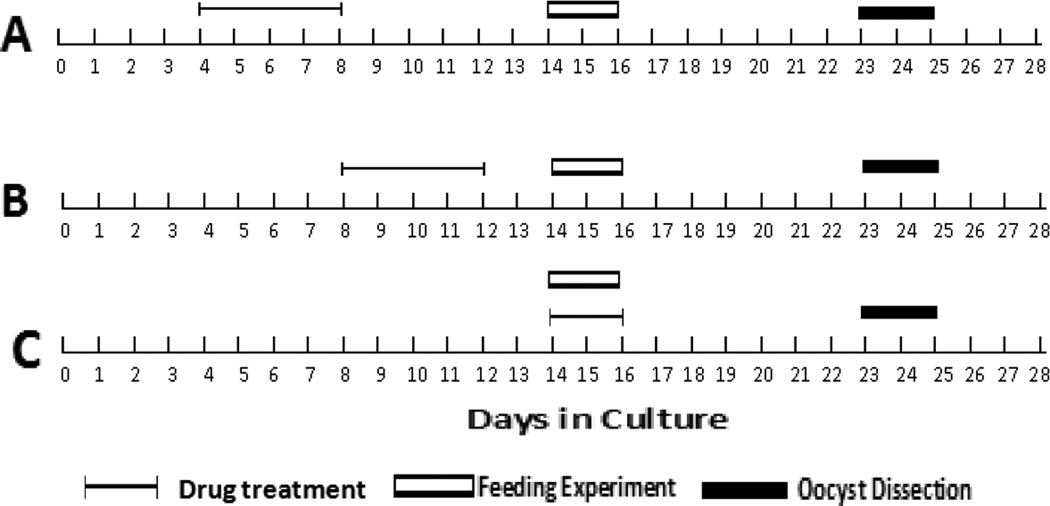
Four days old parasite cultures of NF135 and NF54 were transferred on 24-well plates and submitted to SD3, SD7, SD14, SD21, SD28 or PD3, PD7, PD14, PD21, PD28 for 5 days (Fig. 1A). No drug was added on subsequent days of culture. Gametocytogenesis and gametocytemia were assessed by light microscopy from day 8 onwards. The quality (morphology, pigmentation, overall appearance) and infectivity of the resulting gametocytes were assessed when they reached 14 days of age.
Fig. 1.
Sulfadoxine, pyrimethamine or sulfadoxine-pyrimethamine pressure, mosquito feeding and oocyst measurement days are shown for each experimental design. A) Impact of Sulfadoxine, pyrimethamine or sulfadoxine-pyrimethamine on gametocytogenesis induction; B) impact of Sulfadoxine, pyrimethamine or sulfadoxine-pyrimethamine on gametocyte maturation; C) impact of Sulfadoxine, pyrimethamine or sulfadoxine-pyrimethamine on gametocyte infectivity.
2.5.2. Effect of SP on gametocyte maturation
Eight days old cultures containing early stage II gametocytes of NF135 and NF54 were transferred on 24-well plates and submitted to SD3, SD7, SD14, PD3, PD7 and PD14 (Fig. 1B). Drug pressure was maintained for 5 days after which time normal culture medium was continued. The quality (morphology, pigmentation, overall appearance) and infectivity of the resulting gametocytes were assessed at day 14.
2.5.3. Effect of SP on gametocyte infectivity
Gametocyte production cultures started with the two strains in human RBCs and were maintained for 14 days in a shaking culture system for NF135 and the static culture system for NF54. Standard membrane feeding assays (SMFAs) with laboratory reared A. stephensi were performed as previously described (Ponnudurai et al., 1989a). Briefly, a pre-feed was first performed on a small scale as previously described (Ponnudurai et al., 1989a); 300 ul of culture containing 14–16 days old gametocytes were mixed with 180 ul of RBCs and centrifuged. The supernatant was carefully discarded and the pellet mixed with 150 ul of human serum or FCS. This gametocyte preparation was transferred to a small feeder and fed to 20 A. stephensi mosquitoes which had been starved for 12 h. All operations were performed at 37° C. Twenty-four hours after the feeding, 10 mosquitoes were checked for the presence of round forms using FITC-conjugated anti-Pfs25 monoclonal antibodies.
The SMFA was conducted the next day only f round forms were found in the midgut of 20 dissected mosquitoes. Furthermore, 30 mosquitoes were allowed to feed on each sample but 20 surviving mosquitoes were dissected (Ponnudurai et al., 1989b). Reduction of infectivity is defined as a significant decrease in the prevalence of oocyst positivity among the dissected mosquitoes. Between days 14 and 16, stage V mature gametocytes were tested for exflagellation before proceeding with a SMFA.
S, P and SP were mixed with the gametocyte preparation to yield final concentrations equivalent to SD3, SD7, SD14, PD3, PD7, PD14, SPD3, SPD7 or SPD14 (Fig. 1C). The resulting preparations (mature gametocytes + drug) were used to feed mosquitoes (A. stephensi) through a membrane feeder. For each of three consecutive experiments 20 mosquitoes per strain were dissected on day 8 post-feeding and the presence and the density of oocysts were assessed by light microscopy.
2.6. Mosquito survival
As described in Section 2.1, during the infectivity SMFAs 30 mosquitoes in a cup were fed on gametocyte preparations containing no drug or various concentrations of drugs. Twenty-four hours after the feeding experiments, the number of surviving mosquitoes were counted and recorded for each experiment and each drug concentration.
2.7. Data handling and analysis
The results were recorded on data sheets and entered in Microsoft Access and further analyzed in SPSS vs.12.0. Descriptive statistics provided gametocyte prevalence, gametocyte loads, mosquito oocyst infection rates and loads. A Chi-square test was used to compare prevalence of gametocytes and oocyst infection rates, between comparison groups. Two-way ANOVA or Kruskal-Wallis H tests were used to compare the spread of the oocyst loads between the comparison groups. Experiments were repeated at least once in the same facility, by the same investigators.
3. Results
3.1. Genotyping results
The dhfr and dhps genotypes of the full set of strains analyzed are presented in Table 1. Two strains with the most contrasting genotypes, NF135, (quintuple mutant for dhfr N51I+C59R+S108N and dhps A437G+K540E) and NF54 (wild-type for all but mutant for dhps A437G) were selected for all subsequent gametocyte production and feeding experiments.
Table 1.
Dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps) genotypes of Plasmodium falciparum laboratory strains screened during this study.
| dhfr | dhps | ||||
|---|---|---|---|---|---|
| Codons/P. falciparum strains | 51 | 59 | 108 | 437 | 540 |
| NF-135-W45 | M | M | M | M | M |
| 3D7 | W | W | W | M | W |
| 7G8 | M | W | M | M | W |
| NF-135-T8 | M | M | M | M | M |
| NF-151 | M | W | M | W | W |
| NF-135-T7 | M | M | M | M | M |
| NF-114 | W | M | M | W | W |
| NF-54 | W | W | W | M | W |
| Dd2 | M | M | M | M | W |
M, mutant allele
W, wild-type allele.
3.2. Induction of gametocytes with different concentrations of S and P
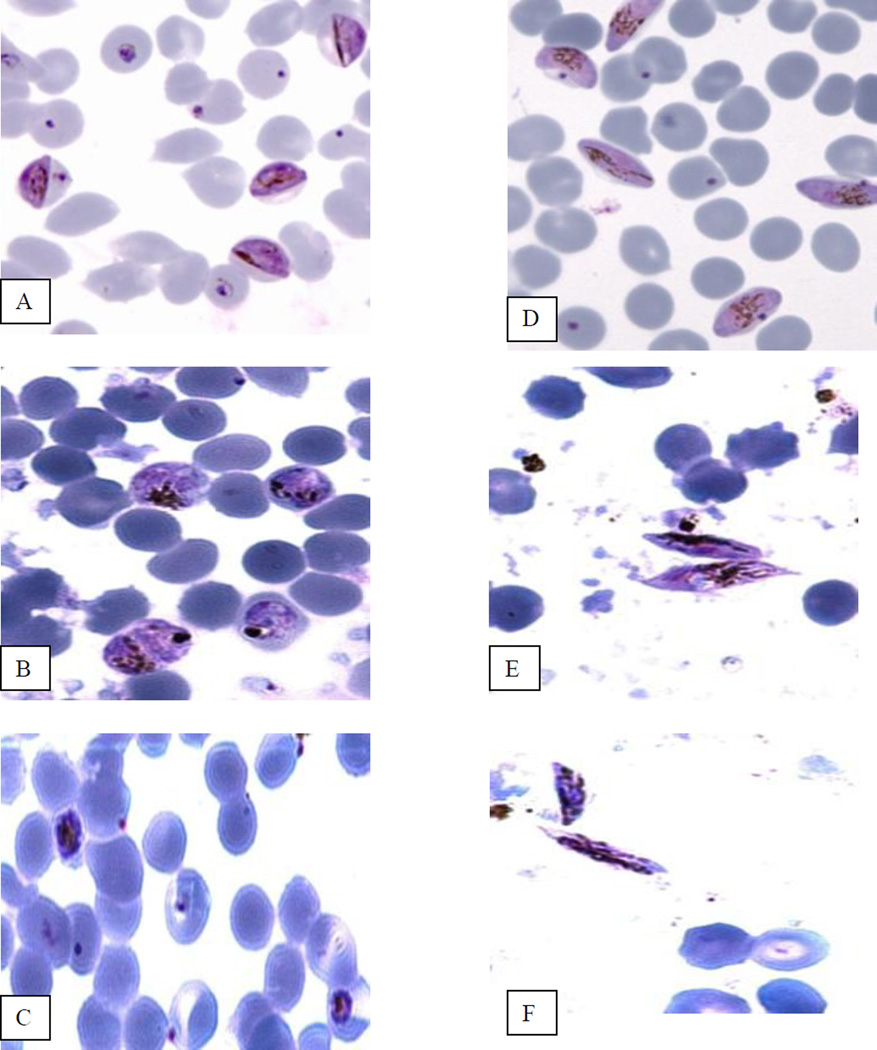
With NF54 and both S and P, while the controls progressed to mature gametocytes (Fig. 2A and D), all drug concentrations lead to parasite degeneration. (Fig. 2B and C).
Fig. 2.
Effect of sulfadoxine and pyrimethamine on Plasmodium falciparum NF 54 strain. A–C) Gametocyte induction experiment. A) Stage II normal gametocytes at day 8; B) cultures treated with pyrimethamine; C: cultures treated with sulfadoxine. D–F) Gametocyte maturation experiment. D) Stage V normal gametocyte at day 14; E) Stage V gametocytes obtained after treatment with pyrimethamine; F) Stage V gametocytes obtained after treatment with sulfadoxine.
In contrast, incubation of asexual stages of NF135 with different concentrations of S and P between days 4 and 8 induced differentiation into gametocyte stage II. These young gametocytes were observed within 24 h of drug treatment for the highest SD3 and PD3 drug concentrations and after 48 h for the lowest SD28 and PD28 drug concentrations. In the control untreated cultures, the first stage II gametocytes appeared on day 8 of culture. The resulting stages IV and V of NF-135 gametocytes showed no differences compared with control cultures. Exflagellation tests were positive for all drug concentrations and the controls. No SMFAs were done with these cultures.
3.3. Gametocyte maturation under S and P pressure
Day 8 early gametocytes of both NF 54 and NF-135 were exposed to the different concentrations of S and P. After 5 days of drug treatment, the resulting mature gametocytes of both strains were atypical. They were smaller, the cytoplasm showed darker and fatter pigments and the extremities were sharper (Fig. 2E and F) compared with control gametocytes (Fig. 2D). These gametocytes were neither able to exflagellate nor to infect mosquitoes.
3.4. Effect of S, P and SP on gametocyte infectivity
Since control NF135 cultures did not produce sufficient numbers of gametocytes, data are shown for NF54.
NF54 produced mature, healthy gametocytes by day 14 in culture (Fig. 2D). All exflagellation tests were positive and control SMFA experiments were successful (Table 2). In a typical experiment (Table 2) physiological Day 3 concentrations of S, P and SP significantly reduced the prevalence of oocysts in A. stephensi. SD7 and SPD7 equally significantly decreased oocyst positivity in the fed mosquitoes while P alone had no effect. Day 14 and lower concentrations had no effect on oocyst numbers. As shown in Table 2, most experiments were repeated once or twice on different days but results remained consistent.
Table 2.
Proportion of oocyst positivity in Anopheles stephensi mosquitoes fed on mature gametocyte preparations in the presence of physiological concentrations of sulfadoxine, pyrimethamine or sulfadoxine-pyrimethamine
| Control | Sulfadoxine | Pyrimethamine | Sulfadoxine- Pyrimethamine |
|||||||||
| Experiment Number |
1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Oocyst + mosquitoes,% (n) | ||||||||||||
| Day 3 concentrations a | 55(20) | 95(20) | 100(20) | 0(14) b | 11(18) b | 0(19) b | 5(20) b | 100(20) | 40(20) b | 0(13) b | 0(16) b | N |
| Day 7 concentrations | 100(20) | 100(20) | N | 0(20) b | 5(20) b | N | 95(20) | 85(20) | N | 0(20) b | N | N |
| Day 14 concentrations | 100(20) | 100(20) | N | 85(20) | N | N | 90(20) | N | N | 95(20) | N | N |
Each value is compared to the control value of the same feeding experiment.
P < 0.001
N = experiment not done.
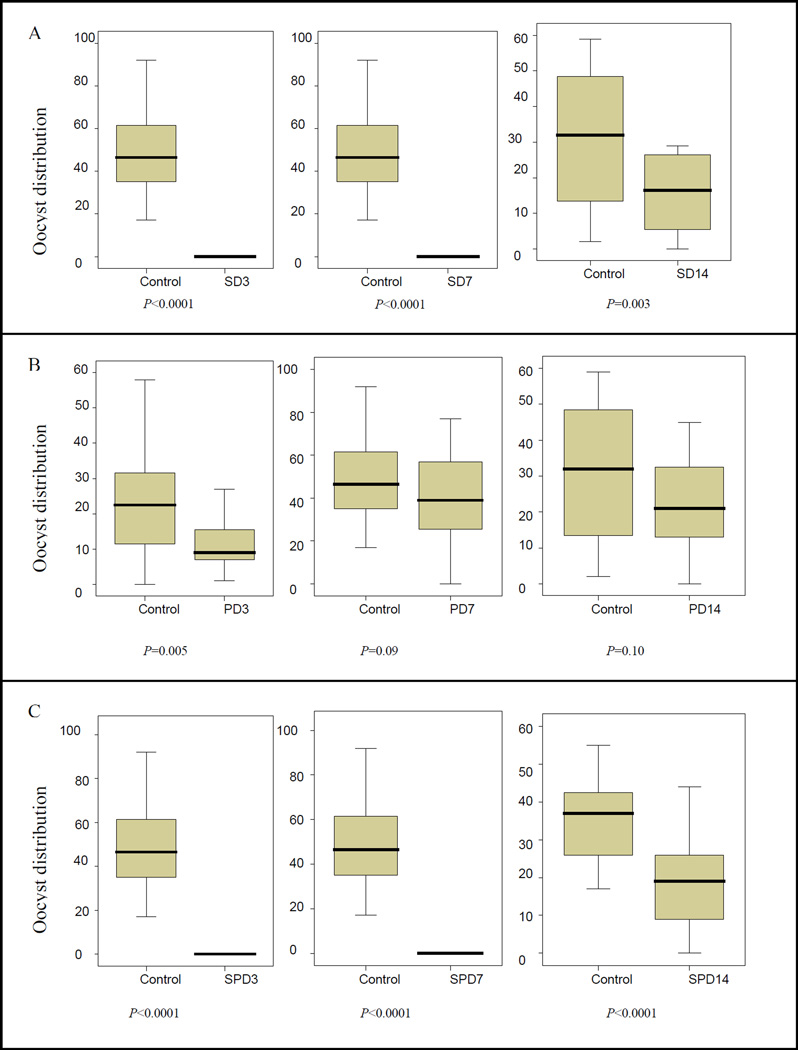
Similarly, SD3, SD7, SD14, SPD3, SPD7 and SPD14 concentrations significantly decreased the density of oocysts (Fig. 3A and C). With P alone, the highest PD3 concentration significantly decreased oocyst density compared with the controls; PD7 concentration showed a trend towards decreased oocyst density but it did not reach statistical significance (P = 0.09). The densities of oocysts with PD14 were similar to controls (Fig. 3B)
Fig. 3.
Effects of various concentrations of drugs on Plasmodium falciparum oocyst density. A) Effects of different Sulfadoxine concentrations on oocyst distributions in mosquitoes. B) Effects of different Pyrimethamine concentrations on oocyst distributions in mosquitoes. C) Effects of different Sulfadoxine462 pyrimethamine concentrations on oocyst distributions in mosquitoes. S, sulfadoxine; P, pyrimethamine; SP, sulfadoxine-pyrimethamine; SD3 = sulfadoxine concentration equivalent to serum levels of sulfadoxine at day 3 following an oral dose of sulfadoxine-pyrimethamine; SD7, sulfadoxine concentration equivalent to serum levels of sulfadoxine at day 7 following an oral dose of sulfadoxine466 pyrimethamine; SD14, sulfadoxine concentration equivalent to serum levels of sulfadoxine at day 14 following an oral dose of sulfadoxine-pyrimethamine; idem for PD3, PD7, PD14, SPD3, SPD7 and SPD14.
3.5. Effect of S, P and SP on mosquito viability
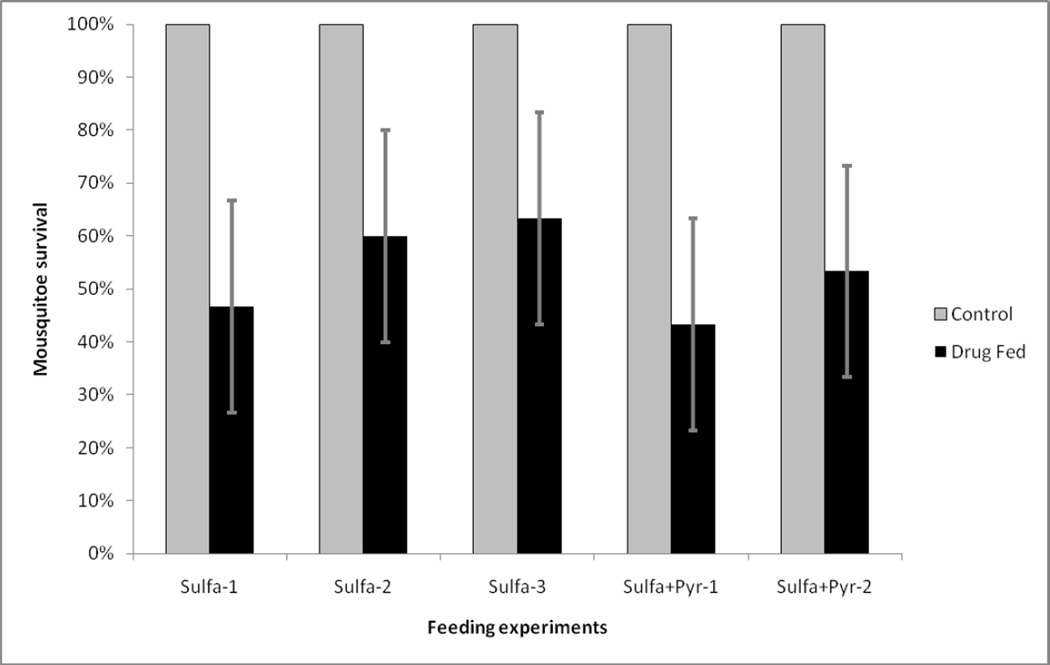
The number of surviving mosquitoes was counted 24 h after membrane feeds containing gametocytes and the different concentrations of S and P. In three separate experiments, SD3 concentrations killed an average of 43.3% (95% Confidence Interval (95% CI): 33.3 – 53.3; n = 90) of the fed mosquitoes (Fig. 4). The addition of P further increased mosquito death rate to an average of 51.7% (95% CI: 39 – 65; n = 60) (Fig. 4). There were no mosquito deaths with P alone nor with the any other drug concentrations (data not shown).
Fig. 4.
Survival of mosquitoes fed with the highest day 3 concentrations of sulfadoxine (three experiments are shown) or sulfadoxine-pyrimethamine (two experiments are presented).
4. Discussion
We showed that S and P have complex effects on the biology of gametocytogenesis. Both drugs induce differentiation of asexual forms into sexual forms, initiating gametocytogenesis. However, treatment of young gametocytes with both drugs impaired their further development into mature stage V gametocytes. The data is consistent with a scenario where the parasites react to the stress imposed by the drugs by committing to their sexual stages. Yet, the drug maintained some of its toxic effect on the newly formed sexual stages leading to odd mature gametocytes. These effects were dose-dependent.
When added to a blood meal together with otherwise healthy and mature gametocytes, S and P significantly reduced the infectivity of the gametocytes to Anopheles mosquitoes. This effect was also dose-dependent since the rate of decrease was higher for the highest dose tested (day 3 drug levels) than for the lower day 7 drug level concentrations. When day 14 drug level concentrations were tested, there were no more differences from controls. Taken together, the above results support previous studies including our own recent report where a single oral dose of SP yielded a sharp increase in gametocyte carriage in the treated patients, but the resulting gametocytes had very low infectivity for Anopheles gambiae by direct feeding (Beavogui et al., 2010). Our data are also consistent with analogous experiments performed in the 1950s with P, suggesting that the low rate of infectivity of post-treatment gametocytes may be due to a sporontocidal effect of the drug that is ingested together with gametocytes during the mosquitoes’ blood meals (Jeffery, 1958a). The effect of drugs on infectivity appeared to be dose-related (Shute and Maryon, 1954; Young and Burgess, 1957a). Furthermore, the sporontocidal effect of P appears to be dependent on the rate of efficacy of the drug (Jeffery, 1958b; Young and Burgess, 1957b). Hogh et al. (1998) found that SP decreased infectivity .
By contrast the effect of S on gametocyte infectivity has been much less studied (Laing, 1965). This may be due to the fact that S and sulfa-drugs were almost never widely used alone for the treatment of malaria in the field. Therefore, our study provides one of the first indications that S could also have a sporontocidal effect. Gametocyte sex ratio is important for effective transmission (Sowunmi and Fateye, 2003a,b) but the nature of the drug does not appear to influence the sex ratio of post treatment gametocytes (Robert et al., 2003; Talman et al., 2004).
The highest day 3 S concentration was lethal to the fed mosquitoes as survival rates were significantly reduced compared with the no drug controls. The same level of lethality was observed when S and P were fed to mosquitoes together with the gametocyte preparation. This shows that, although P alone has no effect on mosquito survival, the addition of P to S tended to increase the lethal effect of S. The mosquitoes were fed with drug and gametocytes and the observed lethal effect could be due to the concomitant presence of both the parasites and the drug. However, it is known that the presence of the gametocytes alone does not kill the mosquitoes and the lethality was only measured 24 h after feeding. Taken together, the data suggest that a significant number of mosquitoes that feed on patients between days 1–3 post-SP treatment would not survive.
When treated with SP, the sensitive NF54 parasites were killed as expected, while the NF-135- resistant parasites were stimulated into gametocytogenesis. Although some parasites died, there were enough asexual forms that could survive to differentiate into gametocytes. In the absence of continuous drug pressure, the resulting gametocytes matured into normal mature gametocytes capable of exflagellation. This is consistent with previous reports showing that mutant/resistant parasites have a longer parasite clearance time which may favor differentiation into gametocytes (Mendez et al., 2002; Tjitra et al., 2002; von Seidlein L et al., 2001b; Ali et al., 2006; Barnes et al., 2008). SP treatment is known to select dhfr and dhps mutant asexual parasites (Tekete et al., 2009) and gametocytes (Beavogui et al., 2010).
This study demonstrates that although SP induces gametocytogenesis, it also kills gametocytes and the malaria vector A. stephensi.
Acknowledgements
We thank Prof. Alassane Dicko and Mr. Zoumana I. Traore for assistance with graphics. Financial support was provided by MIM-UNICEF-UNDP-World Bank- WHO Special Programme for Research and Training in Tropical Diseases (TDR) Grant # A20238, Grant No. 5 D43 TW001589 from the National Institutes of Health, Fogarty International Center (NIH/FIC) and International Atomic Energy Agency (IAEA) RAF/6025. A.A.D. is supported by Howard Hughes Medical Institute International Scholarship (Grant # 55005502).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali E, Mackinnon MJ, bdel-Muhsin AM, Ahmed S, Walliker D, Babiker HA. Increased density but not prevalence of gametocytes following drug treatment of Plasmodium falciparum. Trans.R. Soc. Trop. Med. Hyg. 2006;100:176–183. doi: 10.1016/j.trstmh.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Aponte JJ, Schellenberg D, Egan A, Breckenridge A, Carneiro I, Critchley J, Danquah I, Dodoo A, Kobbe R, Lell B, May J, Premji Z, Sanz S, Sevene E, Soulaymani-Becheikh R, Winstanley P, Adjei S, Anemana S, Chandramohan D, Issifou S, Mockenhaupt F, Owusu-Agyei S, Greenwood B, Grobusch MP, Kremsner PG, Macete E, Mshinda H, Newman RD, Slutsker L, Tanner M, Alonso P, Menendez C. Efficacy and safety of intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in African infants: a pooled analysis of six randomised, placebo-controlled trials. Lancet. 2009;374:1533–1542. doi: 10.1016/S0140-6736(09)61258-7. [DOI] [PubMed] [Google Scholar]
- Barger B, Maiga H, Traore OB, Tekete M, Tembine I, Dara A, Traore ZI, Gantt S, Doumbo OK, Djimde AA. Intermittent preventive treatment using artemisinin-based combination therapy reduces malaria morbidity among school-aged children in Mali. Trop. Med. Int. Health. 2009;14:784–791. doi: 10.1111/j.1365-3156.2009.02294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes KI, Little F, Mabuza A, Mngomezulu N, Govere J, Durrheim D, Roper C, Watkins B, White NJ. Increased gametocytemia after treatment: an early parasitological indicator of emerging sulfadoxine-pyrimethamine resistance in falciparum malaria. J. Infect. Dis. 2008;197:1605–1613. doi: 10.1086/587645. [DOI] [PubMed] [Google Scholar]
- Beavogui AH, Djimde A, Gregson A, Toure AM, Dao A, Coulibaly B, Ouologuem D, Fofana B, Sacko A, Tekete M, Kone A, Niare O, Wele M, Plowe CV, Picot S, Doumbo K. Low infectivity of Plasmodium falciparum gametocytes to Anopheles gambiae following treatment with sulfadoxine-pyrimethamine in Mali. Int. J. Parasitol. 2010 doi: 10.1016/j.ijpara.2010.04.010. [This issue] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckling A, Crooks L, Read A. Plasmodium chabaudi: effect of antimalarial drugs on gametocytogenesis. Exp. Parasitol. 1999;93:45–54. doi: 10.1006/expr.1999.4429. [DOI] [PubMed] [Google Scholar]
- Buffet PA, Briand V, Renia L, Thellier M, Danis M, Mazier D. Intermittent preventive antimalarial treatment to children (IPTc): firebreak or fire trap? Trends Parasitol. 2008;24:482–485. doi: 10.1016/j.pt.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Burkot TR, Williams JL, Schneider I. Infectivity to mosquitoes of Plasmodium falciparum clones grown in vitro from the same isolate. Trans. R. Soc. Trop. Med. Hyg. 1984;78:339–341. doi: 10.1016/0035-9203(84)90114-7. [DOI] [PubMed] [Google Scholar]
- Clarke SE, Jukes MC, Njagi JK, Khasakhala L, Cundill B, Otido J, Crudder C, Estambale BB, Brooker S. Effect of intermittent preventive treatment of malaria on health and education in schoolchildren: a cluster-randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:127–138. doi: 10.1016/S0140-6736(08)61034-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicko A, Sagara I, Djimde AA, Toure SO, Traore M, Dama S, Diallo AI, Barry A, Dicko M, Coulibaly OM, Rogier C, de Sousa A, Doumbo OK. Molecular markers of resistance to sulphadoxine-pyrimethamine one year after implementation of intermittent preventive treatment of malaria in infants in Mali. Malar. J. 2010;9:9. doi: 10.1186/1475-2875-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djimde A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourte Y, Dicko A, Su XZ, Nomura T, Fidock DA, Wellems TE, Plowe CV, Coulibaly D. A molecular marker for chloroquine-resistant falciparum malaria. New. Eng. J. Med. 2001;344:257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- Djimde AA, Fofana B, Sagara I, Sidibe B, Toure S, Dembele D, Dama S, Ouologuem D, Dicko A, Doumbo OK. Efficacy, safety, and selection of molecular markers of drug resistance by two ACTs in Mali. Am. J. Trop. Med. Hyg. 2008;78:455–461. [PubMed] [Google Scholar]
- Hogh B, Gamage-Mendis A, Butcher GA, Thompson R, Begtrup K, Mendis C, Enosse SM, Dgedge M, Barreto J, Eling W, Sinden RE. The differing impact of chloroquine and pyrimethamine/sulfadoxine upon the infectivity of malaria species to the mosquito vector. Am. J. Trop. Med. Hyg. 1998;58:176–182. doi: 10.4269/ajtmh.1998.58.176. [DOI] [PubMed] [Google Scholar]
- Ifediba T, Vanderberg JP. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature. 1981;294:364–366. doi: 10.1038/294364a0. [DOI] [PubMed] [Google Scholar]
- Jeffery GM. Infectivity to mosquitoes of Plasmodium vivax following treatment with chloroquine and other antimalarials. Am. J. Trop. Med. Hyg. 1958a;7:207–211. doi: 10.4269/ajtmh.1958.7.207. [DOI] [PubMed] [Google Scholar]
- Jeffery GM. Infectivity to mosquitoes of Plasmodium vivax following treatment with chloroquine and other antimalarials. Am. J. Trop. Med. Hyg. 1958b;7:207–211. doi: 10.4269/ajtmh.1958.7.207. [DOI] [PubMed] [Google Scholar]
- Kayentao K, Kodio M, Newman RD, Maiga H, Doumtabe D, Ongoiba A, Coulibaly D, Keita AS, Maiga B, Mungai M, Parise ME, Doumbo O. Comparison of intermittent preventive treatment with chemoprophylaxis for the prevention of malaria during pregnancy in Mali. J. Infect. Dis. 2005;191:109–116. doi: 10.1086/426400. [DOI] [PubMed] [Google Scholar]
- Kublin JG, Dzinjalamala FK, Kamwendo DD, Malkin EM, Cortese JF, Martino LM, Mukadam RA, Rogerson SJ, Lescano AG, Molyneux ME, Winstanley PA, Chimpeni P, Taylor TE, Plowe CV. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J. Infect. Dis. 2002;185:380–388. doi: 10.1086/338566. [DOI] [PubMed] [Google Scholar]
- Laing AB. Treatment of acute falciparum malaria with diaphenylsulfone in North-East Tanzania. J. Trop. Med. Hyg. 1965;68:251–253. [PubMed] [Google Scholar]
- Manzi F, Hutton G, Schellenberg J, Tanner M, Alonso P, Mshinda H, Schellenberg D. From strategy development to routine implementation: the cost of Intermittent Preventive Treatment in Infants for malaria control. BMC Health Serv. Res. 2008;8:165. doi: 10.1186/1472-6963-8-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez F, Munoz A, Carrasquilla G, Jurado D, Arevalo-Herrera M, Cortese JF, Plowe CV. Determinants of treatment response to sulfadoxine-pyrimethamine and subsequent transmission potential in falciparum malaria. Am. J. Epidemiol. 2002;156:230–238. doi: 10.1093/aje/kwf030. [DOI] [PubMed] [Google Scholar]
- Peterson DS, Milhous WK, Wellems TE. Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc. Natl. Acad. Sci. USA. 1990;87:3018–3022. doi: 10.1073/pnas.87.8.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnudurai T, Leeuwenberg AD, Meuwissen JH. Chloroquine sensitivity of isolates of Plasmodium falciparum adapted to in vitro culture. Trop. Geograph. Med. 1981;33:50–54. [PubMed] [Google Scholar]
- Ponnudurai T, Lensen AH, Leeuwenberg AD, Meuwissen JH. Cultivation of fertile Plasmodium falciparum gametocytes in semi-automated systems 1 Static cultures. Trans. R. Soc. Trop. Med. Hyg. 1982a;76:812–818. doi: 10.1016/0035-9203(82)90116-x. [DOI] [PubMed] [Google Scholar]
- Ponnudurai T, Lensen AH, Meuwissen JH. An automated large-scale culture system of Plasmodium falciparum using tangential flow filtration for medium change. Parasitology. 1983;87:439–445. doi: 10.1017/s0031182000082962. [DOI] [PubMed] [Google Scholar]
- Ponnudurai T, Lensen AH, van Gemert GJ, Bensink MP, Bolmer M, Meuwissen JH. Infectivity of cultured Plasmodium falciparum gametocytes to mosquitoes. Parasitology. 1989a;98:165–173. doi: 10.1017/s0031182000062065. [DOI] [PubMed] [Google Scholar]
- Ponnudurai T, Lensen AH, van Gemert GJ, Bensink MP, Bolmer M, Meuwissen JH. Sporozoite load of mosquitoes infected with Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 1989b;83:67–70. doi: 10.1016/0035-9203(89)90708-6. [DOI] [PubMed] [Google Scholar]
- Ponnudurai T, Verhave JP, Meuwissen JH. Mosquito transmission of cultured Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 1982b;76:278–279. doi: 10.1016/0035-9203(82)90298-x. [DOI] [PubMed] [Google Scholar]
- Robert V, Awono-Ambene HP, Le Hesran JY, Trape JF. Gametocytemia and infectivity to mosquitoes of patients with uncomplicated Plasmodium falciparum malaria attacks treated with chloroquine or sulfadoxine plus pyrimethamine. Am. J. Trop. Med. Hyg. 2000a;62:210–216. doi: 10.4269/ajtmh.2000.62.210. [DOI] [PubMed] [Google Scholar]
- Robert V, Awono-Ambene HP, Le Hesran JY, Trape JF. Gametocytemia and infectivity to mosquitoes of patients with uncomplicated Plasmodium falciparum malaria attacks treated with chloroquine or sulfadoxine plus pyrimethamine. Am. J. Trop. Med. Hyg. 2000b;62:210–216. doi: 10.4269/ajtmh.2000.62.210. [DOI] [PubMed] [Google Scholar]
- Robert V, Sokhna CS, Rogier C, Ariey F, Trape JF. Sex ratio of Plasmodium falciparum gametocytes in inhabitants of Dielmo, Senegal. Parasitology. 2003;127:1–8. doi: 10.1017/s0031182003003299. [DOI] [PubMed] [Google Scholar]
- Rosario V. Cloning of naturally occurring mixed infections of malaria parasites. Science. 1981;212:1037–1038. doi: 10.1126/science.7015505. [DOI] [PubMed] [Google Scholar]
- Shute PG, Maryon M. The effect of pyrimethamine (daraprim) on the gametocytes and oocysts of Plasmodium falciparum and Plasmodium vivax. Trans. R. Soc. Trop. Med. Hyg. 1954;48:50–63. doi: 10.1016/0035-9203(54)90038-3. [DOI] [PubMed] [Google Scholar]
- Sokhna C, Cisse B, Ba eH, Milligan P, Hallett R, Sutherland C, Gaye O, Boulanger D, Simondon K, Simondon F, Targett G, Lines J, Greenwood B, Trape JF. A trial of the efficacy, safety and impact on drug resistance of four drug regimens for seasonal intermittent preventive treatment for malaria in Senegalese children. PLoS. ONE. 2008;3:e1471. doi: 10.1371/journal.pone.0001471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowunmi A, Fateye BA. Changes in Plasmodium falciparum gametocytaemia in children with chloroquine-sensitive asexual infections. Parasite. 2003a;10:363–369. doi: 10.1051/parasite/2003104363. [DOI] [PubMed] [Google Scholar]
- Sowunmi A, Fateye BA. Plasmodium falciparum gametocytaemia in Nigerian children: before, during and after treatment with antimalarial drugs. Trop. Med. Int. Health. 2003b;8:783–792. doi: 10.1046/j.1365-3156.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- Talman AM, Paul RE, Sokhna CS, Domarle O, Ariey F, Trape JF, Robert V. Influence of chemotherapy on the plasmodium gametocyte sex ratio of mice and humans. Am. J. Trop. Med. Hyg. 2004;71:739–744. [PubMed] [Google Scholar]
- Targett G, Drakeley C, Jawara M, von Seidlein L, Coleman R, Deen J, Pinder M, Doherty T, Sutherland C, Walraven G, Milligan P. Artesunate reduces but does not prevent post treatment transmission of Plasmodium falciparum to Anopheles gambiae. J. Infect. Dis. 2001;183:1254–1259. doi: 10.1086/319689. [DOI] [PubMed] [Google Scholar]
- Tekete M, Djimde AA, Beavogui AH, Maiga H, Sagara I, Fofana B, Ouologuem D, Dama S, Kone A, Dembele D, Wele M, Dicko A, Doumbo OK. Efficacy of chloroquine, amodiaquine and sulphadoxine-pyrimethamine for the treatment of uncomplicated falciparum malaria: revisiting molecular markers in an area of emerging AQ and SP resistance in Mali. Malar. J. 2009;8:34. doi: 10.1186/1475-2875-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temperley M, Mueller DH, Njagi JK, Akhwale W, Clarke SE, Jukes MC, Estambale BB, Brooker S. Costs and cost-effectiveness of delivering intermittent preventive treatment through schools in western Kenya. Malar. J. 2008;7:196. doi: 10.1186/1475-2875-7-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjitra E, Suprianto S, Anstey NM. Higher gametocyte prevalence following failure of treatment of Plasmodium falciparum malaria with sulfadoxine-pyrimethamine and the combination of chloroquine plus sulfadoxine-pyrimethamine: implications for progression of anti-folate resistance. Trans. R. Soc. Trop. Med. Hyg. 2002;96:434–437. doi: 10.1016/s0035-9203(02)90385-8. [DOI] [PubMed] [Google Scholar]
- van der Kolk M, De Vlas SJ, Saul A, van de V, Eling WM, Sauerwein RW. Evaluation of the standard membrane feeding assay (SMFA) for the determination of malaria transmission-reducing activity using empirical data. Parasitology. 2005;130:13–22. doi: 10.1017/s0031182004006067. [DOI] [PubMed] [Google Scholar]
- von Seidlein L, Drakeley C, Greenwood B, Walraven G, Targett G. Risk factors for gametocyte carriage in Gambian children. Am. J. Trop. Med. Hyg. 2001a;65:523–527. doi: 10.4269/ajtmh.2001.65.523. [DOI] [PubMed] [Google Scholar]
- von Seidlein L, Jawara M, Coleman R, Doherty T, Walraven G, Targett G. Parasitaemia and gametocytaemia after treatment with chloroquine, pyrimethamine/sulfadoxine, and pyrimethamine/sulfadoxine combined with artesunate in young Gambians with uncomplicated malaria. Trop. Med. Int. Health. 2001b;6:92–98. doi: 10.1046/j.1365-3156.2001.00683.x. [DOI] [PubMed] [Google Scholar]
- Wang P, Lee CS, Bayoumi R, Djimde A, Doumbo O, Swedberg G, Dao LD, Mshinda H, Tanner M, Watkins WM, Sims PF, Hyde JE. Resistance to antifolates in Plasmodium falciparum monitored by sequence analysis of dihydropteroate synthetase and dihydrofolate reductase alleles in a large number of field samples of diverse origins. Mol. Biochem. Parasitol. 1997;89:161–177. doi: 10.1016/s0166-6851(97)00114-x. [DOI] [PubMed] [Google Scholar]
- Wellems TE, Panton LJ, Gluzman IY, Do Rosario VE, Gwadz RW, Walker-Jonah A, Krogstad DJ. Chloroquine resistance not linked to mdr-like genes in a Plasmodium falciparum cross. Nature. 1990;345:253–255. doi: 10.1038/345253a0. [DOI] [PubMed] [Google Scholar]
- Young MD, Burgess RW. Effect of 25 milligrams of pyrimethamine on the infectivity of Plasmodium vivax, St.Elizabeth strain, to Anopheles quadromaculatus. Am. J. Trop. Med. Hyg. 1957a;6:805–807. doi: 10.4269/ajtmh.1957.6.805. [DOI] [PubMed] [Google Scholar]
- Young MD, Burgess RW. Effect of 25 milligrams of pyrimethamine on the infectivity of Plasmodium vivax, St.Elizabeth strain, to Anopheles quadromaculatus. Am. J. Trop. Med. Hyg. 1957b;6:805–807. doi: 10.4269/ajtmh.1957.6.805. [DOI] [PubMed] [Google Scholar]