Abstract
The proepicardium is a transient extracardiac embryonic tissue that gives rise to the epicardium and a number of coronary vascular cell lineages. This important extracardiac tissue develops through multiple steps of inductive events, from specification of multiple cell lineages to morphogenesis. This article will review our current understanding of inductive events involved in patterning of the proepicardium precursor field, specification of cell types within the proepicardium, and their extension and attachment to the heart.
Introduction
In vertebrates, the proepicardium (PE) is a transient extracardiac embryonic tissue that gives rise to the epicardium and a number of coronary vascular cell lineages [1]. During heart looping, the PE develops as an outgrowth from the right cardiac inflow segment [2]. It is composed of an outer epithelial layer overlaying a core of mesenchymal cells suspended within extracellular matrix [3]. Villous protrusions of the PE extend and attach to the atrioventricular (AV) junction on the inner curvature of the looping stage heart. Following attachment, the outer layer of the PE spreads across the surface of the myocardium to form the epicardium. A subpopulation of epicardial cells undergoes an epithelial-to-mesenchymal transition. Together with mesenchymal cells of the PE core, they invade the myocardium and give rise to coronary smooth muscle cells, perivascular fibroblasts, coronary endothelial cells and erythrocytes [1,4,5]. The PE is thought to also have the capacity to contribute to the cardiomyocyte lineage; however, this remains controversial [6-10].
The inherent complexity of PE development, from specification of multiple cell lineages to morphogenesis, makes the study of PE induction challenging. Because inductive interaction(s) between inducing and responding cells can be a multistep and continuous process during PE development, a number of different events should be investigated. This article will provide a brief overview on inductive events patterning the PE precursor field, specifying cell types within the PE, and promoting extension and attachment to the heart.
PE field
Until recently, little was known about the precise origin of the PE or the developmental field from which PE cells arise. Cre-loxP-based analysis demonstrates that PE cells express Nkx2.5 and Isl1 at some point in their development [7]. Expression of Nkx2.5 and Isl1 delineates the primary and secondary heart fields, respectively [11,12], suggesting that the PE arises from the lateral plate mesoderm (LPM). Data in the zebrafish is also suggestive of LPM origins of the PE [13]. Direct fate mapping or lineage tracing with higher spatial resolution will be required to determine the exact location of PE precursors within the LPM.
High spatial resolution fate mapping data has recently become available in the chick (Figure 1) [14]. These studies identified a previously unrecognized posterior cardiogenic domain defined as the tertiary heart field (Figure 1A). A portion of the PE field was mapped to a location within the LPM directly adjacent to the right tertiary heart field [14]. This cell tracing data provides the first direct evidence for a LPM origin of the PE. PE precursors remain adjacent to, but do not intercalate with, the cardiogenic mesoderm (Figure 1B and 1C), suggesting that topological organization of the PE precursor domain and the tertiary heart field is maintained throughout cardiac morphogenesis. Collectively, these data provide a framework regarding the timing and ontogeny of PE specification, allowing for further assessment of inductive tissue-tissue interactions regulating PE development. Further studies will be necessary to determine the extent of the entire PE field within the LPM.
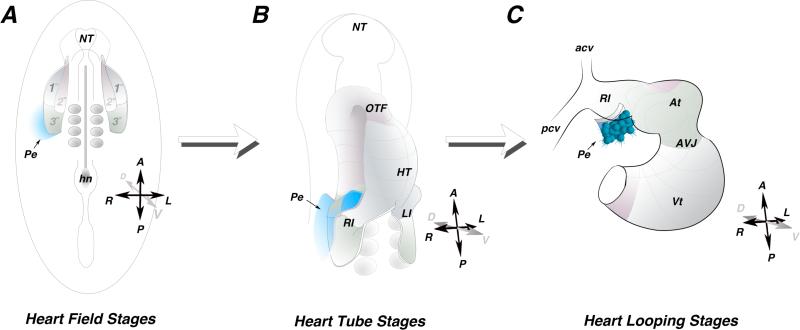
Figure 1.
Proepicardial Field. A) Schematic of an early somite stage chick embryo. Heart precursors occupy bilateral fields within the lateral plate mesoderm. The primary heart field (1°) is indicated in grey, the secondary heart field (2°) is indicated in pink, and the posterior tertiary heart field (3°) is indicated in green. Fate mapping studies indicate the progenitors of the Proepicardium reside outside and adjacent to the tertiary heart field (blue). B) Schematic of a heart tube. The primary heart fields have fused along the midline, while the secondary and tertiary heart field have not yet been incorporated into the heart. The proepicardial precursors maintain their position lateral to the heart field mesoderm (blue). C) Schematic of a looping stage heart. the proepicardium (blue) can be seen extending off the right inflow of the heart. HT - heart tube, At - atria, AVJ - atrioventricular junction, Vt - ventricle, Pe - proepicardium, A - anterior, P -posterior, R - right, L- Left, D - dorsal, V - Ventral.
Molecular induction
A number of different molecular markers are often used to delineate PE identity. These include transcription factors such as Wt1, Tbx18, Tcf21 and signaling components Cfc and Raldh2. These markers are preferentially expressed within epithelial and mesenchymal cells of the PE, but are also expressed in other tissues [6,13,15,16]. Two novel PE markers, Scx and Sema3D, define distinct subpopulations within the mouse PE [17]. Unlike other PE markers, in the heart region expression of Sema3D and Scx is restricted to the PE and epicardium. These molecular markers, alone or in combination, can be used as a readout of PE molecular induction.
How PE identity is induced remains largely unknown; however, the close proximity of the liver bud to the PE is suggestive of inductive interactions between these two tissues . This possibility has been experimentally tested. Ectopic implantation of quail liver bud into posterior lateral regions of chick host embryos induces expression of Wt1, Tbx18 and Tcf21 in adjacent host tissue [18]. Strikingly, other endoderm-derived tissues such as lung bud and stomach do not share this capacity. These results suggest a role of the liver bud in PE molecular induction (Figure 2) but cannot rule out a contribution from the myocardium. However, liver bud implantation in posterior lateral regions far from the myocardium still induces expression of PE markers, suggesting that a myocardium-derived signal is not necessary. To test this possibility more directly, chick non-myocardial mesoderm was co-cultured with quail liver bud, resulting in elevated expression of Wt1 and Tcf21 [18]. A requirement for mature hepatocytes in PE molecular induction has been explored in Hnf1bahi2169 mutant zebrafish in which hepatocyte markers prox1 and hhex are absent [19]. Wt1, Tbx18 and Tcf21 are expressed at wild-type levels in the heart region of the mutant zebrafish, indicating that the completion of liver maturation is not required for PE molecular induction.
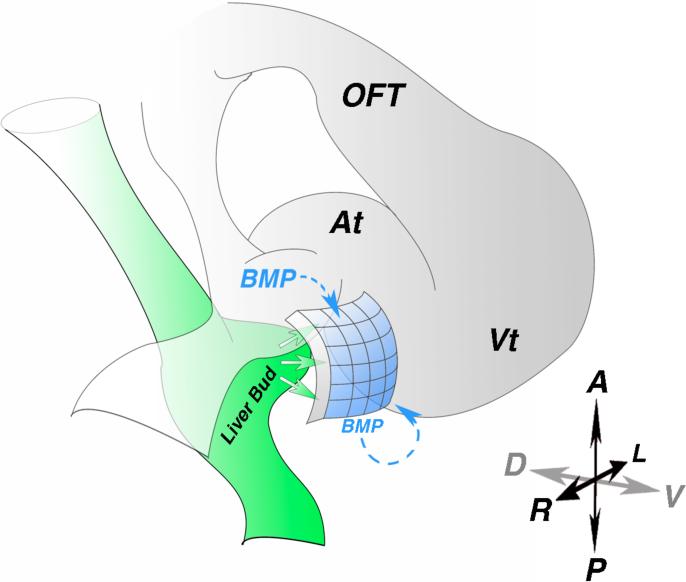
Figure 2.
Poepicardial molecular induction. A) Diagram of an early looping stage heart. Liver bud (green) derived signals induce proepicardial marker expression in the adjacent mesothelium (blue). Paracrine and autocrine BMP signaling is also critical for proepicardial marker gene expression. At - atria, OFT - out flow tract, Vt - ventricle, A - anterior, P - Posterior, R - right, L - left, D - dorsal, V - ventral.
Studies in chick and zebrafish have implicated bone morphogenetic protein (BMP) signaling in PE molecular induction (Figure 2). Both implantation of beads soaked with Noggin, a BMP antagonist, or BMP2-secreting cells, into the right inflow results in loss of Tbx18 expression in the heart region [6]. Consistent with this, in Bmp4 and type 1 BMP receptor mutant zebrafish, expression of Tbx18 and Tcf21 is absent around the heart but unaffected in other tissues [19]. However, the source of BMP signals remains to be determined.
In addition, an indirect effect of fibroblast growth factor (FGF) signaling in PE laterality development has been suggested. FGF8 and Snai1 are asymmetrically expressed in the right side of Hensen's node and the LPM [20]. Inhibition of right-sided FGF8 or Snai1 signaling results in the loss of Tbx18 expression in the right inflow, whereas ectopic left-sided FGF8 or Snai1 signaling leads to bilateral expression of Tbx18 and Wt1 [21]. Taken together, these data suggest that maintenance of laterality is required for proper PE marker expression. The transcription factors Tbx5 and Hand2 appear to be required for PE marker expression. Tbx5a is a member of the T-box transcription factor family that is expressed in the LPM [22]. Expression of Tbx18 and Tcf21 is markedly reduced in the heart region of Tbx5a mutant zebrafish. Introduction of a dominant negative form of Tbx5a at various stages reveals a requirement for Tbx5a prior to heart morphogenesis. Though it is not expressed in the PE, Hand2, a basic helix-loop-helix transcription factor, is necessary for proper development of the epicardium and coronary vasculature [23]. Expression of Tbx18 and Tcf21 is absent in the heart region of Hand2 mutant zebrafish, suggesting a role of Hand2 in PE molecular induction; however, a secondary effect due to extensive myocardial defects cannot be ruled out [19]. Additionally, genetic lineage tracing studies suggest that Hand1-expressing cells of the septum transversum contribute to the PE [23].
Marker expression is an effective readout of inductive interactions; however, additional readouts are required for a more complete understanding of PE induction. Although liver bud and FGF signals induce ectopic expression of PE markers, morphological characteristics such as villous protrusions are not observed. These data suggest that additional signals from the myocardium, or other tissues, may be required for morphogenetic induction.
Morphogenetic induction of the PE
Morphogenetic induction of the PE, or its protrusion and attachment to the heart, is the result of an orchestrated series of cellular events, including cell proliferation and guided protrusion. Several studies have provided different pieces of evidence regarding PE cell protrusion and incorporation to the heart. In the mouse, retinoid X receptor alpha null animals display increased PE cell apoptosis with incomplete formation and distention of the epicardium [24]. Similarly, inhibition of FGF signaling results in reduced PE villous outgrowth associated with increased cell death and decreased cell proliferation [25]. In contrast, Connexin43 alpha 1 null PE explants display increased proliferation and cellular locomotion [26]. In addition to its previously noted role in PE marker expression, Tbx5 appears to also be required for PE cell incorporation into the heart. PE cells infected with retrovirus expressing anti-sense Tbx5 do not contribute to the formation of epicardium and coronary vasculature [22].
Myocardium-derived BMP signals direct protrusion and attachment of the PE to the heart [27]. During PE protrusion, Bmp2 expression is localized to the AV junction. Misexpression or inhibition of Bmp2 results in blocked attachment of the PE to the looping heart. Importantly, perturbation of BMP signaling blocks this morphogenetic process but does not affect PE identity or heart patterning, as revealed by unchanged expression of Tbx18, Wt1, Tcf21, Tbx2 and Cx40. In vitro, PE cells can undergo directional epithelial expansion toward the source of BMPs without the myocardium [27]. Taken together, this study proposes a model in which myocardium-derived paracrine signals induce morphogenetic outgrowth of the PE toward the AV junction.
After attachment, PE cells spread across the surface of the myocardium to form the epicardium. A number of studies have demonstrated the importance of adhesion molecules in this process. Alpha 4 integrin null mice form an epicardial layer at E10.5; however, by E12.5 this layer is completely absent from the ventricles, suggesting defective epicardial attachment to the heart [28]. Similar phenotypes have also been observed in cell surface protein VCAM1 null mice [29].
Together, these studies indicate that myocardium-derived signals are critical to PE morphogenetic induction (Figure 3). These effects may be mediated by a number of cellular functions, including gap junctions, cell adhesion molecules, and growth factors. As the PE undergoes morphogenetic changes, inductive signals must also be acting to specify diverse PE cell fates.
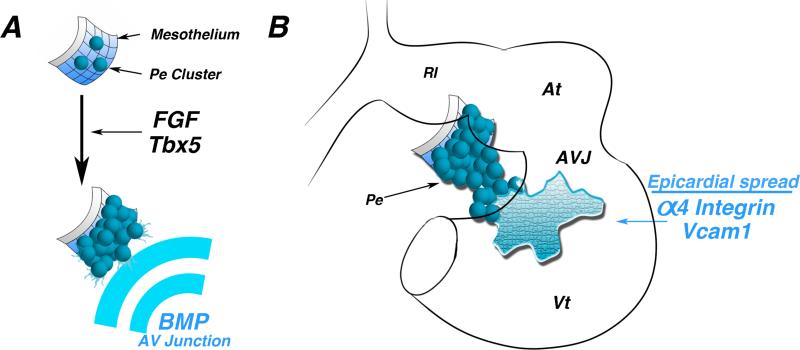
Figure 3.
Proepicardial morphological induction. A) Schematic of early mesothelium transitioning to a “ grape-like” cluster of proepicardial cells. FGF signaling and the transcription factor Tbx5 are required for this transition. BMP secreted by atrioventricular junction myocytes direct oriented proepicardial extension. B) Schematic of a looping stage heart. Following proepicardial extension and attachment to the inner curvature of the heart, Alpha4 integrin and Vcam1 are necessary for proper spreading and adherence of epicardial cells to the myocardium. At -atria, AVJ - atrioventricular junction, Vt - ventricle.
PE cell type diversity
PE cells were originally thought to give rise only to epicardium; however, a series of retroviral-based lineage tracing studies showed that the PE also generates a number of other cardiac lineages, including coronary vascular smooth muscle cells, cardiac fibroblasts and coronary vascular endothelial cells [1,30]. Subsequent studies employing chick/quail chimeras and adenoviral tagging [4,31-33] corroborate these findings.
While in the avian system the PE is capable of generating coronary artery endothelial cells, venous and lymphatic endothelial cells derive from other sources [34,35]. Both in avian and mouse, venous endothelial cells arise from inflow angiogenic sprouts [34,36]. In mouse the origin of coronary artery endothelial cell is less clear, as multiple sources including the sinus venosus, PE and endocardium have been cited to be responsible for this event [17,36-38]. Although multiple cell types arise from the PE, how and when their fate decisions are induced remain unknown.
The distinct cell lineages contributing to the coronary vasculature are distributed in a mosaic pattern within the PE prior to its attachment to the myocardium. Indeed, this expression pattern is observed for cellular and molecular markers [1,17,25,33,38-41].However, it is unknown whether PE cells expressing a single marker give rise to the same cell lineage type in the heart. Previous work suggests that fate segregation towards different cell types occurs within the PE, as retroviral clonal analysis revealed that individual PE cells do not give rise to multiple fates [1]. Alternative evidence shows that epicardial cells expressing Tcf21 give rise to both cardiac fibroblasts and coronary artery smooth muscle cells [42]. This implies that cell fates may not segregate within the PE or that more than one fated cell type is labeled by Tcf21. Uncovering the precise temporal and spatial location of PE cell type segregation is imperative for further investigations on the inductive signals in PE cell type specification.
Current evidence hints to a few factors involved in PE cell type specification. Conditional KO mice lacking b-catenin expression in the PE, display an absence of the main coronary arteries. Vessels are devoid of smooth muscle cells positive for alpha-SMA while retaining PECAM-1 positive endothelial cells [43]. Given that PE migration to the heart and epicardial formation were not affected, the authors concluded that b-catenin has a specific role in induction of the coronary vascular smooth muscle lineage. Similarly, retinoic acid and adenoviral overexpression of Raldh2 in quail PE explants decreases alpha-SMA expression, while inhibition of retinoic acid signaling in vivo leads to increase expression of alpha-SMA. Ectopic VEGF in PE explants results in decreased alpha-SMA expression and modest increase in endothelial QH1 marker [41]. Lastly, Tcf21 positive epicardial cells give rise to coronary artery smooth muscle cells and cardiac fibroblasts. However, in Tcf21 null embryos cardiac fibroblasts markers are absent in the myocardium but retained in the epicardium [42]. Collectively, these studies are beginning to elucidate how diversification of PE cell types is induced during their recruitment to the heart.
Conclusion
Current evidence is starting to reveal critical information regarding PE induction; however, a considerable amount of work is still needed. For instance, the inductive signals that mediate PE field specification are completely unknown. This has been in part due to the lack of information pertaining to the temporal and spatial window of PE field specification. The recent discovery of a previously undescribed PE progenitor field will enable the search for potential inductive signals involved in this process.
The study of PE induction represents an exciting challenge that could potentially result not only in a better understanding of PE developmental biology but also in the implementation of cell based clinical therapies. Currently, myocardium-derived BMP is the only factor known to play a role in PE morphogenetic induction. However, the mechanisms translating paracrine BMP signaling into PE morphogenetic events remain unknown. The contribution of the PE to a number of coronary vascular cell types has been known for more than a decade. While the discovery of inductive PE signals demands diligent work, as many embryological manipulations can cause multiple developmental effects, the determination of events that specify the diverse cell types that arise from the PE is critical to our general understanding of heart development.
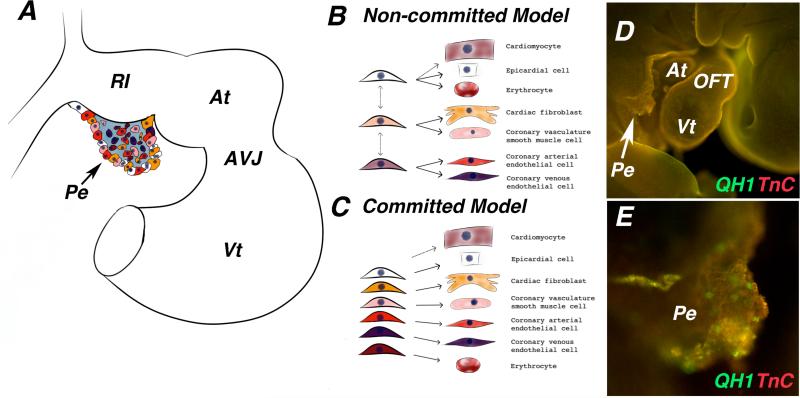
Figure 4.
Proepicardial cell fate induction. A) Schematic of a late looping stage heart. Different colors in the proepicardium represent different cell fate potentials. B. Model of proepicardium cell fate induction in which cell fates have not been definitively established and individual cells within the proepicardium can go on to give rise to multiple cell types. C) Model of proepicardium cell fate induction in which cells within the proepicardium are already restricted to a cell fate prior to the proepicardium binding to the heart. D) Whole mount immunofluorescence staining of a late looping stage quail embryo stained with antibodies against the extracellular matrix protein tenascin-C (red), and the quail endothelial marker QH1 (green). E) Higher magnification image of proepicardium from (D), QH1 positive endothelial cells are clearly present within the proepicardium before it attaches to the heart. At -atria, AVJ - atrioventricular junction, Vt - ventricle, Pe - proepicardium, OFT - outflow tract.
Acknowledgement
The original work presented in this article was supported in part by grants from the NIH (R37HL078921, R01HL092429, and R01HL112268 to TM).
References
- 1.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev. Biol. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 2.Manasek FJ. Embryonic development of the heart. II. Formation of the epicardium. J Embryol Exp Morphol. 1969;22:333–348. [PubMed] [Google Scholar]
- 3.Nahirney PC, Mikawa T, Fischman DA. Evidence for an extracellular matrix bridge guiding proepicardial cell migration to the myocardium of chick embryos. Dev. Dyn. 2003;227:511–523. doi: 10.1002/dvdy.10335. [DOI] [PubMed] [Google Scholar]
- 4.Dettman RW, Denetclaw W, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev. Biol. 1998;193:169–181. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- 5.Tomanek RJ, Hansen HK, Dedkov EI. Vascular patterning of the quail coronary system during development. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:989–999. doi: 10.1002/ar.a.20365. [DOI] [PubMed] [Google Scholar]
- 6.Schlueter J, Männer J, Brand T. BMP is an important regulator of proepicardial identity in the chick embryo. Dev. Biol. 2006;295:546–558. doi: 10.1016/j.ydbio.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 7.Zhou B, Gise, von A, Ma Q, Rivera-Feliciano J, Pu WT. Nkx2-5- and Isl1-expressing cardiac progenitors contribute to proepicardium. Biochem. Biophys. Res. Commun. 2008;375:450–453. doi: 10.1016/j.bbrc.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai C-L, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christoffels VM, Grieskamp T, Norden J, Mommersteeg MTM, Rudat C, Kispert A. Tbx18 and the fate of epicardial progenitors. Nature. 2009;458 doi: 10.1038/nature07916. E8–9–discussion E9–10. [DOI] [PubMed] [Google Scholar]
- 10.Kikuchi K, Holdway JE, Major RJ, Blum N, Dahn RD, Begemann G, Poss KD. Retinoic Acid Production by Endocardium and Epicardium Is an Injury Response Essential for Zebrafish Heart Regeneration. Developmental Cell. 2011;20:397–404. doi: 10.1016/j.devcel.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai C-L, Liang X, Shi Y, Chu P-H, Pfaff SL, Chen J, Evans S. Isl1 Identifies a Cardiac Progenitor Population that Proliferates Prior to Differentiation and Contributes a Majority of Cells to the Heart. Developmental Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu-Issa R, Kirby ML. Patterning of the heart field in the chick. Dev. Biol. 2008;319:223–233. doi: 10.1016/j.ydbio.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serluca FC. Development of the proepicardial organ in the zebrafish. Dev. Biol. 2008;315:18–27. doi: 10.1016/j.ydbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Bressan M, Liu G, Mikawa T. Early mesodermal cues assign avian cardiac pacemaker fate potential in a tertiary heart field. Science. 2013;340:744–748. doi: 10.1126/science.1232877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmona R, Gonzalez-Iriarte M, Perez-Pomares JM, Munoz-Chapuli R. Localization of the Wilms’ tumour protein WT1 in avian embryos. Cell and Tissue Research. 2001;303:173–186. doi: 10.1007/s004410000307. [DOI] [PubMed] [Google Scholar]
- 16.Xavier-Neto J, Shapiro MD, Houghton L, Rosenthal N. Sequential programs of retinoic acid synthesis in the myocardial and epicardial layers of the developing avian heart. Dev. Biol. 2000;219:129–141. doi: 10.1006/dbio.1999.9588. [DOI] [PubMed] [Google Scholar]
- 17.Katz TC, Singh MK, Degenhardt K, Rivera-Feliciano J, Johnson RL, Epstein JA, Tabin CJ. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Developmental Cell. 2012;22:639–650. doi: 10.1016/j.devcel.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishii Y, Langberg JD, Hurtado R, Lee S, Mikawa T. Induction of proepicardial marker gene expression by the liver bud. Development. 2007;134:3627–3637. doi: 10.1242/dev.005280. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Stainier DY. R. Tbx5 and Bmp Signaling Are Essential for Proepicardium Specification in Zebrafish. Circ. Res. 2010 doi: 10.1161/CIRCRESAHA.110.217950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isaac A. Control of Vertebrate Left-Right Asymmetry by a Snail-Related Zinc Finger Gene. Science. 1997;275:1301–1304. doi: 10.1126/science.275.5304.1301. [DOI] [PubMed] [Google Scholar]
- 21.Schlueter J, Brand T. A right-sided pathway involving FGF8/Snai1 controls asymmetric development of the proepicardium in the chick embryo. Proc. Natl. Acad. Sci. U.S.A. 2009;106:7485–7490. doi: 10.1073/pnas.0811944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatcher CJ. A role for Tbx5 in proepicardial cell migration during cardiogenesis. Physiological Genomics. 2004;18:129–140. doi: 10.1152/physiolgenomics.00060.2004. [DOI] [PubMed] [Google Scholar]
- 23.Barnes RM, Firulli BA, VanDusen NJ, Morikawa Y, Conway SJ, Cserjesi P, Vincentz JW, Firulli AB. Hand2 loss-of-function in Hand1-expressing cells reveals distinct roles in epicardial and coronary vessel development. Circ. Res. 2011;108:940–949. doi: 10.1161/CIRCRESAHA.110.233171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkins SJ, Hutson DR, Kubalak SW. Analysis of the proepicardiumepicardium transition during the malformation of theRXRα—/— epicardium. Dev. Dyn. 2005;233:1091–1101. doi: 10.1002/dvdy.20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torlopp A, Schlueter J, Brand T. Role of fibroblast growth factor signaling during proepicardium formation in the chick embryo. Dev. Dyn. 2010;239:2393–2403. doi: 10.1002/dvdy.22384. [DOI] [PubMed] [Google Scholar]
- 26.Li WEI, Waldo K, Linask KL, Chen T, Wessels A, Parmacek MS, Kirby ML, Lo CW. An essential role for connexin43 gap junctions in mouse coronary artery development. Development. 2002;129:2031–2042. doi: 10.1242/dev.129.8.2031. [DOI] [PubMed] [Google Scholar]
- 27.Ishii Y, Garriock RJ, Navetta AM, Coughlin LE, Mikawa T. BMP signals promote proepicardial protrusion necessary for recruitment of coronary vessel and epicardial progenitors to the heart. Developmental Cell. 2010;19:307–316. doi: 10.1016/j.devcel.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 29.Kwee L, Baldwin HS, Shen HM, Stewart CL, Buck C, Buck CA, Labow MA. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995;121:489–503. doi: 10.1242/dev.121.2.489. [DOI] [PubMed] [Google Scholar]
- 30.Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc. Natl. Acad. Sci. U.S.A. 1992;89:9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ. Res. 1998;82:1043–1052. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 32.Männer J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. The Anatomical Record. 1999;255:212–226. doi: 10.1002/(sici)1097-0185(19990601)255:2<212::aid-ar11>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 33.Guadix JA, Carmona R, Muñoz-Chápuli R, Pérez-Pomares JM. In vivo and in vitro analysis of the vasculogenic potential of avian proepicardial and epicardial cells. Dev. Dyn. 2006;235:1014–1026. doi: 10.1002/dvdy.20685. [DOI] [PubMed] [Google Scholar]
- 34.Poelmann RE, Gittenberger-de Groot AC, Mentink MM, Bökenkamp R, Hogers B. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circ. Res. 1993;73:559–568. doi: 10.1161/01.res.73.3.559. [DOI] [PubMed] [Google Scholar]
- 35.Wilting J, Buttler K, Schulte I, Papoutsi M, Schweigerer L, Männer J. The proepicardium delivers hemangioblasts but not lymphangioblasts to the developing heart. Dev. Biol. 2007;305:451–459. doi: 10.1016/j.ydbio.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 36.Red-Horse K, Ueno H, Weissman IL, Krasnow MA. Coronary arteries form by developmental reprogramming of venous cells. Nature. 2010;464:549–553. doi: 10.1038/nature08873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu B, Zhang Z, Lui W, Chen X, Wang Y, Chamberlain AA, Moreno-Rodriguez RA, Markwald RR, O'Rourke BP, Sharp DJ, Zheng D, Lenz J, Baldwin HS, Chang C-P, Zhou B. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell. 2012;151:1083–1096. doi: 10.1016/j.cell.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Combs MD, Braitsch CM, Lange AW, James JF, Yutzey KE. NFATC1 promotes epicardium-derived cell invasion into myocardium. Development. 2011;138:1747–1757. doi: 10.1242/dev.060996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishii Y, Langberg J, Rosborough K, Mikawa T. Endothelial cell lineages of the heart. Cell and Tissue Research. 2009;335:67–73. doi: 10.1007/s00441-008-0663-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kruithof BPT, van Wijk B, Somi S, Kruithof-de Julio M, Pérez Pomares JM, Weesie F, Wessels A, Moorman AFM, van den Hoff MJB. BMP and FGF regulate the differentiation of multipotential pericardial mesoderm into the myocardial or epicardial lineage. Dev. Biol. 2006;295:507–522. doi: 10.1016/j.ydbio.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 41.Azambuja AP, Portillo-Sánchez V, Rodrigues MV, Omae SV, Schechtman D, Strauss BE, Costanzi-Strauss E, Krieger JE, Pérez-Pomares JM, Xavier-Neto J. Retinoic acid and VEGF delay smooth muscle relative to endothelial differentiation to coordinate inner and outer coronary vessel wall morphogenesis. Circ. Res. 2010;107:204–216. doi: 10.1161/CIRCRESAHA.109.214650. [DOI] [PubMed] [Google Scholar]
- 42.Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, Banfi S, Sauer MF, Olsen GS, Duffield JS, Olson EN, Tallquist MD. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development. 2012;139:2139–2149. doi: 10.1242/dev.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zamora M, Männer J, Ruiz-Lozano P. Epicardium-derived progenitor cells require beta-catenin for coronary artery formation. Proc. Natl. Acad. Sci. U.S.A. 2007;104:18109–18114. doi: 10.1073/pnas.0702415104. [DOI] [PMC free article] [PubMed] [Google Scholar]