Abstract
For bacteria, temperature is a fairly reliable cue for entry into a vertebrate host. Many pathogenic bacteria sense elevated temperatures (37°C) and turn on virulence and metabolic genes appropriate for survival and growth within the host. A recent study demonstrated that the expression of a heme uptake protein in Shigella dysenteriae and pathogenic Escherichia coli was dependent on an RNA thermometer.
Keywords: iron, Listeria, Shigella, UPEC, RNA thermometer, PrfA, ShuA, heme
When life is short, the ability to conserve resources and quickly adapt to changing conditions is an existential virtue. Pathogenic bacteria have elaborate mechanisms to detect, respond and adapt to life within the distinct milieus of the host and the environment. Sequences in the mRNA of several heat shock proteins and virulence factors, known as RNA thermometers, rapidly regulate translation in response to altered temperatures.1 These riboswitch sequences in the 5′ untranslated regions (UTR) of mRNAs adopt secondary structures that block ribosome access at low temperatures. At higher temperatures, an open conformation allows ribosome access to the Shine Dalgarno sequences and, consequently, translation of the mRNA. Such structures can respond to alterations in temperatures as low as 1°C. RNA thermosensors also play a role in the preferential translation of some transcripts (e.g., cold shock genes) at lower temperatures.
The expression of PrfA, the global regulator of virulence genes in Listeria monocytogenes, is regulated via multiple mechanisms, including an RNA thermometer.1 At low temperatures (30°C), the extended secondary structure of the 127-nucleotide 5′ UTR of a subset of prfA transcripts (those expressed from the prfAP1 promoter) includes a stem-loop configuration that prevents formation of a translation initiation complex. Destabilization of these secondary structures at higher temperatures (37°C, as might occur within a host) allows for translation initiation and increased levels of PrfA.
While strains harboring constitutively active forms of PrfA (PrfA*, which can function in the absence of a cofactor) are more virulent in mouse models of infection, they exhibit a fitness defect in broth cultures when grown in the presence of wildtype bacteria.2 PrfA*-harboring strains have diminished flagella-dependent swimming motility and an impaired ability to utilize carbon sources such as glucose and cellobiose, and cannot survive long periods of starvation. Thus, the tight regulation of PrfA levels and activity is essential for L. monocytogenes to transition between pathogenic and saprophytic/environmental lifestyles.
In the pathogenic yersiniae, expression of virulence genes is regulated by the transcriptional regulator LcrF.3 A hierarchy of protein and RNA thermosensors regulate LcrF expression at body temperature. While lcrF transcription is regulated by the thermosensitve modulator YmoA, translation is regulated by an RNA thermometer that includes four consecutive uridine residues (“fourU,” Figure 1) that pair with the Shine Dalgarno sequence. Opening of this stem-loop structure at 37°C enables ribosome binding and LcrF translation. Y. pseudotuberculosis strains with stem-loop stabilizing mutations (closed configuration) did not express detectable LcrF at low or high temperatures, and were attenuated in a mouse model of infection. Interestingly, mutant strains harboring a destabilized secondary structure (open configuration), which expressed LcrF at both low and high temperatures, were also impaired for virulence. This suggests that delicate regulation of LcrF levels, by various mechanisms including RNA thermoregulation, is critical for pathogenesis.
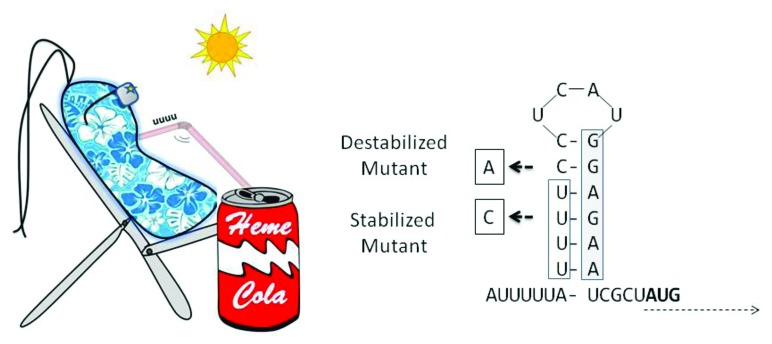
Figure 1. In S. dysenteriae and E. coli, temperature-dependent opening of a fourU structure in the heme uptake-related shuA (or chi) mRNA permits ribosome access to Shine Dalgarno site (boxed) sequences, and consequent translation starting at the downstream AUG site. Mutagenic stabilization of the secondary structure by a U→C mutation prevents ribosome access (and ShuA translation) even at permissive temperatures, while a C→A mutation destabilizes the structure and results in ShuA translation even at low (“Non-permissive”) temperatures.
A recent study explores the role of RNA thermoregulation in iron uptake in Shigella dysenteriae and uropathogenic Escherichia coli.4 Iron is an essential nutrient for most bacteria and, consistent with this, they elaborate multiple, and apparently redundant, iron uptake systems. Thus, none of the single iron transport system mutants of Shigella displayed appreciable defects in intracellular multiplication or cell-to-cell spread, although a mutant deficient in three distinct iron uptake systems failed to replicate within epithelial cells.5 Given its toxicity, however, iron uptake systems are also under tight regulatory controls. Iron transport systems in many bacteria, including Shigella, are regulated by iron and the Fur repressor. Iron-complexed Fur represses transcription of iron-regulated genes by binding to ‘Fur boxes’ in the corresponding promoter regions. In iron-depleted conditions, Fur is released from the promoter regions, and iron uptake genes are expressed.
Ninety five percent of iron in the human body is sequestered in heme, a source utilized by many pathogenic bacteria. The S. dysenteriae outer membrane receptor ShuA (ChuA in E. coli) binds heme and facilitates its transport into the periplasm. Other components of the Shu system mediate heme entry into the cytoplasm, as well as its subsequent degradation to yield nutrient iron. Kouse et al. recently reported that, although iron depletion induced shuA transcription, ShuA protein levels were not increased at 25°C4. At 37°C, however, ShuA protein levels were also elevated.
A fourU RNA thermometer was shown to regulate the expression of ShuA and ChuA, and transfer of a 32-nucleotide shuA 5′UTR region was sufficient to confer temperature-dependent translational regulation to green fluorescent protein expression. A U→C mutation that stabilized the stem-loop structure locked the mRNA in a non-translatable configuration, while a C→A mutation that destabilized the stem-loop structure permitted ShuA translation even at low temperatures. The shuA 5′UTR was found to be conserved among pathogenic E. coli, and the uropathogenic E. coli homolog chuA was shown to be similarly induced by temperature elevation.
A cursory analysis of the 8 open reading frames in the heme uptake cluster reveals the presence of a fourU cluster only in the shuA 5′UTR. The implication of differential regulation of the cluster, if this is indeed the case, to heme uptake and prevention of heme toxicity is unclear at present. While iron deficiency, a signal for Fur-dependent derepression of uptake systems, could occur both in the host and the environment, the utility of ShuA would only be within a host where heme is available. Using a temperature signal to turn on ShuA expression may not be exactly what Pavlov had in mind when he described “conditional reflexes,” but it surely rings a bell.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/25726
References
- 1.Kortmann J, Narberhaus F. Bacterial RNA thermometers: molecular zippers and switches. Nat Rev Microbiol. 2012;10:255–65. doi: 10.1038/nrmicro2730. [DOI] [PubMed] [Google Scholar]
- 2.Xayarath B, Freitag NE. Optimizing the balance between host and environmental survival skills: lessons learned from Listeria monocytogenes. Future Microbiol. 2012;7:839–52. doi: 10.2217/fmb.12.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Böhme K, Steinmann R, Kortmann J, Seekircher S, Heroven AK, Berger E, et al. Concerted actions of a thermo-labile regulator and a unique intergenic RNA thermosensor control Yersinia virulence. PLoS Pathog. 2012;8:e1002518. doi: 10.1371/journal.ppat.1002518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kouse AB, Righetti F, Kortmann J, Narberhaus F, Murphy ER. RNA-Mediated Thermoregulation of Iron-Acquisition Genes in Shigella dysenteriae and Pathogenic Escherichia coli. PLoS One. 2013;8:e63781. doi: 10.1371/journal.pone.0063781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payne SM, Wyckoff EE, Murphy ER, Oglesby AG, Boulette ML, Davies NM. Iron and pathogenesis of Shigella: iron acquisition in the intracellular environment. Biometals. 2006;19:173–80. doi: 10.1007/s10534-005-4577-x. [DOI] [PubMed] [Google Scholar]


