Abstract
Certain therapeutic microbes, including Bifidobacteria infantis (B. infantis) 35624 exert beneficial immunoregulatory effects by mimicking commensal-immune interactions; however, the value of these effects in patients with non-gastrointestinal inflammatory conditions remains unclear. In this study, we assessed the impact of oral administration of B. infantis 35624, for 6‒8 weeks on inflammatory biomarker and plasma cytokine levels in patients with ulcerative colitis (UC) (n = 22), chronic fatigue syndrome (CFS) (n = 48) and psoriasis (n = 26) in three separate randomized, double-blind, placebo-controlled interventions. Additionally, the effect of B. infantis 35624 on immunological biomarkers in healthy subjects (n = 22) was assessed. At baseline, both gastrointestinal (UC) and non-gastrointestinal (CFS and psoriasis) patients had significantly increased plasma levels of C-reactive protein (CRP) and the pro-inflammatory cytokines tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) compared with healthy volunteers. B. infantis 35624 feeding resulted in reduced plasma CRP levels in all three inflammatory disorders compared with placebo. Interestingly, plasma TNF-α was reduced in CFS and psoriasis while IL-6 was reduced in UC and CFS. Furthermore, in healthy subjects, LPS-stimulated TNF-α and IL-6 secretion by peripheral blood mononuclear cells (PBMCs) was significantly reduced in the B. infantis 35624-treated groups compared with placebo following eight weeks of feeding. These results demonstrate the ability of this microbe to reduce systemic pro-inflammatory biomarkers in both gastrointestinal and non-gastrointestinal conditions. In conclusion, these data show that the immunomodulatory effects of the microbiota in humans are not limited to the mucosal immune system but extend to the systemic immune system.
Keywords: B. infantis 35624, inflammation, immunity, microbiota, C-reactive protein
Introduction
There is persuasive evidence from several sources indicating that the gut microbiota has an influence on the development and maintenance, not only of the mucosal,1,2 but also the systemic immune response.3,4 This raises the therapeutic vista of modifying the systemic immune response by enteric microbes. For example, species as varied as Clostridia5 and Bifidobacteria6,7 have been shown to induce regulatory T cells which, theoretically, could modulate immune-inflammatory or autoimmune diseases. The organism Bifidobacterium longum subsp infantis (B. infantis) 35624 induces regulatory T cells in animal models with activity both in the gut and at extra-intestinal sites.7-11 It has also been shown to increase the relative proportion of regulatory T cells in the peripheral blood of healthy human volunteers.12 This raises the question as to whether regulatory T cell induction following administration of B. infantis 35624 would be sufficient to influence inflammatory mediator production in inflammatory disorders both in and beyond the gut in humans.
To address this, we studied cytokine profiles before and after administration of this organism to patients with ulcerative colitis (UC) and two extra-intestinal inflammatory diseases; psoriasis and chronic fatigue syndrome (CFS). The results show that B. infantis 35624-feeding significantly reduced plasma CRP and pro-inflammatory cytokine levels in both gastrointestinal and non-gastrointestinal inflammatory disorders.
Results
Baseline plasma pro-inflammatory biomarkers were elevated in UC and the extra-intestinal conditions psoriasis and CFS compared with healthy volunteers
CRP (p < 0.001), TNF-α (p < 0.001) and IL-6 (p < 0.05), were elevated in patients with psoriasis, CFS and UC compared with healthy volunteers, (Fig. 1). In general, UC patients displayed the highest CRP levels compared with healthy volunteers, while plasma TNF-α and IL-6 levels were comparable for the different disease states.
Figure 1. Plasma pro-inflammatory biomarkers are elevated in patients with inflammatory disorders. Plasma CRP and pro-inflammatory cytokines levels are significantly elevated in chronic fatigue syndrome (CFS) (n = 48), psoriasis (n = 26) and ulcerative colitis (UC) (n = 22) patients compared with healthy volunteers (n = 35). Results are expressed as median values. (Healthy volunteers vs. each inflammatory disorder) (Mann-Whitney U test) p < 0.05 *, p < 0.01 ** p < 0.01 ***.
B. infantis 35624 reduced plasma pro-inflammatory biomarkers in UC and extra-intestinal inflammatory conditions
The administration of B. infantis 35624 was associated with a significant reduction in plasma pro-inflammatory biomarkers in patients with psoriasis, CFS and, to a lesser extent, in those with UC.
CRP
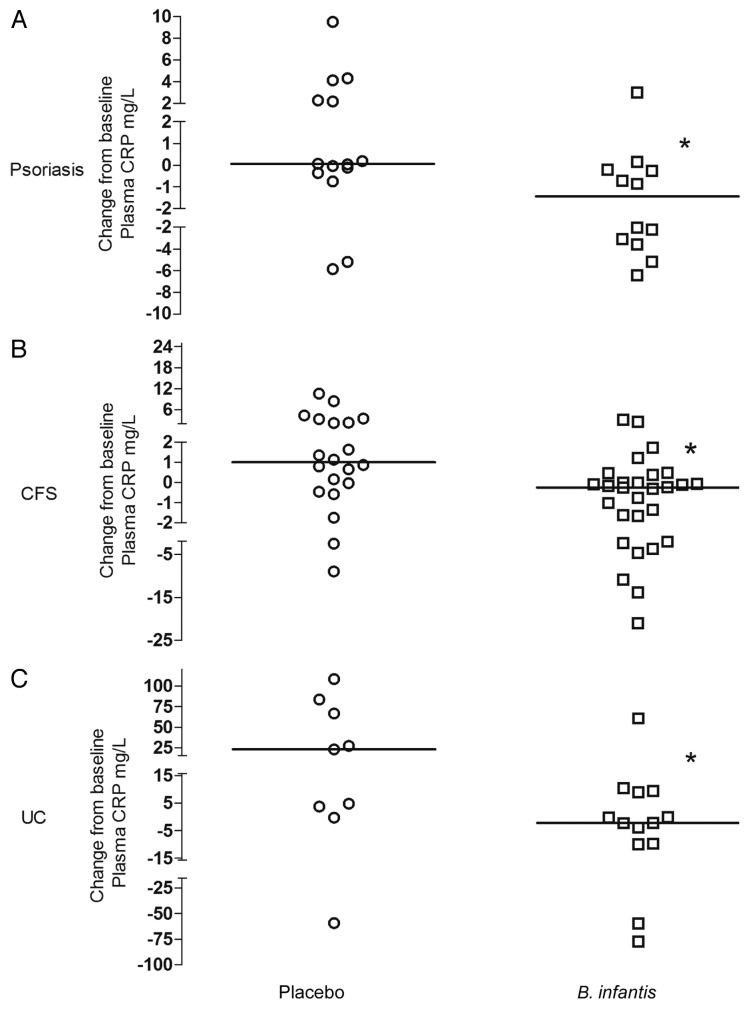
Plasma CRP levels were significantly reduced in B. infantis 35624-fed subjects compared with placebo controls in psoriasis (p = 0.0425), CFS (p = 0.0285) and UC patients (p = 0.0327). Data are expressed as the change from baseline (post-treatment minus pre-treatment level) for each patient (Fig. 2) with the median values (interquartile range) presented in Table 1.
Figure 2.B. infantis 35624-feeding reduces plasma CRP levels compared with placebo. Plasma levels of CRP were significantly reduced in the B. infantis 35624 treated groups compared with placebo treatment following 6–8 weeks of feeding in patients with chronic fatigue syndrome (CFS), psoriasis and ulcerative colitis (UC). Results are expressed as the change from baseline (post-treatment minus pre-treatment level) for each patient. *p < 0.05 vs. placebo; (Mann-Whitney U test).
Table 1.B. infantis 35624-feeding induces a reduction in absolute CRP levels in the majority of inflammatory disorder patients compared with placebo-fed patients. Results expressed as median (interquartile ranges).
| Disease Indication | Treatment | Biomarker | Pre-treatment level (mg/L) | Post-treatment level (mg/L) |
|---|---|---|---|---|
|
Chronic Fatigue Syndrome |
B. infantis |
CRP |
2.35 (1.36–5.65) |
2.07(1.05–4.77) |
| Placebo |
CRP |
1.92 (0.41–9.78) |
3.02 (1.52–9.72) |
|
|
Psoriasis |
B. infantis |
CRP |
4.47 (3.59–7.97) |
2.89 (2.02–5.36) |
| Placebo |
CRP |
2.68 (1.33–4.33) |
3.55 (2.46–5.79) |
|
| Ulcerative Colitis |
B. infantis |
CRP |
3.81 (1.67–10.66) |
1.82 (0.83–15.18) |
| Placebo | CRP | 8.91 (1.18–50.94) | 36.20 (3.26–86.14) |
When comparing pre-and post-feeding levels, plasma CRP was significantly reduced in psoriasis (p = 0.0161) and CFS (p = 0.0393) after eight weeks of feeding B. infantis 35624 but increased slightly in the placebo group after eight weeks of feeding (Fig. 3A and B). There was no statistically significant decrease in CRP levels for UC patients following six weeks B. infantis 35624 feeding; however, CRP levels in the placebo group increased post treatment, likely due to these patients not receiving steroid treatment during the trial period (Fig. 3C).
Figure 3.B. infantis 35624-feeding reduces plasma CRP levels compared with pretreatment level. Placebo-feeding for 8 weeks did not alter plasma CRP levels compared with pretreatment levels in any patients with inflammatory disorders. Plasma CRP levels were significantly reduced following 8 weeks of feeding with B. infantis 35624 compared with pretreatment levels in (A) psoriasis (n = 12). and (B) chronic fatigue syndrome (CFS) (n = 28), However, there was no difference in CRP levels following 6 weeks feeding with B. infantis 35624 in patients with (C) ulcerative colitis (UC) (n = 13) compared with their pretreatment levels. Results are expressed as the individual responses of each patient (mg/L) (pre-treatment vs. post-treatment level) *p < 0.05 (Wilcoxon matched pair test).
TNF-α
Plasma TNF-α levels were significantly reduced in B. infantis 35624-fed subjects compared with placebo controls in psoriasis (p = 0.0405) and CFS (p = 0.0214). However, no significant difference in the levels of TNF-α was observed between B. infantis 35624-fed UC patients compared with placebo following six weeks feeding. Data are expressed as the change from baseline (post-treatment minus pre-treatment level) for each patient (Fig. 4) with the median values (interquartile range) presented in Table 2.
Figure 4.B. infantis 35624-feeding reduces plasma TNF-α levels compared with placebo. Plasma levels of TNF-α were significantly reduced in the B. infantis 35624-treated groups compared with placebo treatment following 8 weeks of feeding in patients with chronic fatigue syndrome (CFS) and psoriasis. However, there was no difference in TNF-α levels following 6 weeks feeding with B. infantis 35624 in patients with ulcerative colitis (UC) compared with placebo treatment. Results are expressed as the change from baseline (post-treatment minus pre-treatment level) for each patient. *p < 0.05 vs. placebo; (Mann-Whitney U test).
Table 2.B. infantis 35624-feeding induces a reduction in absolute IL-6 and TNF-α levels in inflammatory disorder patients compared with placebo-fed patients. Results expressed as median (interquartile ranges).
| Disease Indication | Treatment | Cytokine | Pre-treatment level (pg/ml) |
Post-treatment level (pg/ml) |
|
|---|---|---|---|---|---|
|
Psoriasis |
B. infantis |
IL-6 |
1.62 (1.05–2.96) |
1.48 (0.94–1.93) |
|
| Placebo |
IL-6 |
1.73 (1.32–2.78) |
1.42 (1.12–2.47) |
||
|
Chronic Fatigue Syndrome |
B. infantis |
IL-6 |
0.73(0.60–0.96) |
0.66(0.50–0.85 |
|
| Placebo |
IL-6 |
0.85 (0.70–1.21) |
0.92 (0.83–1.27) |
||
|
Ulcerative Colitis |
B. infantis |
IL-6 |
2.17 (1.05–3.96) |
1.98 (1.29–2.60) |
|
| Placebo |
IL-6 |
3.28 (1.05–4.95) |
5.57(1.39–7.96) |
||
|
Psoriasis |
B. infantis |
TNF-α |
8.20 (7.12–11.05) |
7.67 (6.57–8.43) |
|
| Placebo |
TNF-α |
7.06 (6.48–8.10) |
7.40 (6.35–8.91) |
||
|
Chronic Fatigue Syndrome |
B. infantis |
TNF-α |
7.50 (6.42–7.85) |
6.82 (5.75–9.22) |
|
| Placebo |
TNF-α |
8.01 (6.07–8.90) |
8.36 (6.88–9.84) |
||
|
Ulcerative Colitis |
B. infantis |
TNF-α |
9.38 (7.49–9.84) |
7.12 (8.77–9.84) |
|
| Placebo |
TNF-α |
6.82 (5.75–9.22) |
7.03 (5.48–9.18) |
||
When comparing pre- and post-feeding levels, plasma TNF-α was significantly reduced in psoriasis (p = 0.0269) and CFS (p = 0.0129) after 8 weeks of feeding with B. infantis 35624 but increased slightly in the placebo group (Fig. 5A and B). In UC patients, there was no statistically significant decrease in TNF-α levels following six weeks B. infantis 35624 feeding; however, TNF-α levels in the placebo group increased from pretreatment (Fig. 5C).
Figure 5.B. infantis 35624-feeding reduces plasma TNF-α levels compared with pretreatment level. Placebo treatment did not alter plasma TNF-α levels, compared with pretreatment levels, in all the patient groups tested. Plasma TNF-α levels were significantly reduced following 8 weeks of feeding with B. infantis 35624, compared with pretreatment levels, in (A) psoriasis (n = 12) and (B) chronic fatigue syndrome (CFS) (n = 28). However there was no difference in TNF-α levels following 6 weeks feeding with B. infantis 35624 in patients with (C) ulcerative colitis (UC) (n = 13) compared with their pretreatment levels. Results are expressed as the individual responses of each patient (pg/ml) (pre-treatment vs. post-treatment level) *p < 0.05 (Wilcoxon matched pair test).
IL-6
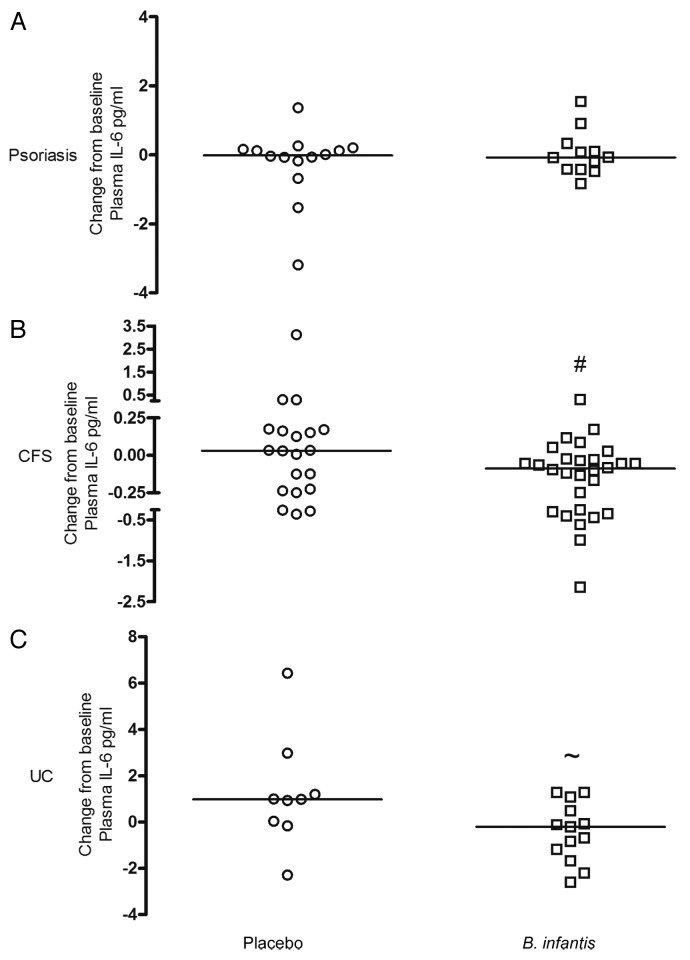
Plasma IL-6 levels were numerically lower in B. infantis 35624-fed patients compared with placebo controls in CFS (p = 0.054) and UC (p = 0.057) but remained unchanged in psoriasis. Data are expressed as the change from baseline (post-treatment minus pre-treatment level) for each patient (Fig. 6) with the median values (interquartile range) presented in Table 2.
Figure 6.B. infantis 35624-feeding reduces plasma IL-6 levels compared with placebo. Plasma levels of IL-6 were reduced in the B. infantis 35624 treated groups compared with placebo treatment following 6–8 weeks of feeding in patients with chronic fatigue syndrome (CFS) (#p = 0.054) and ulcerative colitis (UC) (~p = 0.057). However, there was no difference in IL-6 levels following 8 weeks feeding with B. infantis 35624 in patients with psoriasis compared with placebo treatment. Results are expressed as the change from baseline (post-treatment minus pre-treatment level) for each patient. *p < 0.05 vs. placebo groups (Mann-Whitney U test).
When comparing pre-and post-feeding for CFS patients, plasma IL-6 levels were significantly reduced (p = 0.0021) after eight weeks of feeding B. infantis 35624 but remained unchanged in the placebo group (Fig. 7B). There was no statistically significant decrease in plasma IL-6 in psoriasis and UC following 6‒8 weeks feeding B. infantis 35624 compared with pretreatment levels. However, while plasma IL-6 levels remained unchanged in the psoriasis placebo group, they increased in the UC placebo-fed group (Fig. 7A and C).
Figure 7.B. infantis 35624-feeding reduces plasma IL-6 levels compared with pretreatment level. There was no change in plasma IL-6 levels following 8 weeks feeding with placebo, compared with pretreatment levels, in patients with inflammatory disorders. Plasma IL-6 levels were significantly reduced following 8 weeks of feeding with B. infantis 35624 compared with pretreatment levels in patients with chronic fatigue syndrome (CFS) (n = 28). However, there was no difference in IL-6 levels following 6 or 8 weeks feeding respectively with B. infantis 35624 in patients with ulcerative colitis (UC) (n = 13) or psoriasis (n = 12) compared with their pretreatment levels. Results are expressed as the individual responses of each patient (pg/ml) (pre-treatment vs. post-treatment level) **p < 0.01 (Wilcoxon matched pair test).
Modifications of plasma cytokine and CRP levels are correlated with B. infantis feeding
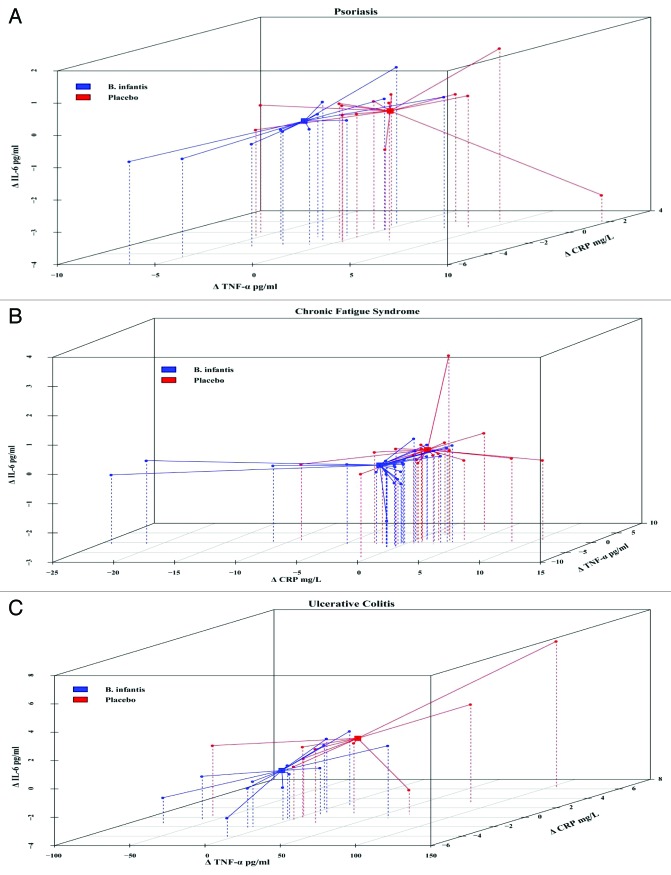
To further explore relationships with B. infantis 35624 feeding and responses in the various inflammatory markers, individual patient changes from baseline values of CRP, TNF-α and IL-6 were plotted in 3D scatter plots for each inflammatory disease; psoriasis, chronic fatigue syndrome and ulcerative colitis. This analysis revealed a separation of B. infantis 35624-fed patients from placebo-fed patients in all three inflammatory diseases (Fig. 8). To demonstrate a unique responder biomarker pattern, the individual CRP, TNF-α and IL-6 levels in both B. infantis 35624 and placebo-fed patients were assessed at the end of feeding. At Week 6–8, B. infantis 35624-feeding reduced plasma levels of CRP, TNF-α and IL-6 as represented by percentage change from baseline in the inflammatory disorder patients compared with placebo-fed patients (Table 3). At week 6–8, 75% of psoriasis patients, 71% of CFS patients and 62% of UC patients displayed decreased levels of CRP, TNF-α and IL-6, when fed B. infantis 35624. In contrast, only 7% of psoriasis patients, 11% of CFS patients and 0% of in the UC patients displayed decreased levels of CRP, TNF-α and IL-6 in the placebo-fed group (Table 4). Furthermore, 70% of all patients fed B. infantis 35624, independent of the nature of their inflammatory disorder, demonstrated a reduction in CRP, TNF-α and IL-6 compared with 9% in the placebo group (Table 4).
Figure 8.B. infantis 35624-feeding reduces pro-inflammatory biomarkers in a highly correlated way in inflammatory disorder patients. 3D Cluster analysis of plasma levels of CRP (z), TNF-α (x) and IL-6 (y) from patients with psoriasis and ulcerative colitis (UC) and of CRP (x), IL-6 (y) and TNF-α (z) from patients with chronic fatigue syndrome (CFS) displayed a separation between the B. infantis 35624 treated groups compared with the placebo treated groups. Results are expressed as the change from baseline (post-treatment minus pre-treatment level) for each patient.
Table 3. In comparison to placebo, B. infantis 35624 feeding reduces plasma CRP, TNF-α and IL-6 levels, expressed as % change from baseline, in inflammatory disorder patients.
| Disease Indication | Treatment | % Change in the level of CRP |
% Change in the level of TNF-α | % Change in the level of IL-6 |
|---|---|---|---|---|
|
Psoriasis |
B. infantis |
-34.0 (-64.0, -7.25) |
-15.5 (-20.0, 3.50) |
-3.0 (-13.0, 16.0) |
| Placebo |
2.0 (-31.75,154.8) |
0.0 (-22.0, 15.0) |
-5.0 (-20.5, 11.25) |
|
|
Chronic Fatigue Syndrome |
B. infantis |
-12.0 (-55.0, 15.75) |
-6.0 (-13.0, 4.0) |
-14.0 (-26.5, -5.0) |
| Placebo |
34.0 (-15.0, 211.3) |
3.0 (-6.0, 17.0) |
4.0 (-10.5, 25.5) |
|
| Ulcerative Colitis |
B. infantis |
-38.0 (-79.0, 178.0) |
-5.0 (-9.50, 8.50) |
-5.0 (-33.0, 36.5) |
| Placebo | 261.0 (19.0, 395.5) | 5.0 (-11.0, 20.0) | 27.0 (-3.5, 137.0) |
Table 4. More patient with inflammatory disorders achieve reductions in plasma CRP, TNF-α and IL-6 levels following feeding with B. infantis 35624 compared with placebo-fed patients.
| Disease Indication | Treatment | Number of subjects ↓IL-6, ↓TNF-α, ↓CRP | Total Number of subjects | % of Subjects ↓IL-6, ↓TNF-α, ↓CRP |
|||
|---|---|---|---|---|---|---|---|
|
Psoriasis |
B. infantis |
9 |
12 |
75% |
|||
| Placebo |
1 |
14 |
7% |
||||
|
Chronic Fatigue Syndrome |
B. infantis |
20 |
28 |
71% |
|||
| Placebo |
3 |
20 |
11% |
||||
|
Ulcerative Colitis |
B. infantis |
8 |
13 |
62% |
|||
| Placebo |
0 |
9 |
0% |
||||
| Inflammatory Diseases |
B. infantis |
37 |
53 |
70% |
|||
| Placebo | 4 | 43 | 9% | ||||
B. infantis 35624 reduces pro-inflammatory biomarkers in healthy subjects
In contrast to the inflammatory disease patients described above, the plasma levels of CRP, TNF-α and IL-6 in healthy human volunteers were unaffected by eight weeks feeding with B. infantis 35624 (data not shown). However, B. infantis 35624 feeding for eight weeks resulted in a reduction in the in vitro secretion of TNF-α (p < 0.05) and IL-6 (p < 0.05) from lipopolysacharide-(LPS) stimulated peripheral blood mononuclear cells (PBMCs) in comparison to those fed placebo (Fig. 9).
Figure 9.B. infantis 35624-feeding reduces pro-inflammatory cytokines levels from LPS stimulated PBMCs in healthy volunteers. In vitro LPS stimulated PBMCs displayed decreased secretion of IL-6 and TNF-α following B. infantis 35624 oral consumption (n = 10) for eight weeks. This finding was not observed in the placebo group (n = 12). Results are expressed as the change from baseline (post-treatment minus pre-treatment level) for each individual. *p < 0.05 vs. placebo group (Mann-Whitney U test).
Discussion
Our findings show that oral administration of B. infantis 35624 modulates the cytokine milieu across both gastrointestinal and non-gastrointestinal inflammatory disorders and healthy subjects. B. infantis 35624 significantly reduced plasma CRP levels in all patient groups, plasma TNF-α in the non-gastrointestinal disorders, psoriasis and CFS, while non-statistically significant trends toward a reduction in levels of IL-6 were seen in patients with CFS and UC. Furthermore, B. infantis 35624 altered responses to inflammatory stimuli in ex vivo cultures from healthy volunteers.
Chronic inflammatory diseases such as UC, CFS and psoriasis are characterized by an over-production of pro-inflammatory cytokines. C-reactive protein (CRP) is an acute phase protein synthesized by hepatocytes and adipocytes in response to increased peripheral pro-inflammatory cytokines, such as TNF-α and IL-6,13,14 and the serum or plasma level is a useful and clinically relevant indicator of systemic pro-inflammatory activity in multiple inflammatory states.15 In this study, plasma CRP was significantly elevated in all conditions investigated compared with healthy controls. These elevated levels of CRP concur with the results of previous studies conducted in CFS,16,17 psoriasis,18-21 and UC patients.22 In standard clinical laboratories the median value of plasma CRP for healthy subjects is < 1mg/L.23-25 Ultrasensitive assays have associated low-grade inflammation (CRP > 2.4 mg/L) with increased risk for coronary artery disease,26 while levels of CRP from 10‒1000 mg/L are associated with overt inflammatory and infectious disorders.25 Interestingly, B. infantis 35624 significantly reduced plasma CRP levels in all three inflammatory conditions examined. Regarding the significance of changes in CRP following treatment, the best illustration is provided by studies in heart disease where very small increments in CRP (1–2 mg/L) were associated with substantial increases in risk for coronary events.27
The effect of other microbes on CRP levels has been quite variable. Studies have shown that microbial treatment reduced CRP levels in the serum or plasma in ulcerative colitis28-30 while others noted an increase in serum CRP levels in a non-gastrointestinal condition eczema dermatitis31,32 or no effect in both gastrointestinal33 and non-gastrointestinal conditions.31,34,35 This suggests that not all commensal microbes can induce this effect in humans.
TNF-α and IL-6 are pro-inflammatory cytokines which are elevated in a variety of inflammatory conditions and involved in transcriptional regulation of CRP.25,36 They are not employed in clinical practice but both of these cytokines have been targeted by aggressive anti-cytokine biologic agent therapy in the treatment of autoimmune diseases.37,38 The attenuation of CRP following B. infantis 35624 treatment in this study is consistent with the reduction in circulating levels of both of those pro-inflammatory cytokines. In general, reduction in inflammatory markers, such as those seen in this study would be regarded as indicative of clinical remission and of a lower risk of relapse.22,39,40
Patients with CFS, psoriasis and UC had higher baseline TNF-α levels compared with healthy controls. These findings are in agreement with other studies.22,41-43 Following eight weeks of treatment with B. infantis 35624, a significant attenuation of TNF-α was observed for CFS and psoriasis patients; no such effect was noted with placebo treatment. A trend toward decreased TNF-α in the UC group was also observed, but this did not reach statistical significance, perhaps due to the shorter treatment time (i.e., 6 weeks). These data implicate TNF-α in the pathophysiology of inflammatory conditions and support the use of specific and well selected therapeutic microbes in alleviating the inflammatory component of such conditions, despite the often conflicting literature.34,35,44-47
Plasma IL-6 levels were significantly higher in CFS, psoriasis and UC patients compared with healthy controls, which is consistent with the findings of previous studies in psoriasis,42,48 CFS43,49 and UC.50 Though previous studies have indicated that certain therapeutic microbes could significantly reduce IL-6 levels in both patients with gastrointestinal inflammatory disorders and healthy controls,44,51-53 plasma IL-6 levels were only marginally decreased following B. infantis 35624 administration in this study. These data reinforce the hypothesis that individual elements of the microbiota influence specific components of the host immune response and not all members of the microbiota, or even not all members of the same species, exert an identical effect.
Overall, 70% of all of the individuals with UC, CFS or psoriasis fed B. infantis 35624 for 6–8 weeks showed marked decreases in all three biomarkers. Despite the limitations of post hoc analysis, this finding supports the concept of B. infantis 35624 acting centrally, perhaps through increased numbers of regulatory T cells, to minimize pro-inflammatory activity in vivo. Furthermore, in the ex vivo studies, secretion of IL-6 and TNF-α from LPS-stimulated peripheral blood mononuclear cells (PBMCs) was significantly reduced by B. infantis 35624 feeding in healthy controls compared with placebo-fed controls.
There are limitations to this study: first, the n value for each clinical condition studied is relatively low; collectively, however, the message is consistent. While the numbers included were sufficient to demonstrate statistical significance for objectively measured biomarkers, larger patient populations would be required to demonstrate clinical efficacy. Second, only one daily dose of this microbe was studied and a dose-dependent anti-inflammatory effect was, therefore, not demonstrated. It is noteworthy in this respect, that many naturally occurring and even biologic agents, such as anti-TNF-α strategies, do not exhibit clear dose-response profiles.
In conclusion, oral administration of a single microbial agent, B. infantis 35624, was sufficient to reduce systemic inflammatory biomarkers in both gastrointestinal and extra-intestinal inflammatory disorders. This is consistent with the hypothesis that certain elements of the enteric microbiota can induce mucosal immunoregulatory responses that can exert an effect systemically.
Methods
Psoriasis patients
Twenty-six male and female patients aged between 18 and 60 y with mild to moderate chronic plaque psoriasis with a psoriasis area severity index (PASI) < 16 were identified from the community by means of local advertising in newspapers and General Practice Clinics.
Ulcerative colitis patients
Twenty-two male and female patients aged between 18 and 75 y with mild to moderate active ulcerative colitis (UC) with a Clinical Activity Index Assessment (CAIA) score ≥ 3 were identified. Subjects were recruited from the inflammatory bowel disease (IBD) Clinic at Cork University Hospital. These subjects had a clinical diagnosis of UC confirmed by clinical, radiological and/or pathological evidence, and their level of disease activity required a change in treatment. All these patients were on the optimal dose of mesalazine (5-ASA) and remained on the same dose throughout the study.
Chronic fatigue syndrome patients
Patients with chronic fatigue syndrome (CFS) meeting the criteria outlined by the Centers for Disease Control (CDC) were recruited from gastroenterology and rheumatology clinics at Cork University Hospital. A total of 48 female patients between 18 and 65 y of age were selected. Those who had other diseases of the gastrointestinal tract, including inflammatory bowel disease (IBD) and clinically significant systemic diseases, individuals diagnosed with lactose intolerance or immunodeficiency, individuals who had undergone any abdominal surgery (with the exception of hernia repair and appendectomy), and those with a psychiatric illness were excluded. The diagnosis of CFS was made using the 1994 Centers for Disease Control and CFS International Study Group diagnostic criteria.54 The international criteria are based on the fulfillment of two major criteria: CFS causing incapacity, lasting more than 6 mo, and the exclusion of associated medical and psychiatric conditions, as well as the concurrence of a series of symptom-based criteria, particularly rheumatologic and neuropsychological symptomatology.
Healthy subject population
35 healthy volunteers (25 female) aged between 18 and 65 were recruited by direct advertisement on the university campus and in a local newspaper. These subjects had no evidence of gastrointestinal tract disease, including irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD), and were also free of any clinically significant systemic diseases including psoriasis and chronic fatigue syndrome. These 35 subjects were used as baseline references for the patients with inflammatory conditions. Twenty-two of the healthy adults were randomized to receive Bifidobacterium infantis 35264 (n = 10) or placebo (n = 12) for eight weeks.
Exclusion criteria
Pregnant or breast feeding females, individuals diagnosed with lactose intolerance or immunodeficiency, individuals who had undergone any abdominal surgery (with the exception of hernia repair and appendectomy) and those with a psychiatric illness or with significant hepatic, renal disease were excluded from all arms of the study, as were patients receiving immunosuppressant therapy or probiotics.
Each potentially eligible patient was evaluated by a full review of clinical history, physical examination, full blood count and routine biochemistry analysis. Subjects with any clinically significant abnormalities in any of these tests were excluded from the study.
Study design
The psoriasis arm of the study was performed in winter and early spring to minimize the confounding effect of ambient UV light; all the other arms of the trial were staggered throughout all seasons of the year. All interventions were performed as randomized, double-blind placebo-controlled studies.
Patients attended for baseline assessment of disease severity and blood was obtained for standard laboratory assessments of full blood count, electrolytes, renal and hepatic function hematology and biochemistry.
Nine milliliters of whole blood was collected in potassium EDTA tubes for biomarker analysis. Samples were centrifuged immediately and plasma frozen at -80°C. Measurements of plasma C-reactive protein (CRP), IL-6 and TNF-α were performed using an electrochemiluminescence multiplex system Sector 2400 Imager from Meso Scale Discovery where antibodies labeled with Sulfo-tag reagents emitted light upon electrochemical stimulation. This is an ultra-sensitive method which has a detection limit for CRP of 0.7ng/ml, IL-6 of 0.3pg/ml and TNF-α of 0.3pg/ml.
Peripheral blood mononuclear cells (PBMCs) were isolated on day -1 (pre-feeding) and day 56 (post-feeding) from healthy volunteers. PBMCs (1 × 106 /mL) were stimulated with 1µg/ml lipopolysaccharide (LPS) for 72 h. PBMC supernatants were stored frozen at -80°C and analyzed for cytokine levels simultaneously using the Meso Scale Discovery multiplex platform.
Treatments
Each patient and 22 healthy controls received sachets containing either 1 × 1010 CFU viable Bifidobacterium infantis 35264 or 5g Maltodextran as placebo per day. Probiotic and placebo were identically packaged. Both patients and investigators were blinded with regard to which treatment was being administered. CFS, psoriasis and healthy controls received either Bifidobacterium infantis 35264 or placebo for eight weeks; UC patients for six weeks.
Statistical analysis
Statistical analysis of cytokine and C-reactive protein levels was undertaken using GraphPad Prism for Windows (Version 5.0). Prior to analysis and to any comparisons being performed, data were examined for normality and homogeneity of variance using the D'Agostino and Pearson omnibus normality test. As there was evidence that the data were not normally distributed,non-parametric statistical methods were, therefore, used in the analysis of the data. When comparing the values for healthy controls vs. inflammatory diseases a Mann-Whitney U test was employed. To compare pre- vs. post-treatment cytokine levels in both B. infantis 35624 and placebo-fed patients a Wilcoxon signed rank test was used. When comparing the effects of B. infantis 35624 feeding and placebo on a patient’s cytokine levels, differences were calculated for each patient as change from baseline (visit 2, week 0) to the end of feeding. The Mann-Whitney U test was then used to compare these changes from baseline in cytokine levels between B. infantis 35624 and placebo-fed subjects in all arms of the study. Differences were considered significant at p < 0.05.
Ethical approval and consent
The study protocol, including all procedures, was approved by the University College Cork Clinical Research Ethics committee of the Cork Teaching Hospitals.
Acknowledgments
We are grateful to Anne Lyons and Dr Paul Scully for their technical assistance in this study. We are appreciative to Carmel Wycherley for the clinical management of the study. We would also like to thank Dr Caroline Brown for reviewing the manuscript and Dr Ian B. Jeffery for creating the three dimensional graphs. Contributions: D.G. performed the laboratory analysis. D.G., F.S., L.O.M., T.D., J.B., B.K. and E.Q. contributed to study concept and design, analysis and interpretation of the data. D.G., F.S., L.O.M., E.M. and E.Q. contributed to the drafting of the manuscript while E.Q. supervised the conduct of these studies.
Glossary
Abbreviations:
- Bifidobacteria infantis
B. infantis
- ulcerative colitis
UC
- chronic fatigue syndrome
CFS
- tumor necrosis factor
TNF-α
- interleukin
IL
- peripheral blood mononuclear cells
PBMCs
- lipopolysaccharide
LPS
- psoriasis area severity index
PASI
- clinical activity index assessment
CAIA
- irritable bowel syndrome
IBS
- inflammatory bowel disease
IBD
Disclosure of Potential Conflicts of Interest
D.G., E.M. and B.K. are employees of the university campus company Alimentary Health Ltd. L.O.M., T.D., F.S. and E.Q. are consultants to Alimentary Health Ltd. F.S. has received research grants from G.S.K. J.B. has no conflict of interest.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/25487
References
- 1.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–63. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 2.Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140:859–70. doi: 10.1016/j.cell.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4615–22. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–27. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–41. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyons A, O’Mahony D, O’Brien F, MacSharry J, Sheil B, Ceddia M, et al. Bacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. Clin Exp Allergy. 2010;40:811–9. doi: 10.1111/j.1365-2222.2009.03437.x. [DOI] [PubMed] [Google Scholar]
- 7.O’Mahony C, Scully P, O’Mahony D, Murphy S, O’Brien F, Lyons A, et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog. 2008;4:e1000112. doi: 10.1371/journal.ppat.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarthy J, O’Mahony L, O’Callaghan L, Sheil B, Vaughan EE, Fitzsimons N, et al. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut. 2003;52:975–80. doi: 10.1136/gut.52.7.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Mahony L, Feeney M, O’Halloran S, Murphy L, Kiely B, Fitzgibbon J, et al. Probiotic impact on microbial flora, inflammation and tumour development in IL-10 knockout mice. Aliment Pharmacol Ther. 2001;15:1219–25. doi: 10.1046/j.1365-2036.2001.01027.x. [DOI] [PubMed] [Google Scholar]
- 10.McKernan DP, Fitzgerald P, Dinan TG, Cryan JF. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterol Motil. 2010;22:1029–35, e268. doi: 10.1111/j.1365-2982.2010.01520.x. [DOI] [PubMed] [Google Scholar]
- 11.Konieczna P, Akdis CA, Quigley EM, Shanahan F, O’Mahony L. Portrait of an immunoregulatory Bifidobacterium. Gut Microbes. 2012;3:261–6. doi: 10.4161/gmic.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konieczna P, Groeger D, Ziegler M, Frei R, Ferstl R, Shanahan F, et al. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut. 2012;61:354–66. doi: 10.1136/gutjnl-2011-300936. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhardt SU, Thiele JR, Bannasch H, Stark GB, Peter K. C-reactive protein: how conformational changes influence inflammatory properties. Cell Cycle. 2009;8:3885–92. doi: 10.4161/cc.8.23.10068. [DOI] [PubMed] [Google Scholar]
- 14.Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, Rousseaux C, Dubuquoy C, Decourcelle C, et al. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn’s disease. Gut. 2012;61:78–85. doi: 10.1136/gutjnl-2011-300370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426–31. doi: 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raison CL, Lin JM, Reeves WC. Association of peripheral inflammatory markers with chronic fatigue in a population-based sample. Brain Behav Immun. 2009;23:327–37. doi: 10.1016/j.bbi.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Spence VA, Kennedy G, Belch JJ, Hill A, Khan F. Low-grade inflammation and arterial wave reflection in patients with chronic fatigue syndrome. Clin Sci (Lond) 2008;114:561–6. doi: 10.1042/CS20070274. [DOI] [PubMed] [Google Scholar]
- 18.Biljan D, Situm M, Kostović K, Batinac T, Matisić D. Acute phase proteins in psoriasis. Coll Antropol. 2009;33:83–6. [PubMed] [Google Scholar]
- 19.Gisondi P, Malerba M, Malara G, Puglisi Guerra A, Sala R, Radaeli A, et al. C-reactive protein and markers for thrombophilia in patients with chronic plaque psoriasis. Int J Immunopathol Pharmacol. 2010;23:1195–202. doi: 10.1177/039463201002300423. [DOI] [PubMed] [Google Scholar]
- 20.Coimbra S, Oliveira H, Reis F, Belo L, Rocha S, Quintanilha A, et al. Psoriasis therapy and cardiovascular risk factors: a 12-week follow-up study. Am J Clin Dermatol. 2010;11:423–32. doi: 10.2165/11319310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Baran W, Szepietowski JC, Mazur G, Baran E. IL-6 and IL-10 promoter gene polymorphisms in psoriasis vulgaris. Acta Derm Venereol. 2008;88:113–6. doi: 10.2340/00015555-0427. [DOI] [PubMed] [Google Scholar]
- 22.Turner D, Mack DR, Hyams J, LeLeiko N, Otley A, Markowitz J, et al. C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) or both? A systematic evaluation in pediatric ulcerative colitis. J Crohns Colitis. 2011;5:423–9. doi: 10.1016/j.crohns.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Hubacek JA, Peasey A, Pikhart H, Stavek P, Kubinova R, Marmot M, et al. APOE polymorphism and its effect on plasma C-reactive protein levels in a large general population sample. Hum Immunol. 2010;71:304–8. doi: 10.1016/j.humimm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–8. [PubMed] [Google Scholar]
- 25.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloyd-Jones DM, Liu K, Tian L, Greenland P. Narrative review: Assessment of C-reactive protein in risk prediction for cardiovascular disease. Ann Intern Med. 2006;145:35–42. doi: 10.7326/0003-4819-145-1-200607040-00129. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Air Force/Texas Coronary Atherosclerosis Prevention Study Investigators Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–65. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 28.Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O’Neil DA, et al. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54:242–9. doi: 10.1136/gut.2004.044834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliva S, Di Nardo G, Ferrari F, Mallardo S, Rossi P, Patrizi G, et al. Randomised clinical trial: the effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment Pharamcol Ther. 2012;35:327–34. doi: 10.1097/01.sla.0000219039.20924.88. [DOI] [PubMed] [Google Scholar]
- 30.Huynh HQ, deBruyn J, Guan L, Diaz H, Li M, Girgis S, et al. Probiotic preparation VSL#3 induces remission in children with mild to moderate acute ulcerative colitis: a pilot study. Inflamm Bowel Dis. 2009;15:760–8. doi: 10.1002/ibd.20816. [DOI] [PubMed] [Google Scholar]
- 31.Viljanen M, Pohjavuori E, Haahtela T, Korpela R, Kuitunen M, Sarnesto A, et al. Induction of inflammation as a possible mechanism of probiotic effect in atopic eczema-dermatitis syndrome. J Allergy Clin Immunol. 2005;115:1254–9. doi: 10.1016/j.jaci.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 32.Marschan E, Kuitunen M, Kukkonen K, Poussa T, Sarnesto A, Haahtela T, et al. Probiotics in infancy induce protective immune profiles that are characteristic for chronic low-grade inflammation. Clin Exp Allergy. 2008;38:611–8. doi: 10.1111/j.1365-2222.2008.02942.x. [DOI] [PubMed] [Google Scholar]
- 33.Reddy BS, Macfie J, Gatt M, Larsen CN, Jensen SS, Leser TD. Randomized clinical trial of effect of synbiotics, neomycin and mechanical bowel preparation on intestinal barrier function in patients undergoing colectomy. Br J Surg. 2007;94:546–54. doi: 10.1002/bjs.5705. [DOI] [PubMed] [Google Scholar]
- 34.Hatakka K, Martio J, Korpela M, Herranen M, Poussa T, Laasanen T, et al. Effects of probiotic therapy on the activity and activation of mild rheumatoid arthritis--a pilot study. Scand J Rheumatol. 2003;32:211–5. doi: 10.1080/03009740310003695. [DOI] [PubMed] [Google Scholar]
- 35.Andreasen AS, Larsen N, Pedersen-Skovsgaard T, Berg RM, Møller K, Svendsen KD, et al. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr. 2010;104:1831–8. doi: 10.1017/S0007114510002874. [DOI] [PubMed] [Google Scholar]
- 36.Choi YS, Hur J, Jeong S. Beta-catenin binds to the downstream region and regulates the expression C-reactive protein gene. Nucleic Acids Res. 2007;35:5511–9. doi: 10.1093/nar/gkm547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldmann M. Many cytokines are very useful therapeutic targets in disease. J Clin Invest. 2008;118:3533–6. doi: 10.1172/JCI37346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–96. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 39.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 40.Glintborg B, Østergaard M, Dreyer L, Krogh NS, Tarp U, Hansen MS, et al. Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor α therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheum. 2011;63:382–90. doi: 10.1002/art.30117. [DOI] [PubMed] [Google Scholar]
- 41.Abanmi A, Al Harthi F, Al Agla R, Khan HA, Tariq M. Serum levels of proinflammatory cytokines in psoriasis patients from Saudi Arabia. Int J Dermatol. 2005;44:82–3. doi: 10.1111/j.1365-4632.2004.02082.x. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi H, Tsuji H, Hashimoto Y, Ishida-Yamamoto A, Iizuka H. Serum cytokines and growth factor levels in Japanese patients with psoriasis. Clin Exp Dermatol. 2010;35:645–9. doi: 10.1111/j.1365-2230.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- 43.Scully P, McKernan DP, Keohane J, Groeger D, Shanahan F, Dinan TG, et al. Plasma cytokine profiles in females with irritable bowel syndrome and extra-intestinal co-morbidity. Am J Gastroenterol. 2010;105:2235–43. doi: 10.1038/ajg.2010.159. [DOI] [PubMed] [Google Scholar]
- 44.Loguercio C, Federico A, Tuccillo C, Terracciano F, D’Auria MV, De Simone C, et al. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540–3. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 45.Fukushima Y, Miyaguchi S, Yamano T, Kaburagi T, Iino H, Ushida K, et al. Improvement of nutritional status and incidence of infection in hospitalised, enterally fed elderly by feeding of fermented milk containing probiotic Lactobacillus johnsonii La1 (NCC533) Br J Nutr. 2007;98:969–77. doi: 10.1017/S0007114507764723. [DOI] [PubMed] [Google Scholar]
- 46.Tandon P, Moncrief K, Madsen K, Arrieta MC, Owen RJ, Bain VG, et al. Effects of probiotic therapy on portal pressure in patients with cirrhosis: a pilot study. Liver Int. 2009;29:1110–5. doi: 10.1111/j.1478-3231.2009.02020.x. [DOI] [PubMed] [Google Scholar]
- 47.Sierra S, Lara-Villoslada F, Sempere L, Olivares M, Boza J, Xaus J. Intestinal and immunological effects of daily oral administration of Lactobacillus salivarius CECT5713 to healthy adults. Anaerobe. 2010;16:195–200. doi: 10.1016/j.anaerobe.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Duffin KC, Krueger GG. Genetic variations in cytokines and cytokine receptors associated with psoriasis found by genome-wide association. J Invest Dermatol. 2009;129:827–33. doi: 10.1038/jid.2008.308. [DOI] [PubMed] [Google Scholar]
- 49.Fletcher MA, Zeng XR, Barnes Z, Levis S, Klimas NG. Plasma cytokines in women with chronic fatigue syndrome. J Transl Med. 2009;7:96. doi: 10.1186/1479-5876-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goral V, Celenk T, Kaplan A, Sit D. Plasma cytokine levels in ulcerative colitis. Hepatogastroenterology. 2007;54:1130–3. [PubMed] [Google Scholar]
- 51.Federico A, Tuccillo C, Grossi E, Abbiati R, Garbagna N, Romano M, et al. The effect of a new symbiotic formulation on plasma levels and peripheral blood mononuclear cell expression of some pro-inflammatory cytokines in patients with ulcerative colitis: a pilot study. Eur Rev Med Pharmacol Sci. 2009;13:285–93. [PubMed] [Google Scholar]
- 52.McNaught CE, Woodcock NP, Anderson AD, MacFie J. A prospective randomised trial of probiotics in critically ill patients. Clin Nutr. 2005;24:211–9. doi: 10.1016/j.clnu.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Kekkonen RA, Lummela N, Karjalainen H, Latvala S, Tynkkynen S, Jarvenpaa S, et al. Probiotic intervention has strain-specific anti-inflammatory effects in healthy adults. World J Gastroenterol. 2008;14:2029–36. doi: 10.3748/wjg.14.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A, International Chronic Fatigue Syndrome Study Group The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]