Abstract
It is well documented that the quality and quantity of prior sleep influences future sleep. For instance, nocturnal sleep restriction leads to an increase in slow wave sleep (SWS) (i.e. SWS rebound) during a subsequent sleep period. However, few studies have examined how prior napping affects daytime sleep architecture. Becuase daytime naps are recommended for management of disrupted sleep, understanding the impact of napping on subsequent sleep may be important. We monitored sleep-wake patterns for one week with actigraphy followed by a 75-minute polysomnographically-recorded nap. We found that greater nap frequency was correlated with increased Stage 1 and decreased SWS. We categorized subjects based on nap frequency during the prior week (0 naps, 1 to 2 naps, and 3 to 4 naps) and found differences in Stage 1, Stage 2, and SWS between groups. Subjects who took no naps had the greatest amount of SWS, those who took 1 to 2 naps had the most Stage 2 sleep, and those who took 3 to 4 naps had the most Stage 1. While correlations were not found between nap frequency and nocturnal sleep measures, frequent napping was associated with increased subjective sleepiness. Therefore, frequent napping appears to be associated with lighter daytime sleep and increased sleepiness during the day. Speculatively, low levels of daytime sleepiness and increased SWS in non-nappers may help explain why these individuals choose not to nap.
Keywords: Sleep architecture, Napping, Slow Wave Sleep, Sleepiness
1. Introduction
The relationship between prior sleep and sleep architecture is well studied and has important implications for nocturnal sleep. The two-process model of sleep regulation examines this relationship in the interaction between circadian and homeostatic processes [1]. The circadian Process (C) is an endogenous, clocklike mechanism whereas the homeostatic Process (S) is a function of waking and prior sleep. The time course of S is derived from EEG slow-wave activity (SWA, spectral power in the 0.75-4.5 Hz range), which increases during waking and dissipates during sleep [1-3]. For example, following repeated partial sleep deprivation, SWA in non-REM sleep increased by approximately 20% and decreased immediately after one recovery night [4]. Additionally, SWA during nighttime sleep is diminished following an early evening nap rich in slow wave sleep (SWS) [5]. Given the close relationship between prior sleep-wake activity and nocturnal sleep architecture, it is logical that this relationship would extend to daytime sleep. Indeed, one study examined naps after varied amounts of prior wakefulness, and found that power spectra in the theta (4-7 Hz) and delta (1-4 Hz) frequencies increased as a function of the duration of prior wakefulness [6].
Prior studies examining the effects of habitual napping on both daytime and nighttime sleep provide evidence that frequent napping alters sleep architecture. However, these studies used short naps (20-40 minutes), which curtailed the ability to determine the effect of prior napping on SWS and rapid eye movement (REM) sleep. Longer sleep periods (60-90 minutes) are required to complete a full sleep cycle. Also, the definition of what constitutes a habitual napper is inconsistent across studies and difficult to classify over a short period of time (please see [7] for a review of habitual napper definitions). No studies have used an objective measurement (i.e. actigraphy) of nap behavior, and only one study used naps subjectively reported in daily diaries to categorize habitual and non-habitual nappers [8]. Evans and colleagues found that appetitive nappers, those who reported napping for pleasure at least once a week, had more Stage 1 and more stage changes fluctuating through light sleep stages during a 40 minute nap compared with replacement and non-habitual nappers [9]. Milner and colleagues defined a habitual napper as someone who reported napping “once or twice a week” or “every day” on a napping behavior survey [10]. In their study, habitual nappers displayed some differences in sleep architecture during a short, 20-min nap, specifically higher theta, alpha, beta, and delta power, and reported being more refreshed post-nap than non-habitual nappers. No sleep stage differences were found in this study, most likely due to using a brief, 20-minute nap duration. Additionally, Dinges found habitual nappers had more Stage 1 and more transitions between stages during a short daytime nap, suggesting a lighter nap that resulted in less sleep inertia upon waking compared to non-habitual nappers [11]. Johnston et al. defined habitual nappers based on nap behavior reported in daily diaries – middle-aged women who recorded at least one nap during a five-day study were considered habitual nappers [8]. Regarding nighttime sleep, they found no differences in nocturnal sleep architecture, mean sleep efficiency (SE), or total sleep time (TST) between habitual and non-habitual nappers. Nonetheless, these nappers reported more daytime sleepiness, suggesting that habitual nap behavior may be a reflection of greater daytime sleepiness. In summary, differences in nap sleep architecture appear to exist between nappers and non-nappers, where nappers remain in lighter sleep stages, compared with non-nappers.
The aim of the present study is to examine how the number of naps taken during a seven-day period affects the sleep architecture of a polysomnographically-recorded daytime nap. Using two measures of daytime sleep (i.e. actigraphy and sleep diaries), we correlated nap frequency and minutes of napping with sleep architecture. We predicted a positive, linear relationship between nap frequency and lighter stages of sleep, such that people who napped more often had more Stage 1 and Stage 2 sleep and less SWS during a daytime nap. We examined nocturnal sleep from a week prior to the experimental nap, to examine how nap frequency affects subsequent nocturnal sleep and how nocturnal sleep affects subsequent daytime nap architecture in our sample. Following from Johnston et al. [8], we predicted greater nap frequency would be associated with increased daytime sleepiness, but not with nocturnal sleep variables.
2. Methods
2.1 Participants
A total of 27 healthy, non-smoking university students between the ages of 18 and 35 gave informed consent to participate in the experiment, which was approved by the University of California, San Diego Human Research Protections Program. Exclusionary criteria included: a) not having a regular sleep-wake schedule (defined as habitually spending 7-9 hrs of time in bed (TIB) per night); b) having a sleep disorder. Sleep disorders were screened by interviewing the subject at the first meeting and asking about potential symptoms of insomnia, apnea, narcolepsy, restless leg syndrome/periodic leg movements;c) any personal or immediate family (i.e., first degree relative) history of diagnosed significant psychopathology; d) personal history of head injury with loss of consciousness greater than 2 minutes or seizures; e) history of substance dependence; f) current use of any psychotropic medications; g) any cardiac, respiratory or other medical condition which may affect metabolism. Subjects received an orientation to the study a week prior to the study day. At this time, they completed the Epworth Sleepiness Scale (ESS) and Horne-Ostberg Morningness-Eveningness Questionnaire (MEQ). The ESS assesses trait daytime sleepiness [12]. The MEQ evaluates circadian phase preference for morningness or eveningness [13]. Subjects were asked to refrain from caffeine, alcohol, and all stimulants for 24hrs prior to and including the study day. Heavy caffeine users (greater than 3 servings/day) were not enrolled to minimize the chance of significant withdrawal symptoms. Average caffeine consumption by our subjects was 0.5 servings of coffee or tea per day.
2.2 Actigraphy and Prior Sleep
Subjects were asked to maintain their usual sleep-wake schedule (7-9 hrs TIB/night) the week prior to the study. There was no suggestion that the subjects should avoid or engage in napping. Subjects filled out sleep diaries and wore actigraph wristwatches (Actiwatch-64, Respironics) as subjective and objective measures of sleep-wake activity, respectively. All of the naps self-reported in sleep diaries were verified by actigraphy, which has been validated to reliably predict TST, SE, and sleep latency (SL) of daytime naps [14]. Actigraphy data were scored using Respironics Actiware version 5.52.003 software. Rest intervals were manually created based on event markers and sleep diaries. Sleep intervals were determined by the software’s “immobile minutes sleep interval” detection algorithm. Prior sleep variables examined from these actigraphy data were Bedtime, Wake Time, TST, sleep latency (SL), wake after sleep onset (WASO), and SE for both nocturnal sleep and naps.
2.3 Protocol
On the experimental day, subjects reported to the Laboratory for Sleep and Behavioral Neuroscience at the San Diego Veterans Affairs Medical Center at 09:00. Subjective sleepiness was measured with the Karolinska Sleepiness Scale (KSS) at 09:00 (KSS1), 11:00 (KSS2), 16:30 (KSS3), and 18:30 (KSS4). The KSS assesses subjects’ momentary state of alertness/sleepiness [15]. At 12:30, electrodes were applied for standard polysomnographic recording. Subjects were in bed by 13:30 and allowed to nap for a maximum of 90 minutes, but given no more than 120 minutes in bed. The mean nap sleep time was 74 minutes and the range of nap total sleep time (TSTnap) was 38 to 90 minutes (Table 1). All subjects napped at the same time of day to control for circadian influences on sleep architecture.
Table 1.
PSG-recorded experimental naps and correlations with nap frequency
| Minutes | r | p | Percent | r | p | |
|---|---|---|---|---|---|---|
| TST | 74.2 (11.3) | 0.064 | 0.75 | - | - | - |
| Stage 1 | 4.6 (5.5) | 0.464* | 0.015 | 6.1 (7.2) | 0.449* | 0.019 |
| Stage 2 | 31.7 (8.3) | 0.210 | 0.29 | 43.2 (11.0) | 0.185 | 0.36 |
| SWS | 25.9 (13.2) | −0.448* | 0.019 | 34.9 (16.3) | −0.491** | 0.009 |
| REM | 12.0 (10.7) | 0.221 | 0.29 | 15.8 (13.8) | 0.199 | 0.32 |
|
Sleep Efficiency |
- | - | - | 81.8 (8.8) | −0.014 | 0.95 |
Displays are means (standard deviations). Statistics report Pearson’s r and p-values for correlations between number of prior naps and experimental nap sleep variables. TST, total sleep time; SWS, slow wave sleep; REM, rapid eye movement.
indicates p<.05
indicates p<.01
2.4 Polysomnography
All PSG data were collected using Astro-Med Grass Heritage Model 15 amplifiers with Grass Gamma software. Scalp EEG and EOG electrodes were referenced to unlinked opposite mastoids (C3/A2, O1/A2, C4/A1, O2/A1, LOC/A2 and ROC/A1) and submental muscle tone EMGs were attached under the chin. The high pass filters were set at 0.3Hz, and the low pass filters at 100Hz for all EEGs and EOGs. A 60Hz notch filter was also utilized to reduce potential background noise. At the beginning of each recording, an internal 50μV calibration signal was generated followed by impedance checks and biocalibrations. EEG data were digitized at a sampling rate of 256 Hz and visually scored in 30-second epochs according to Rechtschaffen and Kales sleep staging criteria [16]. The variables examined from these PSG data were: minutes and percentage of Stage 1nap, Stage 2nap, SWSnap, and REMnap; also, TSTnap and SEnap.
2.5 Statistical Analyses
Pearson correlations were used to examine the relationship between the number (self-reported in diaries) and minutes (from actigraphy) of naps taken the prior week and minutes and percent of sleep stages during the experimental nap. We also correlated the number and minutes of naps with actigraphic nocturnal sleep variables averaged across the week prior to see if daytime napping influences nocturnal sleep. To examine the relationship between nap frequency, chronotype, and daytime sleepiness, we correlated the number of prior naps with MEQ, ESS and each occasion of KSS scores. We also conducted one-way analysis of variance (ANOVA) of each aforementioned dependent variable comparing groups based on the number of prior naps reported in sleep diaries: 0 naps (n=9), 1 to 2 naps (n=11), and 3 to 4 naps (n=7), with post-hoc t-tests to look at specific group differences.
3. Results
3.1 Experimental nap (Table 1)
We correlated the number of naps taken the prior week (reported in sleep diaries) and minutes and percentage of sleep stages in the experimental nap. We found that number of naps was positively correlated with minutes and percentage of Stage 1nap (minutes: r = 0.464, p = 0.015; percent: r = 0.449, p = 0.019) and negatively correlated with minutes and percentage of SWSnap (minutes: r= −0.448, p= 0.019; percent: r= −0.491, p= 0.009) in the experimental nap (Figure 1). Number of naps was not significantly correlated with minutes or percentage of Stage 2nap, REMnap, TSTnap, or SEnap. Total minutes of prior naps (calculated from actigraphy) were not correlated with any experimental nap sleep variable.
Figure 1.
Correlation between the number of naps taken during one week and sleep architecture. There were significant correlations between number of naps and percentage of Stage 1nap (r = 0.449, p = 0.019) and SWSnap (r=-0.491, p=0.009) in the PSG-recorded nap.
One-way ANOVA with nap frequency groups as the independent variable (0 naps, 1-2 naps, 3-4 naps) found significant differences in minutes of Stage 1nap (F2,24 = 3.77, p = 0.04, partial eta2 = 0.24) and marginal differences in percentage of Stage 1nap (F2,24= 3.28, p = 0.06, partial eta2 = 0.22). Subjects who took 3-4 naps the week prior had more Stage 1nap (minutes: t(14)= 2.48, p = 0.03, r = 0.55; percent: t(14)= 2.28, p = 0.04, r = 0.52) than those who took 0 naps. There were differences in percentage of Stage 2nap (F2,24 = 5.65, p = 0.01, partial eta2 = 0.32), and marginal differences in minutes of Stage 2nap (F2,24 = 3.04, p =0.07, partial eta2 = 0.20). Subjects who took 1-2 naps the week prior had a greater percentage of Stage 2nap than those who took 0 naps (t(18)= 3.10, p =0.006, r = 0.59) or 3-4 naps (t(16)= 2.10, p = 0.05, r = 0.46). Additionally, there were significant differences in minutes and percentage of SWSnap (minutes: F2,24 = 3.74, p = 0.04, partial eta2 = 0.24; percent: F 2 2, 24 = 4.77, p=0.02, partial eta =0.29), such that subjects who took 0 naps the week prior had significantly more SWSnap than those who took 1-2 naps (minutes: t(18)= 2.23, p = 0.04, r = 0.47; percent: t(18)= 2.35, p = 0.03, r = 0.48) or 3-4 naps (minutes: t(14)= 2.33, p = 0.04, r = 0.53; percent: t(14)= 2.65, p = 0.02, r = 0.58). Like the overall sample, there were no group differences in minutes or percentage of REMnap, TSTnap, or SEnap based on nap frequency. These results are summarized in Figure 2. 3.2 Prior Sleep (Table 2)
Figure 2.
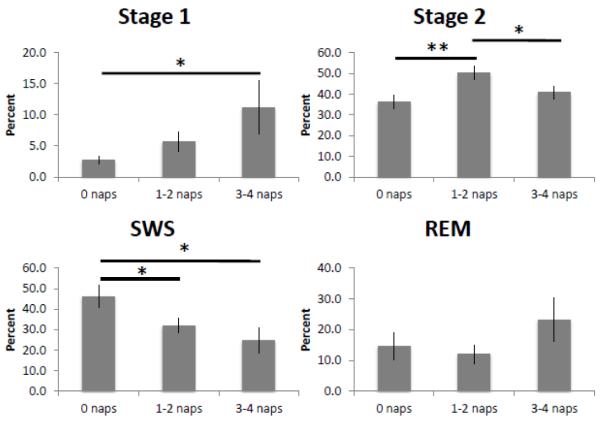
Experimental naps from polysomnography. Percentage of each sleep stage during a PSG-recorded daytime nap based on nap frequency. * indicates p<0.05, ** indicates p<0.01
Table 2.
Relationship between nap frequency and nocturnal sleep
| Mean (SD) | rnum | pnum | rmin | pnum | |
|---|---|---|---|---|---|
| Bedtime | 01:14 (0:55) | 0.289 | 0.16 | 0.294 | 0.27 |
| Wake Time | 08:44 (0:53) | 0.221 | 0.29 | 0.289 | 0.28 |
| TST (min) | 366.6 (40.0) | −0.088 | 0.68 | −0.071 | 0.79 |
| Sleep Latency (min) | 14.6 (7.6) | −0.036 | 0.86 | 0.175 | 0.52 |
| WASO (min) | 64.7 (19.0) | 0.223 | 0.29 | 0.044 | 0.87 |
| Sleep Efficiency (min) | 83.2 (4.19) | −0.237 | 0.25 | −0.053 | 0.84 |
Statistics report Pearson’s r and p-values for correlations between number of prior naps (rnum, pnum), total minutes of prior naps (rmin, pmin) and nocturnal sleep variables. TST, total sleep time; WASO, wake after sleep onset.
We examined naps reported in sleep diaries and recorded by actigraphy. Eighteen participants took at least one nap the week prior (range: 1-4 naps). On average, these naps occurred between 17:02 (±2:08) and 18:10 (±2:07), with an average TST of 56.6 (±22.7) minutes. There were no differences in nap duration or timing between participants who took 1 - 2 naps and those who took 3 - 4 naps during the week.
Although naps are anecdotally asserted to affect nocturnal sleep, nap frequency did not interfere with nocturnal sleep, nor did prior nocturnal sleep affect nap sleep architecture. We correlated the number and minutes of naps during the week with nocturnal sleep variables, however no significant relationships were found. One-way ANOVA did not find any differences in nocturnal sleep based on nap frequency groupings (0 naps, 1-2 naps, 3-4 naps). Additionally, there were no correlations between nocturnal sleep variables and minutes or percentage of sleep stages in the experimental nap.
3.3 Morningness-Eveningness and Subjective Sleepiness (Table 3)
Table 3.
Nap frequency correlations with chronotype and subjective sleepiness
| Scores | Mean (SD) | r | p |
|---|---|---|---|
| MEQ | 44.04 (7.22) | −0.094 | 0.64 |
| ESS | 8.96 (2.97) | 0.425* | 0.027 |
| KSS1 (9:00) | 3.96 (1.34) | −0.048 | 0.81 |
| KSS2 (11:00) | 4.67 (1.59) | 0.366 | 0.060 |
| KSS3 (16:30) | 2.96 (1.45) | 0.339 | 0.084 |
| KSS4 (18:30) | 3.63 (1.31) | 0.418* | 0.030 |
Statistics report Pearson’s r and p-values for correlations between number of prior naps and scores. MEQ, Morningness-Eveningness Questionnaire; ESS, Epworth Sleepiness Scale; KSS, Karolinska Sleepiness Scale.
indicates p<.05
There was no correlation between prior napping and chronotype as measured by the MEQ. We also examined trait (ESS) and state (KSS) measures of subjective sleepiness. Number of naps was correlated with ESS scores (r =0.425, p = 0.02), such that an increase in nap frequency was associated with greater trait sleepiness. We examined KSS scores at four time points across the day. The experimental nap alleviated sleepiness across all subjects, indicated by significantly lower scores at 14:30 (KSS3, t(26) = 5.91, p < 0.001, r = 0.76) and 16:30 (KSS4, t(26) = 4.10, p < 0.001, r = 0.63) compared to pre-nap, KSS2 at 11:00. Number of naps was correlated with increased sleepiness at 11:00 (KSS2, r = 0.36, p=0.06), and 18:30 (KSS4, r = 0.41, p = 0.03). One-way ANOVA found group differences on the ESS (F2,24 = 3.49, p = 0.05, partial eta2 = 0.23) and KSS2 (pre-nap) (F2,24 = 4.24, p = 0.03, partial eta2 = 0.26) based on nap frequency. Participants who took 3-4 naps reported more sleepiness on the ESS than those who took 0 naps (t(14)= 2.67, p = 0.02, r = 0.58). Similarly, participants who napped during the week reported greater state sleepiness prior to the experimental nap compared to those who did not nap (KSS2, 1-2 naps: t(18)= 2.61, p = 0.02, r = 0.52; 3-4 naps: t(14)= 2.39, p = 0.03, r = 0.54).
4. Discussion
Our results show that frequent napping is associated with systematic differences in daytime sleep architecture in our university student population. Specifically, the more individuals nap during a week, the greater the amount of Stage 1 and Stage 2 sleep and the less SWS in their naps. Napping did not have a gross effect on nocturnal sleep (as measured by actigraphy), and conversely, nocturnal sleep did not affect nap sleep architecture. However, nap frequency was positively correlated with subjective sleepiness, such that participants who took more naps during one week reported higher levels of trait and state sleepiness.
The current findings avoid categorical definitions of habitual napping and demonstrate a dose-dependent effect of naps on sleep architecture. Consistent with the two-process model of sleep regulation in which the amount of SWS during a sleep episode is a function of prior sleep and wake [1], these results suggest a relationship between prior napping and amount of SWS during a daytime nap. Similar to studies showing that a nap will decrease the amount of SWA in subsequent nocturnal sleep [5], here napping across one week decreased the amount of SWS in a subsequent nap. Although prior nocturnal sleep can affect the architecture of daytime sleep, nocturnal sleep variables were not associated with any daytime nap architecture differences, isolating nap frequency as the prior sleep variable associated with this effect.
Secondary analyses used nap frequency bins to examine the data in a categorical fashion, similar to prior studies, while still retaining information about how many naps were actually taken. The group analyses show the same effects for Stage 1 and SWS as the correlational analyses. The correlational approach did not find a significant relationship between nap frequency and Stage 2 sleep. However, as seen in Figure 2, this is because there is a quadratic relationship between nap frequency and Stage 2 sleep, where subjects in the 1-2 nap frequency bin had significantly more Stage 2 sleep than subjects who took 3-4 naps or subjects who took zero naps. Within NREM sleep, these data show a progression from deep sleep to lighter and lighter stages of sleep based on nap frequency – people who did not nap the week prior had the highest amount of SWS, people who took 1-2 naps had the most Stage 2, and people who napped the most (3-4 naps) had the most Stage 1 sleep.
We further examined the influence of nap frequency by determining its effect on nocturnal sleep, chronotype, and subjective sleepiness. Consistent with several studies showing that daytime napping does not have a detrimental effect on nocturnal sleep [17, 18], we found no correlation between the number or duration of naps and nocturnal sleep variables collected from actigraphy. Additionally, there were no group differences in nocturnal sleep based on nap frequency. One limitation of this study is that we do not have PSG recordings from nighttime sleep, and cannot consider the effect of nap frequency on specific sleep stages. It is possible that napping could decrease SWA and show a compensatory increase in stage 2 sleep without showing differences in total sleep time [5]. Further studies are required to specifically answer this question. Additionally, although prior studies have associated napping with evening circadian preference [19, 20], our subjects showed no morning or evening-type tendencies based on prior napping. Similar to Johnston et al., nap frequency was associated with increased subjective sleepiness, both trait (ESS) and state (KSS2, KSS4) measures. One way to interpret the fluctuations in state sleepiness across the day is to consider the temporal similarities between KSS1 and KSS3, as well as between KSS2 and KSS4. KSS1 and KSS3 were both taken about 1.5 hours after waking from nighttime and daytime sleep, respectively. KSS2 and KSS4, which were positively correlated with nap frequency, were both taken about 3 hours after waking from these respective sleep periods. These results suggest that people who nap frequently have higher levels of sleepiness during the day, and may be using naps to alleviate this sleepiness.
Why did increased sleepiness in individuals who napped more frequently not result in increased SWS during the experimental nap? One possible explanation is that sleepiness and sleep pressure follow different regulatory mechanisms. Cajochen and colleagues examined frontal low EEG activity (1-7 Hz), core body temperature (CBT), and KSS scores as measures of sleep pressure, circadian rhythm, and subjective sleepiness, respectively [21]. They found that frontal low EEG, measuring sleep pressure, was regulated by sleep-wake-dependent homeostasis. In contrast, modulation of subjective sleepiness levels was strongly circadian, as measured by CBT, even under very low homeostatic pressure. In other words, sleep pressure and subjective sleepiness appear to be dissociated, whereby subjective sleepiness follows circadian fluctuations and sleep pressure is a reflection of the homeostatic process. Together with these results, the increased sleepiness in habitual nappers, shown here and by others [8] may be related to a vulnerability to circadian fluctuations that is independent of homeostatic sleep pressure. Increased sleepiness would therefore result in increased napping, instead of increased SWS.
The effects of napping on daytime sleep architecture shown here may help us understand the development of napping behavior. Napping has been shown to be an effective tool for managing sleep deprivation and dysrhythmia brought about by chronic or acute circadian disruption [22]. The negative physical and psychological symptoms of disrupted sleep are improved by naps during the day or nightshifts [23-27]. Additionally, in healthy, well-rested subjects, napping has been shown to improve performance across a range of memory tasks [28-35]. Despite these benefits of napping, many people do not nap. Anecdotally, the three most-cited reasons non-nappers in our lab avoided daytime sleep include:1) they are not tired, 2) insufficient time, or 3) they wake up confused or groggy. Although this is a correlational study and any causal inference is a matter of speculation, the present results suggest two possible pathways that may drive nap behavior in individuals who choose to nap compared to those who do not. First, individuals may be more inclined to avoid napping due to high levels of SWS that produce unpleasant after-effects. Prior studies have established a link between awakening from SWS or total SWS minutes and the presence of these aversive symptoms, called sleep inertia [36]. Additionally, non-nappers also report that they are not tired and do not need to nap, which informs the second pathway. Individuals who choose to nap may generally be sleepier people as evinced by our correlations between nap frequency and subjective sleepiness. Hayashi and colleagues [37] examined short daytime naps that contained mostly Stage 1 and 2 sleep, and found that these sleep stages are responsible for the recuperative alerting effects of daytime naps. Just as napping is often prescribed as treatment for poor sleep in shift workers or people with narcolepsy [38], individuals who frequently nap may generally be sleepier people who are self-treating their sleepiness with daytime naps. Intriguingly, Milner and Cote [7] also suggest that individuals who nap regularly might be predisposed to be good daytime nappers, or they have learned to become skilled nappers through practice. Future studies should consider the possibility of nap practice or nap training to maximize the benefits of napping, and examine how systematic differences in sleep architecture associated with nap behavior may influence changes in performance following a nap.
Highlights.
-
➢
We monitored sleep-wake patterns for one week with actigraphy.
-
➢
Sleep stages were measured during a polysomnographically-recorded nap.
-
➢
Nap frequency correlated positively with minutes of Stage1 and negatively with SWS.
-
➢
Nap frequency was not associated with changes in nocturnal sleep.
-
➢
Nap frequency was correlated with increased subjective sleepiness.
Acknowledgements
We would like to acknowledge Jennifer Kanady, Denise Cai, and Elizabeth Harrison for study assistance. Research was supported by the UCSD GCRC NIH M01RR00827, NIH K01 MH080992.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- [2].Borbely AA, Achermann P. Concepts and models of sleep regulation: an overview. J Sleep Res. 1992;1:63–79. doi: 10.1111/j.1365-2869.1992.tb00013.x. [DOI] [PubMed] [Google Scholar]
- [3].Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythm. 1999;14:557–68. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- [4].Brunner DP, Dijk DJ, Borbely AA. Repeated partial sleep deprivation progressively changes in EEG during sleep and wakefulness. Sleep. 1993;16:100–13. doi: 10.1093/sleep/16.2.100. [DOI] [PubMed] [Google Scholar]
- [5].Werth E, Dijk DJ, Achermann P, Borbely AA. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am J Physiol. 1996;271:R501–10. doi: 10.1152/ajpregu.1996.271.3.R501. [DOI] [PubMed] [Google Scholar]
- [6].Dijk DJ, Beersma DG, Daan S. EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. J Biol Rhythm. 1987;2:207–19. doi: 10.1177/074873048700200304. [DOI] [PubMed] [Google Scholar]
- [7].Milner CE, Cote KA. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J Sleep Res. 2009;18:272–81. doi: 10.1111/j.1365-2869.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- [8].Johnston SK, Landis CA, Lentz MJ, Shaver JLF. Self-reported nap behaviour and polysomnography at home in midlife women with and without insomnia. Sleep. 2001;24:913–9. doi: 10.1093/sleep/24.8.913. [DOI] [PubMed] [Google Scholar]
- [9].Evans FJ, Cook MR, Cohen HD, Orne EC, Orne MT. Appetitive and replacement naps: EEG and behavior. Science. 1977;197:687–9. doi: 10.1126/science.17922. [DOI] [PubMed] [Google Scholar]
- [10].Milner CE, Fogel SM, Cote KA. Habitual napping moderates motor performance improvements following a short daytime nap. Biol Psychol. 2006;73:141–56. doi: 10.1016/j.biopsycho.2006.01.015. [DOI] [PubMed] [Google Scholar]
- [11].Dinges DF. Adult napping and its effects on ability to function. In: Stampi C, editor. Why we nap: Evolution, chronobiology, and functions of polyphasic and ultrashort sleep. Birkhauser; Boston: 1992. pp. 118–134. [Google Scholar]
- [12].Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- [13].Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- [14].Kanady JC, Drummond SPA, Mednick SC. Actigraphic assessment of a polysomnographic-recorded nap: a validation study. J Sleep Res. 2011;20:214–22. doi: 10.1111/j.1365-2869.2010.00858.x. [DOI] [PubMed] [Google Scholar]
- [15].Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- [16].Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Public Health Service Publications; Washington: 1968. [Google Scholar]
- [17].Campbell SS, Murphy PJ, Stauble TN. Effects of a nap on nighttime sleep and waking function in older subjects. J Am Geriatr Soc. 2005;53:48–53. doi: 10.1111/j.1532-5415.2005.53009.x. [DOI] [PubMed] [Google Scholar]
- [18].Pilcher JJ, Michalowski KR, Carrigan RD. The prevalence of daytime napping and its relationship to nighttime sleep. Behav Med. 2001;27:71–6. doi: 10.1080/08964280109595773. [DOI] [PubMed] [Google Scholar]
- [19].Vela-Bueno A, Fernandez-Mendoza J, Olavarrieta-Bernardino S, Vgontzas AN, Bixler EO, de la Cruz-Troca JJ, et al. Sleep and behavioral correlates of napping among young adults: a survey of first-year university students in Madrid, Spain. J Am Coll Health. 2008;57:150–8. doi: 10.3200/JACH.57.2.150-158. [DOI] [PubMed] [Google Scholar]
- [20].Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep, and daytime behaviour in adolescence. J Sleep Res. 2002;11:191–9. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- [21].Cajochen C, Knoblauch V, Krauchi K, Renz C, Wirz-Justice A. Dynamics of frontal EEG activity, sleepiness and body temperature under high and low sleep pressure. Neuroreport. 2001;12:2277–81. doi: 10.1097/00001756-200107200-00046. [DOI] [PubMed] [Google Scholar]
- [22].Costa G. The impact of shift and night work on health. Appl Ergon. 1996;27:9–16. doi: 10.1016/0003-6870(95)00047-x. [DOI] [PubMed] [Google Scholar]
- [23].Harma M, Knauth P, Ilmarinen J. Daytime napping and its effects on alertness and short-term memory performance in shiftworkers. Int Arch Occup Environ Health. 1989;61:341–5. doi: 10.1007/BF00409390. [DOI] [PubMed] [Google Scholar]
- [24].Macchi MM, Boulos Z, Ranney T, Simmons L, Campbell SS. Effects of an afternoon nap on nighttime alertness and performance in long-haul drivers. Accid Anal Pre. 2002;34:825–34. doi: 10.1016/s0001-4575(01)00089-6. [DOI] [PubMed] [Google Scholar]
- [25].Bonnefond A, Muzet A, Winter-Dill AS, Bailloeuil C, Bitouze F, Bonneau A. Innovative working schedule: introducing one short nap during the night shift. Ergonomics. 2001;44:937–45. doi: 10.1080/00140130110061138. [DOI] [PubMed] [Google Scholar]
- [26].Akerstedt T. Shiftwork and disturbed sleep/wakefulness. Sleep Med Rev. 1998;2:117–28. doi: 10.1016/s1087-0792(98)90004-1. [DOI] [PubMed] [Google Scholar]
- [27].Bonnet MH. The effect of varying prophylactic naps on performance, alertness and mood throughout a 52-hour continuous operation. Sleep. 1991;14:307–15. doi: 10.1093/sleep/14.4.307. [DOI] [PubMed] [Google Scholar]
- [28].Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC. REM, not incubation, improves creativity by priming associative networks. P Natl Acad Sci USA. 2009;106:10130–4. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mednick SC, Nakayama K, Cantero JL, Atienza M, Levin AA, Pathak N, et al. The restorative effect of naps on perceptual deterioration. Nat Neurosci. 2002;5:677–81. doi: 10.1038/nn864. [DOI] [PubMed] [Google Scholar]
- [30].Mednick SC, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nat Neurosci. 2003;6:697–8. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- [31].Mednick SC, Cai DJ, Kanady JC, Drummond SPA. Comparing the benefits of caffeine, naps and placebo on verbal, motor and perceptual memory. Behav Brain Res. 2008;193:79–86. doi: 10.1016/j.bbr.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006;86:241–7. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- [34].Wamsley EJ, Tucker MA, Payne JD, Benavides JA, Stickgold R. Dreaming of a learning task is associated with enhanced sleep-dependent memory consolidation. Curr Biol. 2010;20:850–5. doi: 10.1016/j.cub.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Payne JD, Kensinger EA. Sleep’s role in the consolidation of emotional episodic memories. Curr Dir Psychol Sci. 2010;19:290–5. [Google Scholar]
- [36].Dinges DF, Orne MT, Orne EC. Assessing performance upon abrupt awakening from naps during quasi-continuous operations. Behav Res Meth Ins C. 1985;17:1. [Google Scholar]
- [37].Hayashi M, Motoyoshi N, Hori T. Recuperative power of a short daytime nap with or without stage 2 sleep. Sleep. 2005;28:829–36. [PubMed] [Google Scholar]
- [38].Takahashi M. The role of prescribed napping in sleep medicine. Sleep Med Rev. 2003;7:227–35. doi: 10.1053/smrv.2002.0241. [DOI] [PubMed] [Google Scholar]



