Abstract
A novel sheathless CITP/CZE-MS interface featuring a large i.d. separation capillary and a detachable small i.d. porous ESI emitter was developed in this study to simultaneously achieve large sample loading capacity and stable nanoESI operation. Crucial operating parameters, including sample loading volume, flow rate, and separation window, were systematically investigated to attain optimum CITP/CZE separation efficiency and MS detection sensitivity. The performance of CITP/CZE-nanoESI-MS using the new sheathless interface was evaluated for its achievable low limit of quantification (LOQ) by analyzing targeted peptides, leu-enkephalin and angiotensin II, spiked in a BSA tryptic digest matrix at different concentrations. A linear dynamic range spanning 4.5 orders of magnitude and a 10 pM LOQ with measurement reproducibility at CV < 22% were obtained experimentally for both targeted peptides, representing a 5-fold sensitivity improvement as compared to using the sheath liquid interface developed previously.1
INTRODUCTION
Capillary electrophoresis mass spectrometry (CE-MS) has been an attractive analytical technique since its inception2-4 due to its high separation efficiency, broad sample compatibility and high sensitivity detection. The utility of the CE-MS has been well demonstrated in e.g., proteomics,5-6 metabolomics7-8 and peptidomics,9 for performing glycoform profiling of intact proteins,10-11 metabolomic profiling of clinical samples12-14 and protein phosphorylation studies.15-16 Key reasons that CE-MS is still not considered as the method of choice in the routine chemical or biological sample analysis compared to more conventional liquid chromatography (LC)-MS are its small sample loading capacity and less robust interface. Seeking effective solutions to these two challenge problems continues to be a primary focus of research and development in CE-MS instrumentation.
Capillary electrophoresis is intrinsically a very small scale separation technique with a total volume of typically less than several microliters. The sample loading capacity in a typical capillary zone electrophoresis (CZE) separation is about 1% of the total capillary volume as required to maintain a good separation quality, which limits the sample loading volume to the nanoliter range. While small sample consumption is advantageous when sample amount is strictly limited, it is more often a drawback limiting the measurement dynamic range. To overcome this limitation, transient capillary isotachophoresis (CITP/CZE) was developed to increase the sample loading capacity through in situ sample enrichment.17 A sample loading capacity over 30% of the total separation capillary volume has been demonstrated for CITP/CZE separations,1,17-20 increasing the sample loading volume to several microliters. In addition, the capability of CITP/CZE in selectively enriching low abundance analytes in a complex mixture also makes the technique effective in many biological applications as important disease related biomarkers often exist in a complex biomatrixes at extremely low abundances.18,21 However, in order to achieve microliter sample loading volumes, larger i.d. (> 75 μm) separation capillary are needed, which poses a new challenge to the CITP/CZE-MS interface design. The most efficient CITP/CZE-MS interface benefits from the use of a smaller i.d. (< 30 μm) capillary emitter to operate electrospray ionization (ESI) in the nanoliters per minute flow rate range (nanoESI) as it is well documented that nanoESI both improves analyte ionization efficiency 22 and reduces ionization bias.23, 24
Online coupling of CE with MS is typically achieved by using a sheath liquid2 or a sheathless interface. In the sheath liquid interface, either a sheath liquid flowing coaxially with the separation liquid or a liquid-junction24-26 at the ESI emitter tip is used to provide the electric contact for ESI voltage. Sample dilution due to the use of sheath liquid limits the achievable sensitivity. To overcome this problem, several sheathless interface designs were developed in which the electric contact for ESI voltage was realized by using a special metal,18,27 conductive polymer28 and carbon29 coated ESI emitter or inserting a metal wire into the CE separation capillary.30 Other alternative sheathless interface designs involving the use of a micro-tee31 or a stainless tubing32 to connect the separation capillary with the ESI emitter to close electrical circuit for CE separation voltage and ESI voltage have also been reported. However, these interface designs, sheath liquid or sheathless, all suffer from one or multiple problems including sample dilution, low mechanical robustness, and poor reproducibility. The latest sheathless interface design developed by Moini using a chemically etched porous ESI emitter33 showed a much improved CE-MS performance. This interface design has been recently commercialized by Beckman Coulter (Brea, CA), and has led to several successful applications.10,12,34-40 While this latest sheathless interface design effectively addresses several of the common problems of earlier interface designs, the sample loading capacity is still limited by the need to use small i.d. separation capillary in order to accommodate stable nanoESI operation.
Here we report the development of a new sheathless CITP/CZE-MS interface design that combines the capability for large sample loading volume with stable nanoESI operation. The new interface effectively resolves the mismatch of existing interface designs between the need to use large i.d. separation capillary for large sample loading capacity and small i.d. emitter capillary for stable nanoESI operation. The overall novelty of the interface design includes a large bore separation capillary for large sample loading capacity, a dilution free sheathless interface using porous emitter and a smaller emitter capillary for robust nanoESI operation. Detailed characterization of the new interface was performed to show its achievable sample loading capacity, separation peak capacity, reproducibility, and detection sensitivity. The use of the new sheathless CITP/CZE-MS interface to quantify targeted peptides in bovine serum albumin (BSA) digest matrix and its achievable system limit is also systematically evaluated in the study. Since the main focus of this study is on characterizing the new sheathless CITP/CZE-MS interface technology, samples with intermediate complexity were used in all the experiments. A follow up study will focus on the biological applications of this new interface technology using more complex human serum and plasma samples.
EXPERIMENTAL
Chemicals
BSA tryptic digest standard was purchased from Protea (Morgantown, WV). Bradykinin, angiotensin I, neurotensin, fibrinopeptide, substance P, kemptide, leu-enkephalin, angiotensin II, melittin, renin, ammonium acetate, methanol, acetic acid and hydrofluoric acid (49%) (HF) were purchased from Sigma (St. Louis, MO). Fused silica capillaries were purchased from Polymicro Technologies (Phoenix, AZ). Epoxy (EP42HT-2) was purchased from Masterbond (Hackensack, NJ).
Sample preparation
The solution of 0.1 M acetic acid in deionized water mixed with methanol at a volume ratio 9:1 was used as background electrolyte (BGE) solution. 25 mM ammonium acetate in deionized water with the pH adjusted to pH=4 by adding acetic acid was used as leading electrolyte (LE) solution in all CITP/CZE experiments.
A stock solution of ten peptide mixture (bradykinin, angiotensin I, neurotensin, fibrinopeptide, substance P, kemptide, leu-enkephalin, angiotensin II, melittin, and renin) at 10 μM concentration for each peptide was first prepared in deionized water and stocked in aliquots. The stock solution was subsequently diluted to 3.75 μM using LE solution to serve as a working solution for the performance evaluation and optimization of the online sheathless CITP/CZE-nanoESI-MS platform. The stock solution of ten peptide mixture was further mixed with 50 nM BSA tryptic digest in 25 mM LE at different concentrations ranging from 10 pM to 500 nM for each peptide in the CITP/CZE SRM MS sample quantification experiments. Among the 10 peptides in the sample mixture, leu-enkephalin, angiotensin II and kemptide were selected as representative targets in CITP/CZE-selected reaction monitoring (SRM) MS experiments due to their good signal intensity displayed in our previous study.1
Sheathless CITP/CZE-MS interface
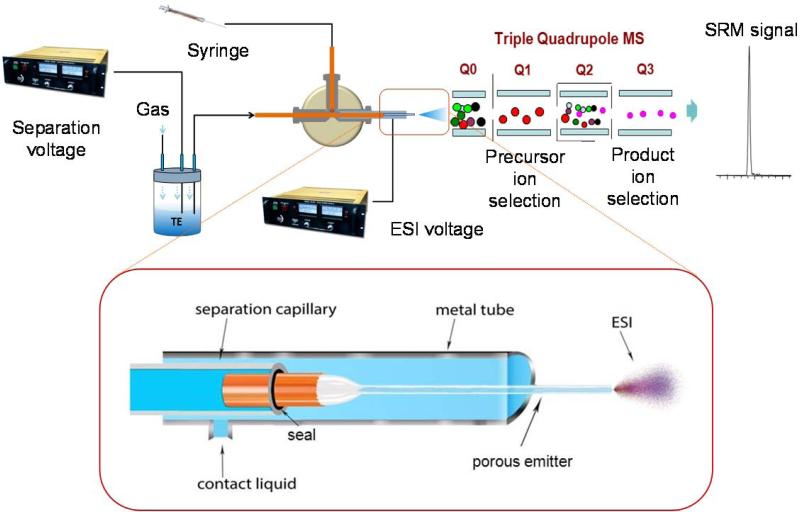
The upper part of figure 1 shows the experimental setup used in this study which is a modified setup used in our previous work.1 Briefly, one end of the separation capillary was inserted in a sealed reservoir for hydrodynamic sample loading or system flushing as well as the application of separation voltage while the joint between the other end of the separation capillary and porous emitter capillary was enclosed in a metal tube containing electrically conductive liquid using a peek cross. A syringe filled with conductive liquid was also connected to the peek cross to fill and refresh the metal tube prior and between each sample analysis. A second ESI voltage was applied directly on the metal tube and the CITP/CZE-nanESI was interfaced with a triple quadrupole mass spectrometry. The new sheathless CITP/CZE-MS interface design, as shown in the lower part of figure 1, involves the use of two different capillaries. A larger i.d. fused silica capillary (360 μm o.d., 100 μm i.d., and 95 cm long) was used as the separation capillary with its inner surface coated with hydroxypropyl cellulose to minimize electroosmotic flow. The coating was prepared by first flushing capillary with 1 mL 1M HCL, by applying 15 psi N2 back pressure to the solvent reservoir, and then flushing 200 μl 5% hydroxypropyl cellulose using 20 psi N2 back pressure. Finally, the capillary was flushed with deionized water to remove excessive hydroxypropyl cellulose. A smaller i.d. capillary (90 μm o.d., 20 μm i.d., and ~4 cm long) was used as ESI emitter. A section of the emitter capillary, ~3 cm long from one end, was chemically etched to make it porous using the method described by Moini 33 to provide electric contact and serve as ESI emitter without further sharpening the emitter tip (etching experiments using HF must be conducted in hood. Goggle, rubber gloves and aprons are required for personnel protection), and the other end of the emitter capillary was subsequently inserted into the separation capillary. The assembly was then sealed by carefully wrapping the acid resistant epoxy around the joint and the epoxy was cured at room temperature overnight. Extended epoxy cure time at elevated temperature may be needed if dealing with stronger acid and is optional with the current setup in this study. The joint was then enclosed in a short metal tube (0.04″ i.d., 1/16″ o.d., and 5 cm long) filled with the contact solution of 2% (by volume) acetic acid in deionized water to form a sheathless CITP/CZE MS interface. The interface contact solution was refreshed after each CITP/CZE MS sample analysis to ensure a good electric contact for the measurement reproducibility. Due to the existence of size change at the capillary joint, it is important to keep all the connecting union and cross clean to prevent capillary blockage. Moreover, buffers and samples should be prepared with filtered deionized water. Prior to each run, the separation capillary was filled with BGE solution and flushed for 15 min by applying 15 psi back pressure in the BEG reservoir (Figure 1). Samples were loaded into the separation capillary at the same back pressure with the loading amount adjusted by varying the length of loading time. After sample loading, the sample reservoir at the capillary inlet was replaced by the BGE reservoir and a 30 kV separation voltage was applied to the sample inlet using a Glassman high voltage power supply. A second voltage varying from 1.5 to 1.7 kV was applied to the metal tube at the emitter end of the capillary for stable electrospray operation using a Bertan high voltage power supply. The ESI voltage was adjusted for each emitter capillary to achieve optimum sensitivity and signal stability at each CITP/CZE flow rate. Position of the emitter relative to the MS inlet was also optimized for best signal intensity and stability. During CITP/CZE analysis, a smaller gas pressure varying from 1 psi to 3 psi, referred as eluting pressure, was applied at the BGE reservoir to maintain a stable flow rate in the low nL/min range.
Figure 1.
Schematic of CITP/CZE-nanoESI-QQQ MS setup used in this study. Lower part shows a detailed view of the sheathless interface design (not to scale).
The new sheathless CITP/CZE-MS interface developed in this study has the benefit of achieving both large sample loading capacity and dilution free nanoESI operation simultaneously for significantly better sensitivity. The detachable design between the large i.d. separation capillary and small i.d. ESI emitter capillary also provides more flexibility in accommodating different experiment requirements. As the separation capillary and emitter capillary are prepared separately, the sizes of the separation capillary and emitter capillary can be altered independently and special treatment of the separation capillary can be performed easily. In addition, the porous emitter can be readily replaced without the need to change the separation capillary with little effort in case of blockage or broken emitter.
For the performance comparison, a sheathless CITP/CZE-MS interface using a single capillary (140 μm o.d., 30 μm i.d., and 95 cm long) was also prepared. The capillary was first neutrally coated with hydroxypropyl cellulose and a 3 cm section from one end of the capillary was then chemically etched to porous. The sample loading volume and eluting pressure was adjusted to obtain the optimum operating conditions for the interface.
Flow rate calibration
To correctly determine the liquid flow rate based on the gas pressure applied at the separation capillary inlet reservoir, the complete CITP/CZE setup was first calibrated experimentally. The liquid flow rates at different gas pressures were determined by using a calibrated pipet (1 to 5 μL, Drummond Scientific, Broomall, PA) to collect the liquid exiting the ESI emitter and measure the time for collecting 1 μL liquid under a given gas pressure. The experimental measurements showed a linear dependence of liquid flow rate on the gas pressure (Supplemental Figure S1).
Prior to each CITP/CZE-ESI-MS experiment, the flow rate at 15 psi gas pressure was measured so that the sample loading time and the eluting pressure could be adjusted accordingly to ensure the reproducibility of the sample loading volume and ESI flow rate.
MS optimization and data processing
All the CITP/CZE-nanoESI-MS analyses were performed using a triple quadruple mass spectrometer (TSQ Quantum ultra, Thermo Fisher Scientific, MA). The inlet capillary of the mass spectrometer was maintained at 200 °C. Unit resolution (0.7 Da peak width) was used for Q3 in full scan MS and for both Q1 and Q3 in SRM operating mode. All the MS spectra were recorded in profile mode. In full scan MS mode, m/z range from 300 to 1400 was used with a scan time of 0.3 sec. Three most intense transitions and their corresponding collision energies for each targeted peptide in CITP/CZE-nanoESI-SRM MS sample quantification experiment were determined by using a fully automated compound optimization procedure in Thermo TSQ Tune view. Table 1 listed all the transitions and their corresponding collision energies for six targeted peptides used for sample quantification in which three peptides from BSA digest were used as quality control analytes to ensure measurement reproducibility.
Table 1.
SRM transitions and parameters used in this study.
| Compound Name | Sequence | Precursor Ion (m/z) | Product Ion (m/z) | Dwell Time (msec) | Collision Energy (V) |
|---|---|---|---|---|---|
| Leu-enkephalin | [YGGFL+H]+ | 556.3 | 397.2 (a4+) | 25 | 19 |
| 556.3 | 425.2 (b4+) | 25 | 16 | ||
| 556.3 | 278.1 (b3+) | 25 | 25 | ||
| Angiotensin II | [DRVYIHPF+2H]2+ | 523.8 | 263.1 (y2+) | 25 | 20 |
| 523.8 | 784.4 (b6+) | 25 | 18 | ||
| 523.8 | 647.4 (b5+) | 25 | 22 | ||
| Kemptide | [LRRASLG+2H]2+ | 386.7 | 567.3 (b5+-NH3) | 25 | 18 |
| 386.7 | 409.3 (b3+-NH3) | 25 | 25 | ||
| 386.7 | 539.4 (a5+-NH3) | 25 | 22 | ||
| BSA peptide I | [LVNELTEFAK+2H]2+ | 582.3 | 951.5 (y8+) | 25 | 18 |
| 582.3 | 595.3 (y5+) | 25 | 20 | ||
| 582.3 | 837.4 (y7+) | 25 | 16 | ||
| BSA peptide II | [HLVDEPQNLIK+2H]2+ | 653.4 | 712.4 (y6+) | 25 | 26 |
| 653.4 | 251.2 (b2+) | 25 | 26 | ||
| 653.4 | 1056.7 (y9+) | 25 | 23 | ||
| BSA peptide III | [CCTESLVNR+2H]2+ | 512.7 | 637.2 (b6+) | 25 | 17 |
| 512.7 | 581.7 (a6+- 28) | 25 | 16 |
The bolded transitions indicate the transition used for quantification of each peptide. The non bolded/italicized transitions were used to confirm the peptide's identity. The italicized transition of BSA peptide was used to monitor the platform's reproducibility and calibration.
All the data analyses of the raw MS files were carried out using Thermo Xcalibur Qual Browser 2.2. In the chromatogram ranges setting, peak algorithm was selected as ICIS. No smoothing or baseline subtraction was used and the default setting was used for mass tolerant. Auto peak detection with default parameters was used for all the peak extraction and peak area measurement while manual integration was also used to verify all the peak area measurements.
RESULTS AND DISCUSSION
Sample loading optimization
The performance of the new CITP/CZE-MS interface was first evaluated for its sample loading capacity and separation quality. CITP/CZE separations were carried out under different sample volumes ranging from 0.27 μL to 3.07 μL using a 10 peptide mixture (3.75 μM concentration for each peptide) in 25 mM ammonium acetate. Sample loading volume was varied by adjusting the loading time at a given gas pressure (15 psi) as calculated according to the flow rate calibration described in the experimental section. Figure 2 shows the extracted ion chromatogram (EIC) at different sample loading volumes with the ESI flow rate measured at 60 nL/min. Well focused narrow peaks were achieved except two late eluting peaks (peak 9 and 10 in figure 2) as focusing factor decreases as the electric mobility of analyte ion decreases. The focusing factor of the later eluting peptides can be further increased by increasing the LE concentration.1 Baseline separation of the ten peptides was achieved at sample loading volumes of 1.39 μL (Figure 2A) and 1.95 μL (Figure 2B), which equal to 18% and 26% of the total separation capillary volume respectively. Good separation was still achieved even at 2.5 μL sample loading volume which accounted for 33% of the total capillary volume (Figure 2C). Significant peak overlapping and loss of separation quality was observed at the sample loadings beyond 2.5 μL due to incomplete CITP sample focusing and insufficient separation time. The loading capacity result shown in Figure 2 is consistent with our previous study using a sheath liquid interface1 in which up to 1/3 of the total separation volume was allowed in CITP/CZE before a significant loss of separation quality was observed. The consistency between these two studies using completely different interfaces implies that the size change from 20 μm to 100 μm at the junction between the emitter capillary and the separation capillary in the current sheathless interface has minor effect on the separation quality. A possible explanation could be that the analyte band speed under the experimental operating condition was significantly faster than the speed of the liquid flow, providing effective analyte peak compression in the ESI process. Despite some overlapping and peak broadening of the late eluting peaks 9 and 10 at 2.5 μL sample loading volume (Figure 2 C), a separation window of more than 20 minutes was observed for all the sample loading volumes shown in Figure 2. In addition, the signal intensity keeps increasing as the sample loading volume increases. Since all of our selected peptide targets in the following sample quantification study elute much earlier than peaks 9 and 10, unless specified otherwise, 2.5 μL (33%) sample loading volume was chosen in all the subsequent experiments to maximize the measurement sensitivity. It was also observed experimentally that the CITP/CZE separation quality at a given sample loading volume improves as the analyte concentration decreases due to improved CTIP focusing1.
Figure 2.
Extracted ion chromatograms (EICs) of CITP/CZE separations using a 10 peptide mixture solution at different sample loading volumes. The labeled peaks from 1 to 10 are melittin, kemptide, substance p, bradykinin, angiotensin I, renin, neurotensin, angiotensin II, leu-enkephalin, and fibrinopeptide A, respectively.
Performance characterization of the sheathless CITP/CZE-MS interface
Following the initial sample loading volume optimization, the CITP/CZE- QQQ MS instrumental platform with the new sheathless interface was characterized systematically using the ten peptides spiked in 50 nM BSA digest in 25 mM LE at different concentrations. The mass spectrometry was operated in a highly sensitive SRM mode in which specific precursor-to-fragment ion transitions were monitored for each analyte in the sample solution. Three peptides (leu-enkephalin, kemptide and angiotensin II) in the sample mixture were chosen as the representative peptide targets in this study due to their good electrospray ionization efficiencies from our previous study1 and three peptides from BSA digest were also chosen to evaluate the stability of the instrument platform. Three most abundant transitions for each selected peptide, as listed in Table 1, were monitored in all the CITP/CZE-SRM MS analyses.
The advantage of analyte focusing was evaluated by comparing CITP/CZE with CZE at the same sample loading volume. With 2.5 μL sample loading and identical experimental setup as well as operating parameters, a broad kemptide peak with a flat top peak intensity caused by the long sample plug was resulted from CZE operation. In contrast, a sharp narrow peak was obtained in CITP/CZE separation mode which displays a 77 times higher peak intensity implying an approximately two orders of magnitude gain in signal intensity in CITP/CZE mode as compared to CZE only mode (Supplemental Figure S2).
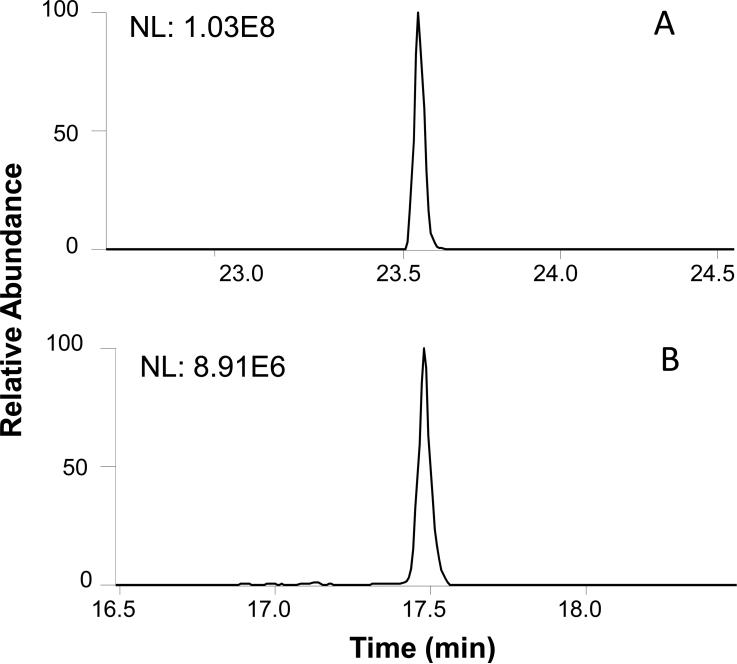
One of the unique features of the new sheathless CITP/CZE-MS interface developed in this study is its capability to use large i.d. separation capillary for increased sample loading volume and small i.d. emitter capillary for stable nanoESI operation simultaneously, both which are critical to the sensitivity achieved. This is evident by comparing the performance of the new interface with a conventional sheathless CITP/CZE-MS interface in which a single capillary (30 μm i.d., 360 μm o.d., and 95 cm long) was used for both CTIP/CZE separation and nanoESI operation.34, 40 The same sample as in the focusing experiment (Supplemental Figure S2) was used for the evaluation. With the same 33% of the sample loading volume with respect to the total capillary volume, 220 nL sample was loaded using the conventional sheathless CITP/CZE-MS interface while 2.5 μL sample was loaded using the new sheathless CITP/CZE interface. Separations in both interface configurations were performed under equal linear velocity of 0.75 cm/min to achieve similar separation quality, corresponding to 60 nL/min in the new interface and 5 nL/min in the conventional single capillary interface. Figure 3 shows the sensitivity comparison between these two interfaces using kemptide EIC. While similar peak widths were observed for both interface configurations, the kemptide peak intensity using the new CITP/CZE-MS interface (Figure 3A) shows a 11 fold improvement as compared to using the conventional sheathless CITP/CZE-MS interface (Figure 3B), which is consistent with the increase of the sample loading volume using the new interface.
Figure 3.
Sensitivity comparison of kemptide EIC using A) the new sheathless CTIP/CZE-MS interface with a 100 μm i.d. separation capillary and a 20 μm i.d. ESI emitter, and B) a conventional sheathless CITP/CZE-MS interface with a single 30 μm i.d. capillary for both CITP/CZE separation and ESI emitter. Sample condition: 50 nM target peptides spiked in 50 nM BSA digest in 25 mM LE.
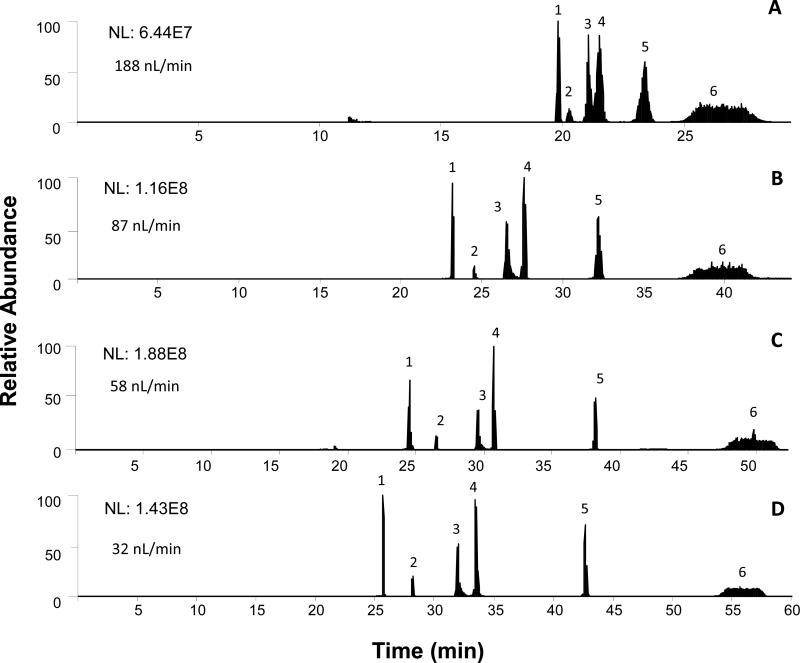
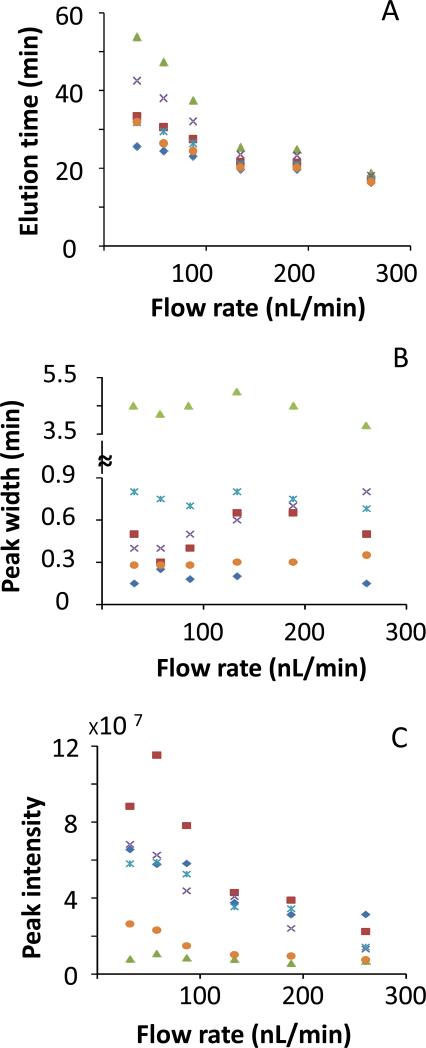
The benefit of operating electrospray at reduced flow rate was further demonstrated by operating the sheathless CTIP/CZE-ESI-SRM MS at different flow rates ranging from 30 nL/min to 377 nL/min. Figure 4 shows the total ion chromatogram (TIC) for kemptide, angiotensin II, leu-enkephalin and three selected BSA peptides from the CTIP/CZE-ESI-SRM MS analyses using the new sheathless interface at four representative ESI flow rates of 188 nL/min, 87 nL/min, 58 nL/min and 32 nL/min, respectively. As the flow rate decreased from 188 nL/min (Figure 4A) to 32 nL/min (Figure 4D), the effective separation window for all six monitored peptides increased from 10 min to 33 min and all the peptide peaks became increasingly separated from each other indicating a higher separation resolution at a lower ESI flow rate. The best CITP/CZE separation was achieved at 32 nL/min while a complete loss of separation was observed at the flow rate exceeding 261 nL/min (Supplemental Figure S3). Figure 5 further shows the change of peak elution time, peak width and peak intensity as a function of ESI flow rate. While the retention time for all the peptide peaks consistently increases with the decrease of the ESI flow rate (Figure 5A), the peak widths remain essentially unchanged (Figure 5B). More importantly, the intensity for all the peptide peaks increases as the flow rate decreases (Figure 5C) down to 58 nL/min because of higher ESI efficiency at lower flow rate. The observed decrease of the peak intensity at 32 nL/min flow rate for angiotensin II and leu-enkephalin relative to the peak intensity at 58 nL/min might be related to the stability of the electrospray implying an even smaller i.d. ESI emitter is needed. The peak capacity of the CITP/CZE separation can be also estimated based on the experimental measurements shown in Figures 4 and 5. In Table S1, the peak capacity at different flow rates was calculated using the average peak width at half peak height for five peptide peaks with Leu-enkephalin peak (peak 6) excluded from the peak width calculation due to its incomplete focusing. A peak capacity as high as over 200 was estimated for the sheathless CITP/CZE separation at 32 nL/min and good separation efficiency can be maintained at ESI flow rate lower than 100 nL/min.
Figure 4.
SRM TIC of CITP/CZE separations at different flow rates. The labeled peaks from 1 to 6 are kemptide, BSA peptide III, BSA peptide II, angiotensin II, BSA peptide I, and leu-enkephalin, respectively. Sample condition: 50 nM target peptides in 50 nM BSA digest and 25 mM LE.
Figure 5.
CITP/CZE peak elution time (A), peak width (B) and peak intensity (C) at different flow rates for kemptide ( ), angiotensin II (
), angiotensin II ( ), leu-enkephalin (
), leu-enkephalin ( ), BSA peptide I (
), BSA peptide I ( ), BSA peptide II (
), BSA peptide II ( ), and BSA peptide III (
), and BSA peptide III ( ).
).
Targeted peptide quantification
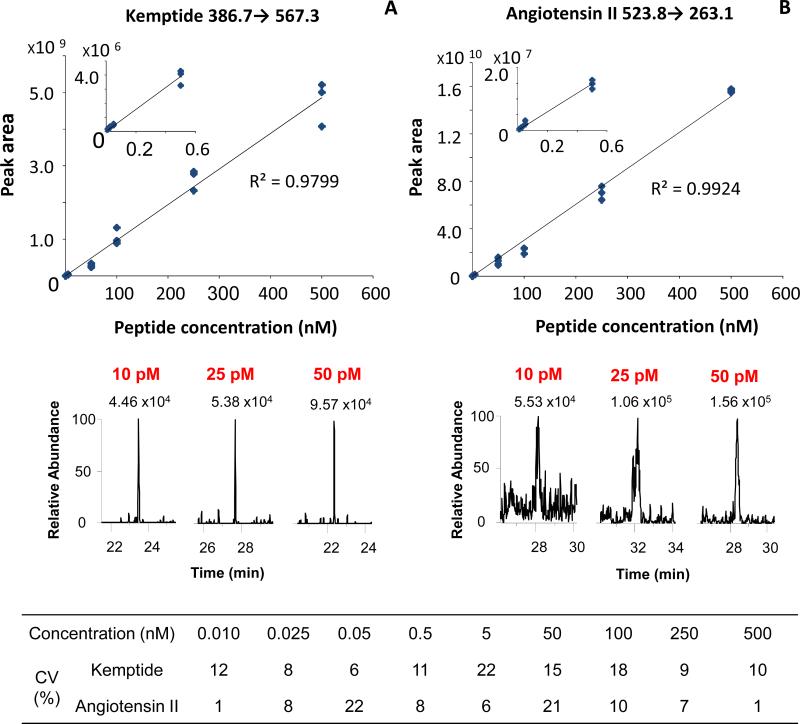
The analytical utility of the sheathless CITP/CZE-SRM MS was further evaluated for its linear dynamic range, limit of quantification (LOQ), and reproducibility. Triplicate analyses were performed using peptides (Table 1) spiked in 50 nM BSA digest at different concentrations from 10 pM to 500 nM. Flow rate of 60 nL/min was used in the CITP/CZE-SRM MS quantification due to the overall good ESI stability, peak capacity and sensitivity as discussed above. Quantification of each peptide was carried out using the most abundant transition (bolded in Table 1). One additional BSA peptide (italicized in Table 1) was also monitored as “internal standard” during each CITP/CZE-ESI-MS analysis to ensure the measurement stability and correct the small variations of run to run sample loading volume and analyte peak intensity. Figure 6 shows the CITP/CZE-nanoESI-SRM MS quantification results for kemptide (Figure 6A) and angiotensin II (Figure 6B). Good linearity (R2 > 0.97) for both targeted peptides between the concentration and peak area was observed in the concentration range from 10 pM to 500 nM, indicating 4.5 orders of magnitude linear dynamic range. Based on the signal-to-noise ratio (S/N) and the measurement CV shown in Figure 6, The LOQs for both kemptide and angiotensin II is estimated to be at 10 pM which corresponds to 25 attomoles total sample loading. The LOQ for kemptide is significantly better than the LOQ for angiotensin II mainly due to the better CITP focusing and lower chemical background for kemptide. The LOQ for leu-enkephalin was estimated to be about 500 pM (data not shown) due to insufficient CITP focusing. Good measurement reproducibility was also observed as the CVs for all the peak area and peak elution time measurements were less than 22 % and less than 10% (Supplemental Table S2), respectively.
Figure 6.
CITP/CZE-SRM MS quantification of kemptide (A) and angiotensin II (B) in BSA digest matrix. Top: Calibration curve between peak area and sample concentration; Middle: EICs from SRM measurements at 10 pM, 25 pM, 50 pM sample concentrations; Bottom: peak area CV at different concentrations.
CONCLUSIONS
A detailed experimental evaluation of the new sheathless CITP/CZE-MS interface in this study showed its capabilities of both high sample loading capacity and stable nanoESI operation and demonstrated its utility in achieving high sensitivity CITP/CZE-nanoESI SRM MS sample quantification. Sample loading volume in μL range for CITP/CZE separation is provided by using a large bore separation capillary in the new interface without the degradation of separation quality, enabling a sample loading capacity comparable to that in typical nanoLC separations. The new interface also allows stable, dilution free ESI operation at low nanoliters per minute flow rate which was shown to simultaneously improve the CITP/CZE separation quality and MS detection sensitivity. The high accuracy for sample quantification (CV < 22%) and the low picomolar LOQ using the new sheathless CITP/CZE-ESI-SRM MS instrument platform make it suited for high sensitivity quantitative sample analysis. This sheathless CITP/CZE-MS interface design can be easily coupled to multiple mass spectrometers or used in different CE operation modes for ultrasensitive analysis. It may also provide a basis for coupling with other separation methods to perform multidimensional separations. Our future work will focus on exploring the potential of the new sheathless CITP/CZE-MS for high sensitivity proteomics measurements.
Supplementary Material
ACKNOWLEDEGEMENTS
This work was partially supported by grants from the National Institutes of Health: National Cancer Institute (1R33CA155252), National Institute of General Medical Sciences (8 P41 GM103493-10), National Cancer Institute (R21 CA143177), and National Institute of General Medical Science (R21 GM103536). All the experiments were performed in the Environmental Molecular Sciences Laboratory, a U.S. DOE national scientific user facility located at the Pacific Northwest National Laboratory (PNNL) in Richland, Washington. PNNL is a multi-program national laboratory operated by Battelle for the DOE under Contract DE-AC05-76RL01830.
Footnotes
Supporting information available: This material is available free of charge via the internet at http://pubs.acs.org
REFERENCES
- 1.Wang C, Lee CS, Smith RD, Tang K. Anal Chem. 2012;84:10395–10403. doi: 10.1021/ac302616m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith RD, Barinaga CJ, Udseth HR. analytical chemistry. 1988;60:1948–1952. [Google Scholar]
- 3.Smith RD, Olivares JA, Nguyen NT, Udseth HR. analytical chemistry. 1988;60:436–441. [Google Scholar]
- 4.Olivares JA, Nguyen NT, Yonker CR, Smith RD. analytical chemistry. 1987;59:1230–1232. [Google Scholar]
- 5.Ramautar R, Heemskerk AAM, Hensbergen PJ, Deelder AM, Busnel J-M, Mayboroda OA. J Proteomics. 2012;75:3814–3828. doi: 10.1016/j.jprot.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 6.Krenkova J, Foret F. PROTEOMICS. 2012;12:2978–2990. doi: 10.1002/pmic.201200140. [DOI] [PubMed] [Google Scholar]
- 7.Barbas C, Moraes EP, Villasenor A. J Pharm Biomed Anal. 2011;55:823–831. doi: 10.1016/j.jpba.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Ramautar R, Mayboroda OA, Somsen GW, de Jong GJ. ELECTROPHORESIS. 2011;32:52–65. doi: 10.1002/elps.201000378. [DOI] [PubMed] [Google Scholar]
- 9.Herrero M, Ibañez E, Cifuentes A. ELECTROPHORESIS. 2008;29:2148–2160. doi: 10.1002/elps.200700404. [DOI] [PubMed] [Google Scholar]
- 10.Haselberg R, de Jong GJ, Somsen GW. Analytical Chemistry. 2013;85:2289–2296. doi: 10.1021/ac303158f. [DOI] [PubMed] [Google Scholar]
- 11.Thakur D, Rejtar T, Karger BL, Washburn NJ, Bosques CJ, Gunay NS, Shriver Z, Venkataraman G. Analytical Chemistry. 2009;81:8900–8907. doi: 10.1021/ac901506p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramautar R, Busnel J-M, Deelder AM, Mayboroda OA. Analytical Chemistry. 2011;84:885–892. doi: 10.1021/ac202407v. [DOI] [PubMed] [Google Scholar]
- 13.Lee R, West D, Phillips SM, Britz-McKibbin P. Analytical Chemistry. 2010;82:2959–2968. doi: 10.1021/ac9029746. [DOI] [PubMed] [Google Scholar]
- 14.Ibáñez C, Simó C, Martín-Álvarez PJ, Kivipelto M, Winblad B, Cedazo-Mínguez A, Cifuentes A. Analytical Chemistry. 2012;84:8532–8540. doi: 10.1021/ac301243k. [DOI] [PubMed] [Google Scholar]
- 15.Bachmann S, Bakry R, Huck CW, Polato F, Corradini D, Bonn GK. ELECTROPHORESIS. 2011;32:2830–2839. doi: 10.1002/elps.201000653. [DOI] [PubMed] [Google Scholar]
- 16.Dong Y-M, Chien K-Y, Chen J-T, Lin S-J, Wang T-CV, Yu J-S. Journal of Separation Science. 2013 n/a-n/a. [Google Scholar]
- 17.Stegehuis DS, Irth H, Tjaden UR, Van der Greef J. J Chromatogr. 1991;538:393–402. doi: 10.1016/s0021-9673(01)88860-9. [DOI] [PubMed] [Google Scholar]
- 18.An Y, Cooper JW, Balgley BM, Lee CS. Electrophoresis. 2006;27:3599–3608. doi: 10.1002/elps.200600093. [DOI] [PubMed] [Google Scholar]
- 19.Wen Y, Li J, Ma J, Chen L. ELECTROPHORESIS. 2012;33:2933–2952. doi: 10.1002/elps.201200240. [DOI] [PubMed] [Google Scholar]
- 20.Kašička V. ELECTROPHORESIS. 2012;33:48–73. doi: 10.1002/elps.201100419. [DOI] [PubMed] [Google Scholar]
- 21.Fang X, Wang W, Yang L, Chandrasekaran K, Kristian T, Balgley BM, Lee CS. Electrophoresis. 2008;29:2215–2223. doi: 10.1002/elps.200700609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilm MS, Mann M. International Journal of Mass Spectrometry and Ion Processes. 1994;136:167–180. [Google Scholar]
- 23.Bahr U, Pfenninger A, Karas M, Stahl B. Analytical Chemistry. 1997;69:4530–4535. doi: 10.1021/ac970624w. [DOI] [PubMed] [Google Scholar]
- 24.Maxwell EJ, Zhong X, Zhang H, van Zeijl N, Chen DDY. Electrophoresis. 2010;31:1130–1137. doi: 10.1002/elps.200900517. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Wojcik R, Dovichi NJ, Champion MM. Anal Chem. 2012;84:6116–6121. doi: 10.1021/ac300926h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wojcik R, Dada OO, Sadilek M, Dovichi NJ. Rapid Communications in Mass Spectrometry. 2010;24:2554–2560. doi: 10.1002/rcm.4672. [DOI] [PubMed] [Google Scholar]
- 27.Wahl JH, Gale DC, Smith RD. Journal of Chromatography A. 1994;659:217–222. doi: 10.1016/0021-9673(94)85026-7. [DOI] [PubMed] [Google Scholar]
- 28.Maziarz EP, Lorenz S, White T, Wood T. Journal of the American Society for Mass Spectrometry. 2000;11:659–663. doi: 10.1016/s1044-0305(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 29.Chang YZ, Her GR. Analytical Chemistry. 1999;72:626–630. doi: 10.1021/ac990535e. [DOI] [PubMed] [Google Scholar]
- 30.Cao P, Moini M. Journal of the American Society for Mass Spectrometry. 1997;8:561–564. doi: 10.1016/S1044-0305(98)00081-6. [DOI] [PubMed] [Google Scholar]
- 31.Tong W, Link A, Eng JK, Yates JR. Analytical Chemistry. 1999;71:2270–2278. doi: 10.1021/ac9901182. [DOI] [PubMed] [Google Scholar]
- 32.Figeys D, Ducret A, Yates JR, Aebersold R. Nat Biotech. 1996;14:1579–1583. doi: 10.1038/nbt1196-1579. [DOI] [PubMed] [Google Scholar]
- 33.Moini M. Anal Chem. 2007;79:4241–4246. doi: 10.1021/ac0704560. [DOI] [PubMed] [Google Scholar]
- 34.Faserl K, Sarg B, Kremser L, Lindner H. Analytical Chemistry. 2011;83:7297–7305. doi: 10.1021/ac2010372. [DOI] [PubMed] [Google Scholar]
- 35.Haselberg R, Ratnayake CK, de Jong GJ, Somsen GW. Journal of Chromatography A. 2010;1217:7605–7611. doi: 10.1016/j.chroma.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Ramautar R, Busnel J-M, Deelder AM, Mayboroda OA. Anal Chem. 2012;84:885–892. doi: 10.1021/ac202407v. [DOI] [PubMed] [Google Scholar]
- 37.Ramautar R, Shyti R, Schoenmaker B, Groote L, Derks RE, Ferrari M, Maagdenberg AJM, Deelder A, Mayboroda O. Anal Bioanal Chem. 2012;404:2895–2900. doi: 10.1007/s00216-012-6431-7. [DOI] [PubMed] [Google Scholar]
- 38.Busnel J-M, Schoenmaker B, Ramautar R, Carrasco-Pancorbo A, Ratnayake C, Feitelson JS, Chapman JD, Deelder AM, Mayboroda OA. Analytical chemistry. 2010;82:9476–9483. doi: 10.1021/ac102159d. [DOI] [PubMed] [Google Scholar]
- 39.Heemskerk AA, Wuhrer M, Busnel JM, Koeleman CA, Selman MH, Vidarsson G, Kapur R, Schoenmaker B, Derks RJ, Deelder AM. Electrophoresis. 2013;34:383–387. doi: 10.1002/elps.201200357. [DOI] [PubMed] [Google Scholar]
- 40.Heemskerk AA, Busnel J-M, Schoenmaker B, Derks RJ, Klychnikov O, Hensbergen PJ, Deelder AM, Mayboroda OA. Analytical chemistry. 2012;84:4552–4559. doi: 10.1021/ac300641x. [DOI] [PubMed] [Google Scholar]
- 41.Wang C, Lee CS, Smith RD, Tang K. Anal Chem. 2012;84:10395–10403. doi: 10.1021/ac302616m. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.