Abstract
Intake of whole grains and other food products high in dietary fiber have long been linked to the prevention of chronic diseases associated with inflammation. A contribution of the gastrointestinal microbiota to these effects has been suggested, but little is known on how whole grains interact with gut bacteria. We have recently published the first human trial that made use of next-generation sequencing to determine the effect of whole grains (whole grain barley, brown rice or a mixture of the two) on fecal microbiota structure and tested for associations between the gut microbiota and blood markers of inflammation, glucose and lipid metabolism. Our study revealed that whole grains impacted gut microbial ecology by increasing microbial diversity and inducing compositional alterations, some of which are considered to have beneficial effects on the host. Interestingly, whole grains, and in particular the combination of whole grain barley and brown rice, caused a reduction in plasma interleukin-6 (IL-6), which was linked to compositional features of the gut microbiota. Therefore, the study provided evidence that a short-term increased intake of whole grains led to compositional alterations of the gut microbiota that coincided with improvements in systemic inflammation. In this addendum, we summarize the findings of the study and provide a perspective on the importance of regarding humans as holobionts when considering the health effects of dietary strategies.
Keywords: inflammation, gut microbiota, metabolic disorders, whole grains
Introduction
A substantial body of evidence indicates that the consumption of whole grains reduces the risk for several major chronic diseases, such as coronary heart disease (CHD), obesity, type 2 diabetes and colon cancer.1 The mechanisms by which whole grains exert their benefits are not fully understood, but the relevance of inflammation in the development of metabolic diseases2 and cancer3 has shifted focus to the anti-inflammatory role of whole grains.4 The epidemiological evidence for an anti-inflammatory effect of whole grains is consistent, and it concurs with the strong epidemiological evidence for a beneficial role in disease prevention.5 However, the evidence stemming from interventional studies regarding both the anti-inflammatory role and health effects of whole grains is inconclusive.4,5 Several reasons for this discrepancy have been debated, but an important confounding factor might be that whole grains differ with respect to their composition and health-promoting characteristics. In addition, the microbial populations in the gastrointestinal tract (the gut microbiota) have been neglected in nutritional studies on whole grains,6 although they are likely to influence the release, fermentation and activation of the bioactive compounds present in whole grains.7 The gut microbiota composition is variable in humans, and it differs functionally across individuals in its ability to ferment dietary carbohydrates,8 constituting a potential confounding factor in studies on the health effects of whole grains.
The gut microbiota has long been hypothesized to play a role in the health effects of whole grains.9 Indeed, recent research revealed that virtually all the diseases that show a negative correlation with whole grain consumption are also connected to the gut microbiota.10 In animal models, the gut microbiota contributes to the development of obesity,11,12 type 2 diabetes,11,12 CHD,13 and colon cancer,14 likely by exacerbating the different subsets of inflammation that contribute to these pathologies.14,15 It is now well established that susceptibility to chronic inflammation is affected by both the microbial communities in the gut and the diet, and evidence has emerged indicating that diet can both increase and decrease the inflammatory potential of the gut microbiota.16-19 A diet high in fat not only causes a microbiota-driven inflammation that precedes obesity and insulin resistance in mice,18 but also decreases intestinal epithelial barrier function leading to endotoxemia due to increased translocation of bacterial lipopolysaccharide (LPS).20 The anti-inflammatory effect of whole grains and their preventive role in diseases exacerbated through a microbiota-driven inflammation suggest that some health benefits of whole grains are due to their effects on the gut microbiome. Despite this emerging link, very little is known about the effect of whole grains on the human gut microbiota and how this effect relates to benefits.
A Human Trial to Determine the Effects of Whole Grains on Both Human Physiology and the Gut Microbiota
We have recently completed the first human trial that employed next-generation sequencing to determine the effect of whole grains on the composition of the fecal microbiota and tested for associations between the bacterial populations and blood markers of inflammation, glucose and lipid metabolism.21 The trial was a non-controlled, randomized crossover study with 28 participants (17 females and 11 males, age 25.9 ± 5.5 y, 13 classified as overweight) with three four-week treatments of either whole grain barley (WGB), brown rice (BR) or a mixture of the two (WGB+BR) in the form of flakes (60 g daily dose). The first week served as a baseline period, and the treatment periods were separated by two-week washout periods (Fig. 1). Participants were advised to incorporate the whole grains into their regular diets. Total body composition was assessed at baseline, and blood samples were drawn at baseline and at the end of each dietary treatment for the determination of fasting glucose and insulin, a lipid profile and the inflammatory markers lipopolysaccharide binding protein (LBP), high-sensitivity CRP (hs-CRP) and IL-6. The glycemic and insulin postprandial response were determined after administration of a standard glucose challenge. Fecal samples were collected within 24 h of blood collection to facilitate the characterization of fecal microbiota composition (by 454 amplicon sequencing of the V1-V3 region of the 16S rRNA gene) and short chain fatty acids (SCFA) in parallel to the host phenotyping.
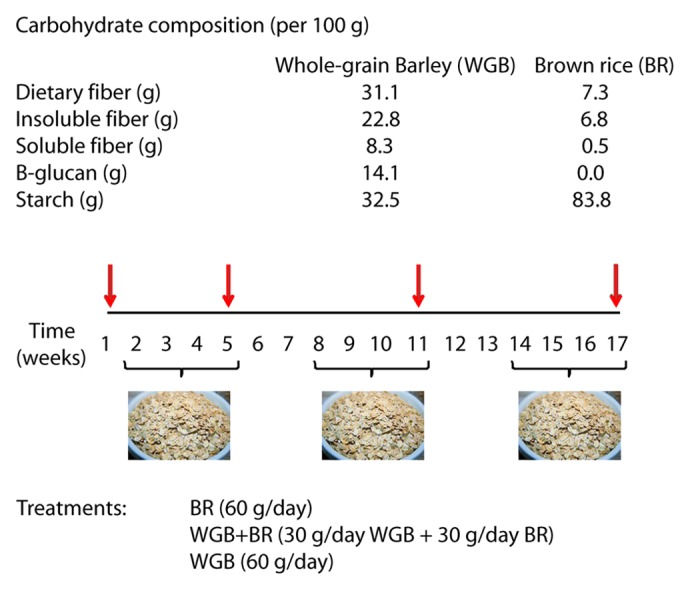
Figure 1. Carbohydrate composition of the whole grains used in the study and trial design. Twenty-eight subjects participated in a randomized 17-week crossover study that investigated the impact of brown rice (BR), whole-grain barley (WGB) and a combination of the two on gut microbiota composition and metabolic and immunological markers. Stool samples and blood samples (indicated by red arrows) were collected during the baseline and at the end of each treatment period.
Highly significant positive correlations between body fat and all three inflammatory markers were detected in the study population at baseline, substantiating the link between adiposity and a low-grade systemic inflammation.2 Interestingly, LBP and hs-CRP were highly correlated (r = 0.90, p < 0.0001), supporting the relation between bacterial LPS and systemic inflammation and the hypothesis of a microbiota-driven endotoxemia.20 Although we did not detect any associations between body mass index and the gut microbiota, all three inflammatory markers showed negative correlations with the family Ruminococcaceae, and this family was also significantly enriched in individuals with low body fat. Within this family, the genera Faecalibacterium and Ruminococcus showed negative correlations with hs-CRP. Faecalibacterium has been shown to be anti-inflammatory in mouse models of colitis,22 has been repeatedly inversely associated with inflammation and has been determined to be less abundant in patients who are obese and diabetic,15 but more research is needed to assess the causation of these associations in humans.
Whole Grain Intake Reduced Systemic Inflammation and Improved Glucose and Lipid Metabolism
The trial revealed that whole grains led to measurable benefits. A significant decrease in plasma IL-6 levels for the WGB+BR treatment vs. baseline values was detected. Quantitatively, this reduction was highest in overweight subjects and in females. Despite not achieving statistical significance due to high inter-individual variation, hs-CRP plasma levels halved during the WGB+BR period when compared with the baseline. Whole grain consumption also improved glucose and lipid metabolism. Postprandial peak glucose levels were significantly lowered in overweight subjects during the WGB+BR period, fasting glucose levels were significantly decreased in women and overweight subjects, and total cholesterol was significantly reduced in females. Interestingly, although all treatments led to immunological and metabolic improvements (especially in females), it was the combination of whole grains that generated the most significant benefits, suggesting a synergistic role between WGB and BR. It is relevant to point out that synergistic activities between whole grain varieties provide a potential explanation for the discrepancy of the health effects of whole grains obtained in epidemiological and interventional studies.4,5,23 Interventional trials might have failed to show benefits because they focused on a limited selection of whole grains, while in epidemiological trials, subjects are likely to consume a diverse set of whole grains which might have synergistic activities.
An explanation for the synergism between the whole grains in our study might be interactions of their components. The WGB and BR flakes differed markedly in their composition, especially in terms of their β-glucan and starch contents (Fig. 1). β-glucan has been shown to modify starch digestibility in humans, resulting in reduced postprandial plasma glucose and insulin concentrations.24,25 Although the reasons for the observed synergism are unknown, we speculate that a possible explanation is that the β-glucan in WGB increased the viscosity of the digesta in the small intestine, which in turn reduced starch digestion rate. The notable consequence of combined WGB and BR intake, could therefore yield increased starch available in the colon, which might have both physiological benefits and an effect on the gut microbiota,26,27 and which might increase microbial butyrate production, an anti-inflammatory bacterial metabolite.28
Effects of Dietary Whole Grain Supplementation on Fecal Microbial Communities
The characterization of the fecal microbiota revealed that all three whole grain treatments induced detectable compositional alterations. All test meals significantly increased the bacterial diversity measured by Shannon’s and Simpson’s indices. Interestingly, intake of prebiotics, resistant starches and dietary fibers, do not seem to increase bacterial diversity in the gut.26,29,30 The compositional complexity of whole grains, which contain a variety of carbohydrates and phytochemicals, could potentially support a wider scope of bacterial taxa when compared with more homogenous carbohydrates, thereby leading to an increase in diversity.
Despite substantial inter-individual variation in fecal microbiota composition, whole grain intake induced statistically significant changes across the subject population. The proportion of the phyla Firmicutes and Bacteroidetes increased and decreased, respectively, with virtually no differences between the treatments. While the decrease in phylum Bacteroidetes was to a large degree due to a reduction of the genus Bacteroides, the expansion within the Firmicutes was more diverse. All whole grain treatments increased the abundance of the genus Blautia and two species within this genus, although WGB propitiated a larger increase. Proportions of the genera Roseburia, Bifidobacterium and Dialister and the species Eubacterium rectale, Roseburia faecis and Roseburia intestinalis increased through WGB intake, and several of these taxa showed a gradual increase according to WGB dose.
Interestingly, the changes in gut microbial ecology induced through whole grains appeared to reflect some of the characteristics of the fecal microbiota associated with long-term diets rich in plant derived carbohydrates. Individuals from non-westernized societies in Burkina Faso, Malawi and the Amazon rain forest of Venezuela, whose regular diet is rich in grains, dietary fibers and vegetables, harbor gut bacterial communities that are more diverse and contain lower proportions of Bacteroides than individuals from western countries.31,32 In addition, US citizens on long-term carbohydrate-rich diets also have decreased levels of Bacteroides when compared with individuals with diets high in protein and animal fat.33 However, short-term intake of whole grains did not increase the abundance of the genus Prevotella, which is enriched in non-westernized societies and US individuals on long-term diets rich in polysaccharides.31-33
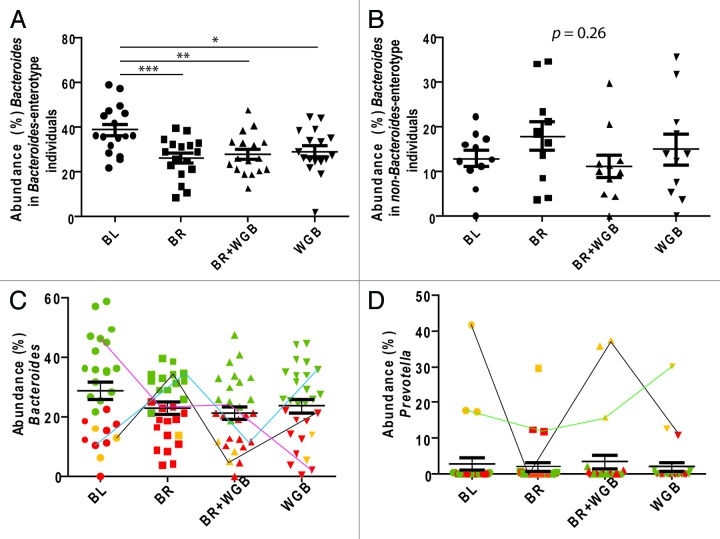
Prevotella and Bacteroides appear to exclude each other in the human gut,32 and they are key members of recently described distinct clusters within the fecal microbiota referred to as enterotypes.34 Enterotypes have been linked to long-term dietary preferences,33 and we therefore determined how whole grains influence enterotypes using a clustering analysis. This analysis confirmed the presence of three clusters each dominated either by the genera Bacteroides, Ruminoccus or Prevotella,34 although the clusters were not discrete, as shown by others,35 and instead follow gradients in the abundance of the three defining bacterial species. While whole grains reduced the amount of Bacteroides in the study population, they had no effect on enterotype membership. However, only the group of subjects that belonged to the “Bacteroides-enterotype” at baseline showed a significant reduction in Bacteroides (Fig. 2A and B). Interestingly, our analysis revealed that enterotype membership within individuals was not necessarily stable; some individuals switched enterotypes during the study period due to substantial shifts in the genera Bacteroides and Prevotella that seemed unrelated to the dietary treatments (Fig. 2C and D). Therefore, our data suggests that enterotypes, if they exist, might not be a fixed trait of an individual and that factors that remain yet unknown can shift cluster identity over time.
Figure 2. Effect of whole grains on the fecal microbiota in relation to bacterial taxa linked to enterotypes. Impact of dietary treatments on the abundance of Bacteroides based on whether the subject harbored (A) the enterotype dominated by Bacteroides or (B) the other non-Bacteroides two enterotypes (Ruminococcus and Prevotella) at baseline. The abundance of the genera (C)Bacteroides and (D)Prevotella in fecal samples of all subjects throughout the treatments. Dots represent populations within individual fecal samples, color-coded according to enterotypes (green = Bacteroides-dominant, red = Ruminococcus-dominant, yellow = Prevotella-dominant). Samples of subjects that showed changes in enterotype identity are linked with lines.
Mechanisms by Which Whole Grains Might Influence Gut Microbial Ecology
Whole grains contain an array of non-digestible carbohydrates that reach the colon where they can be fermented by bacteria. Dietary carbohydrate intake has been linked to higher levels of taxa within the phylum Firmicutes,36 and the increase in this phylum through whole grains is likely due to increased availability of carbohydrates in the colon. Although we did not detect an increase in fecal SCFA (probably because of their absorption by the host), increased carbohydrate fermentation is likely to have resulted in SCFA production, and several of the taxa that increased, such as Eubacterium rectale, Roseburia faecis, Roseburia intestinalis and bifidobacteria, produce SCFA. Consequently, the luminal pH is likely to have dropped through whole grain consumption. This might have caused the decrease in the proportion of Bacteroides, which have been shown to be inhibited under acidic conditions (Fig. 3).37 The high content of β-glucans in WGB probably explains the expansion of bacterial taxa that have the enzymatic capability to utilize this substrate, such as Eubacterium rectale and Roseburia intestinalis, which showed a dose response with increased consumption of WGB. In contrast to WGB, BR induced no significant changes at the species level. This may be explained by the fact that BR does not contain a single carbohydrate in high amounts and might be more heterogeneous as a substrate, leading to insufficient fractional increases of the individual fibers.38 Accordingly, wheat bran has not shown significant changes in the gut microbiota in a previous dietary study.27 The expansion of the genus Blautia, which are acetogens that utilize hydrogen, might have been caused by an increase in hydrogen production induced through higher levels of glycan fermentation.39
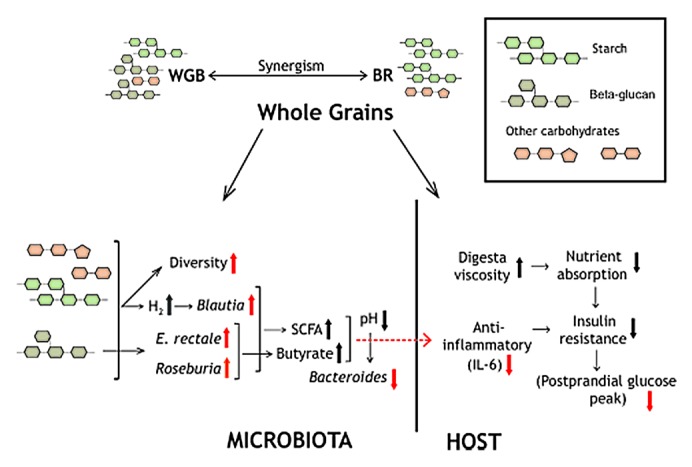
Figure 3. Schematic summary depicting possible mechanisms by which whole grains influence the human host and the gut microbiota (see text for details). Red arrows indicate findings obtained in the study,21 and black arrows indicate hypothetical changes.
Links Between Whole Grain-Induced Benefits on the Host and Fecal Bacterial Communities
To determine whether effects of whole grains on host phenotypes were associated with the fecal microbiota, we first performed a correlation analysis of shifts in bacterial abundance and changes in host markers during the WGB+BR period (the treatment that induced the most significant improvements). This analysis revealed that increases in the abundance of Eubacterium rectale correlated with improvements in postprandial glucose and insulin response. Second, we compared the baseline microbiota in subjects that showed different degrees of response to the whole grains. This analysis revealed that individuals with the greatest reduction in plasma IL-6 concentration had significantly higher proportions of Dialister and a lower abundance of the Coriobacteriaceae at baseline.
From the data generated in our study, we can only speculate about the role of the gut microbiota in the health benefits of whole grains. Intake of whole grains did not affect the Ruminococcaceae or the genus Faecalibacterium, which showed an association with systemic inflammation in the baseline population. However, Eubacterium rectale might exert metabolic and immunological effects through the production of butyrate.28 Furthermore, the whole grain-induced increases of bifidobacteria and Roseburia species, which have been suggested to affect immune/inflammatory and metabolic functions in animal models,12,40 might benefit the host. The associations of baseline populations of Dialister and Coriobacteriaceae with IL-6 response suggest that these taxa may condition the capability of an individual to be immunologically responsive to whole grains. It has been suggested that most of the phytochemicals present in whole grains are bound to carbohydrates and are not absorbed unless released through bacterial action in the colon,23 and Dialister might possess such activities. Coriobacteriaceae have been shown to be increased in patients with Crohn disease and colitic mice and might be pro-inflammatory.41
Conclusion and Perspective
Our study21 has provided the first look into the relationship between whole grains, the gut microbiota and host metabolism and showed that whole grain-induced alterations in the characteristics and composition of the fecal microbiota coincided with immunological and metabolic benefits. The associations between the reduction of IL-6 and the presence of certain bacterial taxa (Dialister, Coriobacteriaceae) indicate a functional role of gut bacteria in the physiological effects of whole grains, which has long been hypothesized.9,23
However, this study had limitations in the elucidation of mechanistic interactions and causation in the detected associations among diet, the gut microbiome and host metabolism. By relying solely on 16S rRNA tag sequencing, our study could not provide insight into the impact of whole grains on the metabolism of the gut microbiota and the microbial transformation of the bioactive phytochemicals. Future research should therefore combine metagenomic and metabolomic approaches with physiological studies of cultural isolates to characterize how bioactive compounds in whole grains become metabolized, released and modified by bacteria in the colon and how this correlates with effects on the host. Moreover, several of the beneficial effects of whole grains might be mediated without microbial contributions and correlations could result from the host and the microbiome responding independently to the diet. Therefore, the impact of whole grain diets (and other nutritional strategies) on host health should be compared in conventional and germ-free animals to separate direct vs. microbiota-mediated effects. Such a tractable model would then allow addition of cultured strains with specific characteristics to identify bacterial contributors and the underlying mechanisms.
The findings obtained in this study suggest that the gut microbiota might condition the health effects of whole grains. As the human gut microbiota is individualized, variations in the microbial communities could have functional consequences in terms of the host’s ability to process and release the bioactive compounds, phytochemicals and antioxidants present in whole grains. This would lead to higher levels of variation in intervention trials, and it clearly poses hurdles to developing universal dietary recommendations. Future studies should be aimed to determine bacterial groups that are linked to the health benefits of specific whole grains and to elucidate the underlying mechanisms. Such knowledge will ultimately allow us to develop more personalized nutritional strategies that consider the individual’s microbiota.
It is now becoming apparent that humans can be seen as holobionts that function and evolve not as single organisms but as an amalgam of host and symbiotic microbial communities, with their interactions influencing all aspects of human biology and health.42 Diet is a key factor that shapes the gut microbiota, which is the densest and probably the most important of the host-associated microbial communities. It is striking that whole-grains and resistant starches, both which provide substrates for the bacteria in the colon, show metabolic and immunological benefits related to the chronic diseases linked to the microbiome. The field of Nutrigenomics, which aims to use an individual’s genetic makeup to optimize nutrition, should therefore be extended to include the metagenome as well. This would ultimately result in something we might want to call “Holobiont Nutrition”, a holistic approach that considers not only our own nutritional requirements but also that of our symbiotic microbial communities and the interactions between the two entities. Given the importance of the microbiome for health, this approach has the potential to improve human nutrition substantially.
Acknowledgments
The original study was supported by ConAgra Foods (Omaha, Nebraska) and matching funds through the United States Department of Agriculture (USDA), Midwest Advanced Food Manufacturing Alliance (MAFMA) program.
Disclosure of Potential Conflicts of Iterest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/24707
References
- 1.Slavin J. Whole grains and human health. Nutr Res Rev. 2004;17:99–110. doi: 10.1079/NRR200374. [DOI] [PubMed] [Google Scholar]
- 2.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 3.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefevre M, Jonnalagadda S. Effect of whole grains on markers of subclinical inflammation. Nutr Rev. 2012;70:387–96. doi: 10.1111/j.1753-4887.2012.00487.x. [DOI] [PubMed] [Google Scholar]
- 5.Jonnalagadda SS, Harnack L, Liu RH, McKeown N, Seal C, Liu S, et al. Putting the whole grain puzzle together: health benefits associated with whole grains--summary of American Society for Nutrition 2010 Satellite Symposium. J Nutr. 2011;141:1011S–22S. doi: 10.3945/jn.110.132944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121:2126–32. doi: 10.1172/JCI58109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adom KK, Sorrells ME, Liu RH. Phytochemicals and antioxidant activity of milled fractions of different wheat varieties. J Agric Food Chem. 2005;53:2297–306. doi: 10.1021/jf048456d. [DOI] [PubMed] [Google Scholar]
- 8.Ze X, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012;6:1535–43. doi: 10.1038/ismej.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slavin J. Why whole grains are protective: biological mechanisms. Proc Nutr Soc. 2003;62:129–34. doi: 10.1079/PNS2002221. [DOI] [PubMed] [Google Scholar]
- 10.Aitken JD, Gewirtz AT. Gut microbiota in 2012: Toward understanding and manipulating the gut microbiota. Nat Rev Gastroenterol Hepatol 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–3. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–9. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 16.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–83. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 17.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487:104–8. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5:e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–6. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cani PD, Delzenne NM. Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr Opin Pharmacol. 2009;9:737–43. doi: 10.1016/j.coph.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Martínez I, Lattimer JM, Hubach KL, Case JA, Yang J, Weber CG, et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013;7:269–80. doi: 10.1038/ismej.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–6. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris KA, Kris-Etherton PM. Effects of whole grains on coronary heart disease risk. Curr Atheroscler Rep. 2010;12:368–76. doi: 10.1007/s11883-010-0136-1. [DOI] [PubMed] [Google Scholar]
- 24.Behall KM, Scholfield DJ, Hallfrisch JG. Barley beta-glucan reuces plasma glucose and insulin responses compared with resistant starch in men. Nutr Res. 2006;26:644–50. doi: 10.1016/j.nutres.2006.10.001. [DOI] [Google Scholar]
- 25.Regand A, Chowdhury Z, Tosh SM, Woleyer TMS, Wood PJ. The molecular weight, solubility and viscosity of oat beta-glucan affect human glycemic response by modifying starch digestibllity. Food Chem. 2011;129:297–304. doi: 10.1016/j.foodchem.2011.04.053. [DOI] [PubMed] [Google Scholar]
- 26.Martínez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS One. 2010;5:e15046. doi: 10.1371/journal.pone.0015046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–30. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McOrist AL, Miller RB, Bird AR, Keogh JB, Noakes M, Topping DL, et al. Fecal butyrate levels vary widely among individuals but are usually increased by a diet high in resistant starch. J Nutr. 2011;141:883–9. doi: 10.3945/jn.110.128504. [DOI] [PubMed] [Google Scholar]
- 29.Davis LM, Martínez I, Walter J, Goin C, Hutkins RW. Barcoded pyrosequencing reveals that consumption of galactooligosaccharides results in a highly specific bifidogenic response in humans. PLoS One. 2011;6:e25200. doi: 10.1371/journal.pone.0025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van den Abbeele P, Gérard P, Rabot S, Bruneau A, El Aidy S, Derrien M, et al. Arabinoxylans and inulin differentially modulate the mucosal and luminal gut microbiota and mucin-degradation in humanized rats. Environ Microbiol. 2011;13:2667–80. doi: 10.1111/j.1462-2920.2011.02533.x. [DOI] [PubMed] [Google Scholar]
- 31.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–6. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. MetaHIT Consortium Enterotypes of the human gut microbiome. Nature. 2011;473:174–80. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koren O, Knights D, Gonzalez A, Waldron L, Segata N, Knight R, et al. A guide to enterotypes across the human body: meta-analysis of microbial community structures in human microbiome datasets. PLoS Comput Biol. 2013;9:e1002863. doi: 10.1371/journal.pcbi.1002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol. 2007;73:1073–8. doi: 10.1128/AEM.02340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duncan SH, Louis P, Thomson JM, Flint HJ. The role of pH in determining the species composition of the human colonic microbiota. Environ Microbiol. 2009;11:2112–22. doi: 10.1111/j.1462-2920.2009.01931.x. [DOI] [PubMed] [Google Scholar]
- 38.Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol Res. 2013;69:52–60. doi: 10.1016/j.phrs.2012.10.020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Nakamura N, Lin HC, McSweeney CS, Mackie RI, Gaskins HR. Mechanisms of microbial hydrogen disposal in the human colon and implications for health and disease. Annu Rev Food Sci Technol. 2010;1:363–95. doi: 10.1146/annurev.food.102308.124101. [DOI] [PubMed] [Google Scholar]
- 40.Neyrinck AM, Possemiers S, Verstraete W, De Backer F, Cani PD, Delzenne NM. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin-glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J Nutr Biochem. 2012;23:51–9. doi: 10.1016/j.jnutbio.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, Zheng Z, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139:1844–, e1. doi: 10.1053/j.gastro.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 42.Zilber-Rosenberg I, Rosenberg E. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol Rev. 2008;32:723–35. doi: 10.1111/j.1574-6976.2008.00123.x. [DOI] [PubMed] [Google Scholar]