Summary
This review provides a framework for development of an operational definition of sarcopenia and of the potential endpoints that might be adopted in clinical trials among older adults.
Introduction
While the clinical relevance of sarcopenia is widely recognized, there is currently no universally accepted definition of the disorder. The development of interventions to alter the natural history of sarcopenia also requires consensus on the most appropriate endpoints for determining outcomes of clinical importance which might be utilised in intervention studies.
Methods and results
We review current approaches to the definition of sarcopenia, and the methods used for the assessment of various aspects of physical function in older people. The potential endpoints of muscle mass, muscle strength, muscle power and muscle fatigue, as well as the relationships between them, are explored with reference to the availability and practicality of the available methods for measuring these endpoints in clinical trials.
Conclusions
Based on current evidence, none of the four potential outcomes in question is sufficiently comprehensive to recommend as a uniform single outcome in randomised clinical trials. We propose that sarcopenia may be optimally defined (for the purposes of clinical trial inclusion criteria, as well as epidemiological studies) using a combination of measures of muscle mass and physical performance. The choice of outcome measures for clinical trials in sarcopenia is more difficult; co-primary outcomes, tailored to the specific intervention in question, may be the best way forward in this difficult but clinically important area.
Keywords: sarcopenia, muscle mass, muscle strength, muscle power, muscle fatigue, older adults
Introduction
Conceptually, the term sarcopenia refers to an age-related loss of skeletal muscle mass and function. Between the ages of 20 and 80 years, a decline in muscle fibre size and number causes a loss of muscle mass of approximately 30%, together with a 20% reduction in mid-thigh cross-sectional area [1,2]. Muscle strength and muscle power also decrease with advancing age, particularly in the lower body, and to a greater degree than muscle mass [3]. The age-associated decline in isometric knee extensor strength has been estimated at between 55% and 76% [4,5].
The origins of sarcopenia are multifactorial and correlates include muscle disuse, endocrine dysfunction, chronic disease, inflammation and nutritional deficiencies [6]. While the clinical relevance of sarcopenia is widely recognized, there remains no universally accepted definition of the term. In addition, there are no agreed endpoints to determine adverse or beneficial outcomes of clinical importance in human intervention studies. This poses problems for the development of pharmacologic interventions to alter the natural history of the disorder. Indeed, a number of potential drug targets have been identified as a result of improved understanding of the functional and structural changes seen in sarcopenia at the molecular level, but there is no precedent for any intervention in terms of gaining regulatory approval. In the absence of widely accepted, clinically meaningful and easily measurable outcomes, little progress can be made in establishing regulatory guidance for the development of agents in this area.
In moving towards an operational definition of sarcopenia, there are analogies with osteoporosis. According to World Health Organization criteria, “osteoporosis is characterized by low bone mass and microarchitectural deterioration of bone tissue, leading to bone fragility and a consequent increase in risk of fracture” [7]. Operationally, however, it is defined in terms of bone mineral density (BMD) using dual energy X-ray absorptiometry (DXA) since measuring quality of bone is not feasible in daily clinical practice. In the context of clinical trials, patients are recruited on the basis of BMD, and the outcome of interest is fracture, for which BMD is a surrogate [8].The question in sarcopenia, then, is whether there are similar markers that can be used to define the disorder, characterise its progress, and provide outcome measurements that would fulfil regulatory requirements. This paper explores the operationalization of the current definition of sarcopenia, and the potential inclusion criteria and clinical outcomes to be considered when designing clinical trials in this context.
Toward an operational definition of sarcopenia
Early attempts to define sarcopenia were based on measurements of skeletal muscle mass with DXA in relation to body size. Calculated as the appendicular fat-free mass of the upper and lower limbs divided by body height squared, a patient’s muscle mass index indicated sarcopenia if it was >2 standard deviations (SD) below the sex-specific average in healthy young men or women [9]. With this definition, the prevalence of sarcopenia was 53% in men and 43% in women over the age of 80 years. The diagnostic criterion was later refined to be appendicular fat-free mass adjusted for height and body fat mass, which provided a stronger association with functional performance using the same thresholds [3].
Since 2005, there have been renewed efforts to define sarcopenia by several groups from the US and Europe [6,9-14]. To date, many of these groups have published definitions of sarcopenia, each one recommending diagnostic criteria based on various combinations of measures of muscle strength, function and physical performance with muscle mass (Table 1). These definitional approaches have paralleled a growing interest in the potential use of simple muscle strength tests (such as handgrip strength), or physical performance tests (such as gait speed, sit-to-stand time and standing balance) as objective screening measures to identify patient groups who might benefit from targeted interventions [15, 16]. Indeed, some of these measures have been widely proposed as diagnostic criteria for sarcopenia and frailty [6, 16]. Most recently, an International Working Group on Sarcopenia presented four recommendations for identifying sarcopenia in clinical practice: (a) Assess patient for reduced physical capability (or weakness); (b) Consider sarcopenia in patients who are non-ambulatory or who cannot rise from a chair unassisted; (c) Assess usual walking pace (habitual gait speed) over a 4-m course; (d) Patients with a habitual gait speed <1.0 m/s should be considered for quantitative measurement of body composition by DXA [6]. A ‘sarcopenia with limited mobility’ syndrome has also been described, indicating a need for therapeutic intervention in people with a habitual gait speed <1.0 m/s, or who walk under 400 m in a 6-minute walk test, in conjunction with an appendicular fat-free mass >2 SD below that of healthy 20–30 year olds of the same ethnic group [11]. In this context, a clinically significant intervention would result in either a 50-m increase in 6-minute walk distance or an increase in gait speed of 0.1 m/s [11].
Table 1.
Diagnostic criteria for sarcopenia: suggested approaches*
| Study group | Definition | Criteria |
|---|---|---|
| ESPEN Special Interest Groups [12] |
“Sarcopenia is a condition characterized by loss of muscle mass and muscle strength. Although sarcopenia is primarily a disease of the elderly, its development may be associated with other conditions that are not exclusively seen in older persons, like disuse, malnutrition and cachexia. Like osteopenia, it can be also be seen in those with inflammatory diseases.” |
|
| European Working Group on Sarcopenia in Older People [10] |
“Sarcopenia is a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength with a risk of adverse outcomes such as physical disability, poor quality of life and death.” The condition is called primary sarcopenia when the cause is aging per se, and secondary sarcopenia when disease, inactivity, or malnutrition contribute |
Reference population of healthy young subjects using cutoff points <2 SDs below mean. Criterion 1 and Criterion 2 or 3. |
| International Working Group on Sarcopenia [6] |
“Sarcopenia is defined as the age-associated loss of skeletal muscle mass and function. The causes of sarcopenia are multifactorial and can include disuse, altered endocrine function, chronic disease, inflammation, insulin resistance, and nutritional deficiencies. While cachexia may be a component of sarcopenia, the two conditions are not the same.” |
|
| Society of Sarcopenia, Cachexia and Wasting Disorders [11] |
“Sarcopenia with limited mobility is a specific condition with clear loss of muscle mass and a clear target for intervention. As such it differs from the more general concept of frailty.” “The limitation in mobility should not be clearly attributable to the direct effect of specific disease, such as peripheral vascular disease with intermittent claudication, or central and peripheral nervous system disorders (such as stroke, Parkinson’s disease, spinal cord disease, or motor neuron disease), dementia, or cachexia.” |
|
Other study groups, such as the Biomarkers Consortium, have convened for the same purpose of developing a consensus statement but have not yet published their findings
Gait speed, sit-to-stand time and standing balance are measures of functional performance which rely on strength and motor control, and, with the exception of standing balance, muscle power. Studies of these measures have been undertaken exclusively in older populations, and few have recorded long-term outcomes. The existing data, however, do show an association between each of these measures and all-cause mortality. In meta-analyses of studies of gait speed and sit-to-stand time, this association was consistent and showed a graded effect [15]. More recently, a pooled analysis indicated a strong association (p<0.001) between gait speed and survival in nine cohort studies, with significant increments in survival per unit increase in gait speed [17].
In clinical practice, gait speed (timed 4-metre walk), sit-to-stand time, and standing balance are often measured within the context of the Short Physical Performance Battery (SPPB) [18, 19]. This battery of tests has been validated in large-scale epidemiological studies and characterizes lower extremity functional performance using timed measures of standing balance (side-by-side stand, tandem and semi-tandem positions), gait speed (timed 4-metre walk), and lower extremity strength (timed test of five chair rises). Scores obtained on a 12-point summary scale indicate a gradient of functional decline that is highly predictive of subsequent mobility-related disability, institutionalization, and mortality [19-21]. It is generally accepted that a total SPPB score ≤10 indicates functional impairment in older populations (each test is scored from 0 to 4) and is strongly predictive of the loss of ability to walk 400 metres [22, 23]. The reproducibility of the SPPB can be enhanced through the use of standardised equipment and an appropriate standard operating procedure.
Muscle mass, strength, power and fatigue
Muscle mass is a well characterized endpoint that can easily be measured using DXA (Table 2). Loss of muscle mass is associated with an increased risk for developing chronic metabolic disease, such as type 2 diabetes [24], but an increase in muscle mass may not always translate into an improved level of physical functioning. As a result, care is required in the design of clinical trials that a lack of improvement in muscle function is not masked by an increase in muscle mass alone [25]; the situation is analogous to that in osteoporosis trials, where certain drugs may increase bone mass but fail to reduce the incidence of fracture [26].
Table 2.
Potential endpoints in trials of interventions for sarcopenia
| Advantages | Disadvantages | |
|---|---|---|
| Muscle mass |
|
|
| Muscle strength |
|
|
| Muscle power |
|
|
| Muscle fatigue |
|
|
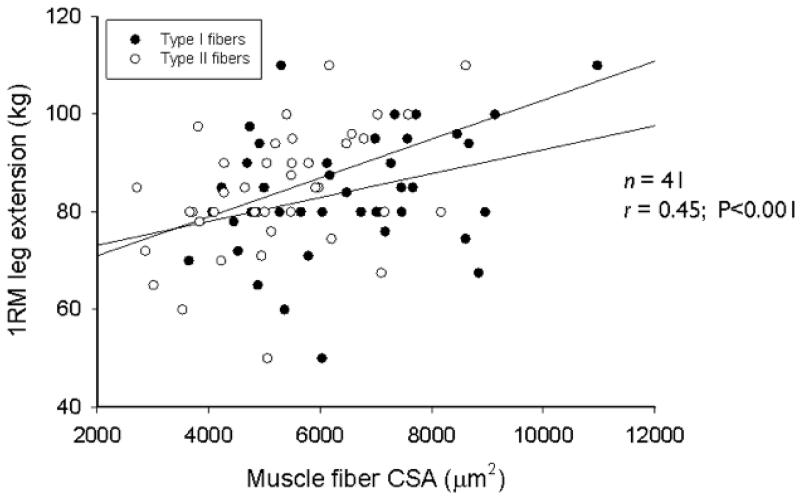
A better predictor of muscle function in the general population is muscle strength, though it may be less useful in certain subgroups (for example, patients with arthritis). In elderly men, muscle strength (the maximum capacity of a muscle to generate force) is positively correlated with muscle mass, as well as muscle fibre cross-sectional area (type I and II muscle fibres) (Figure 1) and myonuclear and satellite cell content [27]. Although decline in strength is associated with loss of lean muscle mass in older adults, the former occurs much more rapidly than the latter in both men and women (Figure 2) [28]. Muscle strength is also predictive of mortality; in the Health, Aging and Body Composition Study low muscle strength was strongly associated with mortality, independently of low muscle mass [29].
Fig. 1.
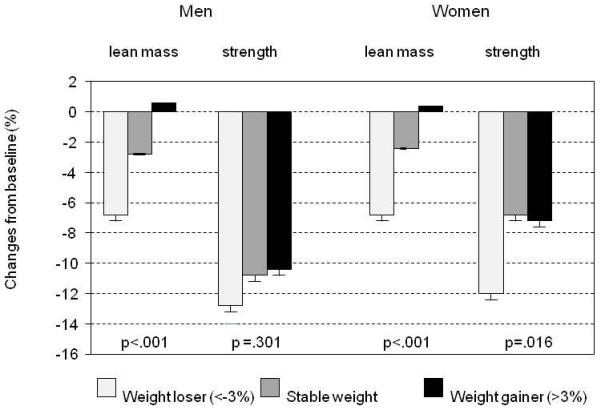
Mass/strength divergence in older men and women taking part in the Health, Aging and Body Composition Study.Maintenance or gaining lean muscle mass did not prevent aging-associated declines in muscle strength, with men losing almost twice as much strength as women. Reprinted from [28] with permission.
Fig.2.
Muscle fibre size is positively correlated with muscle strength. Among 41 older men, 1-RM leg extension (kg) was significantly associated with muscle fibre cross-sectional area (r=0.45; p<0.001).Muscle fibre cross-sectional area was significantly lower in type II than in type I fibres (p<0.01). Greater muscle fibre cross-sectional area was associated with greater thigh muscle area and muscle strength (0.30 ≤ r ≤ 0.45; p<0.05). Reprinted from [27] with permission.
Muscle power (the maximum rate of work undertaken by a muscle per unit time) appears to be better still at predicting functional status as it includes a neuromuscular component that provides information from pathways that are not captured by measures of muscle mass and strength [30]; muscle power is a strong predictor of functional mobility and risk of falling among older adults [31]. The fourth potential endpoint for sarcopenia trials is muscle fatigue, an important determinant of force production that has been defined as “the inability of the muscle to generate or maintain the levels of strength required for a given work rate” [32]. The remainder of this review will summarise current approaches to the measurement of these four indices of muscle function.
Measuring muscle mass
If muscle mass is to be a part of the diagnostic definition of sarcopenia, then measuring it needs to be feasible for both research and in clinical practice, and feasible in older people. Unsuitable methods include isotope dilution (used to measure total body water), in vivo neutron activation, and measurements of potassium-40 isotope, underwater weighing and urine metabolites (creatinine or 3-methyhistidine in 24-hour urine) [33, 34]. Anthropometric methods (e.g. body mass index, arm and calf circumference and arm muscle cross-sectional area as a function of arm muscle circumference and skinfold thickness) are simple but lack precision and are prone to overestimation. Although some anthropometric methods correlate highly with appendicular muscle mass, substantial individual prediction errors are observed in some patients even when combined with grip strength [9]. Bioelectrical impedance is a popular alternative, despite lack of a standardized methodology [35, 36], and is easy to use in both research and clinical settings [37]. However, bioelectrical impedance may be considered more as a surrogate of muscle mass than a direct measurement. Air-displacement plethysmography is a highly reproducible method of measuring body composition but it relies on an assumption that density of fat mass and fat-free mass is the same in all patients [38]. The accuracy and precision of methods used for assessment of muscle mass are shown in Table 3.
Table 3.
Accuracy and precision of methods utilised for the assessment of muscle and fat mass
| Muscle | Fat | |||
|---|---|---|---|---|
| Accuracy | Precision | Accuracy | Precision | |
| Anthropometry | + / − | + / − | + / − | + / − |
| Bioelectrical impedance |
+ / − | + | + / − | + |
| Air-displacement plethysmography |
NA | NA | + | ++ |
| DXA | ++ | ++ | ++ | ++ |
| CT / MRI | ++ | ++(+) | ++ | ++(+) |
CT, computed tomography; DXA, dual-energy X-ray absorptiometry; MRI, magnetic resonance imaging; NA, not applicable.
To obtain a complete picture of body composition, a four-component model comprising total body water, protein, mineral and fat mass is required; however, this is a highly intensive and costly procedure [39]. As a three-component model (combining protein and minerals into ‘solids’) DXA is superior to standard densitometry (which differentiates only between fat mass and fat-free mass), and has been widely adopted. However, DXA is unable to evaluate intramuscular fat, which can account for 5–15% of observed muscle mass in obese people [39]. In the context of research, computed tomography (CT) is often used to assess total and fat-free muscle area, with a smaller margin of error than that seen with DXA [40]. However, due to the large amount of radiation involved, full-body CT has limited utility [41]. Magnetic resonance imaging (MRI) has similar accuracy and reproducibility for fat and muscle as CT and can be used for whole-body imaging. Both CT and MRI are more sensitive to small changes in muscle mass than DXA [42, 43].
Few studies have been done to compare changes in muscle mass as assessed by DXA, CT and MRI in older adults. Hansen et al. reported poor correlation between DXA and CT estimates of change in thigh muscle mass in older patients recovering from hip fracture (r2 = 0.28, p = 0.04) [40]. In a comparable study recruiting relatively healthy older patients to a 10-week muscle training programme and comparing change in thigh muscle mass in trained and untrained legs, DXA tended to overestimate the improvements in the trained legs [43]. The limitations of CT localization and the prohibitive cost and accessibility of MRI as a screening tool in clinical practice should also be taken into account when developing a diagnostic approach to sarcopenia.
Measuring muscle strength
Isokinetic dynamometry is the recognized gold standard for measuring muscle strength, but its use is limited by the cost and availability of expensive equipment. Testing for 1 repetition maximal strength (1-RM) using generic resistance-type exercise equipment offers a reliable alternative that correlates well with strength assessed by means of dynamometry (r=0.88 [44]). However, a disadvantage of using 1-RM strength with generic resistance type exercise equipment is that absolute values of 1-RM strength are not comparable between different sets of equipment. In addition, two measurement sessions are required to accurately assess 1-RM strength: one to estimate and one to finally determine actual 1-RM strength [45].
Low handgrip strength has consistently been linked with poor health outcomes (long-term disability onset, increased risk of complications, extended hospitalization) [15, 46, 47] The first systematic review of objectively measured muscle strength to include a meta-analysis reported a reduction in mortality risk for every 1 kg increase in grip strength across 13 studies involving 44,638 participants [15]. The recommended procedure for measuring grip strength is to take the highest recording out of three repeated tests in the left hand and three in the right hand, but variation in clinical practice is widespread, making comparisons between studies difficult [48]. At a population level, too, there is considerable variation in grip strength with age; in extreme cases, a 70 year old man may have the same handgrip strength of a 20 year old [49]. The Jamar dynamometer is the reference standard for measuring grip strength; however, its design may limit its use in some patients, for example those with advanced arthritis. In these instances, the Martin vigorimeter, which measures grip strength using rubber balls available in three different sizes, may be a suitable alternative [50].
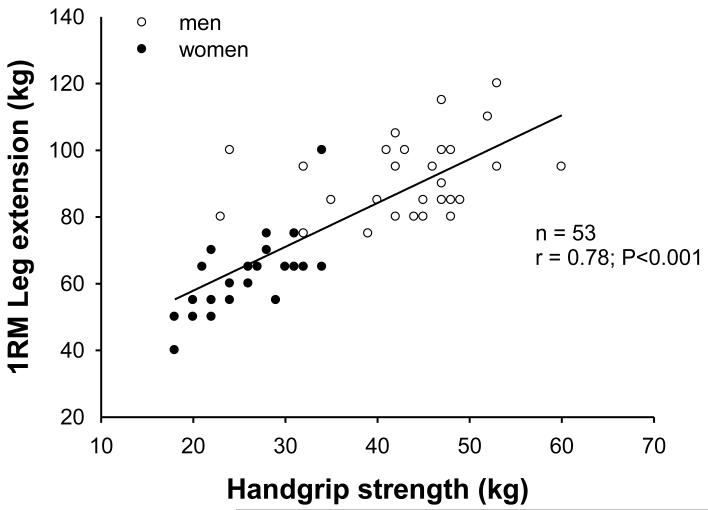
Trials of resistance exercise training in a frail older population have shown significant increases in 1-RM leg strength and improvements in SPPB scores, largely attributable to reductions in sit-to-stand time due to greater leg strength [6]. Despite these increases in leg strength and functional capacity, significant changes in handgrip strength were not observed during the entire training intervention. These observations contrast with the recent findings in a healthy older population, where 1-RM leg extension strength was shown to correlate well with handgrip strength (Figure 3). Thus, handgrip strength may be useful as an inclusion criterion when designing clinical trials, but may be less suitable for use as an outcome measure to assess changes in muscle strength or function in the individual patient.
Fig.3.
1-RM leg extension vs handgrip strength in healthy elderly men and women. Scatter plot for correlation of 1-RM leg extension with handgrip strength in elderly men (open circles) and elderly women (filled circles). Line represents the fitted regression. Pearson correlation coefficient was 0.78 (p<0.001)
Measuring muscle power
Muscle power (maximum rate of work per unit time) has the potential to be more sensitive to age-related physiological change than traditional measures of muscle strength (maximum capacity to generate force). Studies have shown muscle power to be highly predictive of physical capability in older people [51, 52], and this has been linked to age-related impairments in neuromuscular activation [53], tendon stiffness [54], muscle contractile speed [55], and changes in muscle architecture [54]. Peak skeletal muscle power achieved during leg press or knee extension high-velocity resistance training has been validated as a reliable and functionally relevant outcome in older populations [30]. However, as an outcome measure of sarcopenia for use in clinical practice, muscle power is potentially limited by the need for expensive equipment. A recent pilot study proposed that multivariate linear regression equations could be used to accurately predict both average and peak power from a simple sit-to-stand test within 20 seconds that could be conducted in any setting without much preparation [56]. However, such estimates are not able to assess change in muscle power over time, limiting their usefulness in intervention studies. Finally, measurements of muscle power are inappropriate for use in people with arthritis.
Measuring muscle fatigue
Several scales incorporate fatigue or exhaustion in definition of the frail older population, for example the adapted Fried criteria [57] and the Canadian Study of Health and Aging 70-item frailty index [58]. However, there is no gold standard and little consistency between the scales in terms of the questions asked. Muscle fatigue can be divided into central and peripheral components, the latter of which can be measured by a variety of methods (Table 4) [59]; this is usually done in the leg, but there is little published research linking fatigue to sarcopenia and results are inconsistent.
Table 4.
Methods for assessment of peripheral muscle fatigue. Reproduced from [59] with permission.
| Method | Assessment | Protocol | Outcome |
|---|---|---|---|
| MVC and SVC | Measures MVC and SVC until exhaustion |
Sustainment of MVC or SVC at 20–60% of MVC until failure (↓50%) |
↓ isometric muscle strength and endurance |
| Isokinetic measurements | Measures isometric torque, isokinetic torque and total work performed |
5 contractions at an angular velocity of 60– 90’/s; 15–30 contractions at a velocity of ~300’/s |
↓ isometric PT, ↓ isokinetic PT and ↓ total work generated |
| Surface electromyography |
Analyzes the myoelectrical manifestation of fatigue during muscle contractions |
Used during MVC and SVC |
↓ in-muscle activation, ↓ muscle fatigue, ↓ SRM and altered M-wave |
| Twitch interpolation | Differentiates fatigue of central origin from that of peripheral origin |
MVC associated with nerve stimulation; failure if the difference between MVC and twitch is >15% |
↓ contractile activity and transmission or central activation failure |
| Critical power | Assesses the ability to sustain exercise under anaerobic conditions |
Series of short-duration, high-intensity exercises determines critical power (fatigue threshold) |
↓ exercise tolerance, ↓ fatigue threshold |
| Borg scale or VAS | Assesses the perception of fatigue using scales |
Borg scale (0–10), VAS (0–100 mm) |
↑ scores for lower limb fatigue |
| 31P-MRS | Directly and noninvasively measures intramuscular metabolism |
Repetitive localized exercise of MMII, in the MRS system, assesses high-energy compounds |
↓ levels of high-energy phosphates at rest, during exercise and during recovery |
| Biopsy | Identifies the microstructural and bioenegery characteristics of the muscles |
Collection of vastuslateralis muscle samples |
↑ strength/frequency ratio, ↓ % of type I fibres, ↓ CSA fibres, ↓ capillary/fibre ratio; ↓ mitochondrial density |
| Determination of lactate and ammonia levels |
Assesses the inability to convert oxygen into energy in acid solutions |
Collection of venous, arterial or arterialized blood samples at rest, during exercise and during recovery |
↑ lactate and serum ammonia levels during and after exercise |
CSA, cross-sectional area; MMII, lower limb muscles; MRS, magnetic resonance spectroscopy; MVC, maximal voluntary contraction; P, phosphorus; PT, peak tension; SRM, square root of the mean; SVC, submaximal voluntary contraction
Discussion
The establishment of translational clinical pipelines for the prevention and treatment of sarcopenia requires a coherent, consensual approach to criteria for definition of the disorder, and pre-specified outcome measures for regulatory approval. For any given clinical research programme, it is worth considering whether the putative intervention is likely to change muscle mass as well as muscle strength. Muscle strength is variably included within many proposed academic definitions of sarcopenia: the European Working Group on Sarcopenia in Older People has suggested that the diagnosis be extended to include patients with normal gait speed, low muscle mass and low muscle strength as well as those with low gait speed and low muscle mass (Table 1) [10]. The Special Interest Group on Nutrition in Geriatrics of ESPEN has taken a similar approach, including impaired muscle function (as indicated by a 4-metre gait speed <1 metres/second) alongside the traditional thresholds for muscle mass [12]. It is unlikely that regulatory authorities will regard improved functional parameters alone (for example habitual gait speed) as validated, clinically meaningful outcomes in trials of agents for sarcopenia.
Ultimately, the definition of sarcopenia for randomised clinical trials will need to be tailored to the precise nature of the intervention. There are several examples of pharmacological interventions which increase muscle mass, but do not influence muscle strength. It is also clear that muscle mass and muscle strength predict longer-term outcomes of clinical importance such as functional impairment, likelihood of frailty, and in some studies, even mortality. However, there are clearly difficulties in the adoption of any one of these harder outcomes as uniform single measures of the effectiveness of a treatment aimed at sarcopenia.
Based on current knowledge, co-primary outcomes might be the best way forward in this difficult but clinically important area. For agents which are known to influence muscle mass, and for whom phase I and II studies demonstrate commensurate improvements in muscle strength, muscle mass may provide an option for a valid and repeatable co-primary outcome. In such circumstances, muscle strength and physical function (SPPB) could be used as secondary outcome measures or could be combined into a co-primary outcome package. In other circumstances, where questions still remain about the translation from muscle mass to muscle strength, it seems more prudent to reserve muscle strength and physical function as the best co-primary outcome measures. The consequence of this second approach, however, will require the methodology of muscle strength assessments to be clearly defined, both anatomically and physiologically, as well as accepting the exclusion of substantial proportions of older people who have comorbidities such as osteoarthritis, which confound the assessment methodology current available.
Whichever strategy is adopted, flexibility of approach will be essential, according to the pharmacological and biological characteristics of the intervention being evaluated. Regardless of the choice of outcome measure for clinical trials of sarcopenia, highly accurate and precise methodology is available for the assessment of muscle mass and this may serve as a key defining characteristic of sarcopenia in clinical practice, irrespective of the choice of outcome measure adopted in clinical trials.
Acknowledgements
This review was based on a workshop supported by the European Society for the Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the International Osteoporosis Foundation (IOF).
Footnotes
Disclosure of financial interests
WD is an employee and stockholder of Amgen, Inc; WE is an employee of GlaxoSmithKline; BM is an employee and shareholder of Eli Lilly and Company; YT is an employee of Servier. YR has spoken for and prepared an education module with Nutricia and is on the expert board of Cheisi and Lactalis. SB is senior clinical investigator of the Fund for Scientific Research, Flanders, Belgium and holder of the Leuven University Chair in Gerontology and Geriatrics.
Contributor Information
Professor C Cooper, MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton General Hospital, Southampton SO16 6YD, UK.
Professor R Fielding, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA 02111-1524, USA. Tel: +1 (617) 556-3016; Fax: +1 (617) 556-3083; roger.fielding@tufts.edu.
Professor M Visser, Department of Health Sciences, VU University Amsterdam and EMGO+ Institute, VU University Medical Center, Amsterdam, The Netherlands. m.visser@vu.nl.
Professor LJ van Loon, Department of Human Movement Sciences, Maastricht University Medical Centre, PO Box 616, 6200 MD Maastricht, The Netherlands. Tel: +31 43 388 1397; Fax: +31 43 367 0976. L.vanLoon@maastrichtuniversity.nl.
Professor Y Rolland, Gérontopôle de Toulouse, Department of Geriatric Medicine, Toulouse University Hospital, Toulouse, France; INSERM Unit 1027. rolland.y@chu-toulouse.fr.
Professor E Orwoll, Department of Medicine, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd, Portland, Oregon 97239-3098, USA. orwoll@ohsu.edu.
Dr K Reid, Nutrition, Exercise Physiology and Sarcopenia Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA.Tel: +1 (617) 556-3016; Fax: +1 (617) 556-3083, Kieran.Reid@tufts.edu.
Professor S Boonen, Division of Gerontology and Geriatrics & Center for Musculoskeletal Research, Leuven University Department of Experimental Medicine; Division of Geriatric Medicine & Center for Metabolic Bone Diseases, Leuven University Hospital Department of Internal Medicine, Leuven, Belgium. Tel: +32(0)1634 2648. steven.boonen@uz.kuleuven.be.
Dr W Dere, Amgen Ltd, Uxbridge, UK. wdere@amgen.com.
Professor S Epstein, Division of Endocrinology, Albert Einstein Medical Center, York and Tabor Roads, Philadelphia, Pennsylvania 19141, USA. bonedocsol@aol.com.
Dr B Mitlak, Lilly Research Laboratories, Eli Lilly & Co, Indianapolis, Indiana, USA. mitlak_bruce_h@lilly.com.
Dr Y Tsouderos, Institut de Recherches Internationales Servier, 50 rue Carnot, 92284 Suresnes Cedex, France. yannis.tsouderos@fr.netgrs.com.
Professor AA Sayer, MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton General Hospital, Southampton SO16 6YD, UK. aas@mrc.soton.ac.uk.
Professor R Rizzoli, Division of Bone Diseases, Geneva University Hospitals, Geneva, Switzerland. Tel: +41 (0) 22 372 99 50; Fax +41 (0) 22 382 99 73. rene.rizzoli@unige.ch.
Professor JY Reginster, Bone and Cartilage Metabolism Unit, CHU Centre-Ville, Liège, Belgium. jyreginster@ulg.ac.be.
Professor JA Kanis, WHO Collaborating Centre for Metabolic Bone Diseases, University of Sheffield Medical School, Beech Hill Road, Sheffield S10 2RX, UK; Tel: +44 114 285 1109; Fax: +44 114 285 1813. w.j.Pontefract@sheffield.ac.uk.
References
- 1.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-year longitudinal study. J Appl Physiol. 2000;88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 2.Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastuslateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 3.Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, et al. Health ABC Study Investigators Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 4.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 5.Rolland YM, Perry HM, 3rd, Patrick P, Banks WA, Morley JE. Loss of appendicular muscle mass and loss of muscle strength in young postmenopausal women. J Gerontol A BiolSci Med Sci. 2007;62:330–335. doi: 10.1093/gerona/62.3.330. [DOI] [PubMed] [Google Scholar]
- 6.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. International Working Group on Sarcopenia Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Consensus Development Conference Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organisation . Guidelines for preclinical evaluation and clinical trials in osteoporosis. WHO; Geneva: 1998. [Google Scholar]
- 9.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 10.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–409. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Studenski S. Evidence based criteria for sarcopenia with clinically important weakness. Sem Arthritis Rheum. 2012 doi: 10.1016/j.semarthrit.2012.07.007. e-pub Sep 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67:28–40. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper R, Kuh D, Hardy R, Mortality Review Group; FALCon and HALCyon Study Teams Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, Wolfson C. Frailty: an emerging research and clinical paradigm – issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institute on Aging [Accessed 21 February 2012];Assessing Physical Performance in the Older Patient. 2012 www.grc.nia.nih.gov/branches/ledb/sppb.
- 19.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 20.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LIFE Study Investigators Effects of a physical activity intervention on measures of physical performance: results of the Lifestyle Interventions and Independence for Elder Pilot (LIFE-P) Study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 23.Vasunilashorn S, Coppin AK, Patel KV, Lauretani F, Ferrucci L, Bandinelli S, Guralnik JM. Use of the short physical performance battery score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J Gerontol A BiolSci Med Sci. 2009;4A:223–229. doi: 10.1093/gerona/gln022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeBrasseur NK, Walsh K, Arany Z. Metabolic benefits of resistance training and fast glycolytic skeletal muscle. Am J Physiol Endocrinol Metab. 2011;300:E3–10. doi: 10.1152/ajpendo.00512.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wittert GA, Chapman IM, Haren MT, Mackintosh S, Coates P, Morley JE. Oral testosterone supplementation increases muscle and decreases fat mass in health elderly males with low-normal gonadal status. J Gerontol A BiolSci Med Sci. 2003;58:618–625. doi: 10.1093/gerona/58.7.m618. [DOI] [PubMed] [Google Scholar]
- 26.Haguenauer D, Welch V, Shea B, Tugwell P, Wells G. Fluoride for treating postmenopausal osteoporosis. Cochrane Database Syst Rev. 2000;(4):CD002825. doi: 10.1002/14651858.CD002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verdijk LB, Snijders T, Beelen M, Savelberg HH, Meijer K, Kuipers H, Van Loon LJ. Characteristics of muscle fiber type are predictive of skeletal muscle mass and strength in elderly men. J Am Geriatr Soc. 2010;58:2069–2075. doi: 10.1111/j.1532-5415.2010.03150.x. [DOI] [PubMed] [Google Scholar]
- 28.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 29.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the Health, Aging and Body Composition Study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 30.Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40:4–12. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reid KF, Callahan DM, Carabello RJ, Phillips EM, Frontera WR, Fielding RA. Lower extremity power training in elderly subjects with mobility limitations: a randomized controlled trial. Aging Clin Exp Res. 2008;20:337–343. doi: 10.1007/bf03324865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vøllestad NK. Measurement of human muscle fatigue. J Neurosci Methods. 1997;74:219–227. doi: 10.1016/s0165-0270(97)02251-6. [DOI] [PubMed] [Google Scholar]
- 33.Woodrow G. Body composition analysis techniques in the aged adult: indications and limitations. Curr Opin Clin Nutr Metab Care. 2009;12:8–14. doi: 10.1097/MCO.0b013e32831b9c5b. [DOI] [PubMed] [Google Scholar]
- 34.Lustgarten MS, Fielding RA. Assessment of analytical methods used to measure changes in body composition in the elderly and recommendations for their use in phase II clinical trials. J Nutr Health Aging. 2011;15:368–375. doi: 10.1007/s12603-011-0049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol. 2000;89:465–471. doi: 10.1152/jappl.2000.89.2.465. [DOI] [PubMed] [Google Scholar]
- 36.Kyle UG, Genton L, Hans D, Pichard C. Validation of a bioelectrical impedance analysis equation to predict appendicular skeletal muscle mass (ASMM) Clin Nutr. 2003;22:537–543. doi: 10.1016/s0261-5614(03)00048-7. [DOI] [PubMed] [Google Scholar]
- 37.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr. 2004;23:1226–1243. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Fields DA, Goran MI, McCrory MA. Body-composition assessment via air-displacement plethysmography in adults and children: a review. Am J Clin Nutr. 2002;75:453–467. doi: 10.1093/ajcn/75.3.453. [DOI] [PubMed] [Google Scholar]
- 39.Plank LD. Dual-energy X-ray absorptiometry and body composition. Curr Opin Clin Nutr Metab Care. 2005;8:305–309. doi: 10.1097/01.mco.0000165010.31826.3d. [DOI] [PubMed] [Google Scholar]
- 40.Hansen RD, Williamson DA, Finnegan TP, Lloyd BD, Grady JN, Diamond TH, et al. Estimation of thigh muscle cross-sectional area by dual-energy X-ray absorptiometry in frail elderly patients. Am J Clin Nutr. 2007;86:952–958. doi: 10.1093/ajcn/86.4.952. [DOI] [PubMed] [Google Scholar]
- 41.Marin D, Nelson RC, Rubin GD, Schindera ST. Body CT: technical advances for improving safety. AJR Am J Roentgenol. 2011;197:33–41. doi: 10.2214/AJR.11.6755. [DOI] [PubMed] [Google Scholar]
- 42.Chen Z, Wang Z, Lohman T, Heymsfield SB, Outwater E, Nicholas JS, et al. Dual-energy X-ray absorptiometry is a valid tool for assessing skeletal muscle mass in older women. J Nutr. 2007;137:2775–2780. doi: 10.1093/jn/137.12.2775. [DOI] [PubMed] [Google Scholar]
- 43.Delmonico MJ, Kostek MC, Johns J, Hurley BF, Conway JM. Can dual energy X-ray absorptiometry provide a valid assessment of changes in thigh muscle mass with strength training in older adults? Eur J Clin Nutr. 2008;62:1372–1378. doi: 10.1038/sj.ejcn.1602880. [DOI] [PubMed] [Google Scholar]
- 44.Verdijk LB, van Loon L, Meijer K, Savelberg HH. One-repetition maximum strength test represents a valid means to assess leg strength in vivo in humans. J Sports Sci. 2009;27:59–68. doi: 10.1080/02640410802428089. [DOI] [PubMed] [Google Scholar]
- 45.Mayhew JL, Prinster JL, Ware JS, Zimmer DL, Arabas JR, Bemben MG. Muscular endurance repetitions to predict bench press strength in men of different training levels. J Sports Med Phys Fitness. 1995;35:108–113. [PubMed] [Google Scholar]
- 46.Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther. 2008;31:3–10. doi: 10.1519/00139143-200831010-00002. [DOI] [PubMed] [Google Scholar]
- 47.Rantanen T. Muscle strength, disability and mortality. Scand J Med Sci Sports. 2003;13:3–8. doi: 10.1034/j.1600-0838.2003.00298.x. [DOI] [PubMed] [Google Scholar]
- 48.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 49.Aadahl M, Beyer N, Linneberg A, Thuesen BH, Jørgensen T. Grip strength and lower limb extension power in 19-72-year-old Danish men and women: the Health2006 study. BMJ Open. 2011;1:e000192. doi: 10.1136/bmjopen-2011-000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Desrosiers J, Hébert R, Bravo G, Dutil E. Comparison of the Jamar dynamometer and the Martin vigorimeter for grip strength measurements in a healthy elderly population. Scand J Rehabil Med. 1995;27:137–143. [PubMed] [Google Scholar]
- 51.Bean JF, Kiely DK, Herman S, Leveille SG, Mizer K, Frontera WR, Fielding RA. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50:461–467. doi: 10.1046/j.1532-5415.2002.50111.x. [DOI] [PubMed] [Google Scholar]
- 52.Foldvari M, Clark M, Laviolette LC, Bernstein MA, Kaliton D, Castaneda C, Pu CT, Hausdorff JM, Fielding RA, Singh MA. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2000;55:M192–199. doi: 10.1093/gerona/55.4.m192. [DOI] [PubMed] [Google Scholar]
- 53.Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA. Muscle performance and physical function are associated with voluntary rate of neuromuscular activation in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:115–121. doi: 10.1093/gerona/glq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reeves ND, Narici MV, Maganaris CN. Myotendinous plasticity to ageing and resistance exercise in humans. Exp Physiol. 2006;91:483–498. doi: 10.1113/expphysiol.2005.032896. [DOI] [PubMed] [Google Scholar]
- 55.Roos MR, Rice CL, Connelly DM, Vandervoort AA. Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve. 1999;22:1094–1103. doi: 10.1002/(sici)1097-4598(199908)22:8<1094::aid-mus14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 56.Smith WN, Del Rossi G, Adams JB, Abderlarahman KZ, Asfour SA, Roos BA, Signorile JF. Simple equations to predict concentric lower-body muscle power in older adults using the 30-second chair-rise test: a pilot study. Clin Interv Aging. 2010;5:173–180. doi: 10.2147/cia.s7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 58.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rondelli RR, Dal Corso S, Simões A, Malaguit C. Methods for the assessment of peripheral muscle fatigue and its energy and metabolic determinants in COPD. J Bras Pneumol. 2009;35:1125–1135. doi: 10.1590/s1806-37132009001100011. [DOI] [PubMed] [Google Scholar]