Summary
Currently, it remains controversial how vascular endothelial progenitor cells (angioblasts) establish their arterial or venous fates. We show using zebrafish that the arterial progenitors of the major axial vessels originate earlier and closer to the midline than the venous progenitors. Both medial and lateral progenitor populations migrate to distinct arterial and venous positions and not into a common precursor vessel as previously suggested. Overexpression of VEGF or Hedgehog (Hh) homologs results in the partially randomized distribution of arterial and venous progenitors within the axial vessels. We further demonstrate that the function of the Etv2 transcription factor is required at earlier stages for arterial development than for venous. Our results argue that the medial angioblasts undergo arterial differentiation because they receive higher concentration of Vegf and Hh morphogens than the lateral angioblasts. We propose a revised model of arterial-venous differentiation that explains how angioblasts choose between an arterial and venous fate.
Introduction
During the formation of the major blood vessels, vascular endothelial progenitor cells (angioblasts) are thought to adopt an arterial or venous identity prior to the initiation of circulation (Wang et al., 1998; Zhong et al., 2001). Hedgehog (Hh), Vascular Endothelial Growth Factor (VEGF), and Notch signaling pathways have been implicated in proper arterial-venous specification (Cleaver and Krieg, 1998; Jin et al., 2005; Lawson et al., 2001, 2002; Shoji et al., 2003; Swift and Weinstein, 2009; Weinstein et al., 1995) in studies using multiple vertebrates including mice, frog Xenopus laevis, and zebrafish. According to the current model, notochord-derived Sonic Hedgehog (Shh) induces expression of VEGF within the medial part of the somites. VEGF signaling induces phospholipase C gamma-dependent extracellular signal-regulated kinase phosphorylation and activates the expression of Notch and its ligand Delta within the arterial precursors. This signaling pathway then acts to promote arterial expression and repress venous expression. However, it is not well understood how endothelial progenitor cells decide to differentiate as an arterial or venous cell.
The zebrafish represents an ideal model system to study arterial-venous differentiation due to its optical transparency and amenability to genetic techniques (Laale, 1977). In zebrafish, similar to other vertebrates, angioblasts originate within the lateral plate mesoderm (LPM) and migrate over the endoderm to the dorsal midline where they coalesce to form the dorsal aorta (DA) and the posterior cardinal vein (PCV) (Childs et al., 2002; Eriksson and Löfberg, 2000; Jin et al., 2005; Lawson and Weinstein, 2002; Williams et al., 2010). Angioblasts have been described to migrate in two waves to the dorsal midline. It has been suggested that the angioblasts in the first migrating wave coalesce and contribute to the DA, while the second-wave angioblasts contribute to the PCV (Fouquet et al., 1997; Jin et al., 2005; Williams et al., 2010). In addition, it was shown that at least some of the venous endothelial cells migrate to the PCV after 21 hpf from the dorsally positioned vascular cord by the mechanism of ventral sprouting (Herbert et al., 2009). However, definitive evidence for the contribution of the first-and second-wave angioblasts to the DA and PCV is still missing.
The origin of arterial and venous progenitors and their assembly into the major axial vessels remain controversial. Earlier studies have suggested that the arterial-venous fates of angioblasts are predetermined prior to migration and that the progeny of a single angioblast can contribute to either an arterial or venous lineage, but never both (Zhong et al., 2001). It was suggested that angioblasts migrate directly to either the position of the DA or the PCV and coalesce to form two distinct vessels. Recently, it was proposed that all angioblasts initially coalesce into a single vascular cord located at the position of the DA, and at 21 hpf angioblasts located in the DA primordia ventrally sprout to form the first embryonic vein through the mechanism of ventral sprouting (Herbert et al., 2009). Despite these different models for vessel formation, it is still not known how angioblasts coalesce to form the DA and PCV, and whether arterial and venous precursors originate from a common location or from distinct regions within the LPM.
In addition to the extrinsic molecular signals such as Hh and VEGF, cell-autonomous factors also play an important role in the differentiation and migration of endothelial progenitors. We and others have demonstrated that a transcription factor, Etv2/Etsrp/ER71, functions as a master regulator controlling vasculogenesis (Lee et al., 2008; Pham et al., 2007; Sumanas et al., 2005, 2008; Sumanas and Lin, 2006; Wong et al., 2009). Etv2 encodes an ETS domain transcription factor and is one of the earliest markers expressed in endothelial progenitor cells. Etv2 function is required to initiate expression of multiple endothelial-specific genes including VEGF receptor kdrl/flk1/vegfR2. In etv2 morphants, angioblasts fail to differentiate, migrate, or coalesce to form functional vessels, demonstrating the importance of this transcription factor in axial vessel development (Pham et al., 2007; Sumanas and Lin, 2006).
In this study, we performed fate mapping and lineage tracing in live zebrafish embryos to determine the origin of arterial and venous progenitors of the major axial vessels, and their pattern of migration and assembly into the DA and the PCV. Contrary to the current model suggesting that angioblasts originate from a common location within the LPM, we show that two distinct endothelial progenitor populations (medial and lateral) are present within this region. The medial angioblasts migrate to the midline first, followed by the lateral angioblasts. Cell tracking of migrating medial and lateral angioblasts shows that the majority of endothelial progenitor cells directly migrate from the LPM to distinct dorsal and ventral positions and do not coalesce into a common precursor vessel as previously suggested (Herbert et al., 2009). We show through fate mapping and lineage tracing that the arterial and venous cells of the DA and the PCV originate from different pools of endothelial progenitors, medial and lateral angioblasts. The medial angioblasts contribute almost exclusively to the DA, while the lateral angioblasts give rise to the PCV. Our results further argue that the medial angioblasts acquire arterial fate because they are closer to the midline and are exposed to the higher concentrations of Vegf and Hh morphogens; overexpression of Vegf and Hh partially randomizes the contributions of medial and lateral angioblasts to the DA and the PCV. Therefore, this study revises the current model of arterial-venous differentiation and explains the mechanism by which endothelial progenitors decide between arterial and venous fate during the formation of the major axial vessels.
Results
Two Distinct Endothelial Progenitor Populations Originate in the LPM
In this study, we aimed to identify when and where arterial and venous precursors of the major axial vessels originate, and to determine how they migrate and coalesce to form the DA and the PCV. To observe angioblasts during the earliest stages of development, we utilized the expression of a transcription factor, Etv2/Etsrp/ER71, which is one of the earliest markers expressed in the angioblasts, and functions as a master regulator of vasculogenesis (Lee et al., 2008; Pham et al., 2007; Sumanas et al., 2005, 2008; Sumanas and Lin, 2006; Wong et al., 2009). Knockdown of etv2 results in the downregulation of multiple vascular markers including flk1/kdrl, arterial-specific gridlock/hey2(grl) and ephrin B2a (efnb2a), and venous-specific mannose receptor C1(mrc1) and ephB4a (Figures S1C–S1F available online) (Pham et al., 2007; Sumanas and Lin, 2006; Wong et al., 2009). From in situ hybridization (ISH) analysis, etv2 expression is observed in a single line of angioblasts within the LPM of the trunk region as early as the 4-somite stage (Figures S1A and S1B) (Sumanas and Lin, 2006). However, beginning at the 10-somite stage a second line of etv2-expressing angioblasts is observed positioned further away from the midline (Figures 1A, 1D, 1G, 1J, and 1M–1O). The more medially located angioblasts migrate to the midline first starting between the 9- to 10-somite stages (Figure 1A), and continue to migrate (Figures 1M–1O) until the 15-somite stage when nearly all medial angioblasts in the trunk region have reached the midline (Figure 1D). Interestingly, the more posterior medial angioblasts remain bilaterally located within the LPM (Figure 1D) and migrate later than the anterior angioblasts. The lateral angioblasts also migrate to the midline beginning at the 16-somite stage, and most complete their migration by the 22-somite stage (Figures 1D, 1G, and 1J). These data suggest that the earlier and later migrating angioblasts originate at distinct times and locations.
Figure 1. Medial and Lateral Angioblasts Originate in Two Distinct Regions, as Observed by ISH Analysis for etv2Expression.
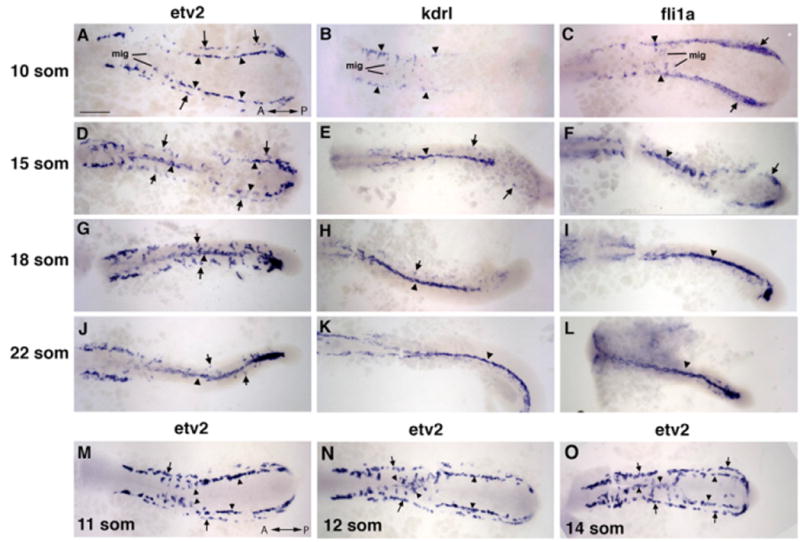
(A–L) etv2, flk1/kdrl, and fli1a expression analysis in trunk angioblasts at the 10-, 15-, 18-, and 22-somite stages. etv2 expression is observed in the medial (arrowheads) and lateral (arrows) angioblasts. flk1/kdrl and fli1a expression is initially restricted to the medial angioblasts (B and C) with very weak observed expression in the lateral angioblasts in 15- to 22-somite stage embryos (E–K; F–L).
(M–O) etv2 expression in migrating medial angioblasts at the 11- to 14-somite stages. Note that the migration wave progresses in the anterior to posterior direction. The lateral angioblasts migrate later at the 16- to 22-somite stages (G and J). Dorsal view of the trunk and tail region of flatmounted embryos. Anterior is to the left. Scale bar, 100 μm.
See also Figures S1 and S2, and Movies S1, S2, S3, and S4.
From flk1/kdrl (Figures 1B, 1E, 1H, and 1K) expression analysis at the 10-somite stage (Thompson et al., 1998), only the medial angioblasts are observed (Figure 1B), and expression in the lateral angioblasts is not apparent until the cells have initiated migration (Figures 1E, 1H, and 1K). As evident from two-color ISH (Figure 2C), etv2 and flk1/kdrl expression overlaps in the medial angioblasts at the 16-somite stage. The lateral angioblasts, however, show strong etv2 expression and weak to no flk1/kdrl expression. Midline etv2 expression also overlaps with arterial grl/hey2 (Zhong et al., 2000) expression (Figures 2E and 2F), while the lateral angioblasts are grl negative (arrows). Interestingly, expression of the erythroid-specific markers scl/tal1 and gata1 (Detrich et al., 1995; Gering et al., 1998; Liao et al., 1998) is positioned between the medial and lateral lines of etv2-expressing angioblasts (Figures 2A, 2B, and 2D). Similar to flk1/kdrl expression, the vascular endothelial marker fli1a (Thompson et al., 1998) is initially restricted to the medial angioblasts at the 10-somite stage (Figure 1C), with very weak expression in the lateral angioblasts in 15- to 22-somite stage embryos (Figures 1F, 1I, and 1L). The wider fli1a expression at the 10-somite stage is likely due to its early expression in both endothelial and erythroid precursors.
Figure 2. Two-Color ISH Expression Analysis of etv2and Other Endothelial and Hematopoietic Markers.
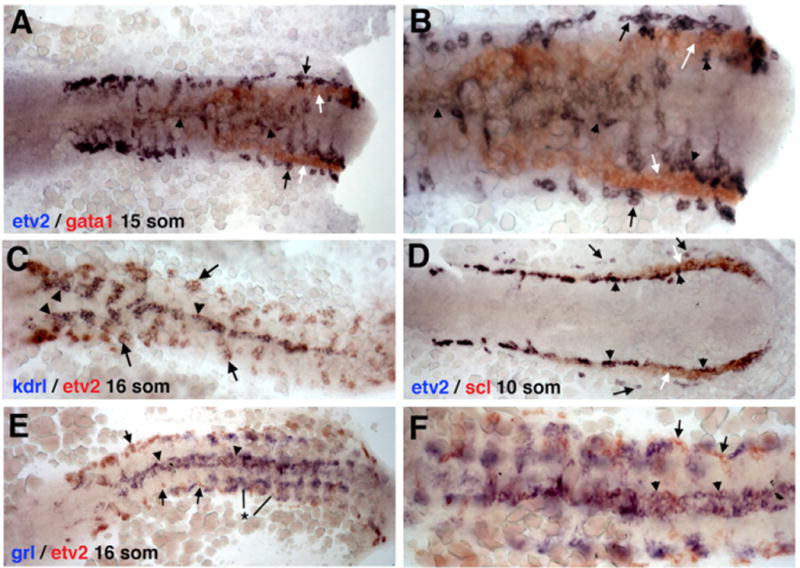
ISH expression of (A and B) etv2 and gata1 at the 15-somite stage, (C) etv2 and kdrl at the 16-somite stage, (D) etv2 and scl at the 10-somite stage, and (E and F) etv2 and grl at the 16-somite stage.
(A and B) Erythroid-specific gata1 expression (white arrows) is positioned between the medial (arrowheads) and lateral (black arrows) etv2-expressingangioblasts; (B) higher magnification view.
(C) etv2 and kdrl expression overlaps at the midline (arrowhead) in the medial angioblasts. While strong etv2 expression is seen in the lateral angioblasts (arrow), no or very little lateral kdrl expression is observed.
(D) Relative to vascular endothelial progenitor cells, erythroid-specific scl expression (white arrows) is positioned between the medial (arrowheads) and lateral (black arrows) etv2-expressing angioblasts.
(E and F) Similar to (C), grl expression overlaps with etv2 expression in medial angioblasts at the midline (arrowheads). Lateral angioblasts are grl-negative (arrows). Note that the strong bilateral grl expression (*) does not correspond to endothelial cells and is within the somitic tissue as reported previously (Zhong et al., 2000).
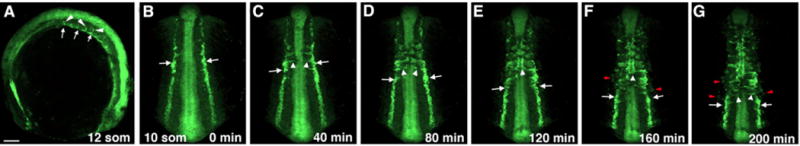
To study angioblast migration, we utilized an established etv2:GFP transgenic reporter line that differentially labels all angioblasts (Proulx et al., 2010). While GFP expression is readily apparent in the medial angioblasts (Figure 3; Movie 1), there is a delay in GFP expression in the lateral precursors (Figures 3F and 3G). This is presumably due to the additional time required for the translation and folding of GFP protein in the lateral angioblasts that originate later. Interestingly, endothelial precursor cells migrate intersomitically to the midline in an anterior to posterior sequence (Figures 1A 1M–1O,, and 2B–2G; Movies 1 and 2). Initially angioblasts cluster around somites, and at the time of migration ingress between the boundaries of adjacent somites (Figure S2A). This intersomitic migration of angioblasts was confirmed by photoactivating angioblasts in embryos injected with Kaede mRNA, thereby converting Kaede protein in the cell from green to red fluorescence (Ando et al., 2002; Hatta et al., 2006; Stark and Kulesa, 2007; Watanabe et al., 2007) (Figures S2B–S2F; Movie 3). Such pattern of migration can also be observed in the fli1a:GFP reporter line (Lawson and Weinstein, 2002) (Figures S2G–S2J; Movie 4). However, fli1a:GFP is also expressed in erythroid precursors during early somitogenesis stages, and time-lapse imaging would capture both cell populations.
Figure 3. The Migration of Trunk Angioblasts in Live etv2:GFP-Transgenic Embryos.
Medial Angioblasts Contribute to the DA, while Lateral Angioblasts Give Rise to the PCV
Based on the etv2 expression pattern, we hypothesized that the medial angioblasts differentiate as arterial progenitors and contribute to the DA, while the lateral angioblasts undergo venous differentiation and contribute to the PCV. To perform fate mapping of the medial and lateral angioblasts, we used BAC modification to establish an etv2:Kaede line that expresses the photoconvertible Kaede protein under the etv2 promoter. Similar to etv2:GFP expression, etv2:Kaede expression pattern was localized to vascular endothelial progenitors (Figure S3). Many embryos displayed a mosaic expression pattern, likely due to epigenetic silencing that is commonly observed in transgenic lines (Goll et al., 2009). Because the lateral angioblasts originate later than the medial progenitors, and the Kaede protein requires time to fold, etv2:Kaede expression in the lateral angioblasts was largely not apparent until after the 15-to 16-somite stages (Figure S3). This allowed us to label all medial angioblasts by exposing the whole etv2:Kaede-positive embryo to UV epifluorescence at the 15- to 16-somite stages. The position of the photoconverted cells was subsequently analyzed at 25–28 hpf, after the DA and PCV have formed. The majority (98%) of the vascular endothelial cells present in the trunk region of the DA were fluorescent for both red and green, indicating that they originated prior to the photoconversion and are derived from the medial angioblasts (Figures 4A, 4B, and 4I). Because Kaede synthesis continues after photoconversion, labeled cells display both green and red fluorescence. Only 2% of cells in the trunk region of the DA displayed green-only fluorescence and had no red Kaede protein. In contrast, 92% cells in the PCV displayed green fluorescence only, indicating that they originated after the photoconversion and were derived from the lateral line angioblasts. Only 8% of the cells within the PCV displayed both red and green fluorescence (Figures 4A, 4B, and 4I). In addition to the flat elongated vascular endothelial cells, etv2:Kaede expression was observed in round nonendothelial cells, which most likely correspond to hematopoietic progenitors such as neutrophils (data not shown); these cells were excluded from the analysis.
Figure 4. The Medial Angioblasts Give Rise to the Dorsal Aorta, while the Lateral Angioblasts Give Rise to the Posterior Cardinal Vein.
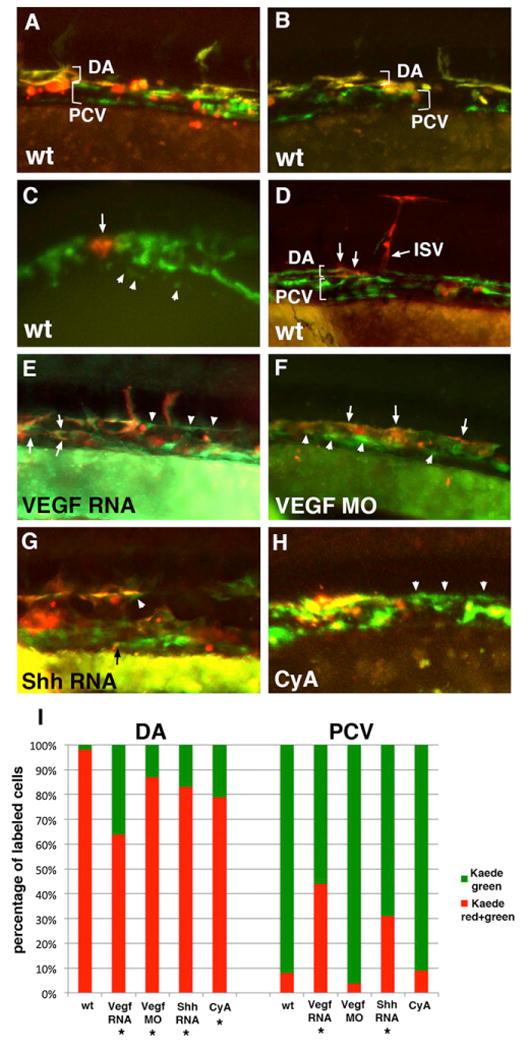
In all experiments, etv2:Kaede embryos were photoconverted at the 14- to 15-somite stages and analyzed at 24–28 hpf. Anterior is to the left; dorsal is up in all panels.
(A and B) Global green to red photoconversion of etv2:Kaede-positive cells demonstrates that all early Kaede+ endothelial progenitor cells that were photoconverted contribute to the DA (observed as an overlap of red and green fluorescence), while all late Kaede+ cells that originated after photoconversion contribute to the PCV (observed as green-only fluorescence). Note that etv2:Kaede expression pattern is mosaic, and not all endothelial cells express Kaede. In addition, etv2:Kaede is expressed in certain hematopoietic lineages such as neutrophils (red round cells in A and B), which were not included in the analysis.
(C and D) Selected groups of medial etv2:Kaede cells were photoconverted using laser confocal microscopy at the 15-somite stage (arrow, C), and their location was analyzed at 26–28 hpf (arrows, D) using fluorescence microscopy. Note that the labeled medial cells exclusively contribute to the DA, including DA-derived intersegmental vessels (ISV, D). Arrowheads in (C) point to the cells of the lateral line. Red round cells apparent on the right side of (D) correspond to etv2:Kaede expression in hematopoietic lineages. Panels in (C) and (D) represent different embryos.
(E) Global photoconversion of etv2:Kaede in VegfA-RNA-overexpressing embryos. Note that the cells in the DA and PCV positions include both double-positive (red and green) medial-line-derived endothelial cells and green-only lateral-line-derived endothelial cells (arrowheads).
(F) VegfA MO-injected embryos display a single vessel, as observed by global etv2:Kaede photoconversion. Double-positive (red and green) medial-line-derived endothelial cells are largely located in the dorsal most part of the vessel, while green-only lateral-line-derived cells are found largely in the ventral part of the vessel.
(G) Shh RNA-injected etv2:Kaede globally photoconverted embryos display a significant number of green-only lateral-line-derived cells in the DA (arrowhead) and occasional medial-line-derived cells in the PCV (arrow, points to a small double-positive endothelial cell).
(H) Cyclopamine (CyA)-treated etv2:Kaede globally photoconverted embryos have disorganized cells within the vascular cord often with no discernable distinction between the DA and the PCV. Higher frequency of green-only cells can be observed in the dorsal part of the vessel (arrowheads).
(I) Average contribution of medial (red) and lateral (green) globally photoconverted etv2:Kaede cells to the DA and the PCV in wild-type (n = 13 embryos and 335 endothelial cells analyzed), VegfA RNA (n = 17 embryos and 417 cells), Vegf MO (n = 10 embryos and 295 cells), Shh RNA-injected (n = 17 embryos and 247 cells), and CyA-treated (n = 8 embryos and 88 cells) embryos. Cell counts for each embryo are approximate estimates. Embryos treated with 1% DMSO (solvent for CyA) displayed distribution of green and red Kaede+ cells, similar to WT (not shown). In addition to the flat elongated vascular endothelial cells, etv2:Kaede expression was observed in round nonendothelial cells, which most likely correspond to hematopoietic progenitors such as neutrophils (data not shown); these cells were excluded from the analysis. *Statistically significant differences, as calculated using Z-test for two proportions to compare the percentage of green cells contributing to the DA or the percentage of red cells contributing to the PCV between the treated embryos and wild-type controls (p ≤ 0.001).
See also Figure S3.
These results suggest that the medial angioblasts contribute predominantly to the DA, while the lateral progenitors largely give rise to the PCV. The small number of red photoconverted cells present in the PCV are likely either the medial cells migrating to the PCV by the ventral sprouting as previously described (Herbert et al., 2009), or the lateral cells that originated earlier and were labeled during whole-embryo photoconversion. To investigate these possibilities, we photoactivated clusters of the medial angioblasts using a 405 nm laser source (Figure 4C). In all cases (n = 11 embryos analyzed, two to three cell clusters per embryo), the endothelial progeny of the labeled cells were detected exclusively within the DA or in the DA-derived intersegmental vessels (Figure 4D). These results argue that the medial angioblasts give rise to the DA, while the lateral angioblasts form the PCV. Therefore, arterial and venous progenitors of the major axial vessels originate in distinct locations within the LPM.
Hh and Vegf Gradient Is Critical for Arterial-Venous Contribution of the Medial and Lateral Angioblasts
Hh and Vegf signaling pathways have been previously implicated in arterial-venous differentiation (Lawson et al., 2002). In zebrafish, sonic hedgehog (shh) is expressed in the floorplate and the neural tube, while vegfa expression is localized to the hypochord and the ventral part of the somites (Krauss et al., 1993; Liang et al., 1998, 2001). Thus, both VegfA and Shh are localized close to the midline and may function as morphogens. Because medial angioblasts are located closer to the midline, they are likely to be exposed to higher levels of Vegf and Hh concentrations and therefore differentiate as arterial cells, while lateral angioblasts would experience lower concentrations and therefore adopt a venous fate. To test this hypothesis, we altered Vegf and Hh levels by injecting synthetic zebrafish VegfA and Shh mRNAs into etv2:Kaede embryos at the 1-cell stage. As previously reported, VegfA and Shh overexpression resulted in the expansion of arterial markers (data not shown) (Lawson et al., 2002), and Shh-overexpressing embryos displayed additional expected phenotypes such as inhibition of lens differentiation (Ekker et al., 1995). As analyzed by whole-embryo photoconversion assay, both Vegf and Shh overexpression resulted in a more random fate choice of medial and lateral angioblasts. In contrast to wild-type embryos, in Vegf- and Shh-overexpressing embryos photoconverted red and green-positive Kaede cells were present in both the DA and the PCV (Figures 4E, 4G, and 4I). Similarly, green-only lateral endothelial cells were present in both the DA and the PCV as well. Because Vegf- and Shh-overexpressing embryos do not form functional vasculature, the distinction between the DA and the PCV in this analysis is based on the dorsoventral position of the cells within the axial vasculature. We also analyzed dorsoventral distribution within the axial vessels of medial and lateral angioblasts in VegfA morpholino (MO)-inhibited embryos (morphants). Consistent with previous reports (Lawson et al., 2002; Nasevicius et al., 2000), VegfA morphants formed a single vessel and failed to initiate circulation. Interestingly, photoconverted medial angioblasts were predominantly positioned at the dorsal side of this vessel, while lateral angioblasts cells remained ventrally positioned (Figures 4F and 4I). This may be explained by possible redundancy between Hh and Vegf signaling or incomplete inhibition of Vegf function using morpholinos (see Discussion). To test how the inhibition of Hh signaling affected the migration of the medial and lateral angioblasts, we analyzed their position in cyclopamine-treated embryos. The distribution of vascular endothelial cells in the trunk axial vasculature region was disorganized, and in some regions the cells failed to form a distinct DA and PCV. The majority of the red photoconverted cells were positioned at the dorsal side of the disorganized vascular cord, while the green lateralline-derived endothelial cells were present in both dorsal and ventral positions (Figure 4H). Vegf RNA, Vegf MO injection, or cyclopamine treatment did not significantly affect the initial formation of the medial and lateral line angioblasts as observed by etv2 ISH expression analysis at the 10- to 15-somite stages (data not shown). Overall, these results argue that Shh and Vegf concentration is one of the critical factors in determining arterial-venous contribution of angioblasts. RNA overexpression disturbs the normal gradient and leads to the partial randomization of the cell fate choice in comparison to wild-type embryos.
Angioblasts Migrate Directly to Their Arterial and Venous Positions
To determine how medial and lateral angioblasts migrate to their positions within the DA and the PCV, we performed time-lapse imaging of migrating angioblasts in Tg(etv2:GFP) and Tg(etv2:Kaede) reporter lines. At the 10- to 16-somite stages, individual medial angioblasts migrate from the LPM directly to the dorsal position where they coalesce and form the DA (Figure 5; Movies 5, 6, and 7). The lateral angioblasts initiate migration to the midline after the 15-somite stage and migrate directly to a ventral position where they coalesce into the PCV (Figure 5; Movie 7). Interestingly, some lateral angioblasts migrate much later at the 22- to 24-somite stages and intercalate into the ventral part of the already formed venous vascular cord (Movie 8). It is apparent from these data that arterial and venous progenitors originate in distinct locations. This is particularly evident in the anterior region where the common cardinal vein (CCV) forms, which is separated from the DA at a greater distance since CCV progenitors stay bilaterally and do not migrate toward the midline (Figure 5; Movie 7). Based on these cell-tracking results, the majority of the medial and lateral angioblasts migrate from the LPM directly to distinct dorsal and ventral positions, forming two separate vessels.
Figure 5. Migration of Medial and Lateral Angioblasts to the DA and PCV Observed in Live etv2:Kaede Embryos.
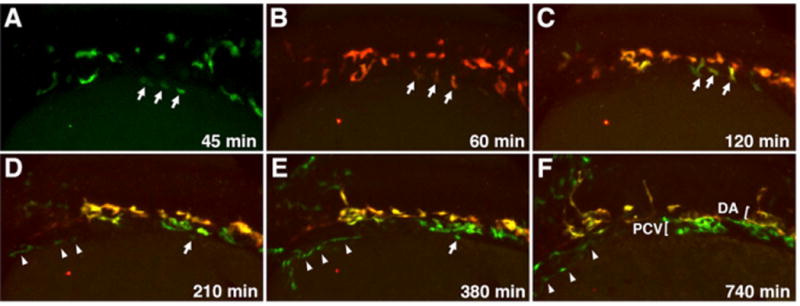
Selected frames from a time-lapse movie of a single etv2:Kaede embryo prephotoconversion at the 14-somite stage (A) and postphotoconversion starting at the 15-somite stage (B) through 28 hpf (F). Arrows mark laterally positioned angioblasts that migrate directly to a ventral position to contribute to the PCV. Arrowheads mark a separate population of lateral angioblasts that contribute directly to the common cardinal vein (CCV). All views are dorsolateral; anterior is to the left, and dorsal is to the top. All selected frames are from Movie 6, embryo 1; imaging (t = 0 min) was started at approximately 13-somite stage.
Etv2 Function Is Required Earlier for Arterial Differentiation than for Venous
If lateral angioblasts preferentially contribute to the venous lineage, then the inhibition of lateral endothelial cell differentiation should result in greater venous patterning defects. Since etv2 expression is not observed in the lateral angioblasts until the 10-somite stage (Sumanas and Lin, 2006) (Figures 1A, S1A, and S1B), suppression of etv2 function at this stage should selectively inhibit the differentiation and migration of the lateral endothelial precursor cells. To inhibit etv2 function at selected developmental stages, we used etv2 MO that was hybridized to a caging strand containing a photocleavable linker (Tomasini et al., 2009). In control experiments, both caged and regular etv2 MOs resulted in similar inhibition of reporter etv2:GFP expression (Figures S4A–S4C). In caged MO-injected embryos that were never photoactivated, some inhibition of etv2:GFP expression was observed; however, these embryos were phenotypically normal and displayed no circulation defects (Figure S4D; data not shown).
We analyzed when etv2 function was required for vascular development. As expected, etv2 caged MO-injected embryos uncaged at the sphere stage (4 hpf) displayed strong downregulation of flk1/kdrl expression throughout the embryo (Figures 6A–6C). Embryos uncaged at the 10-somite and 15-somites stage showed less severe phenotypes (Figures 6D and 6E); however, differential effects on the arterial and venous marker expression were observed. Flk1/Kdrl expression in the DA was weakly affected, while venous expression was greatly reduced (Figure 6D). Uncaging embryos at the 20-somite stage and later resulted in no apparent defects in flk1/kdrl expression, blood circulation, or intersegmental vessel formation at 1–2 dpf (data not shown). This suggests that etv2 may not be required after the 20-somite stage for vasculature formation, or the amount of Etv2 protein already present in the cells might be sufficient for its later function. We have previously shown that etv2 RNA expression is retained or even upregulated in etv2 morphants (Sumanas and Lin, 2006), which can be used to analyze the location of etv2-expressing cells. As analyzed for etv2 expression in etv2 morphants at 22- to 24-somite stages, embryos uncaged at the sphere and 5-somite stages showed complete loss of angioblast migration (Figures S4E–S4H). In embryos that were uncaged at the 10-somite stage, the medial angioblasts migrate normally, while the lateral angioblasts migration is inhibited (Figure S4H). These data suggest that the migration of lateral angioblasts can be selectively inhibited when etv2 MO embryos are uncaged at the 10-somite stage.
Figure 6. Arterial-Venous Differentiation Is Affected by Etv2 Function at Distinct Time Points.
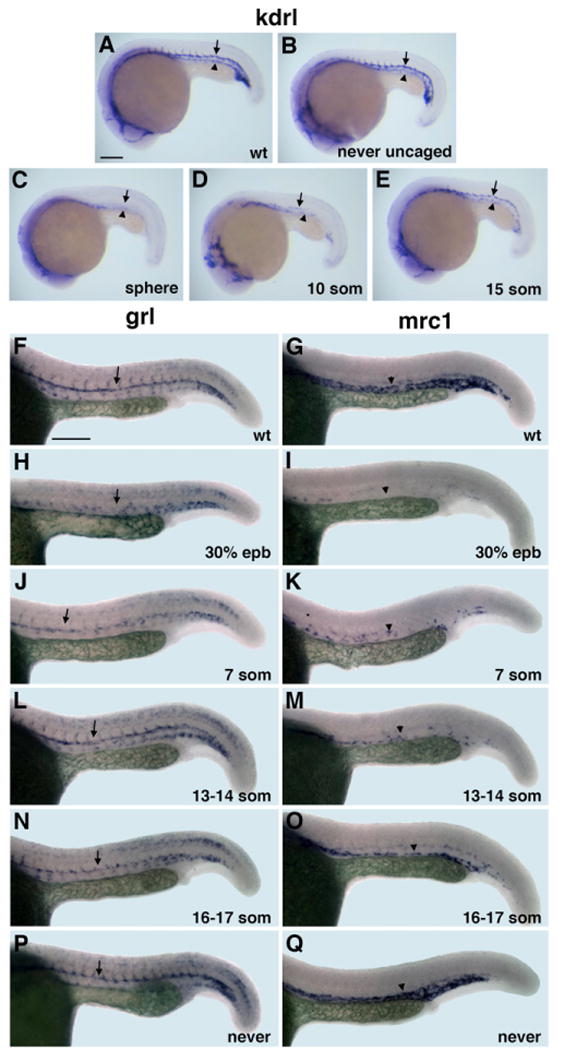
(A–E) flk1/kdrl expression analysis at 22 hpf stage. Control uninjected (A), MO-injected but never uncaged (B), uncaged at the sphere (C), 10-somite (D), and 15-somite (E) stage embryos. Note that both the dorsal aorta (arrows) and the posterior cardinal vein (arrowheads) are absent in (C); the dorsal aorta is partially (D) or fully present (E), while the vein is greatly reduced or absent.
(F–Q) Arterial grl (F, H, J, L, N, and P) and venous mrc1 (G, I, K, M, O, and Q) expression at 24 hpf in photoactivatable etv2 MO-injected embryos. (F and G) Control uninjected, (H and I) uncaged at 30% epiboly, (J and K) 7-somite stage, (L and M) 13 to 14-somite stage, (N and O) 16- to 17-somite stage, and (P and Q) MO-injected but never uncaged embryos. Note that arterial grl expression (arrows) is absent in (H) but is partially present in (J) and not significantly affected when MO is photoactivated at the 13- to 17-somite stages (L and N). Venous mrc1 expression (arrowheads) is absent or strongly reduced in both early (I and K) and late photoactivated embryos (M and O). Scale bar, 100 μm. See also Figure S4.
To determine when etv2 function is required for arterial and venous differentiation, embryos were injected with caged etv2 MO and photoactivated at the 30% epiboly, 7-, 13/14-, and 16/17-somite stages. Activated embryos were raised to 24 hpf and analyzed for arterial expression of hey2/gridlock(grl) and venous expression of mannose receptor C1(mrc1) (Wong et al., 2009; Zhong et al., 2000, 2001) by ISH. As expected, photoactivation at the 30% epiboly stage resulted in the absence of grl or mrc1 expression in the DA or PCV (Figures 6F–6I; Table 1). However, uncaging at the 7-somite stage resulted in the partial formation of the DA (Figure 6J; Table 1), while the PCV was largely absent (Figure 6K; Table 1). Etv2 MO uncaging at the 13- to 17-somite stages had little effect on the patterning of the DA (Figures 6L and 6N; Table 1); however, the PCV remained incompletely formed (Figures 6M and 6O; Table 1). Normal DA and PCV development was observed in embryos that were never uncaged (Figures 6P and 6Q; Table 1). These results demonstrate that the presence of Etv2 protein is required later for venous differentiation than for arterial differentiation, and that lateral angioblasts, which originate later, contribute predominantly to the venous vasculature.
Table 1. Etv2 Function Is Required at Earlier Stages for Arterial grl Expression and at Later Stages for Venous mrc1 Expression.
| grl Expression, 24 hpf | Normal Dorsal Aorta | Reduced/Fragmented Dorsal Aorta | Absent Dorsal Aorta | Number of Embryos |
|---|---|---|---|---|
| Control uninjected | 100% | 0 | 0 | 40 |
| Etv2 MO uncaged at 30% epiboly | 0 | 4% ± 4% | 96% ± 4% | 30 |
| 7-somite | 65% ± 15% | 35% ± 15% | 0 | 23 |
| 13- to 14-somite | 92% ± 2% | 8% ± 2% | 0 | 24 |
| 16- to 17-somite | 100% | 0 | 0 | 23 |
| Never uncaged | 100% | 0 | 0 | 25 |
| mrc1 Expression, 24 hpf | Normal Cardinal Vein | Reduced Cardinal Vein | Absent Cardinal Vein | Number of Embryos |
|---|---|---|---|---|
| Control uninjected | 100% | 0 | 0 | 40 |
| Etv2 MO uncaged at 30% epiboly | 0 | 0 | 100% | 30 |
| 7-somite | 0 | 100% | 0 | 30 |
| 13- to 14-somite | 33% ± 4% | 67% ± 4% | 0 | 25 |
| 16- to 17-somite | 33% ± 8% | 67% ± 8% | 0 | 19 |
| Never uncaged | 88% ± 9% | 12% ± 9% | 0 | 40 |
Data represent an average from two independent experiments. grl and mrc1 expression analyzed at 24 hpf ± refers to the SE.
Discussion
In this study, we demonstrate that etv2 expression marks two populations of angioblasts within the posterior LPM, which originate at distinct time points and are positioned at different distances from the dorsal midline. By performing cell labeling and fate mapping, we show that the medial angioblasts correspond to the arterial progenitors of the DA, while lateral angioblasts give rise to the venous endothelial cells of the PCV. We further show that the majority of angioblasts migrate directly from the LPM either to the DA or to the PCV. Both Vegf and Shh concentrations are important for the proper contribution of both pools of angioblasts to the DA and the PCV.
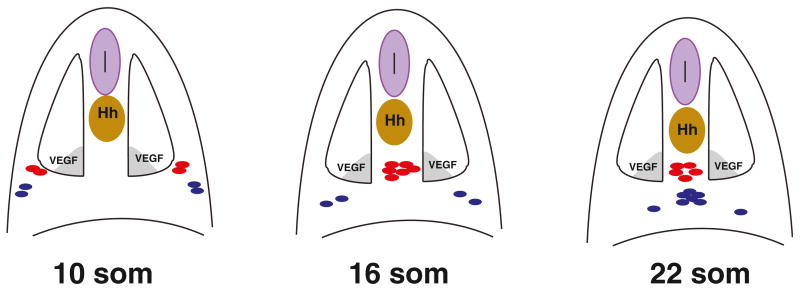
Based on our results, we propose a revised model for arterial-venous differentiation (Figure 7). Angioblasts originate within the LPM in two bilateral lines, medial and lateral. Etv2 expression in the medial angioblasts is observed as early as the 3-somite stage, while expression in the lateral angioblasts is apparent by the 10-somite stage. Beginning at around the 10-somite stage, the medial angioblasts migrate intersomitically to the midline over the endoderm in the anterior to posterior sequence (Figure 7). After the 15-somite stage, the lateral precursors initiate migration, with a migrational behavior similar to that of the medial angioblasts. From the fate-mapping data, the medial angioblasts contribute exclusively or almost exclusively to the DA, while the lateral angioblasts give rise to the PCV. These results argue that arterial and venous progenitors originate at distinct time points and spatial regions.
Figure 7. A Model for Arterial-Venous Differentiation.
At the 10-somite stage endothelial progenitor cells are positioned within the LPM in two bilateral lines, a medial (red) and lateral line (blue). After the 10-somite stage, medial angioblasts migrate intersomitically to the midline directly to the dorsal position where they differentiate as arterial cells. Shortly after the 15-somite stage, the lateral angioblasts migrate to the ventral position at the midline where they differentiate as venous endothelial cells. Hh, expressed in the notochord; VegfA, expressed in the ventral somites function as morphogens and are important for specifying arterial fate in the medial angioblasts.
It is currently unknown when angioblasts commit to an arterial or venous fate. While our data show that arterial and venous progenitors originate from distinct spatial regions, it is likely that angioblasts do not acquire an arterial or venous fate until later developmental stages. Based on our own unpublished observations, and previously described expression patterns, medial progenitors express both arterial (grl, efnb2a, and cldn5b) (Lawson et al., 2001; Thisse and Thisse, 2004; Zhong et al., 2000) and venous (mrc1, flt4, stab1l, and stab2) markers (Hogan et al., 2009; Thompson et al., 1998; Wong et al., 2009) between the 10-to 20-somite stages. Starting at the 20-somite stage in the lateral-line-derived venous progenitors, arterial marker expression is gradually downregulated, while venous markers are upregulated. Notch3 expression is also first apparent in the forming DA at or close to the 20-somite stage (Lawson et al., 2001). Therefore, this time point when the majority of angioblasts have migrated to the midline, likely represents the stage when arterial and venous fates are being specified.
Factors that induce arterial differentiation such as Vegf and Hh are expressed close to the midline. In zebrafish, VegfA is expressed in the medial part of the somites, while Shh is secreted from the notochord and the floorplate (Krauss et al., 1993; Liang et al., 1998, 2001). Until now, it was not known why some angioblasts differentiate as arterial cells and others as venous cells if they are all exposed to similar concentrations of morphogens. Since we demonstrate that arterial precursors originate closer to the midline, while venous progenitors are located further away, this suggests that the medial angioblasts are likely exposed to a higher concentration of Vegf and Hh morphogens, and therefore differentiate as arterial cells. In agreement with this model, both medial and lateral angioblasts migrate more or less randomly to the DA and PCV positions upon Vegf and Hh overexpression. Previous studies have shown that VegfA and Shh overexpression results in the expansion of arterial markers into the PCV and downregulation of venous markers within the DA (Lawson et al., 2002) that, together with our findings, argues that both pools of angioblasts acquire arterial fate upon Vegf and Hh overexpression. The observed more random distribution of angioblasts is likely caused by large amounts of ectopic VegfA and Shh overriding the endogenous gradients. Interestingly, even upon inhibition of VegfA and Hh signaling, many medial angioblasts still migrated to the dorsal position, while lateral angioblasts were preferentially localized to the ventral part of the disorganized vascular cord. Because recent studies indicate that Vegf and Hh have independent roles in arterial-venous differentiation (Wilkinson et al., 2012; Williams et al., 2010), it is possible that there is a redundancy between the two signaling pathways, and just one of them is sufficient to guide angioblasts to the arterial or venous position. In addition, chemical or morpholino-mediated inhibition may not be complete, and minor amounts of these morphogens remaining may be sufficient to establish a partial gradient. An alternative scenario is that neither Vegf nor Hh are absolutely required for angioblasts to migrate to their dorsoventral positions within the DA or PCV. Because of the sphere-like shape of a zebrafish embryo during somitogenesis stages, lateral angioblasts are initially located more ventrally in comparison to the medial angioblasts. Thus, in Vegf and Hhinhibited embryos they may largely stay in the more ventral position corresponding to the PCV after the vascular cord has formed. Nevertheless, profound vascular defects are observed in Vegf- or Hh-inhibited embryos. Our findings support the mechanism that medial angioblasts acquire arterial fate due to their proximity to the sources of Vegf and Hh expression, and thus provide an explanation how angioblasts decide between the arterial and venous fates.
Previous studies have implicated Vegf signaling in multiple steps in cardiovascular development, including arterial-venous differentiation, hematopoietic stem cell (HSC) specification, and angiogenic sprouting of intersegmental vessels (Covassin et al., 2006; Lawson et al., 2002; Leung et al., 2013; Serbedzija et al., 1999; Zygmunt et al., 2011). VegfA performs different functions at distinct time points, possibly mediated by distinct Vegf isoforms. For instance, in Xenopus laevis, the VegfA170 isoform has been implicated in HSC induction (Leung et al., 2013). Based on previous studies and our own unpublished observations, both major VegfA zebrafish isoforms, VegfA121 and VegfA165 can induce arterial differentiation (Lawson et al., 2002; Liang et al., 2001). However, the specific requirement for each isoform in arterial-venous differentiation is unknown.
Although lateral angioblasts are initially positioned further away from the midline, they eventually migrate through the somitic mesoderm where VegfA is highly expressed. What then prevents these angioblasts from acquiring arterial fates during their migration? Because the lateral angioblasts originate and migrate toward the midline later than the medial angioblasts, this timing may be important for arterial-venous specification. Indeed, our results show that Etv2 function is required at distinct time points for arterial and venous specification that correspond to the stages when medial and lateral progenitors undergo endothelial differentiation. Since etv2 function is required to initiate the expression of Vegf receptors such as kdrl (Pham et al., 2007; Sumanas and Lin, 2006), the timing and level of etv2 expression may determine when and which angioblasts are competent to respond to Vegf signaling. Since lateral angioblasts initiate kdrl expression later and at lower levels compared to the medial angioblasts, this may be an important factor that prevents lateral progenitors from acquiring an arterial fate. In addition, medial angioblasts are exposed longer to Vegf signaling before migration, and short exposure of lateral angioblasts during migration may not be sufficient to induce arterial differentiation.
Interestingly, time-lapse imaging of Tg(etv2:GFP) embryos has identified additional migration behaviors. Our results show that the anterior trunk angioblasts migrate to the midline first, followed by the posterior angioblasts. At the onset of migration, angioblasts initially cluster around the somites, and at the time of migration appear to ingress between the boundaries of adjacent somites. This migration behavior was not specific to the Tg(etv2:GFP) line, as the intersomitic migration of endothelial cells was also observed in Tg(fli1a:GFP) embryos. How angioblasts guide between somites is unknown but may be regulated by attraction and repulsion cues between plexin and semaphorin signaling (Gu et al., 2005; Shoji et al., 2003).
Our cell-tracking and fate-mapping data show that the majority of medial and lateral angioblasts migrate directly to dorsoventral positions corresponding to the future DA and PCV location. Although we did not observe any endothelial progenitors undergoing ventral sprouting, we cannot exclude a possibility that some red photoconverted cells observed in the PCV originated from ventral sprouting as reported previously (Herbert et al., 2009). Our analysis did not specifically focus on this event, and the imaging methods, embryonic stages, as well as the region analyzed were different from the previous study (Herbert et al., 2009). Considering that kdrl is only weakly expressed in the venous progenitors, this may explain why direct migration was not previously observed when the kdrl:GFP line was used (Herbert et al., 2009).
In higher vertebrates such as mouse and chick, arteries also differentiate earlier than veins similar to zebrafish. Differently from zebrafish embryos, the DA and PCV are initially paired vessels and fuse only during the later stages (Garriock et al., 2010; Gerety and Anderson, 2002; Meadows et al., 2012; Sabin, 1917). In amniotes, both the paired DA and PCV are thought to originate through vasculogenesis when individual angioblasts coalesce into the axial vessels; however, the details are still poorly understood. Interestingly, the paired PCV are positioned more laterally than the paired DA in amniotes, which is similar to the arrangement of the medial and lateral angioblasts in zebrafish. Although angioblasts coalesce into axial vessels in different anatomical locations in zebrafish compared to amniotes, it is likely that the sites where arterial and venous progenitors originate and the molecular mechanisms of establishing arterial-venous identity are evolutionarily conserved.
This study revises our current understanding of how endothelial progenitor cells assemble into the major axial vessels and defines the molecular mechanism by which they acquire arterial-venous identity. Considering that many of the cellular and molecular mechanisms that govern arterial-venous differentiation are evolutionarily conserved, our findings will be important for future studies of vascular formation in other vertebrate systems.
Experimental Procedures
Fish Lines and Embryos
Tg(etv2:GFP)ci1, Tg(fli1a:GFP)y1(Lawson and Weinstein, 2002), and Tg(kdrl: mcherry)ci5 transgenic lines (Proulx et al., 2010) were used to obtain embryos for imaging. Embryos were raised at 24°C for time-lapse imaging or ISH. To make etv2:Kaede line, the BAC construct was prepared as previously reported (Proulx et al., 2010) with modifications to the pLD53.SC2 shuttle vector. Modifications included the excision of EGFP from pLD53.SC2 using BamHI and SacI. Kaede was PCR amplified from pCS2+:Kaede (kindly donated by Dr. Joshua Waxman, CCHMC) using 5′-gatttggtgacactatag-3′ (fwd) 5′-taa taactcactataggg-3′ (rvr) with flanking BamHI and SacI sites, and directionally cloned into the EGFP site of pLD53.SC2. Modified BAC DNA was linearized with AsiSI and injected into zebrafish embryos. One stable transgenic carrier Tg(etv2:Kaede)ci6 was identified by screening. Zebrafish work was performed under the protocols approved by the Institutional Animal Care and Use Committee (IACUC) within the Cincinnati Children's Hospital Research Foundation.
Etv2 Caged Morpholino Injections and Photoactivation
We mixed 500 μM of Caging strand (Supernova Life Sciences) designed against etv2 MO2 (Sumanas and Lin, 2006) with 50 μM of etv2 MO2 in 1 × Danieau buffer (Nasevicius and Ekker, 2000). The mixture was denatured at 70°C for 30 min and then was allowed to anneal overnight at 4°C. We injected 2.5nl of the etv2 caging strand-MO solution into zebrafish embryos at the 1- to 2-cell stage. All solutions and injections were handled in the dark, and room and microscope lights were equipped with yellow filters to prevent the spontaneous uncaging of caged etv2 morpholino. Injected embryos were kept in the dark until uncaging. Photoactivation was performed by exposing the injected embryos to 365 nm UV light for 30 min. A control etv2 MO2 was prepared in the same way without adding the caging strand.
In Situ Hybridization
In situ hybridization was performed as previously described (Jowett, 1999). Previously published etv2 (Sumanas et al., 2005), kdrl (Thompson et al., 1998), fli1a (Thompson et al., 1998), grl (Zhong et al., 2000), gata1 (Detrich et al., 1995), mrc1 (Wong et al., 2009), efnb2a, and ephb4a (Lawson et al., 2001) probes were used. Prior to imaging, zebrafish embryos were dehydrated in 100% ethanol and flat mounted in araldite. For image acquisition, processed embryos were mounted in 2% methylcellulose or 0.6% low melting agarose and imaged with the AxioImager compound microscope (Carl Zeiss) equipped with a Plan-Neofluar 10×/0.3 NA microscope objective (Carl Zeiss) and an AxioCam Icc3 color camera (Carl Zeiss). During image acquisition a series of z-planes were acquired to produce extended focus projected images. Projected images were produced in AxioVision 4.6 software (Carl Zeiss).
Time-Lapse Confocal and Structured Illumination Imaging
For time-lapse imaging all embryos were mounted in 0.6% low melting agarose containing 0.002% Tricaine (Sigma). Imaging was done at a temperature ranging from 26°C to 32°C. Time-lapse confocal imaging of Tg(etv2:GFP; kdrl:mcherry),Tg(etv2:GFP), and photoconverted etv2:Kaede-injected embryos was performed using the NLO LSM 510 (Carl Zeiss), LSM 510 (Carl Zeiss), LSM 710 (Carl Zeiss), or the Nikon D-Eclipse C1 (Nikon Instruments) microscopes. Confocal images were acquired using the 488 and 561 nm laser lines equipped with a Plan Aprochromat 20×/0.8 NA microscope objective (Carl Zeiss) and a Plan Aprochromat 20×/0.75 NA microscope objective (Nikon Instruments). The captured optical z-slice thickness ranged from 0.3 to 1 μm. Acquired time-lapse movies were either deconvolved using AutoQuant X.2.2 (Media Cybernetics) and saved as maximum intensity projected over time (MIPt), or directly saved as MIPt without deconvolution. Saved MIPt time-lapse files were reviewed in Nikon Elements (Nikon Instruments), LSM viewer (Carl Zeiss), or ImageJ (NIH, USA). Time-lapse structured illumination imaging was done using the Zeiss Apotome unit (Carl Zeiss) equipped with a Plan-Neofluar 5×/0.13 NA microscope objective and an AxioCam MRm monochrome camera (Carl Zeiss). Time-lapse files were processed in LSM viewer and ImageJ. Frame rate adjustments, video rotation, and video sizing were done using VirtualDub (http://www.virtualdub.com) software.
etv2:Kaede Photoconversions
etv2:Kaede embryos were injected at the 1- to 2-cell stage with 50 pg of 1:1 mixture of vegfa121 and vegfa165 RNA, or 200 pg of shh mRNA, or 10 ng vegfa ATG-MO (Nasevicius and Ekker, 2000) or treated in 100 μM cyclopamine from 50% epiboly to 26 somites. Whole-embryo photoconversions were performed at 14- to 15-somites using an AxioImager compound microscope equipped with a Plan-Neofluar 5× objective. Embryos were exposed to the DAPI filter setting for 10–15 s. For photoconversion of groups of medial angioblasts, embryos were mounted in 0.6% low melting agarose containing 0.002% Tricaine. Using an LSM 710 equipped with a Plan Aprochromat 20×/0.8 NA objective, small groups of cells were selected and exposed to 405 nm laser light. Photoconverted embryos (24–26 hpf) were mounted in 0.6% low melting agarose or 3% methylcellulose with 0.004% Tricaine and imaged using either an AxioImager compound microscope equipped with a Plan-Neofluar 10×/0.3 NA objective and an AxioCam MRm camera or a Nikon-D Eclipse C1 confocal microscope. Images were analyzed and processed using AxioVision and Photoshop software. For wild-type and DMSO-treated embryos, cells were categorized based on position within the dorsal aorta or posterior cardinal vein. In experimental embryos that did not form distinct DA and PCV vessels, cells were scored based on their dorsoventral position within the single vessel. Because it was not always possible to clearly distinguish boundaries between the neighboring cells, the cell counts are approximate estimates. Percentage values were calculated for the total number of Kaede cells in all embryos analyzed.
Supplementary Material
Acknowledgments
We thank C. Closson and M. Kofron for assistance with imaging, S.C. Ekker for shh construct, and J. Waxman for ephrin2b probe. This research was supported by NIH grant R01 HL107369-01 to S.S. and AHA Great Rivers Chapter Award 12POST12040123 to V.K. V.K., J.A.S., S.P.D., and K.R. performed experiments, analyzed the resulting data, and provided critical comments; S.S. designed the study, performed experiments, and analyzed the data; V.K., J.A.S., and S.S. wrote the paper.
Footnotes
Supplemental Information: Supplemental Information includes four figures and eight movies and can be found with this article online at http://dx.doi.org/10.1016/j.devcel.2013.03.017.
References
- Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc Natl Acad Sci USA. 2002;99:12651–12656. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs S, Chen JN, Garrity DM, Fishman MC. Patterning of angiogenesis in the zebrafish embryo. Development. 2002;129:973–982. doi: 10.1242/dev.129.4.973. [DOI] [PubMed] [Google Scholar]
- Cleaver O, Krieg PA. VEGF mediates angioblast migration during development of the dorsal aorta in Xenopus. Development. 1998;125:3905–3914. doi: 10.1242/dev.125.19.3905. [DOI] [PubMed] [Google Scholar]
- Covassin L, Amigo JD, Suzuki K, Teplyuk V, Straubhaar J, Lawson ND. Global analysis of hematopoietic and vascular endothelial gene expression by tissue specific microarray profiling in zebrafish. Dev Biol. 2006;299:551–562. doi: 10.1016/j.ydbio.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detrich HW, 3rd, Kieran MW, Chan FY, Barone LM, Yee K, Rundstadler JA, Pratt S, Ransom D, Zon LI. Intraembryonic hematopoietic cell migration during vertebrate development. Proc Natl Acad Sci USA. 1995;92:10713–10717. doi: 10.1073/pnas.92.23.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekker SC, Ungar AR, Greenstein P, von Kessler DP, Porter JA, Moon RT, Beachy PA. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr Biol. 1995;5:944–955. doi: 10.1016/s0960-9822(95)00185-0. [DOI] [PubMed] [Google Scholar]
- Eriksson J, Löfberg J. Development of the hypochord and dorsal aorta in the zebrafish embryo (Danio rerio) J Morphol. 2000;244:167–176. doi: 10.1002/(SICI)1097-4687(200006)244:3<167::AID-JMOR2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Fouquet B, Weinstein BM, Serluca FC, Fishman MC. Vessel patterning in the embryo of the zebrafish: guidance by notochord. Dev Biol. 1997;183:37–48. doi: 10.1006/dbio.1996.8495. [DOI] [PubMed] [Google Scholar]
- Garriock RJ, Czeisler C, Ishii Y, Navetta AM, Mikawa T. An anteroposterior wave of vascular inhibitor downregulation signals aortae fusion along the embryonic midline axis. Development. 2010;137:3697–3706. doi: 10.1242/dev.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerety SS, Anderson DJ. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development. 2002;129:1397–1410. doi: 10.1242/dev.129.6.1397. [DOI] [PubMed] [Google Scholar]
- Gering M, Rodaway AR, Göttgens B, Patient RK, Green AR. The SCL gene specifies haemangioblast development from early mesoderm. EMBO J. 1998;17:4029–4045. doi: 10.1093/emboj/17.14.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll MG, Anderson R, Stainier DY, Spradling AC, Halpern ME. Transcriptional silencing and reactivation in transgenic zebrafish. Genetics. 2009;182:747–755. doi: 10.1534/genetics.109.102079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- Hatta K, Tsujii H, Omura T. Cell tracking using a photoconvertible fluorescent protein. Nat Protoc. 2006;1:960–967. doi: 10.1038/nprot.2006.96. [DOI] [PubMed] [Google Scholar]
- Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, Shokat KM, Stainier DY. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326:294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BM, Bos FL, Bussmann J, Witte M, Chi NC, Duckers HJ, Schulte-Merker S. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat Genet. 2009;41:396–398. doi: 10.1038/ng.321. [DOI] [PubMed] [Google Scholar]
- Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- Jowett T. Analysis of protein and gene expression. Methods Cell Biol. 1999;59:63–85. doi: 10.1016/s0091-679x(08)61821-x. [DOI] [PubMed] [Google Scholar]
- Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- Laale HW. The biology and use of zebrafish, Brachydanio rerio in fisheries research. J Fish Biol. 1977;10:121–173. [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Gomez G, Kyba M, Lin S, et al. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008;2:497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A, Ciau-Uitz A, Pinheiro P, Monteiro R, Zuo J, Vyas P, Patient R, Porcher C. Uncoupling VEGFA functions in arteriogenesis and hematopoietic stem cell specification. Dev Cell. 2013;24:144–158. doi: 10.1016/j.devcel.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Xu X, Chin AJ, Balasubramaniyan NV, Teo MA, Lam TJ, Weinberg ES, Ge R. Cloning and characterization of vascular endothelial growth factor (VEGF) from zebrafish, Danio rerio. Biochim Biophys Acta. 1998;1397:14–20. doi: 10.1016/s0167-4781(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Liang D, Chang JR, Chin AJ, Smith A, Kelly C, Weinberg ES, Ge R. The role of vascular endothelial growth factor (VEGF) in vasculogenesis, angiogenesis, and hematopoiesis in zebrafish development. Mech Dev. 2001;108:29–43. doi: 10.1016/s0925-4773(01)00468-3. [DOI] [PubMed] [Google Scholar]
- Liao EC, Paw BH, Oates AC, Pratt SJ, Postlethwait JH, Zon LI. SCL/Tal-1 transcription factor acts downstream of cloche to specify hematopoietic and vascular progenitors in zebrafish. Genes Dev. 1998;12:621–626. doi: 10.1101/gad.12.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows SM, Fletcher PJ, Moran C, Xu K, Neufeld G, Chauvet S, Mann F, Krieg PA, Cleaver O. Integration of repulsive guidance cues generates avascular zones that shape mammalian blood vessels. Circ Res. 2012;110:34–46. doi: 10.1161/CIRCRESAHA.111.249847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Larson J, Ekker SC. Distinct requirements for zebrafish angiogenesis revealed by a VEGF-A morphant. Yeast. 2000;17:294–301. doi: 10.1002/1097-0061(200012)17:4<294::AID-YEA54>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham VN, Lawson ND, Mugford JW, Dye L, Castranova D, Lo B, Weinstein BM. Combinatorial function of ETS transcription factors in the developing vasculature. Dev Biol. 2007;303:772–783. doi: 10.1016/j.ydbio.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx K, Lu A, Sumanas S. Cranial vasculature in zebrafish forms by angioblast cluster-derived angiogenesis. Dev Biol. 2010;348:34–46. doi: 10.1016/j.ydbio.2010.08.036. [DOI] [PubMed] [Google Scholar]
- Sabin FR. Origin and development of the primitive vessels of the chick and of the pig. Contrib Embryol. 1917;6:61–124. [Google Scholar]
- Serbedzija GN, Flynn E, Willett CE. Zebrafish angiogenesis: a new model for drug screening. Angiogenesis. 1999;3:353–359. doi: 10.1023/a:1026598300052. [DOI] [PubMed] [Google Scholar]
- Shoji W, Isogai S, Sato-Maeda M, Obinata M, Kuwada JY. Semaphorin3a1 regulates angioblast migration and vascular development in zebrafish embryos. Development. 2003;130:3227–3236. doi: 10.1242/dev.00516. [DOI] [PubMed] [Google Scholar]
- Stark DA, Kulesa PM. An in vivo comparison of photoactivatable fluorescent proteins in an avian embryo model. Dev Dyn. 2007;236:1583–1594. doi: 10.1002/dvdy.21174. [DOI] [PubMed] [Google Scholar]
- Sumanas S, Lin S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 2006;4:e10. doi: 10.1371/journal.pbio.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S, Jorniak T, Lin S. Identification of novel vascular endothelial-specific genes by the microarray analysis of the zebrafish cloche mutants. Blood. 2005;106:534–541. doi: 10.1182/blood-2004-12-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S, Gomez G, Zhao Y, Park C, Choi K, Lin S. Interplay among Etsrp/ER71, Scl, and Alk8 signaling controls endothelial and myeloid cell formation. Blood. 2008;111:4500–4510. doi: 10.1182/blood-2007-09-110569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104:576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- Thisse BT, Thisse C. Fast Release Clones: A High Throughput Expression Analysis. ZFIN Direct Data Submission. 2004 http://zfin.org.
- Thompson MA, Ransom DG, Pratt SJ, MacLennan H, Kieran MW, Detrich HW, 3rd, Vail B, Huber TL, Paw B, Brownlie AJ, et al. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev Biol. 1998;197:248–269. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- Tomasini AJ, Schuler AD, Zebala JA, Mayer AN. PhotoMorphs: a novel light-activated reagent for controlling gene expression in zebrafish. Genesis. 2009;47:736–743. doi: 10.1002/dvg.20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Watanabe W, Shimada T, Matsunaga S, Kurihara D, Fukui K, Shin-Ichi Arimura S, Tsutsumi N, Isobe K, Itoh K. Single-organelle tracking by two-photon conversion. Opt Express. 2007;15:2490–2498. doi: 10.1364/oe.15.002490. [DOI] [PubMed] [Google Scholar]
- Weinstein BM, Stemple DL, Driever W, Fishman MC. Gridlock, a localized heritable vascular patterning defect in the zebrafish. Nat Med. 1995;1:1143–1147. doi: 10.1038/nm1195-1143. [DOI] [PubMed] [Google Scholar]
- Wilkinson RN, Koudijs MJ, Patient RK, Ingham PW, Schulte-Merker S, van Eeden FJ. Hedgehog signaling via a calcitonin receptorlike receptor can induce arterial differentiation independently of VEGF signaling in zebrafish. Blood. 2012;120:477–488. doi: 10.1182/blood-2011-10-383729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C, Kim SH, Ni TT, Mitchell L, Ro H, Penn JS, Baldwin SH, Solnica-Krezel L, Zhong TP. Hedgehog signaling induces arterial endothelial cell formation by repressing venous cell fate. Dev Biol. 2010;341:196–204. doi: 10.1016/j.ydbio.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KS, Proulx K, Rost MS, Sumanas S. Identification of vasculature-specific genes by microarray analysis of Etsrp/Etv2 overexpressing zebrafish embryos. Dev Dyn. 2009;238:1836–1850. doi: 10.1002/dvdy.21990. [DOI] [PubMed] [Google Scholar]
- Zhong TP, Rosenberg M, Mohideen MA, Weinstein B, Fishman MC. gridlock, an HLH gene required for assembly of the aorta in zebrafish. Science. 2000;287:1820–1824. doi: 10.1126/science.287.5459.1820. [DOI] [PubMed] [Google Scholar]
- Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock signalling pathway fashions the first embryonic artery. Nature. 2001;414:216–220. doi: 10.1038/35102599. [DOI] [PubMed] [Google Scholar]
- Zygmunt T, Gay CM, Blondelle J, Singh MK, Flaherty KM, Means PC, Herwig L, Krudewig A, Belting HG, Affolter M, et al. Semaphorin-PlexinD1 signaling limits angiogenic potential via the VEGF decoy receptor sFlt1. Dev Cell. 2011;21:301–314. doi: 10.1016/j.devcel.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.