Abstract
Objective
Brain enlargement has been observed in individuals with autism as early as two years of age. Studies using head circumference suggest that brain enlargement is a postnatal event that occurs around the latter part of the first year. To date, no brain imaging studies have systematically examined the period prior to age two. In this study we examine MRI brain volume in six month olds at high familial risk for autism.
Method
The Infant Brain Imaging Study (IBIS) is a longitudinal imaging study of infants at high risk for autism. This cross-sectional analysis examines brain volumes at six months of age, in high risk infants (N=98) in comparison to infants without family members with autism (low risk) (N=36). MRI scans are also examined for radiologic abnormalities.
Results
No group differences were observed for intracranial cerebrum, cerebellum, lateral ventricle volumes, or head circumference.
Conclusions
We did not observe significant group differences for head circumference, brain volume, or abnormalities of radiologic findings in a sample of 6 month old infants at high-risk for autism. We are unable to conclude that these changes are not present in infants who later go on to receive a diagnosis of autism, but rather that they were not detected in a large group at high familial risk. Future longitudinal studies of the IBIS sample will examine whether brain volume may differ in those infants who go onto develop autism, estimating that approximately 20% of this sample may be diagnosed with an autism spectrum disorder at age two.
Keywords: autism, child psychiatry
Introduction
Multiple lines of converging evidence (from post-mortem, magnetic resonance imaging, and head circumference studies) have documented brain and head size enlargement in autism spectrum disorders (ASDs) (1–10). Brain MRI studies have revealed generalized enlargement in cerebral cortical gray and white matter (9–11), and selected subcortical structures (12) that are present as early as 2 years of age. A longitudinal MRI study of two year old children with ASDs followed up at 4–5 years of age, demonstrating enlargement at age two with no increase in growth rate during the subsequent two year interval, was consistent with the conclusion that increased brain volume is the result of overgrowth occurring prior to 2 years of age (10). A recent report on a sample of 2–50 year olds with ASDs (13) has also noted early brain enlargement associated with autism, characterized by an early postnatal period of overgrowth followed by a period of slower or arrested growth.
Longitudinal head circumference studies suggest the onset of brain overgrowth is in the later part of the first year of life (9, 14), and higher rates of macrocephaly has been observed in parents of probands with ASD (7). Data from behavioral studies of infant siblings of autistic individuals (infant siblings who are therefore at high genetic risk for developing an ASDs) suggest that most of the defining features of this condition (e.g., social deficits) are not present at 6 months of age, but are first evident by 12 months of age (15). These two independent lines of research (brain studies, developmental behavioral studies) appear to converge on the latter part of the first year of life as a period of both changes in brain structure and the onset of behavioral symptoms of autism.
Structural imaging studies of early brain development in typically developing children highlight that the first year of life is a time of dynamic growth, where total brain volume increases 100% (16). As autism is not typically diagnosed until about age two years or later, studies of abnormal brain development during infancy in autism must wait until the diagnosis is examined at a later date before reaching conclusions about early brain abnormalities associated with this disorder. Given the high recurrence risk of autism in subsequently born siblings (17), a strategy that has recently been employed is the examination of the infant siblings of autistic children (i.e., infants at risk for autism prior to the diagnosis of autism). Virtually nothing is known about early brain development in infants at risk for autism from neuroimaging studies, including whether there are observable differences in brain volume compared to infants with no known genetic risk for autism (i.e., referred to here as ‘low risk’ subjects who are drawn from the normal population and therefore most often displaying typical development).
The present study reports on volumetric brain MRI measures in a large sample of six month old infants at high genetic risk for autism in comparison to a sample of infants at low risk for autism. The current report is the first to focus on measuring brain volume in six month olds at high familial risk for autism, where approximately 20% may receive a later diagnosis of an ASD, to test whether brain volume differences may be associated with high risk for ASD.
Methods
Sample
This study includes data acquired from an NIH-funded Autism Center of Excellence (ACE) network study. Informally called the Infant Brain Imaging Study (IBIS), the network includes four clinical data collection sites (University of North Carolina at Chapel Hill, University of Washington, Children’s Hospital of Philadelphia, Washington University in St. Louis, a Data Coordinating Center at the Montreal Neurological Institute at McGill University, and two image processing sites (University of Utah and UNC). This ACE Network study is currently ongoing and collecting neuroimaging and behavioral data on infants who are at genetic risk for ASDs by virtue of having an older sibling with autism (referred to as ‘high risk sibs’), with the following design: (1) enroll six month old infants, who are at high genetic risk for autism, to be seen for follow-up assessments at 12 and 24 months of age; (2) enroll high-risk infant sibs who enter the study at 12 months of age and are followed up at 24 months; and (3) enroll a comparison group of typically-developing controls considered to be at low risk for autism (i.e., older sibling is typically developing) seen at 6, 12 and 24 months. Diagnostic outcome data for ASD status is determined at 24 months of age (and again at 36 months of age, depending when the subjects can be seen within the timeline of the project). For the purposes of this paper, we have included cross-sectional data on the six month olds who were scanned from the start of the study through the fall of 2010. Two groups were included: infants at high risk for autism (HR) and typically-developing children considered to be at low-risk for autism (LR). Subjects were combined from all four clinical data collections sites.
The subjects in this sample include all children with imaging data collected and processed thru 9/30/10. There were 318 children who attempted the MRI scans, and 88% HR and 83% LR subjects were successfully scanned, for a total of 276 scans. Due to difficulties in scheduling visits, missed visits (e.g., due to illness, changes in travel plans), some children seen in a wider age window up to 8 months of age. Infants develop rapidly during the first year, and so for the purpose of this analysis, a narrow ‘optimal’ age window was defined as 6 months of age −1 week/+ 3 weeks. There were 134 infants who were in this optimal age window (98 HR and 36 LR) and all results presented below are based on this sample. Of note, the wider age group around the first time point will be included in later longitudinal analyses of these data.
Subjects were characterized as HR if they had an older sibling with a diagnosis of an ASD that was documented in a clinical diagnostic report and confirmed by an Autism Diagnostic Interview (ADI-R) administered at enrollment. Subjects were enrolled in the LR group if they had an older sibling without evidence of ASDs and no family history of a first or second-degree relative with ASDs. Exclusion criteria for both groups included the following: (1) diagnosis or physical signs strongly suggestive of a genetic condition or syndrome (e.g., fragile X syndrome) reported to be associated with ASDs, (2) a significant medical or neurological condition affecting growth, development or cognition (e.g., CNS infection, seizure disorder, congenital heart disease), (3) sensory impairment such as vision or hearing loss, (4) low birth weight (<2000 grams) or prematurity (<36 weeks gestation), (5) possible perinatal brain injury from exposure to in-utero exogenous compounds reported to likely affect brain adversely in at least some individuals (e.g., alcohol, selected prescription medications), (6) non-English speaking families, (7) contraindication for MRI (e.g., metal implants), (8) adopted subjects, and (9) a family history of intellectual disability, psychosis, schizophrenia or bipolar disorder in a first-degree relative.
Assessment Protocols
Behavioral assessment
Infants are assessed directly at ages 6, 12 and 24 months and a phone interview is conducted with parents at 18 months to assess infant development. Direct assessments include brain MRI scans in addition to a battery of behavioral and developmental tests. The assessment battery for the visit at 6 months included the Mullen Scales of Early Learning (Mullen) (18), the Vineland Adaptive Behavior Scales-II (19), the Autism Observation Scale for Infants (20), various questionnaires examining behavior, temperament, and family characteristics, and a medical record review. See Table 1 for a description of subject characteristics.
Table 1.
Subject Descriptives for High Risk (HR) and Low Risk (LR) groups.
| High Risk (N=98) |
Low Risk (N=36) |
Difference p value* |
|||
|---|---|---|---|---|---|
| Sex (% male) | 62% | 58% | .69 | ||
| Gestational age at birth (Week), Mean (SD) | 39.0 | (1.3) | 39.5 | (1.2) | .47 |
| Age at MRI scan (Month), Mean (SD) | 6.2 | (0.3) | 6.3 | (0.2) | .37 |
| Maternal age at birth (Year), Mean (SD) | 33.7 | (4.7) | 32.9 | (5.7) | .37 |
| Mullen ELC **, Mean (SD) | 95.2 | (12.5) | 100.0 | (10.0) | .13 |
Fisher’s test for sex; and ANOVA test for Mullen ELC, gestational age at birth, age at MRI scan, and maternal age at birth while controlling for site differences.
Mullen ELC – Mullen Early Learning Composite
MRI acquisition
All the brain MRI scans were completed at each of the clinical sites on a 3T Siemens Tim Trio scanner with a 12-channel head coil. All scans were completed while infants were naturally sleeping. Specific structured preparation was completed by families at home prior to the scanning, including conditioning to the scanner sounds on a CD played to infants while sleeping. The imaging protocol was designed to maximize tissue contrast for volumetric analysis across three timepoints (ages 6, 12, 24 months). The protocol included (1) a localizer scan, (2) 3D T1 MPRAGE: TR=2400ms, TE=3.16ms, 160 sagittal slices, FOV=256, voxel size = 1mm3, (3) 3D T2 FSE TR=3200ms, TE=499ms, 160 sagittal slices, FOV=256, voxel size = 1mm3, and (4) a 25 direction DTI: TR=12800ms, TE=102ms, slice thickness = 2mm isotropic, variable b value = maximum of 1000s/mm2, , FOV=190.
A number of quality control procedures were employed to assess scanner stability and reliability across sites, time, and procedures. A ‘LEGO’ phantom (21) was scanned monthly at each location and analyzed for image quality (22) and to quantitatively address site specific regional distortions. Two adult subjects (aka ‘human phantoms’) were scanned once per year per scanner (twice in year 1). Phantom data was evaluated for scanner stability across sites and time (23, 24). Results indicated excellent stability across sites, with covariates of variation for intracranial volume below 1%, and with intraclass correlations for intracranial volume at 0.98 for inter-site and 0.99 for intra-site reliability. Finally, all scans were blindly reviewed for image quality by a single rater (D. Louis Collins, McGill), and again rated by a single reviewer (Rachel Gimpel Smith, UNC) in a pre-processing stage prior to image analysis.
Radiologic Review
All scans were reviewed locally by a pediatric neuroradiologist for radiologic findings that, if present, could be communicated to the participants. In addition, a board certified pediatric neuroradiologist (R.C.M., Washington University) blindly reviewed all MRI scans across the IBIS network and rated the incidental findings. A third neuroradiologist (D.W.W.S., University of Washington) provided a second blind review for the Washington University site, and contributed to a final consensus rating if there were discrepancies between the local site reviews and the network review. The final consensus review was used to evaluate whether there were group differences in the number and/or type of incidental findings.
Image Processing
The following brain volumes were obtained: intracranial volume (ICV), total brain volume = gray matter (GM) plus white matter (WM), total cerebrospinal fluid (CSF), cerebrum, cerebellum, and lateral ventricles. There is limited tissue contrast in the 6 month old brain due to ongoing myelination and maturation. As a result of the resulting reduced MRI tissue contrast at that age, reliable separation of WM and GM tissue is highly limited. Thus, we chose to initially focus on the total brain volume measure without separating WM and GM. We also parcellated the brain into large regions (e.g., cerebrum, cerebellum, hemispheres) but omitted a fine-grained lobar or cortical segmentation which is less reliable at this young age.
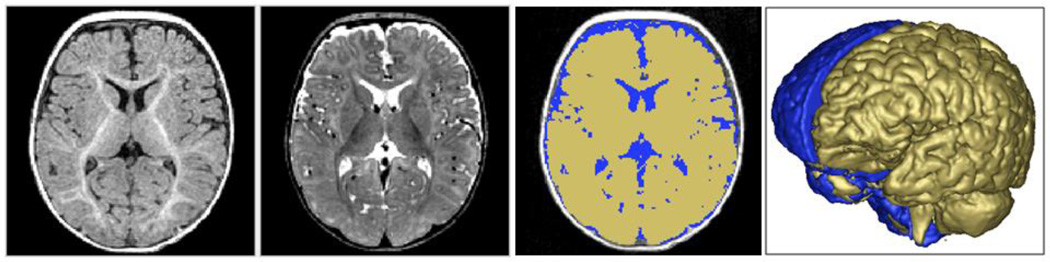
The brain volume segmentations were obtained following a rigid transformation to a normative brain space and pre-processing for bias correction and intensity normalization. These steps are part of the expectation-maximization (EM) tissue segmentation method (25) via the AutoSeg tool (26). Tissue probability maps for WM, GM, and CSF were obtained. ICV was defined to be the sum of WM, GM, and CSF and an example of the segmentation label is shown in Figure 1.
Figure 1. Brain segmentation at six months of age.
Figure 1 displays T1w and T2w axial MRI images of a 6 month infant are displayed in panels 1 and 2, in panel 3 with label maps for total brain (yellow) and CSF (blue) segmentations, and in panel 4 with a 3-D rendering of intracranial volume (ICV) (back, blue) and brain surface (front, yellow).
Measures of head circumference were obtained using a semi-automated image processing tools using an established protocol (9). Head circumference was measured by a single rater using the HeadCirc tool developed by collaborators at UNC, which computes head circumference by extracting the appropriate contour from head segmentation of MRI (using Fourier harmonics to parameterize the contour of the head on MRI). Intra- and inter-rater reliability for the head circumference measures on the six month old dataset was exceptional (ICC = .99 for each). The ITK-SNAP tool (27) was used by a single rater to obtain measurement of the lateral ventricles. Intra- and inter-rater reliability for the lateral ventricles was ICC = .99 (this was true for combined volume, and right/left hemisphere). AutoSeg, HeadCirc and ITK-SNAP tools are available at http://www.ia.unc.edu/dev/download and http://www.nitrc.org.
There were 12 total cases that were excluded based on failing to pass the initial scan review and/or pre-processing steps. Failures included misaligned images (2), off-center/distortions (2), registration (3), or poor image quality/artifact (5). There were no group differences in the rate or type of failures.
Statistical Analysis
Power Consideration
The design of the study was based on findings from our previous examination of 2–4 year olds with ASD compared to TYP controls (10). Percent increase in the volume of relevant structures (e.g., cerebral cortex, cerebral cortical WM and temporal lobe WM) has been reported to range from 4 to 9% with effect size Cohen’s d from 0.59 to 0.95 in cross-sectional samples of 45–51 cases versus 15–26 controls. With our current sample size of 36 LR and 98 HR, we are expecting to have 85% −99% power to identify the similar effect size as observed in the sample of 2–4 year old children with ASD.
Analysis
Our research question was to examine whether there were significant group differences in head circumference, brain volumes, and radiologic findings in a group of infants at high genetic risk for autism. Group differences were tested with Fisher exact test in sex, and separate ANOVA models were used for group differences in Mullen ELC, gestational age at birth, age at MRI scan, and maternal age at birth while controlling for site differences. Cross-sectional group difference in brain volumes were analyzed using a General Linear Model (GLM) in SAS. Covariates included in the model were age at scan, sex (male/female), site, and the Mullen Early Learning Composite standard score. Dependent variables of interest included Intracranial Volume (ICV), Total Tissue (GM+WM), Cerebral Spinal Fluid (CSF), Cerebrum (left/right, total tissue+CSF), Cerebellum (left/right, total tissue+CSF), head circumference, and lateral ventricles (left/right). To test the potential sex differences on group effect, the additional interaction term (Group X Sex) was included in the model.
Results
There were no significant group differences for age (p=.37), sex (p=.69), Mullen Early Learning Composite standard score (p=.13), gestational age (p=.47), maternal age at childbirth (p=.37), or SES (p=.76), as measured by parental report of family income. There was a significant difference between groups in level of maternal education (p=.01), with the HR group containing more college educated mothers (42% compared to 22% LR). However, it was also noted that the LR group had a higher rate of mothers with graduate degrees (42% compared to 18% HR).
Radiologic Reviews
Scans were rated as either normal, abnormal, or noted with incidental findings. One child was excluded from the study for a radiologic abnormality (Chiari I malformation = 5mm). In the HR group, 44% of scans were read as normal and 56% with incidental findings. The LR group had 39% read as normal and 61% with incidental findings. There were no differences observed between the HR and LR groups in proportion of these scans (Chi-Square = .27, 1 DF, p=.60).
Types of incidental findings observed included: enlargement of perivascular spaces (PVS) (LR=4, 11%; HR=5, 5%), enlargement of subarachnoid spaces(SAS) (LR=17, 47%; HR=49, 50%), Chiari I malformation < 5mm (LR=1, 3%), pineal cyst (LR=1, 3%), cavum septum pellucidum (LR=1, 3%), plagiocephaly (LR=1, 3%), cavum velum interpositum (HR= 2, 2%), and pars intermedia cyst of the pituitary (HR=1, 1%). There were 6 HR (6%) and 3 LR (8%) cases who had more than 1 atypicality observed (included in the list above). Specific ratings were given to the PVS (0,1, or 2) and SAS (0,1,2, or 3) to indicate the degree/severity of enlargement so that groups could be compared on the size of perivascular and subarachnoid space enlargement. See Figure 2. No group differences in the degree of SAS enlargement (Chi-Square = 3.1, DF=3, p=.37) or increase in PVS (Chi-Square = 2.5, DF=2, p=.29) were found.
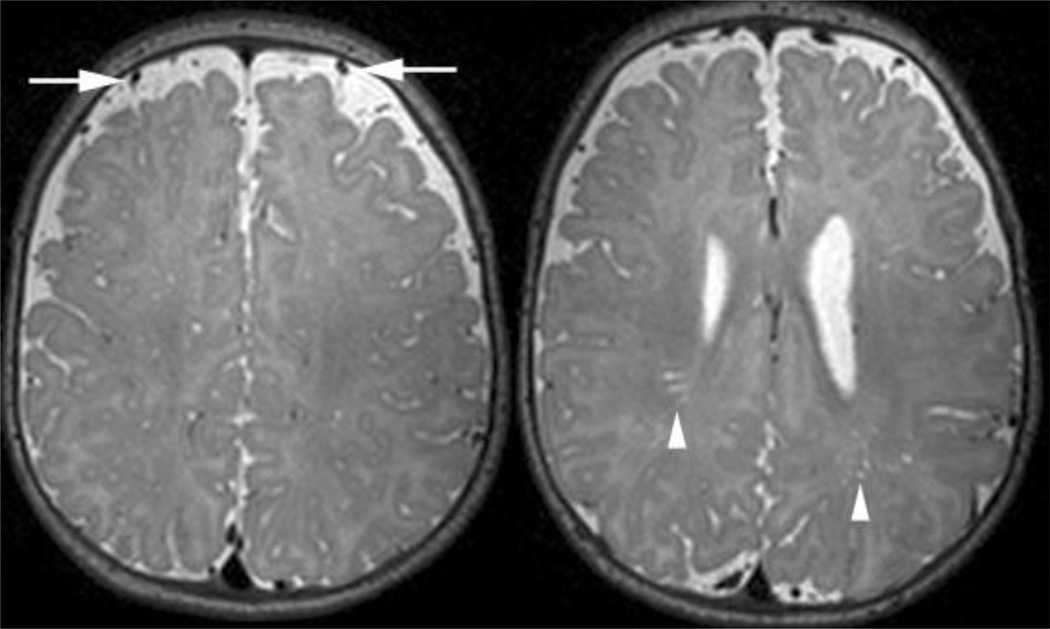
Figure 2. Grading of subarachnoid spaces (SAS) and periventricular spaces (PVS).
The image on the left demonstrates mild enlargement of the subarachnoid spaces. Minimal enlargement is defined as one vessel width of SAS and brain that does not touch the inner table of the calvaria on multiple consecutive sections. Mild SAS has a vessels floating in CSF space over the convexities more than one vessel thick. Moderate enlargement of the SAS extends to significantly enlarge the CSF space between the hemispheres along the falx cerebri. Marked enlargement of the SAS would be profound pathological enlargement for the CSF spaces over and between the hemispheres.
The image on the right demonstrates mild enlargement of the perivascular spaces. Minimal enlargement of the PVS is defined as fewer than 5 small, linear T2 hyperintensities or 1–2 larger foci. Mild enlargement is 5–10 small, linear or 3–5 larger, rounded hyperintensities. Moderate enlargement of the PVS is when there are more than 10 linear or 5–10 larger, rounded hyperintensities. Marked enlargement of the PVS involves pathological number, size and location (e.g., corpus callosum) of the hyperintensities associated with white matter volume loss.
Head Circumference
After adjusting for age and site, HR and LR groups did not differ in head circumference (p=.55). Mean head circumference for the HR group was 44.5 cm (SD 1.41) and for the LR group was 44.6 cm (SD 1.34). There were no group X sex differences in head circumference (p=.36). See boxplot in Figure 3 and scatterplot in Figure 4.
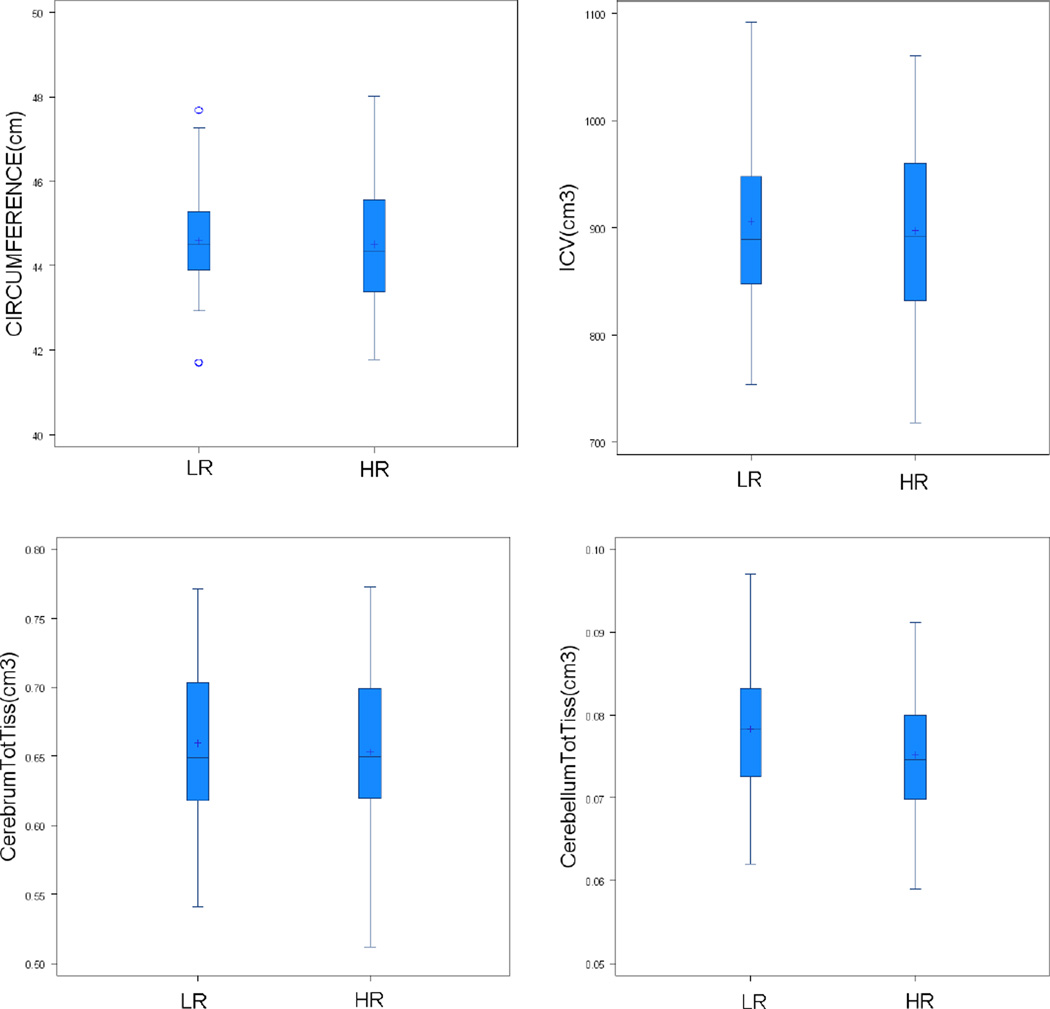
Figure 3. Boxplots of head circumference and total brain volumes for HR and LR subjects.
Figure 3 shows plots of head circumference (panel 1), intracranial volume (ICV) (panel 2), and total tissue volumes for cerebrum (panel 3) and cerebellum (panel 4). Head circumference is displayed in centimeters (cm) and brain volumes shown in centimeters squared (cm3).
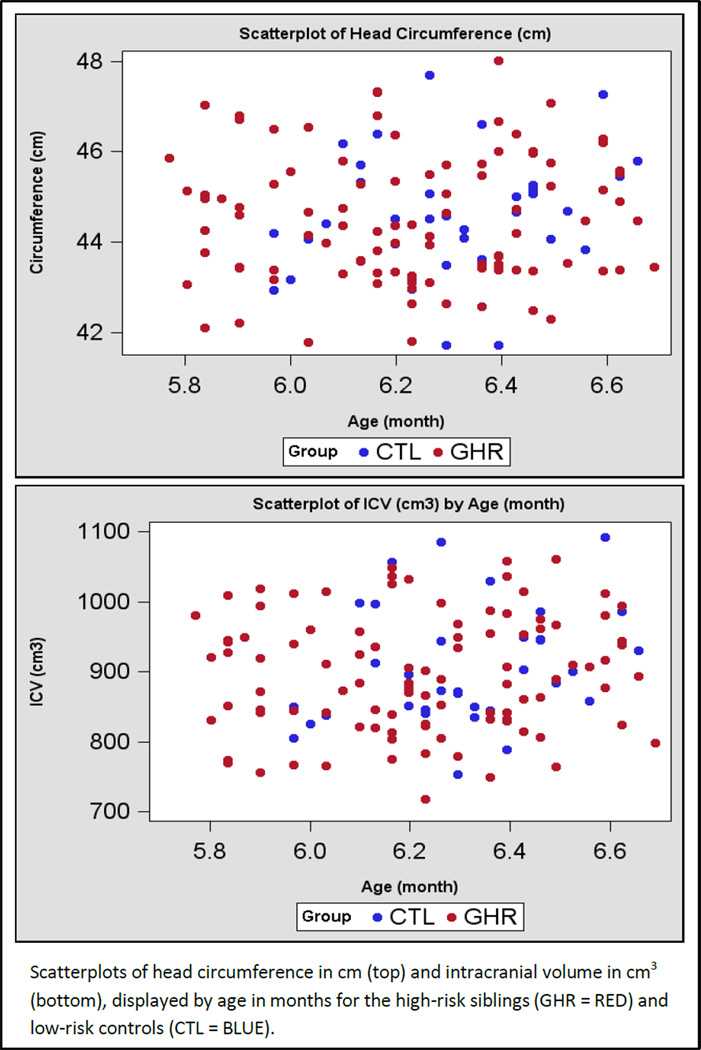
Figure 4.
Scatterplots of head circumference and intracranial volume for HR and LR subjects.
Total brain volume (ICV), Total Tissue, CSF
There were no significant differences between the HR and LR groups for any of the global measures of brain volume after adjusting for age, sex, site, and cognitive development. There were no significant group differences in total brain volume (ICV), total tissue, or total CSF (see Table 2 for mean volumes and comparisons). Boxplots displaying ICV and total tissue for cerebrum and cerebellum for the HR and LR subjects is displayed in Figure 3. A scatterplot for ICV is shown in Figure 4.
Table 2.
Adjusted mean volume differences for cerebrum, cerebellum, and lateral ventricles.
| Low Risk | High Risk | Group Difference | ||||
|---|---|---|---|---|---|---|
| Measurement Region (cm3) | LSMean | (SE) | LSMean | (SE) | F(1, 125) | p |
| Intracranial Volume (ICV) | 897.2 | 12.1 | 889.5 | 7.9 | .28 | .60 |
| Total Tissue (GM+WM) | 737.9 | 9.2 | 730.9 | 6.0 | .39 | .53 |
| Total CSF | 159.3 | 5.1 | 158.6 | 3.3 | .02 | .90 |
| Cerebrum | ||||||
| Total (Tissue + CSF) | 798.6 | 11.4 | 792.6 | 7.4 | .19 | .66 |
| Left (GM+WM) | 326.1 | 4.3 | 323.1 | 2.8 | .36 | .55 |
| Right (GM+WM) | 327.4 | 4.2 | 325.4 | 2.8 | .16 | .69 |
| Total CSF | 145.0 | 5.1 | 144.1 | 3.3 | .02 | .89 |
| Cerebellum | ||||||
| Total (Tissue + CSF) | 89.0 | 1.2 | 87.3 | .8 | 1.26 | .26 |
| Left (GM+WM) | 38.5 | .6 | 37.6 | .4 | 1.65 | .20 |
| Right (GM+WM) | 38.2 | .6 | 37.3 | .4 | 1.8 | .18 |
| Total CSF | 12.3 | .4 | 12.4 | .3 | .05 | .82 |
| Lateral Ventricles | ||||||
| Left | 5.6 | .4 | 5.7 | .2 | .04 | .84 |
| Right | 5.1 | .3 | 4.9 | .2 | .22 | .64 |
adjusted for age, sex, Mullen ELC, and site
Cerebral cortex/Cerebellum
Total tissue volume (GM+WM) and total CSF volume for the cerebrum and cerebellum were examined, as well for each hemisphere (right/left) of the cerebrum and cerebellum. The groups did not differ significantly in any of the measures, and there were no significant group X sex effects. Mean group volumes for these measures are displayed in Table 2.
Lateral Ventricles
Volume of the lateral ventricles was compared between groups for left and right ventricle and group means are presented in Table 2. There were no group differences for either left or right ventricular volume, or when examining group X sex effects (left p=.80, right p=.46).
Discussion
First degree relatives of autistic individuals are reported to have high rates of macrocephaly (6). In addition, it is anticipated that approximately 20% of HR infants in this sample will meet criteria for an autism spectrum disorder by 36 months of age (17). In this first report of brain volume in 6 month old infants at high familial risk for autism from an ongoing longitudinal neuroimaging study, we find no differences in overall brain tissue volumes, head circumference, or ventricular volumes compared to LR peers.
This investigation does not address the question as to whether early brain volume differences can be observed in those HR children who are later diagnosed with an ASD, which will be examined in the longitudinal dataset from this study. Rather our intent was to study whether differences in brain volume could be detected in a sample of children at high genetic risk for autism, an approach that has been used previously to examine familial rates of macrocephaly in ASD (7). There is, therefore, a chance that using outcome data we will be able to detect significant differences at 6 months once we are able to examine trajectories of growth in the various subgroups at 24 months of age.
The absence of brain volume differences in six month olds at high familial risk for autism in this large cohort is consistent with our hypothesis that brain enlargement in autism as well as possibly in those with high genetic liability is a later occurring post-natal process. Disruptions of cortical maturation related to impaired experience-dependent synaptic development have been observed in mouse models of both Angelman Syndrome (28) and Fragile X syndrome (29), neurogenetic developmental disorders that have behavioral features which overlap with ASDs. There is also the report of mutations associated with the defective expression of activity-driven genes in individuals with ASDs (30), although it is unclear whether this mechanism accounts for more than a very small proportion of genetic variance underlying ASDs. Other hypotheses about brain overgrowth, based on the observation that early brain overgrowth is associated with increases in cortical surface area (10), suggest that brain overgrowth in the latter part of the first year may be the result of an increase in neuronal precursors, perhaps related to aberrant molecular regulatory processes (31). The findings from the current study suggest that early postnatal events may underlie latter occurring brain overgrowth and raise the optimistic possibility that there is a window of opportunity where early postnatal intervention, during a period of tremendous brain plasticity, may have an important impact on later emergence of autistic behavior.
Studies examining early behavioral markers of autism have shown infants who later go on to receive a diagnosis of an ASDs do not exhibit overt autistic behaviors at 6 months of age (15, 17). However, by 12 months of age, a time when we anticipate brain differences may begin to emerge, behavioral deficits can be detected in those infants who are later diagnosed with autism (15, 17, 32). Work by our group examining the behavioral characteristics of our sample is underway. In the current study, the HR group likely includes children who later receive a diagnosis of autism. With the later addition of diagnostic outcome data on this sample, it will be possible to examine whether brain volumes for those children who are HR and eventually manifest ASDs differ from those HR children who do not go on to develop ASDs, to specifically test our hypothesis of brain overgrowth in the latter part of the first year of life or early second year of life.
We also did not detect any clinically significant radiologic findings specific to the HR group. The types and rate of incidental findings observed was the same between groups, as was the degree of enlargement for the subarachnoid spaces and the periventricular spaces. Most reports looking for evidence of clinical neuroradiologic abnormalities in individuals with autism have not detected a significant type or pattern of findings associated with the disorder. Scattered reports of individuals with neuronal migration abnormalities (33), as well as subcortical abnormalities in the corpus callosum (34) and increased rates of dilated Virchow-Robin spaces (35) exist. However, numerous methodological shortcomings such as the etiologic heterogeneity known to be inherent in autism, small sample sizes, variations in subject age and inconsistent findings across studies have complicated interpretation of this research. One large-scale retrospective study (36) observed elevated rates clinically reported abnormalities in children with autism compared to medical patients. Forty-eight percent of the ASD cases were rated as having abnormalities. The primary abnormalities observed included white matter signal intensity abnormalities, dilated Virchow-Robin spaces, and temporal lobe abnormalities (e.g., subcortical hyperintensities in the temporal poles). White matter abnormalities (e.g., punctate or posterior T2 hyperintensities) were present in younger children (e.g., 5 year olds versus 7 year olds), while the temporal lobe abnormalities showed no developmental trend. It should be noted that the current study differs from this report by Boddaert (36) as we do not explicitly examine children (2 to 16 years of age) with ASDs, but rather we examine infants at high risk for autism. The developmental nature of incidental findings we observed and their association with ASDs therefore remains a question worthy of future investigation.
A limitation of this study, due in part to our focus on six month olds, is that, due to the difficulty associated with segmenting gray and white matter at this early age in development, we are unable to define regionally-specific brain volumes. White matter structure and myelination may best be captured with other methods, such as diffusion tensor imaging (DTI), and we have recently reported significant differences in white matter development in HR infants who later receive a diagnosis of ASD (37). More dynamic methods like resting state functional MRI (fcMRI) may also detect early functional changes prior to the occurrence of more grossly observable changes in volume. These methodologies may be more sensitive to early brain differences and may be able to detect changes in structural or functional development that are not possible using standard tissue classification methods. Lastly, our current study is cross-sectional and at this point our sample is characterized in terms of HR and LR status. Only a portion of our current HR group may later be diagnosed with an ASD. We will include diagnostic outcome data and longitudinal imaging data from later timepoints (e.g., 12, 24 and/or 36 months of age) to examine the groups for more specific insights into the behavioral and biological mechanisms underlying the development of ASDs.
Acknowledgements
We wish to acknowledge the work of SunHyung Kim, Rachel Gimpel Smith, Michael Graves, and Ryan Scotton for their assistance in processing this data and to Penelope Kostopoulus and Samir Das for their assistance with database management. Most importantly, we extend our sincere appreciation to the families who have participated in this study.
This work was supported by an NIH Autism Center of Excellence grant (NIMH and NICHD #HD055741 to J. Piven) and funding in support of this work from Autism Speaks and the Simons Foundation. Further support was provided by the National Alliance for Medical Image Computing (NA-MIC), funded by the NIH through grant U54 EB005149.
Footnotes
Previous Presentation: IMFAR 2011
Disclosures
The authors report no competing interests.
References
- 1.Piven J, Nehme E, Simon J, Barta P, Pearlson G, Folstein SE. Magnetic resonance imaging in autism: Measurement of the cerebellum, pons, and fourth ventricle. Biol Psychiatry. 1992;31:491–504. doi: 10.1016/0006-3223(92)90260-7. [DOI] [PubMed] [Google Scholar]
- 2.Piven J, Ardnt S, Bailey J, Andreasen N. Regional brain enlargement in autism: A magnetic resonance imaging study. J Am Acad Child Adol Psychiatry. 1996;35:530–536. doi: 10.1097/00004583-199604000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 4.Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59(2):184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 5.Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. Autism as a strongly genetic disorder: Evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- 6.Lainhart JE, Piven J, Wzorek M, Landa R, Santegelo SL, Coon H, Folstein SE. Macrocephaly in children and adults with autism. J Am Academy Child Adol Psychiatry. 1997;36:282–289. doi: 10.1097/00004583-199702000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, Deutsch CK, Dunn M, Estes A, Tager-Flusberg H, Folstein S, Hepburn S, Hyman S, McMahon W, Minshew N, Munson J, Osann K, Ozonoff S, Rodier P, Rogers S, Sigman M, Spence MA, Stodgell CJ, Volkmar F. Head circumference and height in autism: A study by the collaborative program of excellence in autism. Am J Med Genet Part A. 2006;140A:2257–2274. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey A, Luthert P, Bolton P, LeCouteur A, Rutter M. Autism and megalencephaly. Lancet. 1993;34:1225–1226. doi: 10.1016/0140-6736(93)91065-t. [DOI] [PubMed] [Google Scholar]
- 9.Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- 10.Hazlett HC, Poe MD, Gerig G, Styner M, Chappell C, Smith RG, Vachet C, Piven J. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry. 2011;68(5):467–476. doi: 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akschoomoff N, Pierce K, Hagler D, Schork N, Lord C, Courchesne E. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30(12):4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosconi MW, Hazlett HC, Poe MD, Gerig G, Smith RG, Piven J. Longitudinal study of amygdala volume and joint attention in 2- to 4-year old children with autism. Arch Gen Psychiatry. 2009;66(5):509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Courchesne E, Campbell K, Solso S. Brain growth across the life span in autism: Age-specific changes in anatomical pathology. Brain Research. 2011;22:138–145. doi: 10.1016/j.brainres.2010.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Constantino JN, Majmudar P, Bottini A, Arvin M, Virkud Y, Simons P, Spitznage E. Infant head growth in male siblings of children with and without autism spectrum disorders. J Neurodev Disord. 2010;2(1):39–46. doi: 10.1007/s11689-009-9036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brain J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23(2):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28(47):12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Bryson S, Carver LJ, Constantino J, Dobkins K, Hutman T, Iverson JM, Landa R, Rogers SJ, Sigman M, Stone WL. Recurrence risk for autism spectrum disorders: a baby siblings research consortium study. Pediatrics. 2011 doi: 10.1542/peds.2010-2825. August release. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeegers M, Van Der Grond J, Durston S, Ievelstein RJ, Witkamp T, Van Daalen E, Buitelaar J, Engeland HV. Radiological findings in autistic and developmentally delayed children. Brain Dev. 2006;28(8):495–499. doi: 10.1016/j.braindev.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Steiner CE, Guerreiro MM, Marques-de-Faria AP. Brief report: Acrocallosal syndrome and autism. J Autism Dev Disord. 2004;34:723–726. doi: 10.1007/s10803-004-5292-0. [DOI] [PubMed] [Google Scholar]
- 20.Taber KH, Shaw JB, Loveland KA, Pearson DA, Lane DM, Hayman LA. Accentuated Virchow-Robin spaces in the centrum semiovale in children with autistic disorder. J Comput Assit Tomogr. 2004;28:263–268. doi: 10.1097/00004728-200403000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Boddaert N, Zilbovicius M, Philipe A, Robel L, Bourgeois M, Barthelemy C, Seidenwurm D, Meresse I, Laurieer L, Desguerre I, Bahi-Buisson N, Brunelle F, Munnich A, Samson Y, Mouren M-C, Chabane N. MRI findings in 77 children with non-syndromic autistic disorder. PLoS ONE. 2009;4(2):e4415. doi: 10.1371/journal.pone.0004415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullen EM. The Mullen Scales of Early Learing. Circle Pines, MN: American Guidance Service, Inc.; 1995. [Google Scholar]
- 23.Sparrow SS, Balla D, Cicchetti DV. Vineland adaptive behavior scales, second edition. Second ed. Shoreview, MN: American Guidance Service; 2005. [Google Scholar]
- 24.Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, Brian J. The autism observation scale for infants: Scale development and reliability data. J Autism Dev Disord. 2008;38(4):731–738. doi: 10.1007/s10803-007-0440-y. 2008. [DOI] [PubMed] [Google Scholar]
- 25.Liao H, Edwards P, Pan X, Fan Y, Yang G-Z, Fonov V, Janke A, Caramanos Z, Arnold D, Narayanan S, Pike GB, Collins DL. Improved precision in the measurement of longitudinal global and regional volumetric changes via a novel MRI gradient distortion characterization and correction technique. Lecture Notes in Computer Science, Medical Imaging and Augmented Reality. 2010;6326:324–333. [Google Scholar]
- 26.Fonov V, Janke AL, Caramoanos Z, Arnold DL, Narayanan S, Pike GB, Collins DL. Improved precision in the measurement of longitudinal global and regional volumetric changes via a novel MRI gradient distortion characterization and correction technique. MIAR. 2010:324–333. [Google Scholar]
- 27.Gerig G, Gouttard MS, Styner M, Dager S, Collins DL, Fonov V, Evans A, McKinstry RC, Botteron KN, Schultz RT, Paterson JS, Hazlett HC, Piven J. Reliability in a multi-site longitudinal magnetic resonance imaging study: The importance of a priori and ongoing assessment and standardization. Submitted. [Google Scholar]
- 28.Gouttard S, Styner M, Prastawa M, Piven P, Gerig G. Lecture Notes in Computer Science. Vol. 5242. Springer Verlag; 2008. Assessment of Reliability of Multi-site Neuroimaging via Traveling Phantom Study. Proc. MICCAI ’08; pp. 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Leemput K, Maes F, Vandermeulen D, Suetens P. Automated model-based tissue classification of MR images of the brain. IEEE Trans Medical Imaging. 1999;18:897–908. doi: 10.1109/42.811270. [DOI] [PubMed] [Google Scholar]
- 30.Gouttard S, Styner M, Joshi S, Smith RG, Hazlett HC, Gerig G. Subcortical structure segmentation using probabilistic atlas priors. SPIE Medical Imaging 2007: Image Processing. 2007 Mar;6512:88. [Google Scholar]
- 31.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficacty and reliability. Neuroimage. 2006;31(3):1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Yashiro K, Riday TT, Condon KH, Roberts AC, Bernardo DR, Prakash R, Weinberg RJ, Ehlers MD, Philpot BD. Ube3a is required for experience-dependent maturation of the neocortex. Nat Neuroscience. 2009;12(6):777–783. doi: 10.1038/nn.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56(6):955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RD, Mukaddes NM, Balkhy S, Glascon G, Hashmi A, Al-Saad S, Ware J, Joseph RM, Greenblatt R, Gleason D, Ertelt JA, Apse KA, Bodell A, Partlow JN, Barry B, Yao H, Markianos K, Ferland RJ, Greenberg ME, Walsh CA. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321(5886):218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 36.Wetherby AM, Brosnan-Maddox S, Peace V, Newton L. Validation of the Infant—Toddler Checklist as a broadband screener for autism spectrum disorders from 9 to 24 months of age. Autism: The International Journal of Research & Practice. 2008;12(5):487–511. doi: 10.1177/1362361308094501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, Dager SR, Dawson G, Estes AM, Evans A, Hazlett HC, Kostopoulos P, McKinstry RC, Paterson SJ, Schultz RT, Zwaigenbaum L, Piven J. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psych. doi: 10.1176/appi.ajp.2011.11091447. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]