Abstract
Ten prepubertal girls and 15 young women were tested for maximal torque, peak rate of torque development, electro-mechanical delay (EMD), and time to peak rate of torque development during isometric elbow flexion. Absolute peak torque (17.0 ± 7.7 vs. 40.5 ± 8.3 Nm) and peak rate of torque development (105.9 ± 58.6 vs. 297.2 ± 113.0 Nm·s−1) were lower in the girls (p < .05). Normalized to muscle cross sectional area, torque was similar (8.27 ± 2.74 vs. 8.44 ± 1.65 Nm·cm−2), as was peak rate of torque development, normalized to peak torque (6.21 ± 1.94 vs. 7.30 ± 2.26 Nm·s−1/Nm). Both, time to peak rate of torque development (123.8 ± 36.0 vs. 110.5 ± 52.6 ms) and EMD (73.2 ± 28.6 vs. 51.9 ± 25.6 ms), were longer in the girls, although EMD’s difference only approached statistical significance (p = .06). Age-related isometric strength differences in females appear to be mainly muscle-size dependent. However, the time to peak torque and EMD findings suggest differential motor-unit activation which may functionally manifest itself in fast dynamic contractions.
Numerous studies have demonstrated that children’s maximal muscle strength is lower than that of adults (5,18,42). In males, muscle strength is consistently lower in boys even when normalized for body size (5,42). In females, age-related differences are inconsistent. While some studies report a lower body size-normalized strength in girls compared with women (29), others do not (5,28,31,38,43).
Several factors, besides body size may contribute to age-related differences in maximal strength, including differences in muscle activation, fiber composition, and agonist-antagonist cocontraction. The scant available literature on muscle fiber composition suggests that the latter is similar in children and adults (12). This is supported by the similar contractile characteristics (contraction time and half-relaxation time) during electrically evoked twitch contraction in children and adults (4,8,9,22,35,39). Although few studies have suggested a lower percentage of type II muscle fibers in boys compared with men (15,34), the difference is too small to account for the age-related muscle strength differences observed between boys and men. No comparison of muscle fiber-type composition has been reported between girls and women.
The rate or degree of muscle activation has been suggested to be lower in boys compared with men, based on indirect evidence and varying techniques (1,2,4,5,23,25,33,39,45). However, all these comparisons were made with heterogeneous or male-only samples. There are no comparisons of muscle activation indices or examination of their roles in girl-woman strength differences.
Differences in the rate of force development are likely manifestations of the nature of muscle activation or its composition. It too appears to be lower in children than in adults, whether measured during maximal voluntary (1,21), or electrically stimulated twitch contractions (4,22), but has been demonstrated only in males. No age comparisons have been published for females. As is the case with maximal force, explanations for children’s lower rate of force development may involve differences in muscle fiber composition and in muscle activation. Other factors capable of affecting rate of force development include musculo-tendinous stiffness, excitation-contraction coupling, and muscle-fiber conduction velocity, through muscle-fiber diameter. These have been examined in children only to a limited extent and with inconsistent results (7,22,33). All the cited studies have examined male-only or heterogeneous subject samples.
Another factor that could affect maximal muscle strength is agonist-antagonist coactivation. Some studies (19,23,33), although not all (3,32,37), demonstrate greater coactivation in children. At least in part, differences are due to the type of examined contractions. Apparently, dynamic/locomotive muscular activity is associated with higher degrees of coactivation, particularly in children, while isometric contractions induce little coactivation across age groups. All the mentioned studies examined the lower limbs only and it is not known whether their findings apply to the upper limbs as well.
The aim of this study was to compare the maximal torque, rate of torque development, and electro-mechanical delay (EMD) of prepubertal girls vs. young women during maximal isometric elbow flexion. There is indirect evidence suggesting sex-differences in neuromuscular development during growth and maturation (41). However, as indicated above, none of the studies which examined muscle strength in girls employed EMG. Therefore, in view of the scant available data, it was hypothesized that peak torque and rate of torque development would be lower and EMD longer in girls compared with women.
Methods
Participants
Ten prepubertal girls and 15 adult women, whose physical characteristics are shown in Table 1, were recruited to participate in this study, approved by the University’s Research Ethics Board. All participants were right-handed, healthy individuals with no upper-limb injuries. All were physically active but none was a competitive athlete, or participated in a unilateral sport on a regular basis. Written informed consent was obtained from all participants and the children’s parents, before the study’s onset.
Table 1.
Participants Characteristics. Values Are Expressed As Mean ± SD (Minimum to Maximum)
| Variable | Women | Men |
|---|---|---|
|
| ||
| n | 15 | 10 |
| Age (yrs)* | 21.5 ± 0.6(20.6–22.8) | 9.1 ± 1.4(7.3–11.8) |
| Years from PHV (yrs) | — | −2.6 ± 1.2(−4.2– −0.2) |
| Body mass (kg)* | 67.0 ± 10.7(53.9–94.0) | 27.7 ± 4.5(21.1–34.7) |
| Height (cm)* | 170.6 ± 6.6(160.2–181.1) | 133.4 ± 11.5(116.3–155.6) |
| Body fat (%)* | 26.6 ± 2.9(22.2–33.5) | 17.3 ± 3.8(12.0–22.3) |
| Lean body mass (kg)* | 49.0 ± 6.0(40.1–62.5) | 22.9 ± 3.9(17.0–28.6) |
| Biceps skinfold thickness (mm)* | 8.1 ± 3.1 (4.9–17.9) | 6.0 ± 1.5 (3.5–8.5) |
| Triceps skinfold thickness (mm)* | 18.0 ± 3.1(13.0–24.5) | 10.9 ± 2.3(7.8–14.5) |
| Biceps CSA (cm2)* | 4.83 ± 0.70(3.50–6.27) | 2.02 ± 0.52(1.05–2.61) |
| Triceps CSA (cm2)* | 1.53 ± 0.47(0.79–2.36) | 0.68 ± 0.25(0.35–1.07) |
Note. CSA = cross sectional area, PHV = age of peak height velocity
significant difference between girls and women (p ≤.05)
Anthropometric Measurements
Standing and seated heights (for girls only) were measured using a wall-mounted stadiometer (Length Boards, Ellard Instrumentation, Ltd., Monroe, WA). Seated height was used to estimate the age of peak height-velocity (36), an indicator of somatic maturity. Body mass was measured on a digital scale (EKS Electronic Scales, France). Skinfold thicknesses were measured in triplicate, using skinfold calipers (RH15 916, Harpenden, England) at the biceps, triceps, subscapular, and suprailiac sites on the right side of the body. The median value at each site was used for calculating body fat percentage using the equations of Durnin & Womersley (14) for women and Slaughter et al. (44) for girls.
Muscle CSA was calculated from muscle diameter, as determined by B-Mode, real-time ultrasonography. Muscle thickness (diameter) measurements of the biceps brachii and the lateral head of the triceps brachii were taken over the muscle bellies via B-mode Ultrasound (System 5, GE Vingmed, Horten, Norway, 5.0-MHz probe). For both measurements, the elbow was held at 90°, in a relaxed state while the scan head was kept perpendicular to the dermal surface. A water-soluble transmission gel was applied over the scan head to improve the ultrasound image. The ultrasound recording sites for muscle diameter corresponded to the electrode placement for the electromyographic (EMG) recording. Three measurements were taken at each site, recorded on a PC computer and analyzed off-line. The mean values were used.
Experimental Protocol
All participants visited the laboratory on two occasions at least three days apart. On the first visit participants were informed of the study’s procedures, anthropometric measurements were taken, and questionnaires were filled out (facilitated by a researcher for the girls). Physical activity levels were determined using the Godin-Shephard Leisure-Time Exercise Questionnaire. The girls self-assessed their pubertal stage in accordance with secondary sex characteristics (pubic hair), as described by Tanner (13,46). Then, the complete test protocol was practiced to familiarize the participants with all test procedures. The actual test protocol was performed on the second visit.
The Biodex System 3 torque dynamometer (Biodex, Shirley, NY) was used to measure torque during isometric contractions of the elbow flexors and extensors. Isometric contractions were chosen to minimize antagonist involvement so as to make torque measurements attributable to agonist action as much as possible. The participant sat upright on the dynamometer seat with the right shoulder at 90° of flexion, resting her upper arm on a proprietary armrest adjusted for the subject’s height and upper-arm length. With the participant’s hand supinated, grasping the dynamometer’s lever grip, both elbow and the lever arm were set at 90°.The dynamometer’s axis of rotation was visually aligned with the lateral epicondyle of the right humerus. The participants was then stabilized in the dynamometer’s chair by 2 straps secured diagonally across the chest in an ‘X’ fashion, and one strap across the hips.
The preparatory warm-up consisted of 3–5 three-second maximal voluntary contractions (MVC) of flexion and extension. Ten seconds separated successive contractions. Participants were given explicit standardized instructions to contract from a relaxed state. Online visual representation of the dynamometer’s torque signal was available for the participants on a PC screen. Participants were instructed to pull toward the body/shoulder (elbow flexion) as hard and as fast as possible and to keep the torque output on the display as high as possible. Participants were also instructed to “explode into the contraction.” These instructions were given for maximizing the rate of torque development. Following a 2–3-min rest, the participant performed two sets of contractions. Instructions were identical to those of the warm-up. Participants completed 2 sets of 5 MVCs with a 30-s rest period between contractions and at least a 2-min break between sets (totaling 10 MVCs). Participants were verbally encouraged to exert maximal effort throughout each contraction. Maximal isometric elbow extensions were performed in a similar manner to obtain maximal triceps EMG data for the calculation of antagonist coactivation. The timing of testing within the menstrual cycle was not standardized as previous research has demonstrated no effect of menstrual cycle on isometric strength (40).
Electromyography
Surface EMG signals were collected from 2 sites, at the belly midsections of the biceps brachii and the lateral head of the triceps brachii. The bipolar surface electrodes (DE-2.1, DelSys Inc., Boston, MA, interelectrode distance of 1 cm) were placed in line with the muscle fibers away from the estimated motor point (10). The reference electrode was placed over the middle of the right clavicle. Electrode sites were prepared by shaving the skin when necessary and thoroughly rubbing the skin with alcohol. Raw EMG signals were amplified by a DelSys amplifier (10–500 Hz band-pass filter, Bagnoli-4 EMG System, Boston, MA), A-to-D converted (DI-205-C, DATAQ Instruments, Akron, OH), and recorded at 1000 Hz acquisition rate (WinDaq Pro Data Acquisition, DATAQ Instruments, Akron, OH).
Data Analysis
Torque and EMG traces were visually scrutinized for execution errors, deviations in EMG baseline, torque and EMG amplitude artifacts, and tracing irregularities. Faulty data sets were eliminated and out of the remaining sets, the best five repetitions, based on highest peak torques and torque development rates, were assigned for analysis. Using MatLab (The MathWorks, Natick, MA), several variables were calculated separately for flexion and extension. Peak agonist and antagonist EMG amplitudes were determined and averaged over the best five trials. These were used to calculate the coactivation index (see below). Mean traces were created for torque, agonist EMG, and antagonist EMG. These traces were used to calculate rate of torque development and EMD for both muscle groups. Peak rate of torque development was calculated by taking the maximum of the 1st derivative of the torque signal (20). EMD was defined as the time lapse between the onsets of EMG and torque generation and calculated in the agonist muscle. The onset of torque was defined as the point in time in which 5% of peak torque was attained, while the onset of EMG was defined as the time at which the rectified linear envelope EMG rose above the 95% confidence interval for baseline noise and stayed above that point for more than 20 ms (11,27). The time of peak rate of torque development was calculated as the time delay between the onset of torque generation and the occurrence of peak rate of torque development. Coactivation was calculated as the ratio between the antagonist’s EMG amplitude divided by its EMG amplitude as an agonist (i.e., triceps EMG amplitude during flexion/triceps EMG amplitude during extension).
Statistical Analysis
Data for both girls and women are presented as means and standard deviations. Correlation between variables was examined using the Pearson correlation coefficient. An unpaired two-tailed student’s t test was used to compare the means between the two groups. A covariate analysis of variance was used to control for variables which correlated with the dependent variable (e.g., body mass). Difference between groups were considered significant at p < .05. All analyses were performed using SPSS 16.0.
Results
Participant characteristics appear in Table 1. All body size indices were markedly smaller in the girls, as expected. It should be noted that this was also the case for body-fat percentage and skinfold thickness over the biceps and triceps muscles.
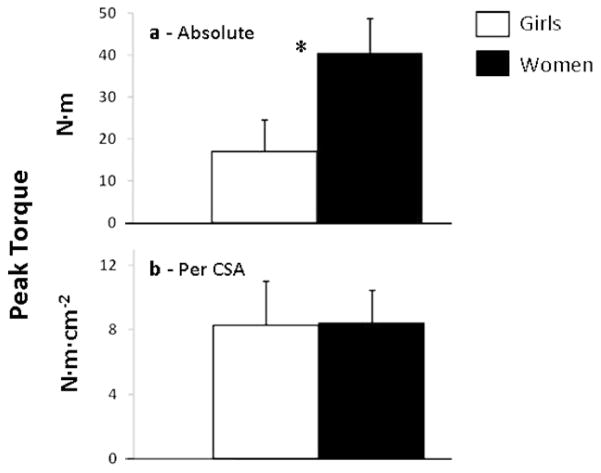
Expectedly, girls’ absolute peak torque was significantly lower than the women’s (Figure 1 a). However, this difference was no longer apparent when torque was normalized to body-mass (0.60 ± 0.21 vs. 0.61 ± 0.09 N·m·kg−1 for girls and women, respectively), and to biceps CSA. Likewise, when these anthropometric measures were used as covariates, no group difference in peak torque was apparent.
Figure 1.
Elbow flexion peak torque in absolute values (upper), relative to biceps cross sectional area (middle), and relative to biceps EMG activity (lower)—*p ≤ .05.
Girls exhibited significantly lower absolute peak rate of torque development values, compared with women. Peak rate of torque development was significantly related to peak torque (r = .77 in girls, r = .63 in women). The girl-woman difference in peak rate of torque development disappeared when peak rate of torque development was normalized for peak torque (Figure 2 b). Time to peak rate of torque development, relative to the onset of torque, occurred significantly later in the girls (123.8 ± 36.0 ms) than in the women (110.5 ± 52.6 ms).
The girls’ agonist EMD (73.2 ± 28.6 ms) was 41% longer than the women’s (51.9 ± 25.6 ms) but the difference only approached statistical significance (p = .06). Antagonist EMD followed a similar pattern (28.5% difference) that did not reach statistical significance (44.6 ± 31.3 and 34.7 ± 42.5 ms, respectively).
The coactivation index was similar in girls and women (0.30 ± 0.12 vs. 0.30 ± 0.20, respectively).
Discussion
This study compared prepubertal girls to adult women in maximal voluntary isometric torque and contractile characteristics of elbow flexion. While absolute peak torque and peak rate of torque development were much lower in girls, the differences disappeared when peak torque was normalized to the biceps CSA (Figure 1 a, b), and peak rate of torque development was normalized to peak torque (Figure 2 b). Thus, it appears that in females, age-related differences in isometric muscle strength are largely determined by muscle size rather than by differences in its activation.
Most previous studies have examined only males. Utilizing differing analytical approaches the reported results with respect to CSA-normalized peak torque are inconsistent (8,25,30,37,47). A few female studies reported the absence of age-related differences in CSA-normalized peak torque (8,31). It is interesting to note that in dynamic knee extensions Kanehisa et al. (29) showed body-size normalized maximal torque to be similar in girls and women during slow contractions (rad·s−1), but significantly lower in girls at higher contraction velocities. Thus, our findings are supported by previous studies in suggesting that in females, body size accounts for most of the observed child-adult differences in maximal isometric muscle strength. This may differ in fast, dynamic contractions, possibly due to maturation-dependent differences in motor-unit recruitment or functional changes in the higher-threshold (fast-twitch) motor units.
Our participants were instructed to contract as hard and as fast as possible, to facilitate proper examination of the maximal rates of torque development. While absolute peak rate of torque development was lower in girls, no age-related differences were observed when peak rate of torque development was normalized to peak torque (Figure 2 b). We are aware of no other study that examined age-related differences in peak rate of torque development in females. However, in a previous study in our laboratory (48), peak rate of torque development in isometric elbow-flexion, normalized to peak torque, was lower in boys than in men. Similar results were reported by Asai & Aoki (1). Thus, age-related differences in peak rate of torque development are apparent in males but not in females. As suggested above, age-related differences in peak rate of torque development may be more evident in fast dynamic contractions.
No differences were apparent in coactivation between the two groups. This is in agreement with previous findings of similar coactivation in girls and women during isokinetic knee extension (32) and during isometric elbow flexion in boys and men (48). Thus, during single joint movements, coactivation does not appear to differ between girls and women and could not therefore have differentially affected the measured torques.
Both, EMD and time to peak rate of torque development are expected to shorten with increasing involvement of fast-twitch motor units. While only differences in the latter variable reached statistical significance, both these variables, as well as the antagonist EMD were consistently longer in girls. This finding is in agreement with previous findings in boys (1,48) and in a mixed sample of boys and girls (22). Cavanagh & Komi (6) suggested that in adults, the series-elastic component (muscle-tendon stiffness) is the main determinant of EMD. The musculo-tendinous stiffness has not been specifically investigated in girls. However, in a mixed sample of 7–10 yr-old children, musculo-tendinous stiffness in plantar flexion (33) was found lower in children compared with adults. On the other hand, in elbow flexion, Cornu & Goubel (7) found no age-related differences in musculo-tendinous stiffness. Moreover, in a recent study that specifically addressed stiffness-EMD relationships in adults, Grosset et al. (24) showed only 14–19% of the EMD variance was explained by stiffness. Therefore, while musculo-tendinous stiffness is likely a major determinant of EMD, other important factors must be involved.
Thus, the girl’s longer EMD (as well as their longer time to peak rate of torque development) may be reflecting lower recruitment or utilization of the faster, higher-threshold (type II) motor units, or possibly immature (yet-undifferentiated) contractile characteristics of those motor units. This possibility has not been directly studied in children but has been suggested, based on indirect evidence (1,25). The examination of recruitment of specific motor units presently requires the use of indwelling electrodes, a technique which cannot be ethically justified in children. However, the possibility of lesser utilization of type-II fibers in children is consistent with children’s physical performance characteristics such as lower size-normalized anaerobic power (16), and faster recovery from intense, short-term exercise (17,26).
In summary, unlike previous findings in males, size-normalized maximal voluntary isometric torque and rate of torque development did not show age-related changes in females. However, as in males, both EMD and the time to peak rate of torque development were consistently longer in girls compared with women—a fact that could possibly have functional implications in fast dynamic contractions. This needs be addressed by future research.
Acknowledgments
The study was partly funded by the Canadian Institute for Health Research.
We would like to thank the participants and their parents for their cooperation and enthusiasm in the project. We would also like to thank Jen Tyrrell and Katie Banks for their technical assistance in data collection and reduction.
Contributor Information
Bareket Falk, Dept. of Physical Education and Kinesiology, Faculty of Applied Health Sciences, Brock University, St. Catharines, Ontario L2S 3A1, Canada.
Laura Brunton, Dept. of Health and Rehabilitation Services, University of Western Ontario, London, Ontario, Canada N6A 4B8.
Raffy Dotan, Dept. of Physical Education and Kinesiology, Faculty of Applied Health Sciences, Brock University, St. Catharines, Ontario L2S 3A1, Canada.
Charlotte Usselman, Dept. of Kinesiology, University of Western Ontario, London, Ontario, Canada N6A 4B8.
Panagiota Klentrou, Dept. of Physical Education and Kinesiology, Faculty of Applied Health Sciences, Brock University, St. Catharines, Ontario L2S 3A1, Canada.
Davie Gabriel, Dept. of Physical Education and Kinesiology, Faculty of Applied Health Sciences, Brock University, St. Catharines, Ontario L2S 3A1, Canada.
References
- 1.Asai H, Aoki J. Force development of dynamic and static contractions in children and adults. Int J Sports Med. 1996;17:170–174. doi: 10.1055/s-2007-972827. [DOI] [PubMed] [Google Scholar]
- 2.Asmussen E. Growth in muscular strength and power. In: Rarick G, editor. Physical Activity, Human Growth and Development. London: Academic Press; 1973. pp. 60–79. [Google Scholar]
- 3.Bassa E, Patias D, Kotzamanidis C. Activation of antagonist knee muscles during isokinetic efforts in prepubertal and adult males. Pediatr Exerc Sci. 2005;17:65–75. [Google Scholar]
- 4.Belanger AY, McComas AJ. Contractile properties of human skeletal muscle in childhood and adolescence. Eur J Appl Physiol Occup Physiol. 1989;58:563–567. doi: 10.1007/BF00418500. [DOI] [PubMed] [Google Scholar]
- 5.Blimkie CJ. Age- and sex-associated variation in strength during childhood: Anthropometric, morphologic, neurologic, biomechanical, endocrinologic, genetic, and physical activity correlates. In: Gisolfi CV, editor. Perspectives in Exercise Science and Sports Medicine, Vol. 2: Youth, Exercise and Sports. Indianapolis, IN: Benchmark Press; 1989. pp. 99–163. [Google Scholar]
- 6.Cavanagh PR, Komi PV. Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1979;42:159–163. doi: 10.1007/BF00431022. [DOI] [PubMed] [Google Scholar]
- 7.Cornu C, Goubel F. Musculo-tendinous and joint elastic characteristics during elbow flexion in children. Clin Biomech (Bristol, Avon) 2001;16:758–764. doi: 10.1016/s0268-0033(01)00076-6. [DOI] [PubMed] [Google Scholar]
- 8.Davies CT. Strength and mechanical properties of muscle in children and young adults. Scand J Sports Sci. 1985;7:11–15. [Google Scholar]
- 9.Davies CT, White MJ, Young K. Muscle function in children. Eur J Appl Physiol Occup Physiol. 1983;52:111–114. doi: 10.1007/BF00429036. [DOI] [PubMed] [Google Scholar]
- 10.Delagi EF, Perotto A. Anatomic guide for the electromyographer. Springfield, IL: CC Thomas; 1980. [Google Scholar]
- 11.Di Fabio RP. Reliability of computerized surface electromyography for determining the onset of muscle activity. Phys Ther. 1987;67:43–48. doi: 10.1093/ptj/67.1.43. [DOI] [PubMed] [Google Scholar]
- 12.Dubowitz V. Enzyme histochemistry of skeletal muscle. J Neurol Neurosurg Psychiatry. 1965;28:516–524. doi: 10.1136/jnnp.28.6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duke PM, I, Litt F, Gross RT. Adolescents’ self-assessment of sexual maturation. Pediatrics. 1980;66:918–920. [PubMed] [Google Scholar]
- 14.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 15.Elder GC, Kakulas BA. Histochemical and contractile property changes during human muscle development. Muscle Nerve. 1993;16:1246–1253. doi: 10.1002/mus.880161116. [DOI] [PubMed] [Google Scholar]
- 16.Falk B, Bar-Or O. Longitudinal changes in peak aerobic and anaerobic mechanical power of circumpubertal boys. Pediatr Exerc Sci. 1993;5:318–331. [Google Scholar]
- 17.Falk B, Dotan R. Child-adult differences in the recovery from high-intensity exercise. Exerc Sport Sci Rev. 2006;34:107–112. doi: 10.1249/00003677-200607000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Froberg K, Lammert O. Development of muscle strength during childhood. In: Bar-Or O, editor. The Encyclopedia of Sports Medicine. The Child and Adolescent Athlete. London: Blackwell Scientific Publications; 1995. pp. 25–41. [Google Scholar]
- 19.Frost G, Dowling J, Dyson K, Bar-Or O. Cocontraction in three age groups of children during treadmill locomotion. J Electromyogr Kinesiol. 1997;7:179–186. doi: 10.1016/s1050-6411(97)84626-3. [DOI] [PubMed] [Google Scholar]
- 20.Gabriel DA, Basford JR, An K. Training-related changes in the maximal rate of torque development and EMG activity. J Electromyogr Kinesiol. 2001;11:123–129. doi: 10.1016/s1050-6411(00)00041-9. [DOI] [PubMed] [Google Scholar]
- 21.Going SB, Massey BH, Hoshizaki TB, Lohman TG. Maximal voluntary static force production characteristics of skeletal muscle in children 8–11 years of age. Res Q Exerc Sport. 1987;58:115–123. [Google Scholar]
- 22.Grosset JF, Mora I, Lambertz D, Perot C. Age-related changes in twitch properties of plantar flexor muscles in prepubertal children. Pediatr Res. 2005;58:966–970. doi: 10.1203/01.PDR.0000181375.61935.7D. [DOI] [PubMed] [Google Scholar]
- 23.Grosset JF, Mora I, Lambertz D, Perot C. Voluntary activation of the triceps surae in prepubertal children. J Electromyogr Kinesiol. 2008;18:455–465. doi: 10.1016/j.jelekin.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Grosset JF, Piscione J, Lambertz D, Perot C. Paired changes in electromechanical delay and musculo-tendinous stiffness after endurance or plyometric training. Eur J Appl Physiol. 2008 doi: 10.1007/s00421-008-0882-8. [DOI] [PubMed] [Google Scholar]
- 25.Halin R, Germain P, Bercier S, Kapitaniak B, Buttelli O. Neuromuscular response of young boys versus men during sustained maximal contraction. Med Sci Sports Exerc. 2003;35:1042–1048. doi: 10.1249/01.MSS.0000069407.02648.47. [DOI] [PubMed] [Google Scholar]
- 26.Hebestreit H, Mimura K, Bar-Or O. Recovery of muscle power after high-intensity short-term exercise: comparing boys and men. J Appl Physiol. 1993;74:2875–2880. doi: 10.1152/jappl.1993.74.6.2875. [DOI] [PubMed] [Google Scholar]
- 27.Hodges PW, Bui BH. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol. 1996;101:511–519. doi: 10.1016/s0013-4694(96)95190-5. [DOI] [PubMed] [Google Scholar]
- 28.Ikai M, Fukunaga T. Calculation of muscle strength per unit cross-sectional area of human muscle by means of ultrasonic measurement. Int Z Angew Physiol. 1968;26:26–32. doi: 10.1007/BF00696087. [DOI] [PubMed] [Google Scholar]
- 29.Kanehisa H, Ikegawa S, Tsunoda N, Fukunaga T. Strength and cross-sectional area of knee extensor muscles in children. Eur J Appl Physiol Occup Physiol. 1994;68:402–405. doi: 10.1007/BF00843736. [DOI] [PubMed] [Google Scholar]
- 30.Kanehisa H, Ikegawa S, Tsunoda N, Fukunaga T. Strength and cross-sectional areas of reciprocal muscle groups in the upper arm and thigh during adolescence. Int J Sports Med. 1995;16:54–60. doi: 10.1055/s-2007-972964. [DOI] [PubMed] [Google Scholar]
- 31.Kanehisa H, Yata H, Ikegawa S, Fukunaga T. A cross-sectional study of the size and strength of the lower leg muscles during growth. Eur J Appl Physiol Occup Physiol. 1995;72:150–156. doi: 10.1007/BF00964130. [DOI] [PubMed] [Google Scholar]
- 32.Kellis E, V, Unnithan B. Co-activation of vastus lateralis and biceps femoris muscles in pubertal children and adults. Eur J Appl Physiol Occup Physiol. 1999;79:504–511. doi: 10.1007/s004210050545. [DOI] [PubMed] [Google Scholar]
- 33.Lambertz D, Mora I, Grosset JF, Perot C. Evaluation of musculotendinous stiffness in prepubertal children and adults, taking into account muscle activity. J Appl Physiol. 2003;95:64–72. doi: 10.1152/japplphysiol.00885.2002. [DOI] [PubMed] [Google Scholar]
- 34.Lexell J, Downham D. What determines the muscle cross-sectional area? J Neurol Sci. 1992;111:113–114. doi: 10.1016/0022-510x(92)90119-6. [DOI] [PubMed] [Google Scholar]
- 35.McComas AJ, Sica RE, Petito F. Muscle strength in boys of different ages. J Neurol Neurosurg Psychiatry. 1973;36:171–173. doi: 10.1136/jnnp.36.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirwald RL, Baxter-Jones AD, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34:689–694. doi: 10.1097/00005768-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 37.Morse CI, Tolfrey K, Thom JM, Vassilopoulos V, Maganaris CN, Narici MV. Gastrocnemius muscle specific force in boys and men. J Appl Physiol. 2008;104:469–474. doi: 10.1152/japplphysiol.00697.2007. [DOI] [PubMed] [Google Scholar]
- 38.Nevill AM, Holder RL, Baxter-Jones A, Round JM, Jones DA. Modeling developmental changes in strength and aerobic power in children. J Appl Physiol. 1998;84:963–970. doi: 10.1152/jappl.1998.84.3.963. [DOI] [PubMed] [Google Scholar]
- 39.Paasuke M, Ereline J, Gapeyeva H. Twitch contraction properties of plantar flexor muscles in pre- and post-pubertal boys and men. Eur J Appl Physiol. 2000;82:459–464. doi: 10.1007/s004210000236. [DOI] [PubMed] [Google Scholar]
- 40.Petrofsky JS, LeDonne DM, Rinehart JS, Lind AR. Isometric strength and endurance during the menstrual cycle. Eur J Appl Physiol Occup Physiol. 1976;35:1–10. doi: 10.1007/BF00444652. [DOI] [PubMed] [Google Scholar]
- 41.Quatman CE, Ford KR, Myer GD, Paterno MV, Hewett TE. The effects of gender and pubertal status on generalized joint laxity in young athletes. J Sci Med Sport. 2008;11:257–263. doi: 10.1016/j.jsams.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sale DG, Spriet LL. Skeletal muscle function and energy metabolism. In: Bar-Or O, DRL, Clarkson PM, editors. Exercise and the Female – A Life Span Approach. Carmel, IN: Cooper Publishing Group; 1996. pp. 289–359. [Google Scholar]
- 43.Seger JY, Thorstensson A. Muscle strength and electromyogram in boys and girls followed through puberty. Eur J Appl Physiol. 2000;81:54–61. doi: 10.1007/PL00013797. [DOI] [PubMed] [Google Scholar]
- 44.Slaughter MH, Lohman TG, Boileau BA. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–723. [PubMed] [Google Scholar]
- 45.Streckis V, Skurvydas A, Ratkevicius A. Children are more susceptible to central fatigue than adults. Muscle Nerve. 2007;36:357–363. doi: 10.1002/mus.20816. [DOI] [PubMed] [Google Scholar]
- 46.Tanner JM. Growth at Adolescence. Oxford: Blackwell Scientific Publications; 1962. [Google Scholar]
- 47.Tonson A, Ratel S, Le Fur Y, Cozzone P, Bendahan D. Effect of maturation on the relationship between muscle size and force production. Med Sci Sports Exerc. 2008;40:918–925. doi: 10.1249/MSS.0b013e3181641bed. [DOI] [PubMed] [Google Scholar]
- 48.Usselman C, Gabriel DA, Dotan R, Brunton L, Klentrou P, Falk B. Muscle activation patterns in prepubertal boys and young men. Appl Physiol Nutr Metab. 2008;32:S88. [Google Scholar]