Abstract
Background
Red blood cell (RBC) transfusion is a life-saving intervention for critically ill patients; however, it has been linked to increased morbidity and mortality. We hypothesize that a number of important proteins accumulate during routine storage of RBCs, which may explain some of the adverse effects seen in transfused patients.
Study Design
Five RBC units were drawn and divided (half pre-storage leukoreduced (LR-RBC) and half left as an unmodified control (RBC). The supernatant was separated on days 1 and 42 of storage and proteomic analyses completed with in-gel tryptic digestion and nano-liquid chromatography tandem mass spectrometry.
Results
In RBC supernatants, 401 proteins were identified: 203 increased with storage, 114 decreased, and 84 were unchanged. In LR-RBC supernatant, 231 proteins were identified: 84 increased with storage, 30 decreased, and 117 were unchanged. Prestorage leukoreduction removed many platelet- and leukocyte-derived structural proteins; however, a number of intracellular proteins accumulated including: peroxiredoxins (Prdx) 6 and latexin. The increases were confirmed by immunoblotting, including the T-phosphorylation of Prdx-6, indicating that it may be functioning as an active phospholipase. Active matrix metalloproteinase-9 also increased with a coinciding decrease in the metalloproteinase inhibitor 1 and cystatin C. We conclude that a number of proteins increase with RBC storage, which is partially ameliorated with leukoreduction, and transfusion of stored RBCs may introduce mediators that result in adverse events in the transfused host.
Keywords: transfusion, proteins, red blood cell, supernatant, storage, leukoreduction
Introduction
Transfusion of red blood cells (RBC supernatant) is a lifesaving step in critical care medicine that seeks restitution of oxygen carrying capacity in patients with a diverse range of clinical abnormalities. Many of the acute adverse events of transfusion, both hemolytic and non-hemolytic are well known, and mitigation strategies have decreased fatal transfusion reactions especially those secondary to major blood group incompatibilities and to transfusion-related acute lung injury (TRALI) [1]. Although much has been learned about transfusions through serologic testing as well as the role of donor antibodies in TRALI, many adverse events are poorly understood [1]. Of primary interest is the link between transfusion of older stored RBC supernatant and increases in acute lung injury, post-injury multiple organ failure, and increased morbidity and mortality [2–6].
Pre-storage leukoreduction, whether by filtration or buffy coat removal, significantly decreases leukocytes and platelets, HLA antigen exposure, and the production of leukocyte- and platelet-derived mediators, namely interleukin-18 (IL-18) and soluble CD40 ligand. Respectively these mediators decrease febrile transfusion reactions and has been linked to decreases in other non-hemolytic transfusion reactions [7–10]. Despite these beneficial effects, lipids accumulate during routine storage of both RBC supernatant and LR-RBC supernatant, which are present in the plasma fraction, and have been implicated in TRALI and the untoward effects of stored blood especially in injured patients [11–14]. Although lipids and other soluble mediators in the supernatant of LR-RBC, and RBCs have been implicated in transfusion reactions, the proteins present in the supernatant and those that accumulate during routine storage have not been well characterized. We hypothesize that there are a number of important proteins that accumulate in the supernatant during routine storage of RBC units, whether pre-storage leukoreduced or not, that may have profound effects based upon their accumulation or production of other mediators which are present in the plasma fraction.
Materials and Methods
Reagents
Bovine serum albumin (BSA), ammonium bicarbonate, dithiothreitol (DTT), and iodoacetamide were all purchased from Sigma-Aldrich (St. Louis, MO). Formic acid (FA) was obtained from Fluka (Buchs, Switzerland), and acetonitrile was from Burdick and Jackson (Morristown, NJ). Trypsin (sequencing grade, l-1-tosylamido-2-phenylethyl chloromethyl ketone-treated) was from Promega (Madison, WI). Antibodies for immunoblotting were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) or, in the case of Prdx6, a rabbit polyclonal antibody was employed, as previously described [15].
Preparation of RBC supernatant
One unit of whole blood (500±50 mL) was collected from five healthy donors per AABB/FDA recommendations, using a CPD with Optisol™ collection bag system (Teruflex, Terumo Corporation, Tokyo, Japan). Plasma was separated from RBCs by centrifugation followed by expression, employing an automated closed system, Compomat G4 (Fresenius-Kabi, Schweinfurt, Germany) and AS-5 (Optisol) was added to a final hematocrit of 50–60%. The estimated amount of residual plasma was 5–10 ml/unit [16]. Half of each RBC unit was pre-storage leukoreduced via filtration (LR-RBCs) using a Pall BPF4 leukoreduction filter (Westbury, NY) by weight with the other half left as an unmodified control (RBCs). The RBCs/LR-RBCs were stored at 1–6°C, and samples were obtained through sterile couplers on day (D)1 and D42 (the last day a unit can be transfused). The supernatant was isolated via centrifugation (5000xg for 7 min) followed by a second spin at 12,500xg for 5 min to sediment residual cellular material and contaminating platelets [17]. The plasma fraction was stored at −80°C, and all proteomic analyses were complete within 2 months of storage.
Immunoaffinity Depletion of High-Abundance Proteins
The 14 most abundant proteins (albumin, IgG, α1-anti-trypsin, IgA, transferrin, haptoglobin, fibrinogen, α2-macroglobulin, α1-acid glycoprotein, IgM, apolipoprotein AI, apolipoprotein AII, complement C3, and transthyretin) were depleted from the RBC plasma fraction using an antibody-based multiple affinity removal spin cartridge (Agilent Technologies, Santa Clara, CA, USA) [18, 19]. Depleted samples were concentrated using spin concentrators (5000 molecular weight cutoff; Agilent Technologies, Santa Clara, CA, USA), and the protein concentrations of these depleted, concentrated samples were determined by the bicinchoninic acid assay (BCA), Accurate Chemical (Westbury, NY) with absorbance read at 595 nm.
One-dimensional gel electrophoresis, in-gel tryptic digestion and liquid chromatography–tandem mass spectrometry
Proteins (30 μg) were separated using polyacrylamide gel electrophoresis [18, 19]. Proteins were visualized using Coomassie Blue stain, and following destaining, each lane was cut into 10 equal-sized pieces covering the same MW range for all samples. The collected extractions were pooled with the initial digestion supernatant, concentrated under vacuum to approximately 20 μL, and subjected to liquid chromatography tandem mass spectroscopy (LC-MS/MS) analysis [18, 19].
Database searching, protein identification
MS/MS spectra were extracted from raw data files, converted into .mgf files using an in-house script, and Mascot (version 2.2) was used to perform database searches against the human subset SwissProt database of the extracted MS/MS data as reported [18, 19]. Scaffold (version 2) was used to validate MS/MS based peptide and protein identifications. Unique peptides were calculated for each sample and normalized to total peptides across samples, and the total fragment (MS/MS) ion intensities were summed for all peptides assigned to a given protein and normalized between samples. These values were used to calculate a relative fold change of a given protein over 42 days of storage [20]. Both methods have demonstrated good agreement between observed and expected protein ratios [21]. Heat maps were created for the normalized (z-score, mean 0 and standard deviation 1) spectral counts using matrix2png [22]. Values are from the data acquired on the LTQ-FT instrument.
Western Blot Analysis
Samples (20 μl each) of the supernatant from D1 or D42 LR-RBCs were separated via 10% or 15% sodium-dodecyl-sulfate- (SDS) polyacrylamide gels, and transferred to nitrocellulose as described [23]. Immunoblots were performed with antibodies specific for Prdx6 and latexin, and the Prdx6 immunoblots were stripped and re-probed with specific antibodies to phospho-threonine [23]. Blots were also stripped and probed for albumin to ensure equal loading.
Detection of RBCs supernatant matrix metalloproteinase activity using zymography
Samples (20 μg) were diluted into NuPAGER LDS Sample Buffer (4X) (Invitrogen, Paisley, Renfrewshire, UK) and loaded onto a 7.5% SDS-polyacrylamide gel containing 0.1% gelatin [24]. Proteolytic activities were detected as clear bands against the blue background, indicating areas where the gelatin was degraded by the enzymes. The molecular weights were calculated using protein standards.
Statistical Analysis
The data is presented as the ratio of the fold increase or decrease in the amounts of proteins as the result of pre-storage leukoreduction and storage. Among-group comparisons were determined by a student’s t-test in order to classify sets of proteins that showed a statistically significant difference with a confidence level of p<0.005 (denoted by: *). A preliminary power analysis was done employing the data from plasma which compared male-donor to female donor plasma [19]. A sample size of 5 was shown to impart a statistical power of 80%.
Results
Proteomics analysis of RBC/LR-RBC supernatants
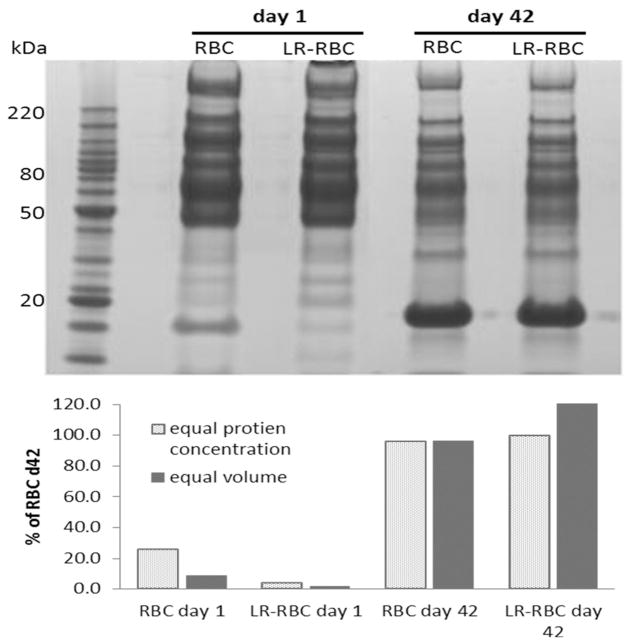
A representative SDS-PAGE image of RBC supernatants is illustrated in Figure 1 with the hemoglobin band quantified below. A complete total proteomic signature was obtained using two different methods with the LTQ-FT instrument (Supplementary File). A total of 412 proteins with 99% confidence (FDR<1%) and minimum of 2 distinct peptides were identified from 5 units of RBC supernatant and LR-RBC supernatant after days 1 and 42 of storage, generated from the FT platform. A total of 401 proteins were identified in RBC supernatant: a) 205 increased (fold change > 2) during storage with 125 unique to plasma from day 42, b) 98 decreased (fold change < 2), 49 unique to day 1 plasma samples, and c) the remaining 98 were unchanged. Likewise, in the LR-RBC supernatant 231 proteins were identified: a) 83 increased (fold change > 2) with routine storage with 42 unique to plasma from day 42, b) 24 decreased in concentration (fold change < 2), 7 of those only present in plasma samples from day 1, and c) 124 remain unchanged (Figure 2). In both RBC supernatant and LR-RBC supernatant the amount of protein increased 2 to 3 fold during storage. Importantly, preliminary data from three RBC/LR-RBC units from 3 female donors showed the identical proteins with the only novel protein being pregnancy zone protein which is increased due to gender [19].
Figure 1. Representative 1D-PAGE profile of RBC and LR-RBC supernatants after days 1 and 42 of storage.
Quantitative densitometric analysis of the ~16 kDa MW band (hemoglobin band) by equal protein concentration (textured fill), and corrected for equal volume of supernatants (solid fill). The numbers of the left side of the gel indicate molecular size in kDa.
Figure 2. Venn diagram of total proteins identified in the supernatant of RBCs (A) and LR-RBCs (B) stored days 1 and 42.
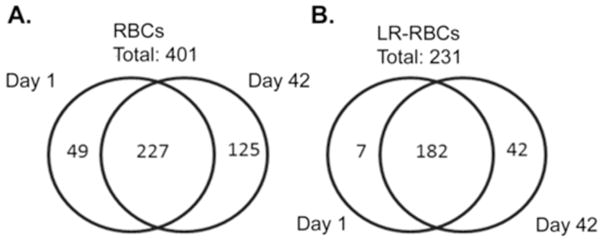
These diagrams represent a breakdown of the total proteins identified. The numbers on the left (D1) and right (D42) are the number of protein unique to the RBC or LR-RBC supernatant. The numbers in the center are the total number of proteins that are in both D1 and D42 supernatant samples.
Leukocyte- and Platelet-derived proteins
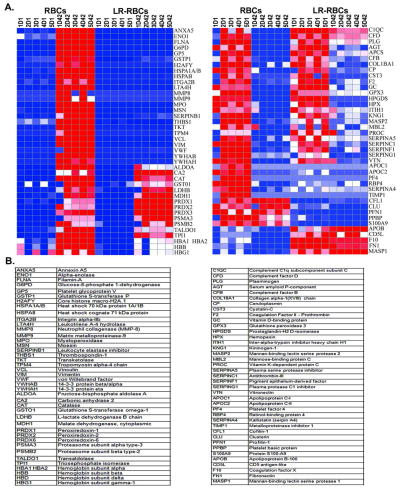
In RBC supernatant there was a significant accumulation of leukocyte- and platelet-derived proteins including: neutrophil cytosol factors 2 & 4, moesin, talin, matrix metalloproteinase-8 (MMP-8), MMP-9 from leukocytes; and platelet glycoprotein V, thrombospondin 1, and von Willebrand factor from platelets (Figure 3, Supplementary File). Platelet glycoprotein V and von Willebrand factor were not found in the pre-storage leuko- and platelet-reduced identical units; moreover, other filament-binding and integrin- related proteins are also increased including α-actinin, cofilin, etc. In addition, a number of cellular structural proteins were increased during RBC storage that did not demonstrate the same accumulation in LR-RBC supernatant, including: filamin, tropomyosin, parvin, and vinculin. Transaldolase, fructose-bisphosphate aldolase, phosphoglycerate kinase and α-enolase, all glycolytic enzymes, significantly accumulated during RBC storage and were more pronounced in LR-RBC supernatant (Figure 3, Supplementary File).
Figure 3. Heat map of select identified proteins across RBC and LR-RBC supernatants.
A. Proteins are grouped by patterns of relative abundance change for both RBC and LR-RBC supernatants at day (D1) and D42 of 5 stored units. Gene names are on the right of the heat maps with the protein identification below (B). The map reads from the very little (blue) to a 1:1 ratio (white) to the greatest amount (red).
RBC-related proteins
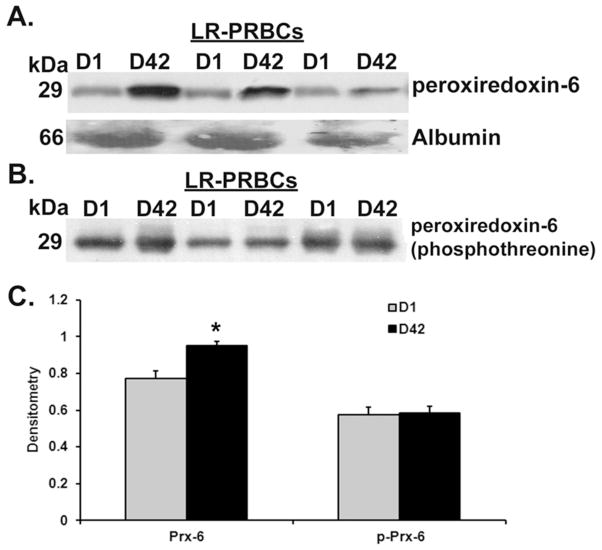
Numerous RBC proteins increased with storage including 14-3-3 proteins, band 3, ribose-5- phosphate isomerase, and erythrocyte band 7 integral membrane protein (Figure 3, Supplementary File). In addition, there were a number of proteins that increased with storage of both RBC supernatant and LR-RBC supernatant that were unexpected and are most likely present due to either release from, or lysis of RBCs including: glutathione-S-transferase (a family of enzymes required, with glutathione, to limit oxidative damage), and catalase (a ubiquitous enzyme which catalyzes the decomposition of hydrogen peroxide) [25–28]. Lactate and malate dehydrogenases, triosephosphate isomerase and additional enzymes involved in glycolysis. Latexin, also known as endogenous carboxypeptidase inhibitor (ECI), is another protein that increased during storage, and has been implicated as a mediator of the hematopoietic stem cell compartment [29]. Using data from the LTQ subset (supplementary files), latexin increased in D42 samples by more than 10-fold in all 3 LR-RBC samples. This was confirmed with western blot analysis showing latexin levels elevated in LR-RBC supernatant (Figure 4) with identical results for RBC supernatant (data not shown). Furthermore, Prdx1, Prdx2, and Prdx6 all increased during storage of both RBC supernatant and LR-RBC supernatant, and a significant (p<0.05, n=3) increase in Prdx6 was confirmed by immunoblotting (Figure 5A & C). Unlike Prdx1 or Prdx2, Prdx6 contains a phospholipase domain which requires either acidic pH or T-phosphorylation for activity [30–32]. The immunoblot in Figure 5A was stripped and reprobed with a specific phospho-threonine antibody. The results suggest that the released Prdx6 was T-phosphorylated on both D1 and D42 indicating that it may be functioning as an active, atypical phospholipase (Figure 5B).
Figure 4. Latexin accumulates in the plasma fraction during routine storage of LR-RBC supernatant.
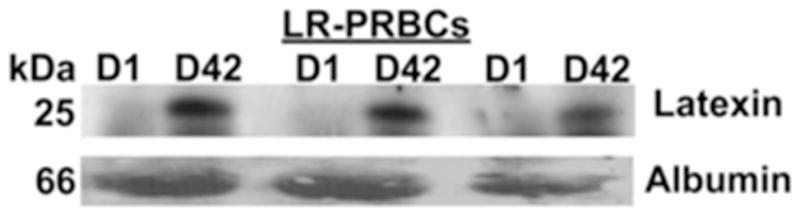
A representative immunoblot for latexin demonstrated significant immunoreactivity in the plasma fraction of stored, day (D)42, LR-RBC supernatant which was not present on D1 in the identical samples. Albumin is shown as loading control. These data illustrated that latexin accumulates during routine storage of LR-RBC supernatant and is likely a red blood cell protein (n=3).
Figure 5. Peroxiredoxin 6 accumulates in the plasma fraction during routine storage of LR-RBC supernatant, and is T-phosphorylated.
Panel A: a representative immunoblot which demonstrates minimal amounts of Prdx6 in the plasma fraction of day (D)1 LR-RBC supernatant. The immunoreactivity significantly increases (C) during storage and is released into the plasma fraction of stored, D42 LR-RBC supernatant indicating it is likely a protein inherent to the red blood cell.so that there are obvious amounts in the plasma fraction. Albumin is shown as a loading control. Panel B: a representative western blot depicts the identical blot in panel A that has been stripped and re-probed with phospho-threonine. There is phospho-threonine immunoreactivity in the bands that demonstrated Prdx6 immunoreactivity suggesting the Prdx6 was T-phosphorylated and may be an active phospholipase. Panel C: The mean densitometry for panels A and B calculated with ImageJ software.
Lastly, a number of proteins decreased with storage, most likely due to increased protease activity. There was a significant accumulation of MMP-8 and MMP-9, which display extracellular protease activity, most proteasome subunits [33, 34] and a drastic decrease in cystatin C, and a range of protease inhibitors in the serpin superfamily (Figure 3, Table S1). In addition, the amount of protein in the plasma fraction increased 2–3-fold during storage and thus, relative protein ratios may falsely appear to decrease.
Detection of matrix metalloproteinase activity
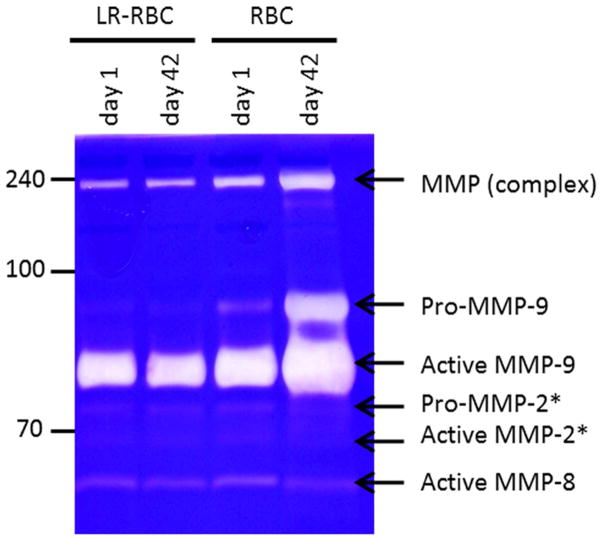
To further evaluate the changes in matrix metalloproteinases activity in RBC supernatant and LR-RBC supernatant units over the 42 days of storage we employed a gelatin zymography assay (Figure 6). RBC supernatant produced four major bands suggesting the presence of the MMP-9 & NGAL complex (240 kDa), pro-MMP-2 (92 kDa), act-MMP-9 (82 kDa) and act-MMP-8 (50kDa). Levels of pro-MMP-9 expression/release increase in RBC supernatant over time without LR. This is consistent with our proteomic results. Zymography showed no bands for pro-MMP-9 in LR-RBC supernatant at days 1 and 42 of storage and exhibited only a faint band for RBC supernatant at day 1. Elevated levels of act-MMP-9 were observed in RBC supernatant stored for 42 days. There was minimal decrease in act-MMP-9 in the stored LR-PBCs samples based on the zymography results, and an apparent decrease based on the proteomic results, but this observation did not reach statistical significance. The concentration of MMP-8 did not change drastically by zymography and showed an increase by the label free quantification method. Consistent with our mass spectrometry results that showed a significant increase of neutrophil gelatinase-associated lipocalin (NGAL, 6-fold), the high molecular weight MMP complex that has been reported to contain NGAL increased and the difference in the RBC and LR-RBC supernatant samples at D42 is also significant (23-fold, data not shown).
Figure 6. Gelatin zymograph showing gelatinase activity during RBC storage.
Representative matrix metalloproteinase activity, MMP-8 and MMP-9, in RBC supernatant and LR-RBC supernatant units as measured on days 1 and 42 of storage.
Discussion
The variables studied in the storage of RBC units were pre-storage leukoreduction and the storage time. It was necessary to deplete the 14 most abundant proteins because the relative concentration of these proteins compromises the identification of other proteins, especially the detection of lower-abundance proteins. The combination of protein depletion and 1D PAGE fractionation followed by LC-MS/MS analyses reported here has expanded the protein coverage compared to a previous 2D PAGE based approach [35]. Because of the stochastic nature of the analytical platforms, two different methods were employed to obtain a complete total proteomic signature. Significantly more proteins were present in the supernatant of RBCs compared to LR-RBC supernatant at D1 and D42 of storage as would be expected due to leukocyte and platelet contamination. The amount of protein increased two- to three-fold during storage. Importantly, the immuno-affinity chromatography employed does not totally remove the targeted abundant proteins; rather it reduces their relative concentrations so as not to interfere with identification and quantification of other proteins that are present in lesser concentrations. As a result peptides from several of the depleted proteins were identified and appear in Table S1.
Many proteins identified in this study were common plasma proteins, and in both types of RBC supernatant there was an increase in RBC-related proteins including: structural proteins, protein 14-3-3, band 3, etc., the α- and β-hemoglobin chains, and enzymes involved in essential metabolic function such as glycolysis and redox cycling to protect against oxidative damage. Furthermore, storage of unmodified RBC supernatant resulted in increased concentrations of both leukocyte specific proteins (moesin, MPO, MMP-8, MMP-9) and platelet specific proteins (integrin alpha 2b, platelet glycoprotein V) which are removed by pre-storage leukoreduction, a process that reduces leukocytes by >3 logs and platelets by ~2 logs [9]. There were also a number of structural proteins including actin, α-actinin-1, vinculin, and talin-1, of which the latter three are important scaffolding proteins for integrin anchoring and presentation [36–38]. In addition, several glycolytic enzymes also increased including α–enolase [39]. It has been reported that α-enolase is involved in plasmin activation and may cause extracellular matrix degradation [40, 41].
A number of proteins also significantly decreased in RBC supernatant, including mannose-binding lectin 2, the complement protein C4b, protein C, and prothrombin. These decreases could lead to a decrease in innate immunity and reinforce the use of plasma and cryoprecipitate for injured patients requiring massive transfusion [42–44]. Such decreases are likely due to proteolysis as there are a number of proteases that increase and protease inhibitors that decrease during RBC storage [35]. However, it is of note that some of the proteins appear resistant to proteolysis, e.g. α-actinin-1, vinculin, and talin-1. These proteins are scaffolding proteins that allow for stable presentation of adhesion molecules and may be expected to be protease-resistant, which would account for the observed increase [36–38].
Fewer proteins in LR-RBC supernatant increased with storage, compared to RBC supernatant, including: proteasome subunits, glycolytic enzymes, redox and other intracellular proteins. Prdx-2, similar to Prdx1, is a 2-cys peroxiredoxin with thiol-dependent hydrogen peroxide scavenging activity that binds to hemoglobin in the cytosol to prevent its oxidation [45]. The presence of Prdx1, Prdx2 and Prdx6 in LR-RBC supernatant provides confirmatory evidence that these antioxidants are RBC proteins that are released during storage [46]. In addition, Prdx6, is found associated with p67phox in phagocytic cells and translocates to the plasma membrane to enhance NADPH oxidase activity which is related to activation of its phospholipase domain via T-phosphorylation [15, 31]. The accumulation of an active phospholipase during RBC storage explains the production of arachidonic acid (AA), and the 5-LO metabolites 5-, 12-, & 15-HETE via cleavage of lipids from RBC membranes, which have the capacity to cause TRALI in vivo [14].
During LR-RBC storage a number of important proteins decrease in the supernatant, including: cystatin C and Kininogen-1, such that massive transfusion may render the host unable to inhibit cysteine proteases, mannose-binding protein 2, and other effectors in innate immunity [43, 47–54]. Furthermore, decreases in metalloproteinase inhibitor 1 may leave the host susceptible to proteases which may cause pro-inflammatory activation of endothelial beds through PARS activation leading to PMN sequestration and decrease innate immunity [43, 50]. Lastly, there is an increase in the carboxypeptidase inhibitor latexin, which regulates hematopoiesis such that over-expression in rodents decreases the hematopoietic stem cell compartment [29]. This protein may explain the observed aplasia in injured patients requiring massive transfusion, and the preliminary data that increased transfusion of Pediatric patients who receive stem cells for the treatment of malignancies leads to delayed engraftment [55].
Importantly, RBC supernatants only contain the equivalent of 5–10 ml of plasma in one unit such that the volume of the unit is based upon the donor hematocrit and weight [16]. Most of the proteins which accumulate during storage are intracellular proteins, which are either released/secreted or are due to cell lysis which is part of the storage lesion of RBC units. This breakdown of fragile, relatively older RBCs has been well chronicled and is an area of important investigation [56]. Such intracellular proteins may directly affect patients, i.e. latexin, or may provide substrates such that their activity in the RBC unit may lead to the production of mediators. For example Prdx6, may be responsible for the accumulation of lipid mediators in RBC/LR-RBC supernatants which have been linked to TRALI [14, 32]. Many of the described intracellular enzymes, that have defined roles in specific intracellular processes, may act very differently when they are extracellular and may have unexpected effects on plasma proteins [57, 58]. Recent studies that were controlled for the amount of RBCs transfused have shown that older stored RBCs are an independent risk factor for developing multiple organ failure following serious injury [6]. Further work is needed to characterize possible masquerading mediators which may have clinical affects especially when stored RBCs are used for the resuscitation of injured patients that require massive transfusion.
Supplementary Material
Acknowledgments
This work was supported by grants P50-GM049222 from NIGMS, S10RR023015 from the NCRR, and K07-HL088968 from NHLBI, NIH and Bonfils Blood Center.
Footnotes
The authors have no financial conflicts of interest with the submitted manuscript.
References
- 1.Transfusion Reactions. 3. Bethesda: AABB Press; 2007. [Google Scholar]
- 2.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 3.Purdy FR, Tweeddale MG, Merrick PM. Association of mortality with age of blood transfused in septic ICU patients. Can J Anaesth. 1997;44:1256–1261. doi: 10.1007/BF03012772. [DOI] [PubMed] [Google Scholar]
- 4.Silliman CC, Boshkov LK, Mehdizadehkashi Z, et al. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood. 2003;101:454–462. doi: 10.1182/blood-2002-03-0958. [DOI] [PubMed] [Google Scholar]
- 5.Weinberg JA, McGwin G, Jr, Griffin RL, et al. Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma. 2008;65:279–282. doi: 10.1097/TA.0b013e31817c9687. [DOI] [PubMed] [Google Scholar]
- 6.Zallen G, Offner PJ, Moore EE, et al. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 7.Blumberg N, Heal JM, Gettings KF, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50:2738–2744. doi: 10.1111/j.1537-2995.2010.02748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hebert PC, Fergusson D, Blajchman MA, et al. Clinical outcomes following institution of the Canadian universal leukoreduction program for red blood cell transfusions. JAMA. 2003;289:1941–1949. doi: 10.1001/jama.289.15.1941. [DOI] [PubMed] [Google Scholar]
- 9.Khan SY, Kelher MR, Heal JM, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–2462. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyman TH, Dinarello CA, Banerjee A, et al. Physiological levels of interleukin-18 stimulate multiple neutrophil functions through p38 MAP kinase activation. J Leukoc Biol. 2002;72:401–409. [PubMed] [Google Scholar]
- 11.Kelher MR, Masuno T, Moore EE, et al. Plasma from stored packed red blood cells and MHC class I antibodies causes acute lung injury in a 2-event in vivo rat model. Blood. 2009;113:2079–2087. doi: 10.1182/blood-2008-09-177857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silliman CC, Clay KL, Thurman GW, et al. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J Lab Clin Med. 1994;124:684–694. [PMC free article] [PubMed] [Google Scholar]
- 13.Silliman CC, Paterson AJ, Dickey WO, et al. The association of biologically active lipids with the development of transfusion-related acute lung injury: a retrospective study. Transfusion. 1997;37:719–726. doi: 10.1046/j.1537-2995.1997.37797369448.x. [DOI] [PubMed] [Google Scholar]
- 14.Silliman CC, Moore EE, Kelher MR, et al. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2011 doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambruso DR, Ellison MA, Thurman GW, et al. Peroxiredoxin 6 translocates to the plasma membrane during neutrophil activation and is required for optimal NADPH oxidase activity. Biochim Biophys Acta. 2012;1823:306–315. doi: 10.1016/j.bbamcr.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Silliman CC, Kelher M, Ambruso DR. Bioactive lipids from stored cellular blood components: in vitro method is crucial for proper interpretation. Transfusion. 2012;52:1155–1157. doi: 10.1111/j.1537-2995.2012.03564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bercovitz RS, Kelher MR, Khan SY, et al. The pro-inflammatory effects of platelet contamination in plasma and mitigation strategies for avoidance. Vox Sang. 2011 doi: 10.1111/j.1423-0410.2011.01559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dzieciatkowska M, Wohlauer MV, Moore EE, et al. Proteomic analysis of human mesenteric lymph. Shock. 2011;35:331–338. doi: 10.1097/SHK.0b013e318206f654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silliman CC, Dzieciatkowska M, Moore EE, et al. Proteomic analyses of human plasma: Venus versus Mars. Transfusion. 2012;52:417–424. doi: 10.1111/j.1537-2995.2011.03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Old WM, Meyer-Arendt K, Aveline-Wolf L, et al. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol Cell Proteomics. 2005;4:1487–1502. doi: 10.1074/mcp.M500084-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.Griffin NM, Yu J, Long F, et al. Label-free, normalized quantification of complex mass spectrometry data for proteomic analysis. Nat Biotechnol. 2010;28:83–89. doi: 10.1038/nbt.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavlidis P, Noble WS. Matrix2png: a utility for visualizing matrix data. Bioinformatics. 2003;19:295–296. doi: 10.1093/bioinformatics/19.2.295. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin NJ, Banerjee A, Kelher MR, et al. Platelet-activating factor-induced clathrin-mediated endocytosis requires beta-arrestin-1 recruitment and activation of the p38 MAPK signalosome at the plasma membrane for actin bundle formation. J Immunol. 2006;176:7039–7050. doi: 10.4049/jimmunol.176.11.7039. [DOI] [PubMed] [Google Scholar]
- 24.Shim YJ, Kang BH, Jeon HS, et al. Clusterin induces matrix metalloproteinase-9 expression via ERK1/2 and PI3K/Akt/NF-kappaB pathways in monocytes/macrophages. J Leukoc Biol. 2011;90:761–769. doi: 10.1189/jlb.0311110. [DOI] [PubMed] [Google Scholar]
- 25.al-Turk WA, Stohs SJ, el-Rashidy FH, et al. Glutathione, glutathione S-transferase and glutathione reductase in human erythrocytes and lymphocytes as a function of sex. Drug Des Deliv. 1987;1:237–243. [PubMed] [Google Scholar]
- 26.Deisseroth A, Dounce AL. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiol Rev. 1970;50:319–375. doi: 10.1152/physrev.1970.50.3.319. [DOI] [PubMed] [Google Scholar]
- 27.Ketterer B. Glutathione S-transferases and prevention of cellular free radical damage. Free Radic Res. 1998;28:647–658. doi: 10.3109/10715769809065820. [DOI] [PubMed] [Google Scholar]
- 28.Mentzer WCJ. Pyruvate kinase deficiency and disorders of glycolysis. In: Nathan DG, Oski FA, editors. Hematology of Infancy and Childhood. 4. Philadelphia: W.B. Saunders Company; 2012. pp. 634–73. [Google Scholar]
- 29.Liang Y, Jansen M, Aronow B, et al. The quantitative trait gene latexin influences the size of the hematopoietic stem cell population in mice. Nat Genet. 2007;39:178–188. doi: 10.1038/ng1938. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Feinstein SI, Manevich Y, et al. Mitogen-activated protein kinase-mediated phosphorylation of peroxiredoxin 6 regulates its phospholipase A(2) activity. Biochem J. 2009;419:669–679. doi: 10.1042/BJ20082061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chatterjee S, Feinstein SI, Dodia C, et al. Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J Biol Chem. 2011;286:11696–11706. doi: 10.1074/jbc.M110.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leavey PJ, Gonzalez-Aller C, Thurman G, et al. A 29-kDa protein associated with p67phox expresses both peroxiredoxin and phospholipase A2 activity and enhances superoxide anion production by a cell-free system of NADPH oxidase activity. J Biol Chem. 2002;277:45181–45187. doi: 10.1074/jbc.M202869200. [DOI] [PubMed] [Google Scholar]
- 33.Abdul-Hussien H, Soekhoe RG, Weber E, et al. Collagen degradation in the abdominal aneurysm: a conspiracy of matrix metalloproteinase and cysteine collagenases. Am J Pathol. 2007;170:809–817. doi: 10.2353/ajpath.2007.060522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson PL, Xu X, Wilson L, et al. Human neutrophil elastase-mediated cleavage sites of MMP-9 and TIMP-1: implications to cystic fibrosis proteolytic dysfunction. Mol Med. 2010;16:159–166. doi: 10.2119/molmed.2009.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anniss AM, Glenister KM, Killian JJ, et al. Proteomic analysis of supernatants of stored red blood cell products. Transfusion. 2005;45:1426–1433. doi: 10.1111/j.1537-2995.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- 36.Mangeat P, Burridge K. Actin-membrane interaction in fibroblasts: what proteins are involved in this association? J Cell Biol. 1984;99:95s–103s. doi: 10.1083/jcb.99.1.95s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieswandt B, Varga-Szabo D, Elvers M. Integrins in platelet activation. J Thromb Haemost. 2009;7 (Suppl 1):206–209. doi: 10.1111/j.1538-7836.2009.03370.x. [DOI] [PubMed] [Google Scholar]
- 38.Oikonomou KG, Zachou K, Dalekos GN. Alpha-actinin: a multidisciplinary protein with important role in B-cell driven autoimmunity. Autoimmun Rev. 2011;10:389–396. doi: 10.1016/j.autrev.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Mentzer WCJ. Pyruvate kinase deficiency and disorders of glycolysis. In: Nathan DG, Oski FA, editors. Hematology of Infancy and Childhood. 4. Philadelphia: W.B. Saunders Company; 2012. pp. 634–73. [Google Scholar]
- 40.Lopez-Alemany R, Suelves M, Munoz-Canoves P. Plasmin generation dependent on alpha-enolase-type plasminogen receptor is required for myogenesis. Thromb Haemost. 2003;90:724–733. doi: 10.1160/TH03-04-0291. [DOI] [PubMed] [Google Scholar]
- 41.Wygrecka M, Marsh LM, Morty RE, et al. Enolase-1 promotes plasminogen-mediated recruitment of monocytes to the acutely inflamed lung. Blood. 2009;113:5588–5598. doi: 10.1182/blood-2008-08-170837. [DOI] [PubMed] [Google Scholar]
- 42.Blom AM, Villoutreix BO, Dahlback B. Complement inhibitor C4b-binding protein-friend or foe in the innate immune system? Mol Immunol. 2004;40:1333–1346. doi: 10.1016/j.molimm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Dean MM, Flower RL, Eisen DP, et al. Mannose-binding lectin deficiency influences innate and antigen-presenting functions of blood myeloid dendritic cells. Immunology. 2011;132:296–305. doi: 10.1111/j.1365-2567.2010.03365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hathaway WE, Goodnight SH. Disorders of Hemostasis and Thrombosis: A Clinical Guide. 1. New York: McGraw-Hill, Inc; 1993. [Google Scholar]
- 45.Rinalducci S, D’Amici GM, Blasi B, et al. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion. 2011;51:1439–1449. doi: 10.1111/j.1537-2995.2010.03032.x. [DOI] [PubMed] [Google Scholar]
- 46.Antunes RF, Brandao C, Maia M, et al. Red blood cells release factors with growth and survival bioactivities for normal and leukemic T cells. Immunol Cell Biol. 2011;89:111–121. doi: 10.1038/icb.2010.60. [DOI] [PubMed] [Google Scholar]
- 47.Arora P, Ricks TK, Trejo J. Protease-activated receptor signalling, endocytic sorting and dysregulation in cancer. J Cell Sci. 2007;120:921–928. doi: 10.1242/jcs.03409. [DOI] [PubMed] [Google Scholar]
- 48.Babovic-Vuksanovic D, Snow K, Ten RM. Mannose-binding lectin (MBL) deficiency. Variant alleles in a midwestern population of the United States. Ann Allergy Asthma Immunol. 1999;82:134–8. 141. doi: 10.1016/S1081-1206(10)62586-0. [DOI] [PubMed] [Google Scholar]
- 49.Friedland RP. Lipid metabolism, epidemiology, and the mechanisms of Alzheimer’s disease. Ann N Y Acad Sci. 2002;977:387–390. doi: 10.1111/j.1749-6632.2002.tb04842.x. [DOI] [PubMed] [Google Scholar]
- 50.Gomez DE, Alonso DF, Yoshiji H, et al. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- 51.Martin P, Lerner A, Johnson L, et al. Inherited mannose-binding lectin deficiency as evidenced by genetic and immunologic analyses: association with severe recurrent infections. Ann Allergy Asthma Immunol. 2003;91:386–392. doi: 10.1016/S1081-1206(10)61686-9. [DOI] [PubMed] [Google Scholar]
- 52.Nelson AR, Fingleton B, Rothenberg ML, et al. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 53.Schreiber G, Richardson SJ. The evolution of gene expression, structure and function of transthyretin. Comp Biochem Physiol B Biochem Mol Biol. 1997;116:137–160. doi: 10.1016/s0305-0491(96)00212-x. [DOI] [PubMed] [Google Scholar]
- 54.Ye S. Polymorphism in matrix metalloproteinase gene promoters: implication in regulation of gene expression and susceptibility of various diseases. Matrix Biol. 2000;19:623–629. doi: 10.1016/s0945-053x(00)00102-5. [DOI] [PubMed] [Google Scholar]
- 55.Bercovitz RS, Andrews J, Dietz A, et al. Blood component utilization in pediatric stem cell patients in the first 100 days post-transplant. 56. 2011. p. 904. [Google Scholar]
- 56.Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion: role of red blood cell breakdown. Transfusion. 2011;51:844–851. doi: 10.1111/j.1537-2995.2011.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeffery CJ. Moonlighting proteins: old proteins learning new tricks. Trends Genet. 2003;19:415–417. doi: 10.1016/S0168-9525(03)00167-7. [DOI] [PubMed] [Google Scholar]
- 58.Jeffery CJ. Moonlighting proteins--an update. Mol Biosyst. 2009;5:345–350. doi: 10.1039/b900658n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.