Abstract
Background
The major arteries and veins are formed early during development. The molecular tools to identify arterial and venous endothelial cells improve our understanding of arterial-venous differentiation and branching morphogenesis. Compared to arterial differentiation, relatively little is known about what controls venous development, due to a lack of definitive molecular markers for venous endothelial cells.
Results
Here we report that the antibody against EphB1, an EphB class receptor, makes it possible to establish a reliable whole-mount immunohistochemical analysis of venous identity with greater resolution than previously possible in embryonic and adult skin vasculature models. EphB1 expression is restricted to the entire venous vasculature throughout embryonic development to adulthood, whereas the previously established venous marker EphB4 is also detectable in lymphatic vasculature. This venous-restricted expression of EphB1 is established after the vascular remodeling of the primary capillary plexus has occurred. Compared to its venous-specific expression in the skin, however, EphB1 is not restricted to the venous vasculature in yolk sac, trunk and lung.
Conclusions
These studies introduce EphB1 as a new venous-restricted marker in a tissue-specific and time-dependent manner.
Keywords: venous endothelial cell marker, venous development, EphB1, EphB4
INTRODUCTION
Blood vessels form a highly branched hierarchical vascular tree composed of arteries and veins. The primitive vascular network (capillary plexus) is remodeled to form a well-defined, hierarchical branching network in peripheral tissues (Risau, 1997). In this process, vascular endothelial growth factor (VEGF) and Notch signaling directs arterial differentiation (Mukouyama et al., 2002; Visconti et al., 2002; Duarte et al., 2004; Gale et al., 2004), whereas chicken ovalbumin upstream-transcription factor II (COUP-TFII) maintains venous identity by inhibiting Notch activation (You et al., 2005). Our understanding of a genetic program specifying arterial identity has been advanced by the identification and functional analysis of signaling molecules including Neuropilin-1 (Nrp1), Dll4, Notch1, Notch4, Hey1, Hey2, ephrinB2, Cx37, and Cx40 (Rocha and Adams, 2009; Chong et al., 2011). However, relatively little is known about what controls venous development, due to lack of definitive molecular markers for venous endothelial cells.
EphB4 is the first gene to be described that is expressed in a venous–specific manner from early in embryogenesis into adulthood (Wang et al., 1998), and which is functionally essential for angiogenesis as well (Gerety et al., 1999). EphB4taulacZ knock-in mice were used to show EphB4 expression in veins (Gerety et al., 1999). Although EphB4 is widely accepted as a venous marker, weak expression is also detectable in some arterial and lymphatic endothelial cells during development (Shin et al., 2001; Shin and Anderson, 2005; Srinivasan et al., 2007). COUP-TFII (You et al., 2005), Neuropilin-2 (Nrp2, (Yuan et al., 2002), and VEGFR3 (Kaipainen et al., 1995) are more abundantly expressed in veins than arteries, but these genes are not restricted to veins throughout development. Thus, the study of venous branching morphogenesis has been hindered by the confounding specificity of these markers’ expression.
Here we show that EphB1, an EphB class receptor, is specifically expressed by venous endothelial cells, but not arterial and lymphatic endothelial cells, in embryonic and adult skin vasculature. This expression provides a directly observable vascular network with an anatomically recognizable pattern. The antibody against EphB1 makes it possible to establish a reliable whole-mount immunohistochemical analysis of venous identity with greater resolution than previously possible. Interestingly, our extensive comparison of EphB1 and EphB4 expression in embryonic skins reveals that both EphB1 and EphB4 are uniformly expressed in the primitive capillary network at embryonic day (E) 13.5, a stage when no or fewer arterial markers such as ephrinB2 and Nrp1 are expressed. By E14.5, vascular remodeling occurs and both markers are expressed in remodeled veins. By E15.5, EphB1 expression is restricted to the entire venous vasculature, whereas EphB4 is also expressed in lymphatic vasculature. The venous-specific expression of EphB1 persists into adulthood in the skin vasculature. Compared to the venous-specific expression in the skin, however, EphB1 is not restricted to venous vasculature in yolk sac, trunk and lung. These data indeed suggest EphB1 is a new venous-specific marker that functions in a tissue-specific and time-dependent manner.
RESULTS
EphB1 is expressed in veins in the developing skin vasculature
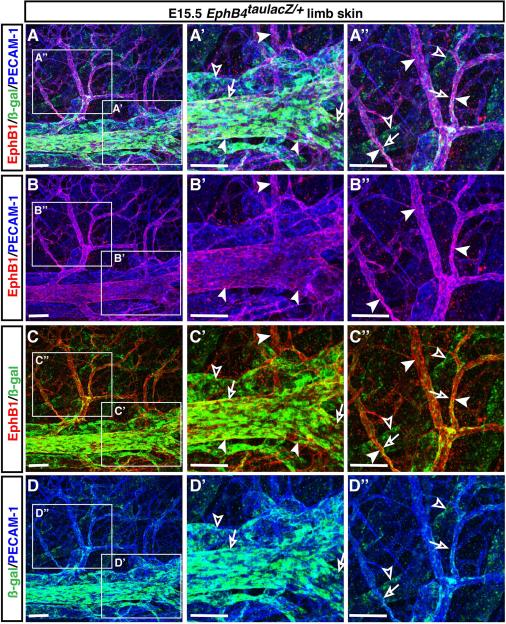
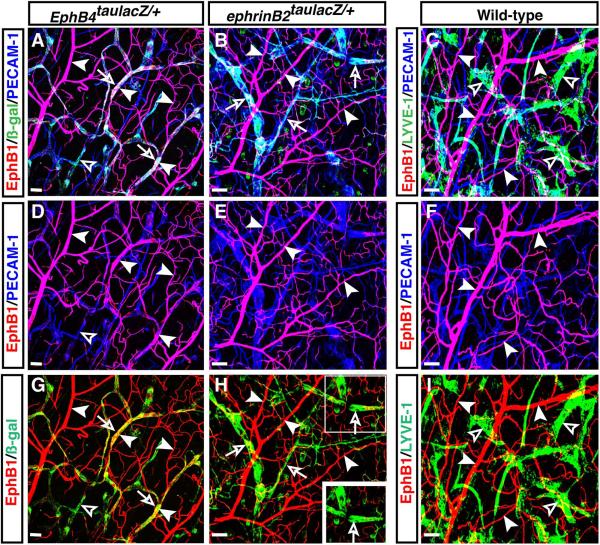
Previous studies demonstrated that ephrinB2, a transmembrane ligand, is expressed by arteries but not veins (Wang et al., 1998), whereas one of its receptors, the tyrosine kinase EphB4, is more abundantly expressed by veins than by arteries (Wang et al., 1998; Gerety et al., 1999). Whole-mount staining of EphB4taulacZ/+ heterozygous limb skin with antibodies to ß-galactosidase (ß-gal) revealed that at embryonic day (E) 15.5, EphB4taulacZ expression was mainly detected in veins (Fig. 1A-A”, C-C”, D-D”, open arrows) but weak EphB4taulacZ expression was also detectable in lymphatic-like vessels which do not form a smooth vessel structure and express relatively weak levels of the pan-endothelial cell marker PECAM-1 (Fig. 1A-A”, C-C”, D-D”, open arrowheads; see Fig. 2A, D, G). This observation prompted us to explore genes specifically expressed in venous endothelial cells in the developing skin vasculature. We initially examined the expression patterns of those EphB receptors (EphB1, EphB2, EphB3) reported to bind the arterial marker ephrinB2 (Flanagan and Vanderhaeghen, 1998), using whole-mount immunohistochemical analysis of limb skins with antibodies to those EphB receptors. Among them, only EphB1 antibody yielded venous-specific staining for the limb skin vasculature (Fig. 1A-A”, B-B”, C-C”, arrowheads). EphB1 expression appeared to overlap with EphB4taulacZ expression in large-diameter veins, whereas EphB1 expression was much more clearly detectable in branched middle-diameter veins and venous capillaries than EphB4taulacZ expression (Fig. 1A-A”, C-C”). Importantly, no weak EphB1 expression was detectable in EphB4taulacZ-expressing lymphatic-like vessels (Fig. 1A-A”, C-C”).
Figure 1. Venous-restricted expression of EphB1 in mouse embryonic limb skin vasculature.
Whole-mount triple immunofluorescence analysis of forelimb skin of E15.5 EphB4taulacZ/+ heterozygous embryos was performed with antibodies to the pan-endothelial marker PECAM-1 (A-B”, D-D”, blue), ß-galactosidase (A-A”, C-D”, ß-gal for EphB4taulacZ, green) and EphB1 (A-C”, red). Expression of EphB4taulacZ was mainly detected in large-diameter veins and some branched veins (A-A”, C-C”, D-D”, open arrows), but its weak expression was also detectable in lymphatic-like vessels (A-A”, C-C”, D-D”, open arrowheads). In contrast, EphB1 expression was clearly restricted to EphB4taulacZ-expressing veins but not found in lymphatic-like vessels (A-A”, B-B”, C-C”, arrowheads). Enlarged images (A’-D”) show the boxed regions in A-D. Scale bars are 100 μm.
Figure 2. EphB1 expression is not detectable in the developing dermal lymphatic vasculature.
Whole-mount triple immunofluorescence analysis of forelimb skin of E15.5 EphB4taulacZ/+ heterozygous embryos (A, D, G) and wild type embryos (B, C, E, F, H, I) was performed with antibodies to ß-galactosidase (A, D, G, ß-gal for EphB4taulacZ, red), lymphatic endothelial cell markers LYVE-1 (A, B, G, H, green) and Nrp2 (C, I, green), in combination with EphB1 (B, C, E, F, H, I red), PECAM-1 (A-F, blue). EphB4taulacZ expression was detectable in LYVE-1+ lymphatic vessels (A, D, G, open arrowheads), but EphB1 expression was not detectable in LYVE-1+ and Nrp2+ lymphatic vessels (B, C, H, I, open arrowheads), and was restricted to veins (B, C, E, F, arrowheads). Scale bars are 100 μm.
EphB1 is expressed by veins but not lymphatics and arteries in the skin vasculature
These observations led us to precisely characterize the expression of EphB1 in combination with the lymphatic endothelial cell markers LYVE-1 and Neuropilin-2 (Nrp2). As mentioned above, EphB4taulacZ expression was also detectable in LYVE-1+ lymphatic vasculature in EphB4taulacZ/+ heterozygous limb skin (Fig. 2A, D, G, open arrowheads). However, no significant expression of EphB1 was detectable in the LYVE-1+ and Nrp2+ lymphatic vasculature in the limb skin at E15.5 (Fig. 2B, C, E, F, H, I, arrowheads vs. open arrowheads).
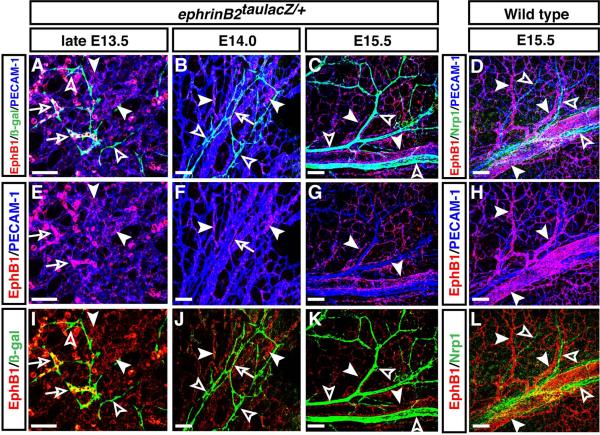
We next sought to examine the expression of EphB1 in arterial vasculature, by performing whole-mount limb skin staining with the arterial endothelial cell markers ephrinB2 and Neuropilin-1 (Nrp1). From late E13.5 onwards, in conjunction with vascular remodeling of the primary capillary plexus, ephrinB2taulacZ expression was detectable in branched vessels in ephrinB2taulacZ/+ heterozygous limb skin (Fig. 3A, B, I, J, open arrowheads). At E14.0, some ephrinB2taulacZ-expressing honeycomb-like vessels appear to be in the process of vascular remodeling and express EphB1 (Fig. 3B, F, J, open arrows). By E15.5, after vascular remodeling was complete, no significant expression of EphB1 was observed in the ephrinB2taulacZ-expressing arterial vasculature (Fig. 3C, G, K, arrowheads vs. open arrowheads). We confirmed that no significant EphB1 expression was detectable in the Nrp1+ arterial vasculature (Fig. 3D, H, L, arrowheads vs. open arrowheads). Taken together, these data clearly demonstrate that EphB1 expression is restricted to the venous vasculature but not the lymphatic and arterial vasculature. Given that none of the commercial anti-EphB4 antibodies was adequately specific for whole-mount staining of limb skin vasculature and that EphB4taulacZ expression is not absolutely restricted to venous endothelial cells, EphB1 represents a venous-restricted marker that allows us to analyze venous identity with a significant resolution.
Figure 3. EphB1 expression is not detectable in the developing dermal arterial vasculature.
Whole-mount triple immunofluorescence labeling of forelimb skin of late E13.5 (A, E, I), E14.0 (B, F, J) and E15.5 (C, G, K) ephrinB2taulacZ/+ heterozygous and E15.5 wild type (D, H, L) embryos was performed with antibodies to ß-gal (A-C, I-K, green) and the arterial marker Nrp1 (D, L, green), in combination with EphB1 (A-L, red) and PECAM-1 (A-H, blue). EphB1 expression was detectable in capillary plexus at late E13.5 (A, E, I, arrowheads) and E14.0 (B, F, J, arrowheads). Some ephrinB2taulacZ-expressing vessels co-express EphB1 at late E13.5 (A, E, I, open arrows) and E14.0 (B, F, J, open arrows), but most ephrinB2taulacZ-expressing vessels do not express EphB1 (A, B, I, J, open arrowheads). At E15.5, no significant expression of EphB1 (C, G, K, arrowheads) was observed in ephrinB2taulacZ-expressing arterial vasculature (C, G, K, open arrowheads). The mutually exclusive expression of EphB1 (D, H, L, arrowheads) and the arterial marker Nrp1 (D, L, open arrowheads) was also observed at E15.5. Scale bars are 100 μm.
Venous differentiation in the developing skin vasculature
We next examined venous fate establishment in the developing skin vasculature. By E13.5, a primary capillary plexus is established in the skin. Subsequently, the primitive vascular system undergoes intensive vascular remodeling triggered by blood flow and develops into a hierarchical vascular branching network including arteries and veins. Our previous whole-mount immunohistochemical studies demonstrated that no vascular expression of arterial markers such as ephrinB2 and Nrp1 is detectable in the primitive vasculature at E13.5 (Mukouyama et al., 2002). Surprisingly, expression of EphB1 and EphB4taulacZ was detectable throughout the primary capillary plexus at E13.5 (Fig. 4A, D, G, J). We further confirmed that EphB1 expression was detectable at earlier stage (Fig. 4D inset, E13.0). Given the prominent expression of EphB1 and EphB4taulacZ in the primary capillary plexus, we cannot presently discern whether this expression may be considered an indicator of immature vessels, or whether this expression pattern correlates with pre-specification of venous identity.
Figure 4. Time-course analysis of EphB1 and EphB4 expression in the developing skin.
Whole-mount triple immunofluorescence analysis of forelimb skin of EphB4taulacZ/+ heterozygous embryos was performed with antibodies to ß-galactosidase (A-C, G-L, ß-gal for EphB4taulacZ, green) in combination with EphB1 (A-I, red) and PECAM-1 (A-F, J-L, blue). At E13.5, the PECAM-1+ capillary plexus appeared to co-express EphB1 and EphB4taulacZ (A, D, G, J). By E15.5, expression of EphB1 (arrowheads) and EphB4taulacZ (open arrows) was detected in veins (B, C, E, F, H, I, K, L), whereas EphB4taulacZ expression was also detected in lymphatic vessels (C, I, L, open arrowheads). Inset in D shows double staining of forelimb skin of E13.0 wild type (WT) embryos with antibodies to EphB1 and PECAM-1. EphB1 expression was detectable in capillary plexus at an earlier stage than E13.5. Scale bars are 100 μm.
By E14.5, vascular remodeling had occurred and arterial markers were detectable in some vessels (Mukouyama et al., 2002). Expression of EphB1 and EphB4taulacZ progressively decreased in the remodeled arterial vessels (Fig. 4B, E, K, PECAM-1 single positive vessels) and became detectable in the developing venous vasculature (Fig. 4B, E, H, K, arrowheads and open arrows). Significant EphB1 expression but weak or faint EphB4taulacZ expression was detectable in some smaller vessels, suggesting that these EphB1+ vessels are in the process of vascular remodeling to form larger-diameter veins (Fig. 4B, E, H). By E15.5, the venous expression of EphB1 and EphB4taulacZ largely persisted (Fig. 4C, F, I, L, arrowheads and open arrows), although EphB4taulacZ expression was also detectable in lymphatic vessels (Fig. 4C, I, L, open arrowheads). Our detailed time-course analysis of vascular EphB1 expression suggests that EphB1 expression is detectable throughout the primary capillary plexus, and after vascular remodeling EphB1 expression is restricted to the venous vasculature in a time-dependent manner. Furthermore, the venous expression of EphB1 allows detection of a previously unrecognized intricate venous network in developing skin vasculature.
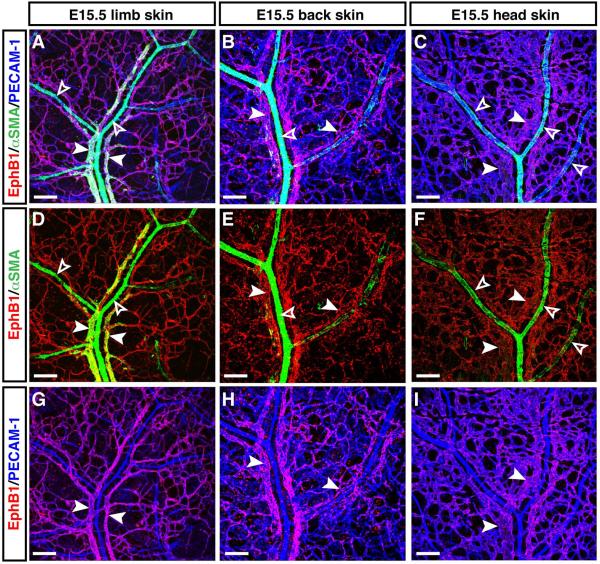
Given that arterial branches are densely covered with αSMA+ vascular smooth muscle cells (VSMCs), whereas less or no VSMC coverage occurs in venous branches (Mukouyama et al., 2002), we further confirmed whether EphB1+ vessels exhibit venous structure. Indeed, EphB1 expression was detectable in a typical venous vasculature with less or no VSMC coverage (Fig. 5A, D, G, arrowheads vs. open arrowheads). In contrast, no significant EphB1 expression was detectable in a typical arterial vasculature with dense VSMC coverage (Fig. 5A, D, open arrowheads). Moreover, venous expression of EphB1 was observed in back and head skin vasculatures (Fig. 5B, E, H, back skin; C, F, I, head skin, arrowheads vs. open arrowheads). Of note, venous remodeling from the primary capillary plexus appears to be in process at E15.5, albeit arterial remodeling has been completed to form a hierarchical arterial branching. These data lead to a more general understanding of the venous expression of EphB1 in embryonic skin vasculature.
Figure 5. Venous expression of EphB1 in embryonic limb, back and head skin vasculatures.
Whole-mount triple immunofluorescence analysis of forelimb skin (A, D, G), back skin (B, E, H), and head skin (C, F, I) of E15.5 embryos was performed with antibodies to the vascular smooth muscle cell (VSMC) marker αSMA (A-F, green) in combination with EphB1 (A-I, red) and PECAM-1 (A-C, G-I, blue). At E15.5, arterial branches are densely covered with αSMA+ VSMCs (A-F, open arrowheads), whereas less or no VSMC coverage occurs in venous branches (A-F, arrowheads). EphB1 expression was detectable in venous vasculature in limb, back and head skin vasculatures. Scale bars are 100 μm.
Venous expression of EphB1 in the adult skin vasculature
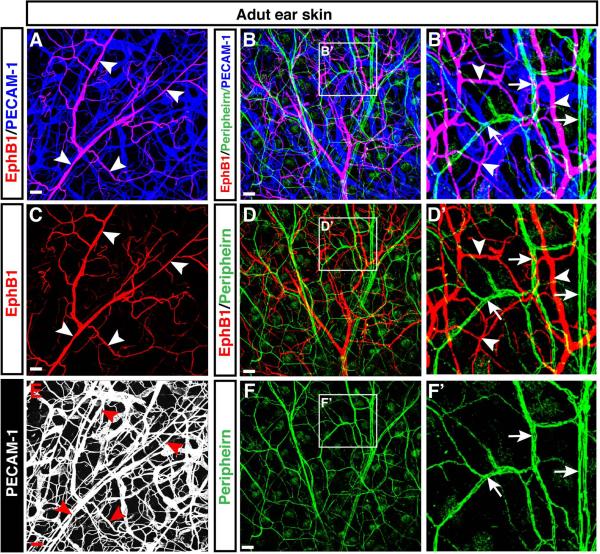
We next examined the expression of EphB1 in adult skin. Our whole-mount immunohistochemical analysis of adult ear skin revealed that EphB1 expression persisted in the adult skin vasculature (Fig. 6A, C, E, arrowheads). The cellular resolution of EphB1 expression afforded by the immunohistochemical staining with the antibody to EphB1 allowed us to visualize the highly branched vascular network. Although EphB1 had previously been reported to be largely expressed in the nervous system (Flanagan and Vanderhaeghen, 1998), no significant expression of EphB1 was detectable in peripherin+ nerves in the adult ear skin (Fig. 6B, B’, D, D’, F, F’, arrows vs. arrowheads).
Figure 6. Expression of EphB1 in the adult skin vasculature.
Whole-mount immunofluorescence analysis of adult ear skin was performed with antibodies to the neuronal marker Peripherin (B, B’, D, D’, F, F’, green, arrows) in combination with EphB1 (A-D’, red, arrowheads) and PECAM-1 (A-B’, blue; E, white). No significant expression of EphB1 was detectable in peripherin+ peripheral nerves in the adult ear skin (arrows and arrowheads). Enlarged images (B’, D’, F’) show the boxed regions in B, D, F. Scale bars are 100 μm.
Some EphB1+ vessels express the venous marker EphB4taulacZ (Fig. 7A, D, G, arrowheads and open arrows). EphB1 expression was detected in the entire venous network (Fig. 7A, D, G, arrowheads), whereas EphB4taulacZ expression was partially detected in venous and lymphatic-like vessels (Fig. 7A, D, G, open arrows and open arrowheads). No significant expression of the arterial marker ephrinB2taulacZ and the lymphatic marker LYVE-1 was detected in EphB1+ vessels (Fig. 7B, E, H, ephrinB2taulacZ, arrowheads vs. open arrows; Fig. 7C, F, I, LYVE-1, arrowheads vs. open arrowheads). Taken together, these data demonstrate that the preferential expression of EphB1 in the skin venous vasculature persists into adulthood.
Figure 7. Venous-restricted expression of EphB1 in the adult skin.
Whole-mount triple immunofluorescence analysis of adult ear skin from EphB4taulacZ/+ heterozygous (A, D, G), ephrinB2taulacZ/+ heterozygous (B, E, H), and wild-type (C, F, I) mice was performed with antibodies to ß-galactosidase (A, G, ß-gal for EphB4taulacZ, green, open arrows; B, H, ß-gal for ephrinB2taulacZ, green; open arrows), or LYVE-1 (C, I, green, open arrowheads) in combination with EphB1 (A-I, red, arrowheads) and PECAM-1 (A-F, blue). Venous-restricted expression of EphB1 persists into adulthood. No significant expression of the arterial marker ephrinB2taulacZ and the lymphatic marker LYVE-1 was detected in EphB1+ vessels. Inset in H shows single plane image of the white boxed region, suggesting that some overlapping signals for EphB1 in ephrinB2taulacZ-expressing vessels is caused by the preparation of compressed z-stack images. Scale bars are 100 μm.
EphB1 expression is not restricted to veins in yolk sac, trunk and lung
The preceding observations raised the question of whether EphB1 expression is restricted to veins in other tissues. To address this question, we examined the expression of EphB1 in the dorsal aorta and cardinal vein in E9.5 and E13.5 mouse embryos (Fig. 8). Double immunohistochemical analysis of transverse sections of E9.5 and E13.5 trunks with antibodies to EphB1 and PECAM-1 revealed that EphB1 expression was detectable in PECAM-1+ endothelial cells in E9.5 trunk vasculature including both the dorsal aorta and cardinal vein (Fig. 8A, D, arrowheads). The pan-endothelial expression of EphB1 persisted in E13.5 trunk vasculature (Fig. 8B, E, arrowheads), but the expression was not detectable in LYVE-1+ lymphatic vessels (Fig. 8C, F, arrowheads vs. open arrowheads).
Figure 8. EphB1 expression is not restricted to cardinal veins.
Double or triple immunofluorescence analysis of trunk sections of E9.5 (A, D) and E13.5 (B, E) wildtype, and E13.5 EphB4taulacZ/+ heterozygous (C, F) embryos was performed with antibodies to EphB1 (A-F, red), PECAM-1 (A, B, blue), ß-gal (C, green) and LYVE-1 (C, F, blue). EphB1 expression was detectable in both the dorsal aorta and cardinal vein (A-F, arrowheads) but was not detectable in LYVE-1+ lymphatic vessels (C, F, open arrowheads). Note that EphB1 expression was also detectable in the perineural vascular plexus (A, B, D, E, open arrowheads) and lung vasculature (B, E, open arrows; See Fig. 9E-H). SP: spinal cord; A: dorsal aorta; V: cardinal vein. Scale bars are 100 μm.
We further examined the expression of EphB1 in yolk sac and lung vasculature (Fig. 9). EphB1 expression was detectable in yolk sac capillary plexus at E8.0, as seen in E13.5 skin capillary plexus (Fig. 9A, C). At E9.5, after the vascular remodeling had occurred, EphB1 expression persisted in all yolk sac vasculature including Nrp1+ remodeled arteries (Fig. 9B, D, open arrowheads), Nrp1− remodeled veins and capillaries (Fig. 9B, D, arrowheads). In embryonic lung vasculature, EphB1 expression was detectable in EphB4taulacZ-expressing vessels, which appear to form the majority of lung microvasculature (Fig. 9E, G, arrowheads), whereas EphB1 expression was also detectable in ephrinB2taulacZ-expressing large-diameter arteries (Fig. 9F, H, open arrowheads).
Figure 9. Expression of EphB1 in yolk sac and lung vasculatures.
(A-D) Whole-mount triple immunofluorescence analysis of E8.0 yolk sac from EphB4taulacZ/+ heterozygous embryos (A, C) and E9.5 yolk sac from wild type embryos (B, D) embryos was performed with antibodies to ß-gal (A, green) or Nrp1 (B, D, green) in addition to EphB1 (A-D, red) and PECAM-1 (A-C, blue). EphB1 expression was detectable in capillary networks in E8.0 yolk sac vasculature, and in all E9.5 yolk sac vasculature including Nrp1+ remodeled arteries (open arrowheads), Nrp1− remodeled veins (arrowheads) and capillaries. (E-H) Triple immunofluorescence analysis of trunk sections of E15.5 EphB4taulacZ/+ heterozygous (E, G) and ephrinB2taulacZ/+ heterozygous (F, H) embryos was performed with antibodies to EphB1 (E-H, red), ß-gal (E-H, green) and PECAM-1 (E, F, blue). EphB1 expression was detectable in EphB4taulacZ-expressing lung microvasculature (E, G, arrowheads) and ephrinB2taulacZ-expressing large-diameter arteries (F, H, open arrowheads). Note that ephrinB2taulacZ expression was detectable in distal epithelial cells (open arrows). SP: spinal cord. Scale bars are 100 μm.
These data suggest that EphB1 may be considered as a tissue-specific venous endothelial cell marker in skin vasculature, rather than as a general venous-restricted marker.
DISCUSSION
In this study, we have described in detail the venous expression of EphB1 in mouse embryonic and adult skin vasculature. Whole-mount staining of skin with the antibody to EphB1 allows us to study venous vessel identity and the intricate branching network with greater resolution. Our findings provide important implications for the use of EphB1 as a venous-restricted marker in the analysis of venous differentiation and branching patterns in the embryonic skin and the analysis of neo-vascularization and tumor angiogenesis in the adult skin.
EphB receptors are expressed in the developing vasculature and play critical roles in embryonic angiogenesis. Previous studies demonstrated that the expression of EphB2, EphB3 and EphB4 mRNA is detectable in embryonic blood vessels (Adams et al., 1999) and the prominent venous expression of EphB4 was detected by anti-ß-galactosidase antibody staining in EphB4taulacZ/+ heterozygous mice (Gerety et al., 1999). Interestingly EphB4taulacZ/taulacZ homozygous mutants (Gerety et al., 1999) and EphB2−/−;EphB3−/− double homozygous mutants (Adams et al., 1999; Williams et al., 2003) exhibit angiogenic remodeling defects, resulting in the failure of organized vessel architecture during early embryogenesis (~E9.5). In contrast, EphB1−/− homozygous mutants do not show any detectable phenotypic defects in vascular development (Williams et al., 2003). Since no vascular defect is detectable in single EphB2−/− or EphB3−/− homozygous mutants as well, these EphB receptors may be functionally redundant such that EphB2, EphB3 or EphB4 may compensate for the lack of EphB1 in the homozygous mutants.
Recent studies have revealed that ephrinB2 regulates the endocytosis of VEGFR2 and VEGFR3 in sprouting angiogenesis and lymphangiogenesis (Sawamiphak et al., 2010; Wang et al., 2010). The internalization of VEGFR2 and VEGFR3 is necessary for activation and downstream signaling of the receptor and is required for VEGF-mediated endothelial sprouting. Given that EphB1 and EphB4 are differentially expressed by blood and lymphatic endothelial cells, one intriguing scenario is that endothelial ephrinB2 may be activated by either EphB1 or EphB4 during sprouting angiogenesis, whereas ephrinB2 activation in lymphatic endothelial cells may be mainly conducted by EphB4 during sprouting lymphangiogenesis.
Our skin vasculature model system provides a directly observable vascular network with an anatomically recognizable pattern (Li and Mukouyama, 2011). The time course analysis allows us to study the process of development from primary vascular network to a well-defined, hierarchical branching network. Previously, we discovered that in embryonic limb skin, arterial differentiation and the branching pattern of arterial vessels are controlled by that of peripheral sensory nerves (Mukouyama et al., 2002). We further discovered that nerve-derived VEGF-A is necessary for proper arterial differentiation (Mukouyama et al., 2005), and nerve-derived Cxcl12 controls vessel branching and alignment with nerves (Li et al., 2013). Given that few molecular tools are available to identify venous endothelial cells, however, what controls venous differentiation and branching patterns still remains elusive. The use of EphB1 makes it possible to image venous endothelial cells in the skin with greater resolution and higher reliability and to delineate defective venous development in mouse mutants.
Our detailed analysis of embryonic EphB1 expression provides the first evidence that in the skin EphB1 is expressed throughout the primitive capillary plexus, but after vascular remodeling has occurred the expression is restricted to veins. EphB1 expression is robustly reduced in the arterial marker ephrinB2+ vessels, underlying the mechanism that negatively regulates EphB1 expression during arterial differentiation. These data raised the question of whether EphB1 expression at the stage of the primitive capillary plexus is correlated with pre-specification of venous identity. One scenario is that venous specification might simply require the maintenance of venous-restricted genes, whereas arterial specification might require the induction of arterial specific genes and the repression of venous- restricted genes. Resolution of this issue will require an extensive comparison of gene expression patterns between EphB1+ endothelial cells from the primary capillary plexus and EphB1+ venous endothelial cells. Overall, the use of EphB1 as a venous-restricted marker in future studies should not only provide insights into our understanding of venous development but also refine our understanding of arterial-venous branching network formation.
EXPERIMENTAL PROCEDURES
Experimental animals
The characterizations of ephrinB2taulacZ/+ mice (Wang et al., 1998), and EphB4taulacZ/+ mice (Gerety et al., 1999) have been reported elsewhere and were used with approval of the NHLBI Animal Care and Use Committee.
Whole-mount immunohistochemistry of limb skin
Staining was performed essentially as described previously (Mukouyama et al., 2002; Li and Mukouyama, 2011). Briefly, forelimb skin tissue was dissected from embryos (E13.5~E15.5), fixed in 4% paraformaldehyde/PBS at 4°C overnight, and dehydrated in 100% methanol at − 20°C. Staining was performed using anti-PECAM-1 antibody (rat monoclonal antibody, clone MEC13.3, BD Pharmingen, 1:300 overnight at 4°C) to detect endothelial cells; anti-Nrp1 antibody (rabbit polyclonal antibody, A.L. Kolodkin, 1:3000, overnight at 4°C) was used as an arterial endothelial cell marker; ß-gal antibody (rabbit polyclonal antibody, Cappel, 1:500, overnight at 4°C) as arterial (ephrinB2taulacZ) or venous (EphB4taulacZ) endothelial cell markers; anti-LYVE1 antibody (rat monoclonal antibody, MBL, 1:300, overnight at 4°C) and anti-Nrp2 (rabbit polyclonal antibody, Cell Signaling 1:100, overnight at 4°C) to detect lymphatic endothelial cells; and anti-EphB1 antibody (goat polyclonal antibody, M-19, Santa Cruz, 1:100) to detect venous endothelial cells. For immunofluorescence detection, either Alexa-488-, Alexa-568-, Cy3- or Dylight 649-conjugated secondary antibodies (Invitrogen 1:250 or Jackson, 1:300, 1 hr at room temperature) were used. All confocal microscopy was carried out on a Leica TCS SP5 confocal (Leica).
Whole-mount Immunohistochemistry of adult ear skin
Murine adult ear skin tissue was dissected and fixed in 4% paraformaldehyde/PBS room temperature for 1 hour, and washed with PBS. Staining procedures were performed essentially as described previously (Feldheim et al., 1998; Flanagan and Vanderhaeghen, 1998; Nagao et al., 2009) and staining with antibodies and use of reagents were performed as described above. All confocal microscopy was carried out on a Leica TCS SP5 confocal (Leica).
Section immunohistochemistry
Section staining was performed as described previously (Mukouyama et al., 2005). In brief, embryos (E9.5 and E13.5) were fixed with 4% paraformaldehyde/PBS at 4°C overnight, immersed in 30% sucrose/PBS at 4°C overnight, and then embedded in OCT compound. Embryos were cryosectioned at 10-12μm thickness and collected on pre-cleaned slides (Matsunami, Japan). Staining was performed using anti-PECAM-1 antibody and anti-EphB1 to detect endothelial cells. For immunofluorescence detection, either Alexa-568- or Dylight 649-conjugated secondary antibodies (Invitrogen 1:250 or Jackson, 1:300, 1 hr at room temperature) were used. All confocal microscopy was carried out on a Leica TCS SP5 confocal (Leica).
Highlights.
The antibody against EphB1 provides a reliable whole-mount immunohistochemical probe for venous identity in the skin vasculature.
EphB1 expression is restricted to the skin venous vasculature after vascular remodeling of the primary capillary plexus has occurred.
Venous-restricted expression of EphB1 persists in the adult skin vasculature
ACKNOWLEDEGEMENTS
We thank S. Motegi and K. Zukosky for technical assistance with whole-mount ear skin staining. We also thank M. Udey, R. S. Adelstein, A. Michelson and R. S. Balaban for invaluable help and discussion, and other members of the Laboratory of Stem Cell and Neuro-Vascular Biology for technical help and thoughtful discussions. Thanks to J. Hawkins and the staff of NIH Bldg50 animal facility for assistance with mouse breeding and care, K. Gill for laboratory management and technical support, M. Conti for editorial advice on the manuscript, and Y. Carter, L. Oundo and R. Reed for administrative assistance. None of the authors have any financial or other conflicts of interest. This work was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH HL005702-07).
REFERENCES
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes & Development. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong DC, Koo Y, Xu K, Fu S, Cleaver O. Stepwise arteriovenous fate acquisition during mammalian vasculogenesis. Developmental dynamics : an official publication of the American Association of Anatomists. 2011;240:2153–2165. doi: 10.1002/dvdy.22706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, Rossant J. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes & Development. 2004;18:2474–2478. doi: 10.1101/gad.1239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim DA, Vanderhaeghen P, Hansen MJ, Frisen J, Lu Q, Barbacid M, Flanagan JG. Topographic guidance labels in a sensory projection to the forebrain. Neuron. 1998;21:1303–1313. doi: 10.1016/s0896-6273(00)80650-9. [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annual Review of Neuroscience. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Molecular Cell. 1999;4:403–414. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Kohara H, Uchida Y, James JM, Soneji K, Cronshaw DG, Zou YR, Nagasawa T, Mukouyama YS. Peripheral Nerve-Derived CXCL12 and VEGF-A Regulate the Patterning of Arterial Vessel Branching in Developing Limb Skin. Developmental Cell. 2013;24:359–371. doi: 10.1016/j.devcel.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Mukouyama YS. Whole-mount Immunohistochemical Analysis for Embryonic Limb Skin Vasculature: a Model System to Study Vascular Branching Morphogenesis in Embryo. Journal of visualized experiments : JoVE. 2011 doi: 10.3791/2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development. 2005;132:941–952. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Nagao K, Ginhoux F, Leitner WW, Motegi S, Bennett CL, Clausen BE, Merad M, Udey MC. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3312–3317. doi: 10.1073/pnas.0807126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Rocha SF, Adams RH. Molecular differentiation and specialization of vascular beds. Angiogenesis. 2009;12:139–147. doi: 10.1007/s10456-009-9132-x. [DOI] [PubMed] [Google Scholar]
- Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465:487–491. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- Shin D, Anderson DJ. Isolation of arterial-specific genes by subtractive hybridization reveals molecular heterogeneity among arterial endothelial cells. Developmental dynamics : an official publication of the American Association of Anatomists. 2005;233:1589–1604. doi: 10.1002/dvdy.20479. [DOI] [PubMed] [Google Scholar]
- Shin D, Garcia-Cardena G, Hayashi S, Gerety S, Asahara T, Stavrakis G, Isner J, Folkman J, Gimbrone MA, Jr., Anderson DJ. Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Developmental Biology. 2001;230:139–150. doi: 10.1006/dbio.2000.9957. [DOI] [PubMed] [Google Scholar]
- Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes & Development. 2007;21:2422–2432. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti RP, Richardson CD, Sato TN. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF). Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8219–8224. doi: 10.1073/pnas.122109599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, Barberis A, Benjamin LE, Makinen T, Nobes CD, Adams RH. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- Williams SE, Mann F, Erskine L, Sakurai T, Wei S, Rossi DJ, Gale NW, Holt CE, Mason CA, Henkemeyer M. Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron. 2003;39:919–935. doi: 10.1016/j.neuron.2003.08.017. [DOI] [PubMed] [Google Scholar]
- You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, Eichmann A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129:4797–4806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]