Abstract
Stress can enhance or suppress immune functions depending on a variety of factors. Our previous studies observed that Toll-like receptor 2 (TLR2) participates in chronic restraint stress-induced immune dysfunction. However, the mechanism by which TLR2 prevents immune suppression remains elusive. Our investigation found that stimulation of TLR2 by peptidoglycan (PGN) significantly attenuates splenocyte apoptosis and markedly blocks alterations of anti-apoptotic and apoptotic proteins. Activation of TLR2 inhibits chronic stress-reduced phosphorylation of c-Jun N-terminal kinase (JNK) and diminishes chronic stress-induced up-regulation of corticosterone production. Additionally, our data show that chronic stress causes a dramatic decrease of cytokine IL-2 level but an increase of IL-4 and IL-17 in CD4+ T cells. Interestingly, PGN could block these alterations of cytokine levels. Collectively, our studies demonstrate that stimulation of TLR2 attenuates chronic stress-induced immune suppression by modulating apoptosis-related proteins and immunoregulatory agents.
Keywords: TLR2, Stress, Apoptosis, Immune response, Corticosterone
1. Introduction
It has been shown that stress can either increase or decrease immune functions depending on a variety of factors including duration of stressful situation. Acute stress results in an enhancement of both cellular and humoral immunity, while chronic stress has significant suppressive effects on the immune system [1–4]. Reduction of lymphocyte numbers is demonstrated in a chronic restraint stress mouse model [1]. A recent study has shown that chronic stress induces a regulatory phenotype in macrophages with decreased tumor necrosis factor (TNF)-α and interleukin 6 (IL-6) but increased IL-10 production [2].
Toll-like receptor 2 (TLR2), a crucial member of TLR family, plays a critical role both in the innate and adaptive immune responses [5–7]. TLR2 is also involved in the regulation of cell survival and apoptosis [8]. As for apoptosis, a variety of molecules, especially caspases and members of the Bcl-2 family, play pivotal roles in this process. Among the caspases, caspase 3 is a key protein and a marker in apoptosis with the ability of cleaving and destroying hundreds of cellular protein substrates, and poly(ADP-ribose) (PARP) is one of the main cleavage targets of caspase 3 [9, 10]. The Bcl-2 family, central regulators of cell survival and apoptosis, is a group of proteins that either promote (eg., Bax and Bak) or prevent (e.g., Bcl-2 and Bcl-xL) apoptosis [11]. We previously observed that TLR2 plays a role in stress-induced reduction in the number of splenocytes [12]. However, the mechanisms by which TLR2 protects against stress-induced loss of splenocytes are still unclear.
C-Jun N-terminal kinase (JNK) is an important mediator of intracellular signaling cascade during innate and adaptive immune responses [13]. As a downstream target of TLR2 signaling pathway, JNK exerts strong regulatory effects on T help 1 (Th1) and Th2 responses [13, 14]. Glucocorticoids (GCs), a class of steroid hormones, are the end products of the hypothalamic-pituitary-adrenal (HPA) axis activity in response to inflammatory and stress-related stimuli [15, 16]. In adaptive immune system, GCs induce selective suppression of Th1 response and lead to a shift towards Th2-mediated humoral immunity [17]. So far, it is unclear whether these two immunoregulatory agents could be modulated by TLR2 stimulation which has been shown to rescue the Th1/Th2 disequilibrium induced by chronic stress.
In the current study, we investigated whether activation of TLR2 could block chronic stress-induced cell apoptosis and immune suppression. Specifically we determined the involvement of anti-apoptotic Bcl-2 and pro-apoptotic Bax. We also examined the involvement of JNK, corticosterone and cytokines.
2. Materials and methods
2.1. Mice
TLR2 knockout (TLR2 KO) mice on a C57BL/6 background and wild type C57BL/6 mice were purchased from Jackson Laboratory and maintained in the Division of Laboratory Animal Resources at East Tennessee State University (ETSU), a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All animal studies were approved by the ETSU Committee on Animal Care.
2.2. Experimental model of restraint stress
Six- to eight-week-old male mice were subjected to an established chronic physical restraint protocol used in our laboratory as well as others [1, 18]. Briefly, mice were placed in a 50-ml conical centrifuge tube with multiple punctures to allow ventilation. Mice were held horizontally in the tubes for 12 h. Control littermates were kept in their original cage without food and water for 12 h. After physical restraint, mice were sacrificed by CO2 asphyxiation, and the spleens were harvested.
2.3. Experimental protocols
To investigate the effects of PGN on splenocyte apoptosis and immune dysfunction in chronic stress, mice were treated with or without PGN (50µg/ 25g body weight, Sigma, St Louis, MO) by intraperitoneal (i.p.) injection 1h before the mice were subjected to restraint stress. To determine whether TLR2 is essential for the effects of PGN, TLR2 KO mice were also administered with or without PGN (50µg/ 25g body weight, i.p.) 1h before the initiation of stress.
2.4. Western blot analysis
Western blotting was performed as described previously [19]. Briefly, the cellular proteins were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred onto Hybond ECL membranes (Amersham Pharmacia, NJ). The ECL membranes were incubated overnight at 4°C with the appropriate primary antibody [anti-TLR2 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-Bcl-2, anti-Bax, anti-phospho-JNK, anti-JNK, anti-cleaved-caspase-3, anti-caspase-3, anti-PARP, anti-GAPDH (Cell Signaling Technology, Beverly, MA) ], followed by incubation with peroxidase-conjugated secondary antibodies (Cell Signaling Technology, Inc.). The blot was again washed three times with TBS before being exposed to the SuperSignal West Dura Extended Duration substrate (Pierce Biotechnology, Rockford, IL). The signals were quantified by scanning densitometry using a Bio-Image Analysis System (Bio-Rad).
2.5. TUNEL staining
TUNEL staining was performed as described previously [20]. Briefly, spleens from mice were fixed in 10% buffered formalin and embedded in paraffin. After deparaffinization and hydration of sections, TUNEL staining for apoptotic nuclei was performed using an In Situ Cell Death Detection kit (Roche Diagnostic, Indianpolis, IN). Briefly, sections were exposed for 10 min to a permeabilization solution (0.1% Triton X-100, 0.1% sodium citrate). After washing, 50µl of TUNEL reaction mixture was placed on the sections and then incubated in a humidified atmosphere for 60 min at 37°C. 50µl of substrate solution was added following convert-AP incubation. Finally, sections were counterstained with haematoxylin. Sections were examined with the light microscope using a 40×objective. The percentage of apoptotic cells was calculated (TUNEL-positive cells/total cells) and averaged across at least five randomly chosen microscopic fields for each slide.
2.6. Determination of corticosterone
Blood was collected from all experimental and control mice immediately after stress. Samples were allowed to clot for 30 minutes at room temperature before centrifugation for 15 minutes at 1000×g. Then serum was removed and stored at −20°C for subsequent assay. The serum level of corticosterone was determined using a corticosterone mouse ELISA kit (IBL America, Minnuapolis, MN).
2.7. Culture and stimulation of CD4+T cells
Splenic CD4+ T cells were negatively selected by using the MagCellect™ Mouse CD4+ T Cell Isolation Kit (R&D Systems, Minneapolis, MN). Isolated CD4+ T cells were plated in 96-well culture plates that were pre-coated with anti-CD3 (BD Biosciences Pharmingen, San Diego, CA) at 5µg/ ml in PBS for 24 h at 4 °C. Cells were seeded at 2.5×105 per well. The culture medium used was Gibco® RPMI Media 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal calf serum (FCS), 2mM L-glutamine, 100U/ml penicillin, 100mg/ ml streptomycin, and 1µg/ ml anti-CD28.
2.8. Enzyme linked immunosorbent assay (ELISA) for cytokines
The supernatants from anti-CD3/anti-CD28-stimulated CD4+ T cells cultures were collected after 48h. The amount of IL-2 and IL-4 in the supernatants was examined by using a Quantikine Mouse ELISA kit (R&D Systems, Minneapolis, MN) [1]. IL-17 was quantified using a mouse IL-17 platinum ELISA kit (eBioscience, Vienna, Austria).
2.9. Isolation of RNA and real-time quantitative RT-PCR
Total RNA was isolated from CD4+ T cells using a RNeasy Plus Mini Kit (QIAGEN Sciences, Maryland, MD) according to the manufacturer’s instructions. The real-time PCR was performed as described previously [1, 21]. Briefly, one microgram of RNA from each sample was used for reverse transcription and synthesis of cDNA using a Reaction Ready™ first strand cDNA synthesis kit (SABiosciences, Frederick, MD). PCR was performed using RT2 real-time™ SYBR Green Fluorescien PCR Master Mix (SABiosciences). GAPDH expression was used as internal control. The primer sequences used were as follows: IL-2 forward 5′-AGC AGC TGT TGA TGG ACC TA-3′, IL-2 reverse 5′- TAC TTG AAC CTG GAG ACG C-3′, IL-4 forward 5'- GGT CTC AAC CCC CAG CTA GT--3′, IL-4 reverse 5′-TAG TGA ACT CTC TCT AGT AGC CG-3′, IL-17 forward 5′- GCC CTC AGA CTA CCT CAA CC-3′, IL-17 reverse 5′-GAA TTC ATG TGG TGG TCC AG-3′, GAPDH forward 5′-TGA CCA CAG TCC ATG CCA TC-3′,GAPDH reverse 5′- GAT GGG GGT TAC ACA GGC AG-3′.
2.10. Statistical analysis
The results were presented as means ± S.D. The data were analyzed using one-way analysis of variance and Student’s t-test. A value of p < 0.05 was considered to be statistically significant.
3. Results
3.1. PGN protects against stress-induced splenocyte apoptosis through TLR2
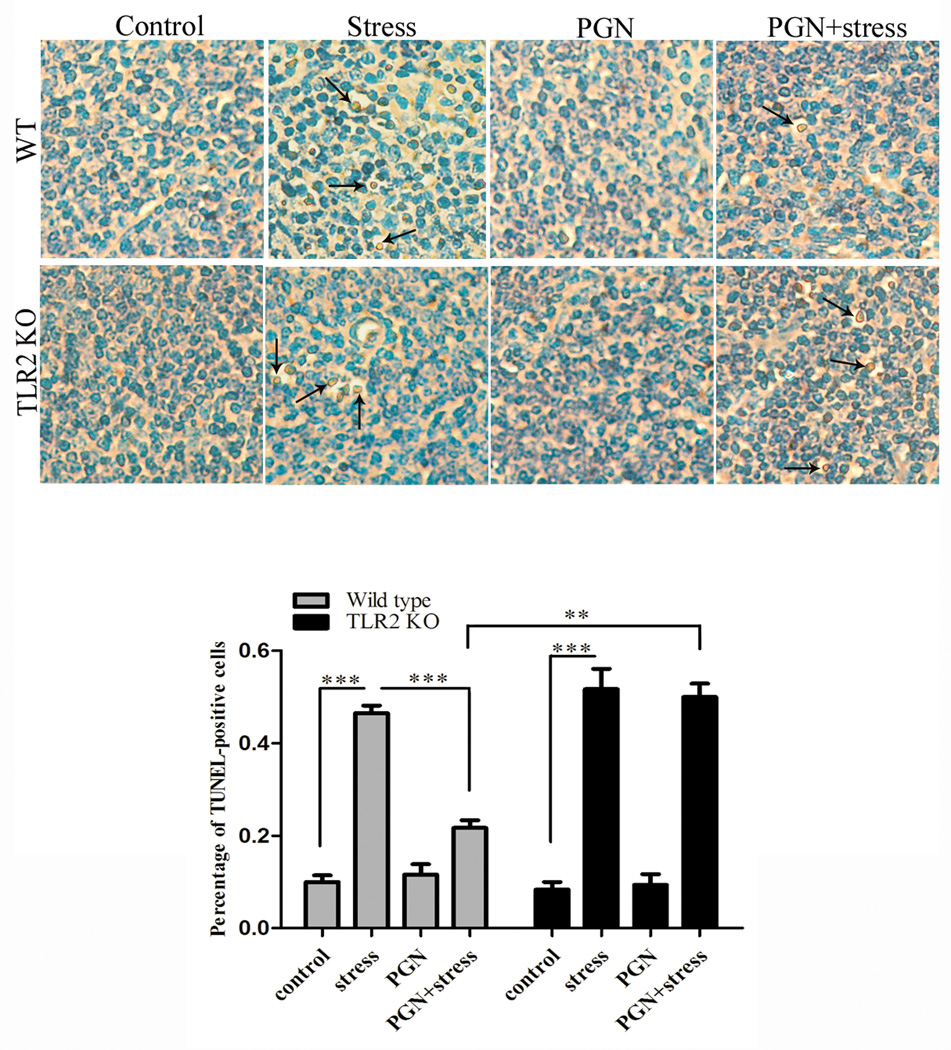
To determine whether PGN can block chronic stress-induced loss of splenocytes, wild type mice were administered with PGN and apoptotic cells in the mouse spleen were assessed by TUNEL assay. As shown in Fig.1, the percentage of TUNEL-positive cells was markedly decreased in PGN-treated stressed mice compared with untreated stressed mice. To evaluate whether this anti-apoptotic effect of PGN is through TLR2, we treated TLR2 KO mice with or without PGN before restraint stress. There was no significant difference in the percentage of apoptotic cells between PGN-treated and untreated TLR2 KO mice following chronic stress, suggesting that PGN exerts anti-apoptotic effect via a TLR2-dependent manner. Thus, we further attempted to determine the mechanisms responsible for the anti-apoptotic action of PGN in our system.
Fig. 1.
TLR2 stimulation by PGN protected against apoptosis of splenocytes following chronic stress. Age-matched TLR2 KO mice and wild type (C57BL/6) mice were administered with or without PGN (50µg/ 25g body weight, i.p.) 1 h before the mice were subjected to restraint stress (n = 5 per group). After 12 h of stress, mouse spleens were harvested and apoptotic cells were determined by TUNEL staining. ** P < 0.01 compared with indicated groups. *** P < 0.001 compared with indicated groups.
3.2. PGN blocks chronic stress-induced activation of caspase-3
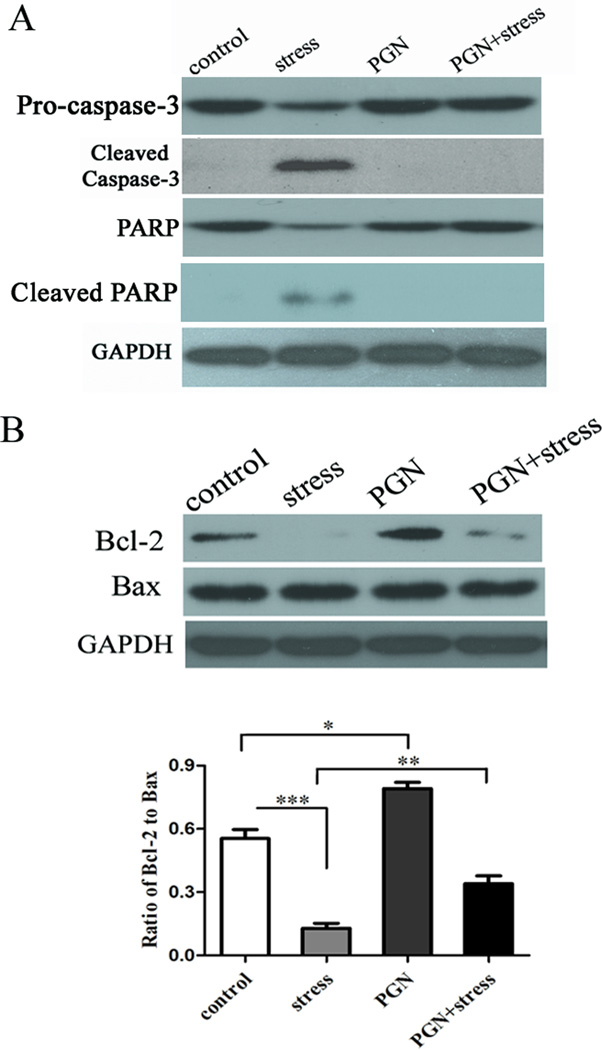
For most types of apoptosis, activation of the caspase family of cell death proteases is essential for the execution of both intrinsic and extrinsic apoptosis pathway [11]. However, we do not know whether caspase-3 involves in the chronic stress induced apoptosis of splenocytes. In this study, we found that the level of cleaved caspase-3 was remarkably increased in stressed mice (Fig. 2A). Congruously, induction of PARP cleavage was also detected in mice following chronic stress (Fig. 2A). Decrease of TLR2 expression was found at as early as 6 h of stress before detection of cleaved caspase-3 (data not shown). Importantly, PGN administration dramatically blocked stress-induced cleavage of caspase-3 as well as PARP to almost control level (Fig. 2A).
Fig. 2.
Administration of PGN modulated apoptosis-related proteins. Six to eight-week-old wild type (C57BL/6) male mice were pre-treated with PGN and stress as described in Fig. 1. At 12 h of stress, spleens were harvested and cellular proteins were extracted. The expression of cleaved caspase-3 and cleaved PARP (A), and Bcl-2/Bax (B) was analyzed by Western blot. *P < 0.05 compared with indicated groups. ** P < 0.01 compared with indicated groups. *** P < 0.001 compared with indicated groups.
3.3. PGN modulates apoptosis related genes
Anti-apoptotic protein Bcl-2 and pro-apoptotic protein Bax were examined in this study to see whether these two apoptosis-related proteins were affected by chronic stress. As shown in Fig. 2B, chronic stressed mice had an appreciably lower expression of Bcl-2 compared with control unstressed mice. By contrast, chronic stress did not alter the level of Bax. PGN significantly enhanced the expression of Bcl-2, but did not change Bax expression. These results suggest that stimulation of TLR2 by PGN promotes anti-appototic effect by modulating Bcl-2/Bax.
3.4. PGN increases the level of phospho-JNK
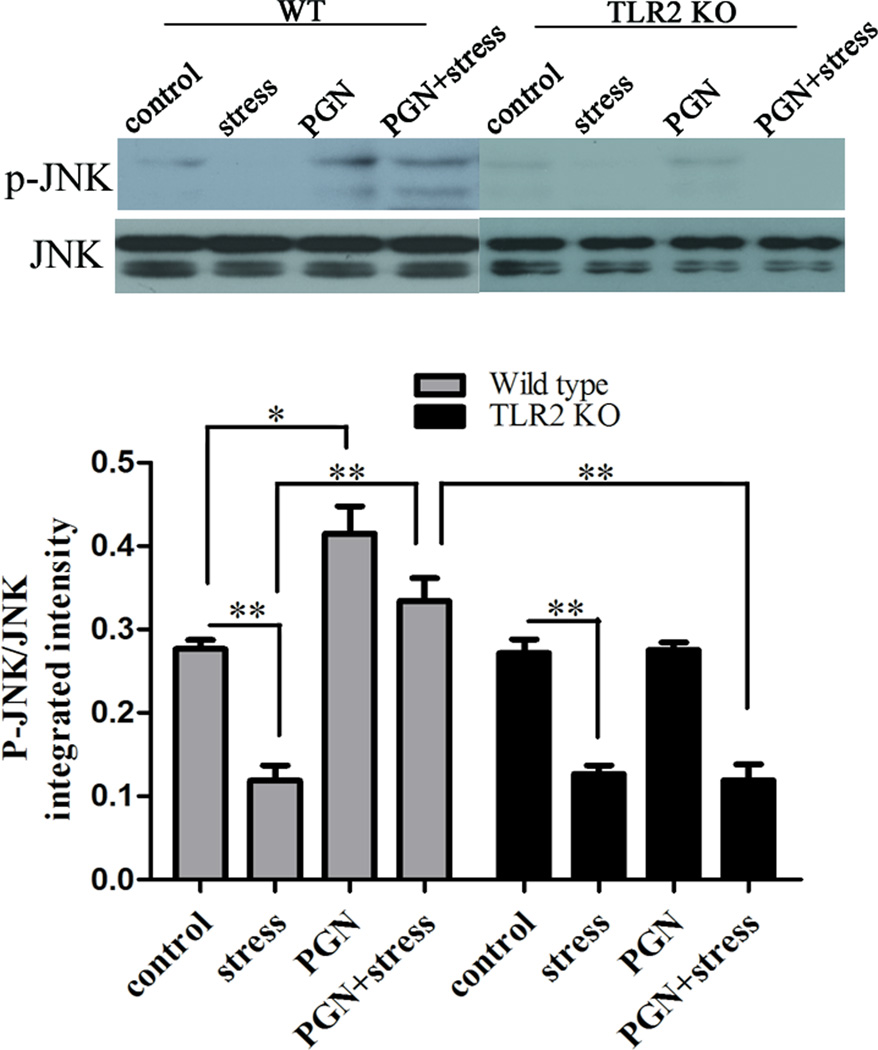
To determine whether immunoregulatory agents could be modulated by chronic stress and TLR2 stimulation. JNK, a member of stress-activated protein kinases (SAPKs), exerts strong regulatory effects during innate and adaptive immune response [13]. It is activated by phosphorylation mediated by mitogen-activated protein kinase (MAPK) kinases. To evaluate whether JNK, as a downstream target of TLRs, could be regulated by chronic stress, we showed that chronic stress decreased the level of JNK phosphorylation (Fig.3). However, administration of PGN markedly increased the ratio of phospho-JNK/JNK in stressed mice compared with untreated stressed mice. Moreover, PGN-induced JNK activation was abolished in TLR2 KO mice, further confirming that PGN promotes JNK phosphorylation through TLR2.
Fig. 3.
PGN treatment increased JNK phosphorylation through TLR2. The enhancement effect was abrogated in TLR2 KO mice. Age-matched TLR2 KO mice and wild type (C57BL/6) mice were administered with PGN and stress as described in Fig. 1. At 12 h of stress, spleens were harvested and cellular proteins were extracted. JNK phosphorylation was examined by Western blot. The intensity ratio of phospho-JNK/ JNK was calculated. *P < 0.05 compared with indicated groups. ** P < 0.01 compared with indicated groups.
3.5. Stimulation of TLR2 by PGN attenuates stress-enhanced corticosterone levels
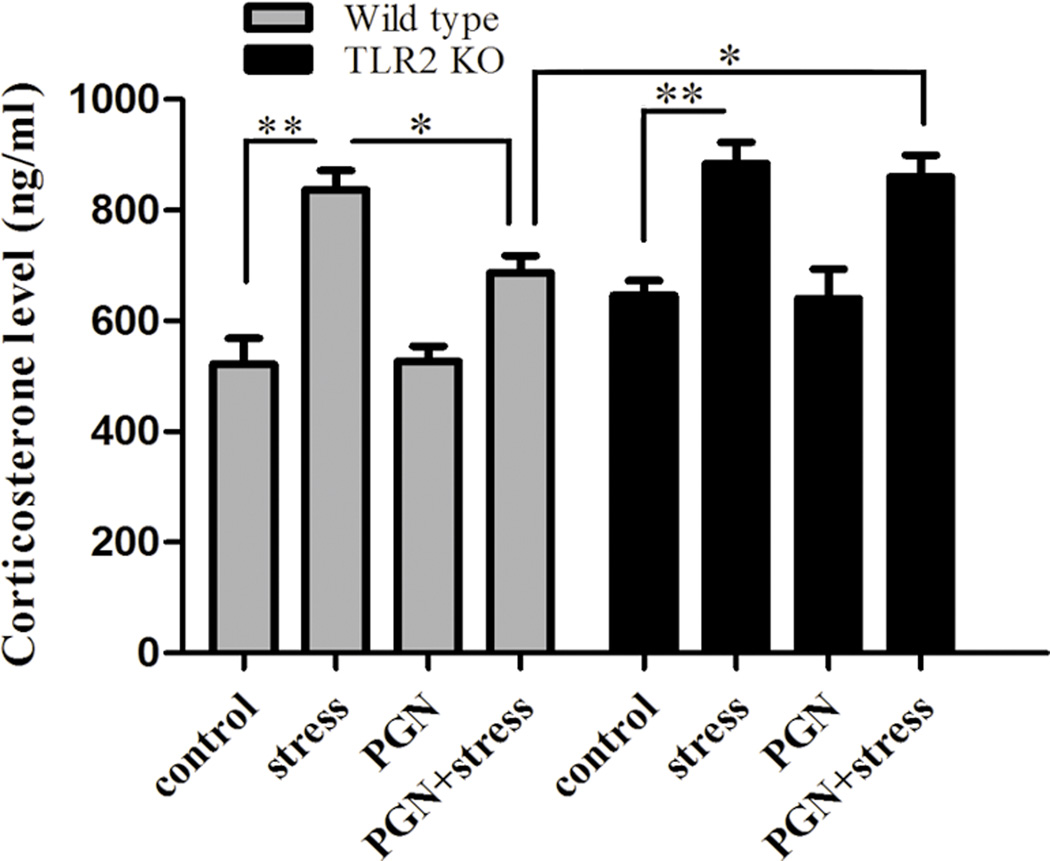
GCs, another immunoregulatory agent, play a critical role in regulation of immune functions as major effectors of HPA axis [16]. We sought to determine whether TLR2 participates in regulation of corticosterone production. As shown in Fig. 4, corticosterone level was higher in stressed mice compared with the controls both in wild type mice and TLR2 KO mice. Interestingly, PGN diminished chronic stress-increased corticosterone production in wild type mice, but not in TLR2 deficient mice (Fig. 4). Our results suggest that TLR2 stimulation by PGN exerts an inhibitory effect on steroid response to chronic stress.
Fig. 4.
PGN administration inhibited stress-induced corticosterone production through TLR2. The suppressive impact was abolished in TLR2 KO mice. Age-matched TLR2 KO mice and wild type mice (C57BL/6) were administered with PGN and stress as described in Fig. 1. After 12 h of stress, serum was collected and corticosterone level was determined by ELISA. *P < 0.05 compared with indicated groups. ** P < 0.01 compared with indicated groups.
3.6. Stimulation of TLR2 by PGN blocks chronic stress-induced changes of cytokine levels
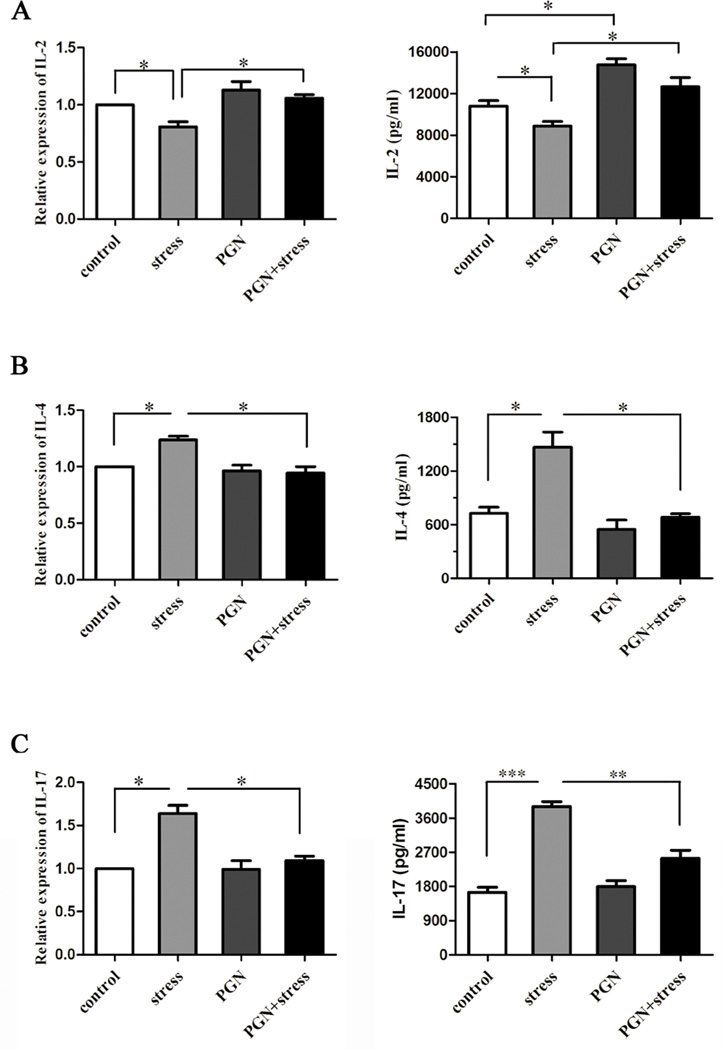
To investigate the effect of PGN on chronic stress-mediated cytokines, we first administered stressed or unstressed control mice with or without PGN and then isolated CD4+ T cells from spleens. Our studies showed that PGN significantly attenuated chronic stress-induced alterations of Th1 IL-2 and Th2 IL-4 in both mRNA and protein (Fig. 5A and 5B). We next determined the level of Th17 cytokine IL-17, a relatively new cytokine. We found that PGN markedly blocked stress-enhanced production of IL-17 in both protein and transcriptional levels (Fig. 5C). However, the changes of IL-4 and IL-7 mRNA levels were less than that of protein levels. IL-4 and IL-17 mRNA was then evaluated at early time points (6 h and 24 h). The results show that the changes at 6 h and 24 h were similar to that at 48 h after in vitro culture with anti-CD3/anti-CD28 antibody stimulation (data not shown). Thus, our studies suggest that PGN possesses the capability of rescuing cytokine disequilibrium induced by chronic stress.
Fig. 5.
PGN treatment suppressed changes of cytokine levels caused by chronic stress. Six to eight-week-old wild type (C57BL/6) male mice were treated with PGN and stress as described in Fig. 1. After 12 h of stress, mouse spleens were harvested, and CD4+ T cells were isolated and cultured with anti-CD3/anti-CD28 antibodies in vitro for 48 h. RNA was extracted from CD4+ T cells and subsequently quantitative real-time PCR was performed. Cell-free supernatants were collected for subsequent ELISA assay.
4. Discussion
As for apoptosis, caspase activation is required for the initiation and execution of apoptosis signaling pathways [11]. In this study, we observed that TLR2 expression was down-regulated at as early as 6 h of stress time period and persisted at a lower level compared with the unstressed control mice (data not shown). However, caspase-3 cleavage was detected at or after 12h of stress. Taken together, our data suggest that chronic stress (more than 6 h), but not acute stress (less than 6 h), enhances activation of caspase-3 and induces cell apoptosis, and TLR2 dysfunction might involve in mediating splenocyte apoptosis. Pam3CSK4, a TLR2 ligand, has been shown to protect against splenocyte reduction [12]. In our current study, we investigated whether the other TLR2 ligand, PGN, exerted a similar effect. Importantly, we utilized PGN to define the mechanisms by which TLR2 modulates cell apoptosis and immune responses. Our data showed PGN significantly diminished the percentage of apoptotic cells induced by chronic stress. TLR2 KO mice abolished the protective actions of PGN, suggesting that PGN protects splenocyte against apoptosis through TLR2. Thus, we next attempted to determine the mechanisms by which PGN exerts the anti-apoptotic effect. Our previous study demonstrated PGN activates PI3K/Akt pathway to promote splenocyte survival [12]. Here, we examined the regulatory effect of TLR2 stimulation by PGN on other apoptosis-related proteins. Interestingly, PGN administration markedly inhibited cleavage of caspase-3 and PARP induced by stress. Moreover, PGN significantly enhanced Bcl-2 expression. By contrast, Bax was not affected either by chronic stress or PGN administration. However, the ratio of Bcl-2/ Bax, rather than their absolute concentrations, determined the susceptibility of cell to death following apoptotic stimuli [22]. Our data in this study showed that PGN increased the Bcl-2/ Bax ratio dramatically, further supporting the concept that PGN protects against apoptosis. Consistently, a recent study also shows treatment of PGN in human polymorphonuclear neutrophils reduces caspase-3 activity and modulates the expressions of Bcl-2 family proteins [23]. Collectively, our data implicate that TLR2 stimulation by PGN protects against stress-induced apoptosis by modulating apoptosis-related proteins. In chronic restraint stress, the regulation between the apoptosis-related markers evaluated in this study remains to be determined in our future research.
Accumulating evidence supports that JNK plays important roles both in the development of lymphocytes and the function of innate and adaptive immune cells [13]. As for the conventional CD4+ T cells, the JNK family promotes Th1 response but inhibits Th2 response. Since there is a skewed Th1/Th2 balance in chronic stress, it’s meaningful to investigate whether JNK activity is modulated in the same situation. Our data in this study demonstrated that there was a diminished activity of JNK in stressed mice, which might help explain the disequilibrium of Th1/Th2 balance in chronic stress. By contrast, the phosphorylated level of p38 MAPK, another member of SAPK family, was elevated in stressed mice (data not shown). This discordance between alterations of JNK and p38 MAPK activity implicates that they play different roles in the chronic restraint stress. Since we observed a paralleled decrease of TLR2 expression and JNK phosphorylation, we speculated that stimulation of TLR2 may retrieve the reduction of JNK activity caused by chronic stress. To evaluate this hypothesis, the mice were pretreated with PGN 1 h before the stress procedure. Our results showed that PGN administration considerably promoted JNK phosphorylation both in unstressed and stressed mice. These results also suggest that TLR2 dysfunction might be responsible for the reduced JNK activity following chronic stress.
GCs, the end products of HPA axis activity, exert a generalized immunosuppressing effect on innate immune system but selective suppression of the Th1 response in adaptive immune system [15–17]. In the present study, increased serum corticosterone level was detected in stressed mice, which is consistent with the shift of Th1/Th2 cytokine balance towards Th2 induced by chronic stress. Recent studies have shown that both TLR2 protein and mRNA expression can be down-regulated by GC treatment [24, 25]. GCs also exhibit an inhibitory effect on activation of JNK in a dual specificity phosphatase 1-dependent manner [26]. Therefore, decrease of TLR2 protein level and diminished activity of JNK in our chronic stress model might be associated with enhancement of GC release. Bornstein et al reported that TLR2 is essential for adrenal GC response to LPS stimulation [27]. In contrast, here we found that TLR2 stimulation by PGN partially impaired GC response to chronic stress in wild type mice, and TLR2 KO mice also had a significant increase of corticosterone following stress. The discrepancy may be explained by that TLR2 play different roles in the modulation of adrenal stress response to different stimulating systems (i.e., LPS and chronic restraint stress). The mechanism for the regulatory effects of TLR2 signal on GC release remains to be determined in future experiments.
In our previous study, mouse splenocytes were isolated, cultured and stimulated with concanavalin A to investigate the suppressive effect of chronic stress on immune functions [12]. However, except for CD4+ T cells, CD8+ T cells can produce IL-2 and IL-4 cytokines upon activation by T cell stimulator [28, 29]. Since Th1 cells and Th2 cells play major roles in the determination of Th1/Th2 cytokine balance in stress [16], we focused on the function of CD4+ T cells in the present study. The results of ELISA assay showed that chronic restraint stress induced a marked decrease of Th1 cytokine IL-2 but an increase of Th2 cytokine IL-4, which were consistent with our previous results based on stimulation of splenocytes with concanavalin A [12], Moreover, the same trend was observed in mRNA level analyzed by quantitative real-time PCR. PGN administration remarkably suppressed these cytokine alterations. Since PGN reversed stress-induced changes in JNK activity and GCs level, it might rescue stress-disrupted Th1/Th2 cytokine balance by modulating these immunoregulatory agents. Previous studies have indicated that IL-17, a relatively new cytokine, is predominantly secreted by Th17 cells [30]. It has been suggested that IL-17 is a strong pro-inflammatory cytokine [31]. However, Ke et al reported that IL-17 inhibits T cell activation and ameliorates the development of experimental autoimmune uveitis [32]. Another study showed that IL-17 suppresses Th1 differentiation and exerts anti-inflammatory effects on H. pylori-induced gastritis[33]. Thus, IL-17 possesses both pro- and anti-inflammatory activities. In our current study, increase of IL-17 was found in stressed mice both at protein and transcriptional levels. TLR signaling pathway has been implicated as an important regulator in the generation, maintenance and expansion of Th17 cells [34, 35]. However, data presented here showed PGN pretreatment ameliorated IL-17 secretion by CD4+ T cells in response to chronic stress, implicating TLR2 has an inhibitory effect on the function of Th17 cells. This effect has been suggested to be mediated by enhancement of IL-27 production [36].
In summary, our data demonstrated that stimulation of TLR2 with PGN protected against splenocyte apoptosis and alterations of Th1, Th2 and Th17 cytokines induced by chronic stress by modulating apoptosis-related factors and immunoregulatory agents. These findings suggest that TLR2 stimulation may be an effective approach for the rescue of stress-induced immune dysfunction.
►Stimulation of TLR2 by PGN protects mouse splenocytes against chronic stress-induced apoptosis. ►TLR2 activation prevents decreased level of JNK phosphorylation following chronic stress. ►TLR2 stimulation inhibits stress-enhanced release of corticosterone. ►TLR2 triggering attenuates chronic stress-induced immune suppression.
Acknowledgments
This work was supported in part by NIH grants NIGM094740 and NIDA020120 and ETSU Major Research Grant 82061 grant to D. Yin. This work was supported in part by the Integrated Chinese and Western Medical Research Foundation 2012Z-Y17 from Hubei Provincial Health Department of China to D. Hu.
Abbreviations
- TLR2
toll like receptor 2
- PGN
peptidoglycan
- JNK
c-Jun N-terminal kinase
- TUNEL
terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Zhang Y, Woodruff M, Zhang Y, Miao J, Hanley G, Stuart C, Zeng X, Prabhakar S, Moorman J, Zhao B, Yin D. Toll-like receptor 4 mediates chronic restraint stress-induced immune suppression. J. Neuroimmunol. 2008;194:115–122. doi: 10.1016/j.jneuroim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sesti-Costa R, Ignacchiti MD, Chedraoui-Silva S, Marchi LF, Mantovani B. Chronic cold stress in mice induces a regulatory phenotype in macrophages: correlation with increased 11beta-hydroxysteroid dehydrogenase expression. Brain. Behav. Immun. 2012;26:50–60. doi: 10.1016/j.bbi.2011.07.234. [DOI] [PubMed] [Google Scholar]
- 3.Dragos D, Tanasescu MD. The effect of stress on the defense systems. J. Med. Life. 2010;3:10–18. [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HR, Moon S, Lee HK, Kang JL, Oh S, Seoh JY. Immune dysregulation in chronic stress: a quantitative and functional assessment of regulatory T cells. Neuroimmunomodulation. 2012;19:187–194. doi: 10.1159/000331586. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan J, Selvarajoo K, Tsuchiya M, Lee G, Choi S. Toll-like receptor signal transduction. Exp. Mol. Med. 2007;39:421–438. doi: 10.1038/emm.2007.47. [DOI] [PubMed] [Google Scholar]
- 6.Oberg HH, Juricke M, Kabelitz D, Wesch D. Regulation of T cell activation by TLR ligands. Eur. J. Cell. Biol. 2011;90:582–592. doi: 10.1016/j.ejcb.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Lin H, Yan J, Wang Z, Hua F, Yu J, Sun W, Li K, Liu H, Yang H, Lv Q, Xue J, Hu ZW. Loss of immunity-supported senescence enhances susceptibility to hepatocellular carcinogenesis and progression in Toll-like receptor 2-deficient mice. Hepatology. 2013;57:171–182. doi: 10.1002/hep.25991. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Jiang S, Tapping RI. Toll-like receptor signaling in cell proliferation and survival. Cytokine. 2010;49:1–9. doi: 10.1016/j.cyto.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter AG. Flipping the safety catch of procaspase-3. Nat. Chem. Biol. 2006;2:509–510. doi: 10.1038/nchembio1006-509. [DOI] [PubMed] [Google Scholar]
- 10.Fuentes-Prior P, Salvesen GS. The protein structures that shape caspase activity, specificity, activation and inhibition. J. Biochem. 2004;384:201–232. doi: 10.1042/BJ20041142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira WO, Amarante-Mendes GP. Apoptosis: a programme of cell death or cell disposal? Scand. J. Immunol. 2011;73:401–407. doi: 10.1111/j.1365-3083.2011.02513.x. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Chen L, Zhang Y, Lesage G, Zhang Y, Wu Y, Hanley G, Sun S, Yin D. Chronic stress promotes lymphocyte reduction through TLR2 mediated PI3K signaling in a beta-arrestin 2 dependent manner. J. Neuroimmunol. 2011;233:73–79. doi: 10.1016/j.jneuroim.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rincon M, Davis RJ. Regulation of the immune response by stress-activated protein kinases. Immunol. Rev. 2009;228:212–224. doi: 10.1111/j.1600-065X.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhu J, Mohan C. Toll-like receptor signaling pathways--therapeutic opportunities. Mediators. Inflamm. 2010:781235. doi: 10.1155/2010/781235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet. Oncol. 2004;5:617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 17.Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann. N. Y. Acad. Sci. 2004;1024:138–146. doi: 10.1196/annals.1321.010. [DOI] [PubMed] [Google Scholar]
- 18.Sheridan JF, Dobbs C, Jung J, Chu X, Konstantinos A, Padgett D, Glaser R. Stress-induced neuroendocrine modulation of viral pathogenesis and immunity. Ann. N. Y. Acad. Sci. 1998;840:803–808. doi: 10.1111/j.1749-6632.1998.tb09618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hua F, Ha T, Ma J, Li Y, Kelly J, Gao X, Browder IW, Kao RL, Williams DL, Li C. Protection against myocardial ischemia/reperfusion injury in TLR4-deficient mice is mediated through a phosphoinositide 3-kinase-dependent mechanism. J. Immunol. 2007;178:7317–7324. doi: 10.4049/jimmunol.178.11.7317. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Sun X, LeSage G, Zhang Y, Liang Z, Chen J, Hanley G, He L, Sun S, Yin D. beta-arrestin 2 regulates Toll-like receptor 4-mediated apoptotic signalling through glycogen synthase kinase-3beta. Immunology. 2010;130:556–563. doi: 10.1111/j.1365-2567.2010.03256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin D, Zhang Y, Stuart C, Miao J, Zhang Y, Li C, Zeng X, Hanley G, Moorman J, Yao Z, Woodruff M. Chronic restraint stress modulates expression of genes in murine spleen. J. Neuroimmunol. 2006;177:11–17. doi: 10.1016/j.jneuroim.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 23.Francois S, El Benna J, Dang PM, Pedruzzi E, Gougerot-Pocidalo MA, Elbim C. Inhibition of neutrophil apoptosis by TLR agonists in whole blood: involvement of the phosphoinositide 3-kinase/Akt and NF-kappaB signaling pathways, leading to increased levels of Mcl-1, A1, and phosphorylated Bad. J. Immunol. 2005;174:3633–3642. doi: 10.4049/jimmunol.174.6.3633. [DOI] [PubMed] [Google Scholar]
- 24.Jin X, Qin Q, Tu L, Qu J. Glucocorticoids inhibit the innate immune system of human corneal fibroblast through their suppression of toll-like receptors. Mol. Vis. 2009;15:2435–2441. [PMC free article] [PubMed] [Google Scholar]
- 25.Du Q, Min S, Chen LY, Ma YD, Guo XL, Wang Z, Wang ZG. Major stress hormones suppress the response of macrophages through down-regulation of TLR2 and TLR4. Surg. J. Res. 2012;173:354–361. doi: 10.1016/j.jss.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, Saklatvala J, Clark AR. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J. Exp. Med. 2006;203:1883–1889. doi: 10.1084/jem.20060336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bornstein SR, Zacharowski P, Schumann RR, Barthel A, Tran N, Papewalis C, Rettori V, McCann SM, Schulze-Osthoff K, Scherbaum WA, Tarnow J, Zacharowski K. Impaired adrenal stress response in Toll-like receptor 2-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2004;101:16695–16700. doi: 10.1073/pnas.0407550101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanciu LA, Shute J, Promwong C, Holgate ST, Djukanovic R. Increased levels of IL-4 in CD8+ T cells in atopic asthma. J. Allergy. Clin. Immunol. 1997;100:373–378. doi: 10.1016/s0091-6749(97)70251-3. [DOI] [PubMed] [Google Scholar]
- 29.Das A, Hoare M, Davies N, Lopes AR, Dunn C, Kennedy PT, Alexander G, Finney H, Lawson A, Plunkett FJ, Bertoletti A, Akbar AN, Maini MK. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J. Exp. Med. 2008;205:2111–2124. doi: 10.1084/jem.20072076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat. Rev. Immunol. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 31.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 32.Ke Y, Liu K, Huang GQ, Cui Y, Kaplan HJ, Shao H, Sun D. Anti-inflammatory role of IL-17 in experimental autoimmune uveitis. J. Immunol. 2009;182:3183–3190. doi: 10.4049/jimmunol.0802487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otani K, Watanabe T, Tanigawa T, Okazaki H, Yamagami H, Watanabe K, Tominaga K, Fujiwara Y, Oshitani N, Arakawa T. Anti-inflammatory effects of IL-17A on Helicobacter pylori-induced gastritis. Biochem. Biophys. Res. Commun. 2009;382:252–258. doi: 10.1016/j.bbrc.2009.02.107. [DOI] [PubMed] [Google Scholar]
- 34.Teixeira-Coelho M, Cruz A, Carmona J, Sousa C, Ramos-Pereira D, Saraiva AL, Veldhoen M, Pedrosa J, Castro AG, Saraiva M. TLR2 deficiency by compromising p19(IL-23) expression limits Th 17 cell responses to Mycobacterium tuberculosis. Int. Immunol. 2011;23:89–96. doi: 10.1093/intimm/dxq459. [DOI] [PubMed] [Google Scholar]
- 35.Yu CF, Peng WM, Oldenburg J, Hoch J, Bieber T, Limmer A, Hartmann G, Barchet W, Eis-Hübinger AM, Novak N. Human plasmacytoid dendritic cells support Th17 cell effector function in response to TLR7 ligation. J. Immunol. 2010;184:1159–1167. doi: 10.4049/jimmunol.0901706. [DOI] [PubMed] [Google Scholar]
- 36.Loures FV, Pina A, Felonato M, Calich VL. TLR2 is a negative regulator of Th17 cells and tissue pathology in a pulmonary model of fungal infection. J. Immunol. 2009;183:1279–1290. doi: 10.4049/jimmunol.0801599. [DOI] [PubMed] [Google Scholar]