Abstract
To test the hypothesis that increasing severity of the fetal inflammatory response would have a dose-dependent relationship with severe neurodevelopmental impairment (NDI) or death in extremely preterm infants.
Study design
We report 347 infants 23 to 28 weeks gestational age admitted to a tertiary neonatal intensive care unit between 2006 and 2008. The primary outcome was death or NDI at 18–22 month follow-up. Exposure status was defined by increasing stage of funisitis (stage 1: phlebitis; stage 2: arteritis with or without phlebitis; stage 3: subacute necrotizing funisitis) and severity of chorionic plate vasculitis (inflammation with or without thrombosis).
Results
A fetal inflammatory response was detected in 110 placentas (32%). Severe NDI/death rate was higher in infants with subacute necrotizing funisitis compared with infants without placental/umbilical cord inflammation (60% vs. 35%; p<0.05). Among infants with stage 1 or 2 funisitis, the presence of any chorionic vasculitis was associated with higher rates of severe NDI/death (47% vs. 23%; p<0.05). After adjustment for confounding factors, only subacute necrotizing funisitis (RR: 1.87; 95% CI: 1.04 – 3.35; p=0.04) and chorionic plate vasculitis with thrombosis (RR: 2.21; 95% CI: 1.10 – 4.46; p=0.03) were associated with severe NDI/death.
Conclusions
Severe fetal inflammatory response characterized by subacute necrotizing funisitis and severe chorionic plate vasculitis with thrombosis are associated with severe NDI/death in preterm infants.
Keywords: chorioamnionitis, infant, premature, prognosis, funisitis, chorionic plate vasculitis
Advances in neonatal care have reduced mortality in extremely preterm infants.1 However, the risk of adverse neurodevelopmental outcomes remains high. Neurodevelopmental impairment (NDI) at 18–22 months of corrected age occurs in nearly 40% of extremely low birth weight (≤ 1.0 kg birth weight) infants.2–4 The incidence of histologically identified chorioamnionitis in preterm births is approximately 50% and is inversely related to gestational age.1,5,6 Chorioamnionitis may induce preterm delivery via a maternal inflammatory response. Exposure to inflammation may also injure the immature brain, particularly when histologic chorioamnionitis is associated with a fetal inflammatory response (FIR).7–11 The association of chorioamnionitis with NDI is inconsistent, perhaps because of varying definitions of intrauterine inflammation.3,9,12–18 It is possible that the maternal inflammatory response and the fetal inflammatory response are associated with different risks for NDI and mortality.3
Any evidence of mural inflammation in the umbilical vessels and the chorionic plate vessels indicates FIR19–22 as these vessels are continuous with the fetal cardiovascular system and are considered to be of fetal origin.6,7 Of preterm placentas with histologically identified chorioamnionitis, 50–70% have funisitis6,15 and approximately 30% have chorionic plate vasculitis.23,24 It has been estimated that FIR occurs in approximately 25–40% of all preterm births.5,6,9,24,25 However, the severity of the FIR has not been consistently reported.3,5,22,23 Evidence suggesting an association between FIR and NDI in preterm infants7,8,10,15,19,20 is difficult to interpret due to limited information regarding the severity of inflammation3 and imprecise measurement of effect, mainly due to sample size limitations.19
We hypothesized that an increasing duration and/or severity of placental histopathology compatible with fetal inflammatory response will have a dose-dependent relationship with the rate of NDI/death in preterm infants.
Methods
This was a retrospective cohort study using prospectively collected data on inborn infants 23 0/7 to 28 6/7 weeks of gestational age admitted to the tertiary neonatal intensive care unit at the University of Alabama at Birmingham (UAB) between 2006 and 2008. The primary composite outcome was severe NDI by 18–22 months’ corrected age or death at any time prior to follow-up time. Composite scores for cognitive, language, and motor domains were determined using the Bayley Scales of Infant Development III (BSID III) by trained and certified examiners at 18–22 months’ corrected age.26 Neurologic examination and ascertainment of vision and hearing were also performed. Severe NDI was defined as one or more of the following: moderate or severe cerebral palsy (CP), BSID III cognitive score <70, Gross Motor Function Classification Level ≥ 2, blindness, and/or hearing loss despite amplification.26, 27
Demographic, clinical, and outcome data were collected prospectively from the infant’s medical chart by trained research nurses. Waiver of authorization and informed consent were obtained from the UAB Institutional Review Board. Infants with major congenital anomalies, without placental evaluation, and those lost to follow-up were excluded.
At our center, placental pathology is routinely performed in cases of preterm delivery. Each placenta and umbilical cord is assessed individually regardless of singleton or multiple gestation. Placentas of extremely preterm infants were evaluated by two pathologists (OMF-P, SDR) using prospectively defined standards within the first 48 hours after delivery.13,21 Any vasculitis in the umbilical cord (funisitis) or vasculitis in the chorionic plate of the placenta was considered to indicate FIR. For analyses of dose-dependence, infants were categorized into 5 different groups based on placental and umbilical cord histopathology by increasing stage and severity of fetal inflammatory response (Table I). Subacute necrotizing funisitis (SNF) is a robust indicator of duration and intensity of fetal inflammation20, 21 and chorionic plate vasculitis is an important gauge of both magnitude and ability of neutrophils to mount a fetal inflammatory response.6,20,21 Severity of maternal response was also analyzed. Severe histologic chorioamnionitis (HCA) was defined as intense inflammation (polymorphonuclear aggregates of ≥ 10–20 cells) with or without microabscesses.20,21 Mild to moderate HCA was defined as HCA without signs of severe inflammation.21
Table 1.
Groups created based on placental and umbilical cord histopathology*
| Group | Description |
|---|---|
| No chorioamnionitis | Absence of membranous, chorionic plate, and umbilical cord inflammation |
| Histologic chorioamnionitis (HCA), no funisitis | Presence of histologic chorioamnionitis alone without chorionic plate vasculitis and/or funisitis.
|
| Histologic chorioamnionis, stage 1 or 2 funisitis without chorionic vasculitis | Funisitis was defined as the presence of neutrophils in the walls of the umbilical vessels and/or Wharton’s jelly
|
| Histologic chorioamnionitis, stage 1 or 2 funisitis with grade 1 chorionic plate vasculitis |
|
| Histologic chorioamnionitis, subacute necrotizing funisitis or grade 2 chorionic vasculitis |
|
Potential covariates included maternal age, gestational age, race/ethnicity, twin and other multiple gestations, clinical chorioamnionitis by obstetrical assessment, premature rupture of membranes > 18 h, maternal preeclampsia, use of antenatal steroids, use of antepartum antibiotics, Apgar score < 3 at 5 min, and birth weight. Secondary outcomes included early onset sepsis (positive blood culture ≤ 72 hours of age), late onset sepsis (positive blood culture ≥ 72 hours of age and treated for > 5 days), severe intraventricular hemorrhage (IVH) defined as grade 3 or 4 IVH,28 and periventricular leukomalacia (PVL) defined as cystic area(s) identified in the brain parenchyma by neuroimaging at 28 days after birth.
Statistical analyses
Prior data indicated that the proportion of extremely preterm infants with severe NDI or death was approximately 38%. Assuming a hypothetical risk ratio (RR) for severe NDI/death in exposed infants relative to unexposed infants of 1.5 (least extreme RR to be detected), we planned a study with a minimum of 89 exposed infants and at least 177 unexposed infants (1:2 ratio of exposed to unexposed infants) for a power of 80% and α 0.05 using χ2 test. Unadjusted comparisons of baseline characteristics were performed using T test and one-way ANOVA (Tukey method) for continuous variables and χ2 test and Fisher exact test for categorical variables. Poisson regression was used to estimate risk ratios (RRs) and 95% confidence intervals (CIs) for the association between severe NDI/death and the severity of placental histopathology with and without adjustment for birth weight (BW), multiple gestation, and preeclampsia. A similar approach was used for secondary outcomes including death, severe NDI, individual components of NDI, and short-term neurologic morbidity (IVH and PVL).
Effect-measure modification between FIR and antenatal steroids for the outcome of NDI/death was assessed by joint exposure in additive and multiplicative scales. For this analysis, FIR and antenatal steroids were analyzed as risk factors. Exposure to FIR and no steroids/incomplete course of antenatal steroids was considered the high-risk category. The reference group (exposure to neither) was used to establish the baseline risk for NDI/death combining all other potential causes of NDI/death and to illustrate the effect of adding the other three categories to the baseline risk (departure from additivity). Under the assumption of a possible biologic interaction, the magnitude of risk observed in the high-risk category was expected to be greater than the relative risks observed in the other two categories after addition and/or multiplication of the obtained values.
All statistical analyses were performed using SAS 9.2 (Cary, NC).
Results
A total of 347 of 399 eligible preterm infants had outcome data at 18–22 months of corrected age (87%). Of these 347, 110 (32%) infants had placental/umbilical cord features indicative of FIR. Baseline characteristics of infants by exposure to FIR are shown in Table II. The mean BW was 830 g and the median gestational age was 26 weeks. The mean duration of gestation was 185 days (26 weeks and 3 days) with a standard deviation of ±12 days. Slightly more than half of the infants were non-Hispanic black infants. Clinical chorioamnionitis, prolonged rupture of membranes, and antepartum antibiotics were more common in infants with FIR. Maternal preeclampsia and multiple gestation were more common in the non-FIR group. Infants with FIR were more likely to be born at lower gestational ages and were more likely to develop severe IVH.
Table 2.
Baseline characteristics
| MATERNAL | FIR group (n=110) | Non-FIR group (n=237) | p |
|---|---|---|---|
| Maternal age, yr (Mean ± SD) | 25±6 | 26±6 | 0.06 |
| Black race (%) | 48 | 53 | 0.39 |
| Multiple gestation (%) | 22 | 30 | 0.13 |
| Preeclampsia (%) | 5 | 17 | 0.003 |
| Full course of antenatal steroids (%) | 68 | 60 | 0.14 |
| Antepartum antibiotics (%) | 86 | 72 | 0.003 |
| Clinical chorioamnionitis (%)§ | 36 | 12 | 0.0001 |
| Rupture of membranes >18 hrs prior to delivery (%) | 73 | 38 | 0.0001 |
| NEONATAL | |||
| Birth weight, grams (Mean±SD) | 842±232 | 824±234 | 0.50 |
| Gestational age, wk (Median, IQR) | 25 (24–27) | 26 (25–28) | 0.002 |
| Male sex (%) | 50 | 50 | 0.97 |
| Apgar score at 5 min (Median, IQR) | 7 (5–8) | 7 (5–8) | 0.17 |
| Apgar scores ≤ 3 at 5 minutes (%) | 9 | 10 | 0.76 |
| Apgar scores < 7 at 5 minutes (%) | 37 | 35 | 0.72 |
| Cord pH (Mean ± SD)** | 7.21±0.08 | 7.25±0.12 | 0.47 |
| Respiratory distress syndrome (%)† | 92 | 88 | 0.36 |
| Prophylactic indomethacin (%) | 59 | 63 | 0.50 |
| Early onset sepsis (%) | 3 | 1 | 0.33 |
| Late onset sepsis (%) | 41 | 35 | 0.36 |
| Intraventricular hemorrhage Grade 3 or 4* (%)28¶ | 23 | 14 | 0.04 |
| Cystic periventricular leukomalacia*(%)‡ | 4 | 4 | 0.91 |
n=332, infants who survived to 28 days of age
n=285, cord arterial pH
Respiratory distress syndrome was defined as clinical features of respiratory distress and/or required oxygen or positive pressure support for more than 6 hours within the first 24 hours
Cystic periventricular leukomalacia was defined by sonogram as any cystic area(s) in the brain parenchyma within 28 days
Clinical chorioamnionitis was considered a medical diagnosis if it was documented in the mother’s medical record by obstetricians, usually based on maternal temperature, uterine tenderness, fetal tachycardia or other clinical variables.
Intraventricular hemorrhage was defined by sonogram as any blood/echo-density in the brain parenchyma (grade 4) or any ventricular size enlargement with our without concurrent or prior intraventicular blood (grade 3).
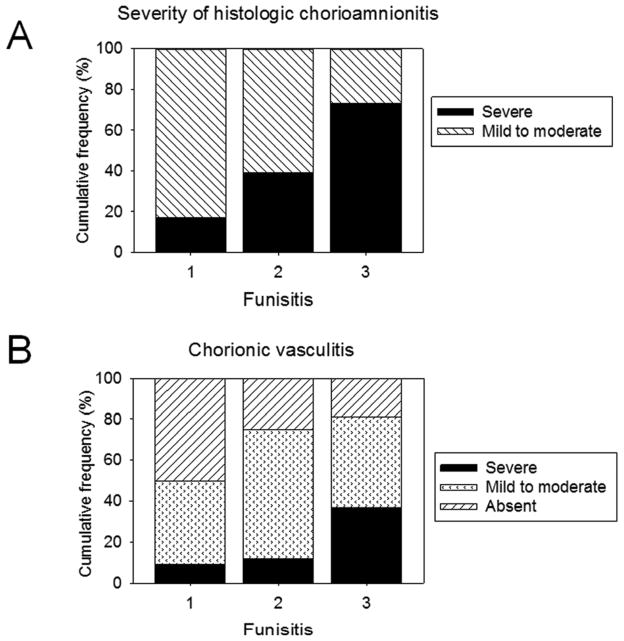
Placental inflammation was observed in 148 placentas (43%). Severe HCA was observed in 30% of placentas with inflammation and in 38% of placentas with features indicative of FIR. Severity of HCA had a strong correlation with funisitis (ranged from 8% in absence of funisitis to 73% in presence of stage 3 funisitis) (Figure). Placental inflammation (n=148) was classified as histologic chorioamnionitis (HCA; maternal response) without funisitis (26%), phlebitis alone (24%), combined arteritis/phlebitis (40%), and subacute necrotizing funisitis (10%). Increasing magnitude of umbilical cord inflammation was associated with increasing severity of chorionic plate vasculitis, suggesting a dose-dependent relationship (p=0.02; Figure). Two cases of chorionic plate vasculitis were excluded from analysis because they could not be categorized accurately, there being no evidence of thrombosis despite intense inflammation.
Figure.
Increasing severity of funisitis was associated with increasing severity of A, histologic chorioamnionitis (Chi-square test; p<0.001) and B, chorionic vasculitis (Chi-square test; p=0.02)
Severe NDI or death occurred in 141 infants (41%); 39% in the non-FIR group and 45% in the FIR group (p=0.31). Severe FIR was associated with higher rates of NDI/death in the crude (p=0.005) and adjusted analysis (p=0.016) (Table III). Infants with subacute necrotizing funisitis and/or severe chorionic plate vasculitis had higher rates of NDI/death compared with infants without HCA (p=0.02). Infants with stage 1 or 2 funisitis and chorionic vasculitis tended to have higher rates of severe NDI/death compared with the no HCA group (p=0.07) but infants who had stage 1 or 2 funisitis without chorionic vasculitis tended to have lower rates of NDI/death compared with the no HCA group (p=0.16). In the subgroup of infants with stage 1 or 2 funisitis, the presence of chorionic vasculitis was associated with higher risk of severe NDI/death (RR: 1.93; 95% CI: 1.002– 3.72; p=0.03). Infants with subacute necrotizing funisitis were more likely to have severe NDI/death compared with infants without placental/umbilical cord inflammation (adjusted RR, ARR: 1.87; 95% CI: 1.04 – 3.35; p=0.04). Similarly, the risk of severe NDI/death was greater in infants with severe chorionic plate vasculitis (ARR: 2.21; 95% CI: 1.10 – 4.46; p=0.03).
Table 3.
Dose-dependent relationship between fetal inflammatory response and NDI/death
| NDI or death | Mortality | NDI | ||||
|---|---|---|---|---|---|---|
| N (%) | Adjusted RR (95%CI)* | N (%) | Adjusted RR (95%CI)* | N (%) | Adjusted RR (95%CI)* | |
| No histologic chorioamnionitis | 70 (35) | Referent | 45 (23) | Referent | 25 (16) | Referent |
| Histologic chorioamnionitis, no funisitis | 22 (58) | 1.50 (1.04 – 2.14) | 16 (42) | 1.62 (1.00 – 2.21) | 6 (27) | 1.88 (0.89 – 3.96) |
| Histologic chorioamnionitis, stage 1 or 2 funisitis without chorionic vasculitis | 6 (23) | 0.81 (0.44 – 1.50) | 5 (19) | 1.12 (0.52 – 2.39) | 1 (5) | 0.59 (0.14 – 2.51) |
| Histologic chorioamnionitis, stage 1 or 2 funisitis with grade 1 chorionic vasculitis | 28 (47) | 1.22 (0.88 – 1.70) | 21 (36) | 1.39 (0.89 – 2.15) | 7 (18) | 1.19 (0.59 – 2.40) |
| Histologic chorioamnionitis, subacute necrotizing funisitis or grade 2 chorionic vasculitis | 15 (60) | 1.94 (1.27 – 2.96) | 10 (40) | 2.01 (1.12 – 3.59) | 5 (33) | 2.52 (1.11 – 5.72) |
Adjusted for 100-g increment in BW, singleton birth, and preeclampsia.
The overall mortality rate was 28% (Table III). Subacute necrotizing funisitis and/or severe chorionic plate vasculitis were associated with higher risk of mortality compared with infants without placental inflammation (p=0.04). Differences in baseline characteristics by groups created based on placental and umbilical cord histopathology are shown in Table IV (available at www.jpeds.com).
Of the 250 infants assessed at follow-up, 44 were diagnosed with severe NDI (18%) (Table III). Subacute necrotizing funisitis and/or severe chorionic plate vasculitis were associated with a greater risk for severe NDI. Subacute necrotizing funisitis and/or severe chorionic plate vasculitis was associated with a higher risk of BSID III language score <70 when compared with the absence of placental inflammation (ARR: 2.57; 95% CI: 1.02 – 6.46; p=0.04) (Table V; available at www.jpeds.com).
Severe IVH was observed in 17% of infants (57 of 332 survivors to 28 days). After adjustment, severe FIR (subacute necrotizing funisitis and/or grade 2 chorionic vasculitis) was not associated with a higher risk of severe IVH (ARR: 1.52; 95%CI= 0.64 – 3.64; p =0.57) or PVL. Early onset sepsis was documented in 1% of infants without evidence of placental inflammation, 3% of infants with HCA alone, and 3% of infants with FIR (p=0.22); however, our study was not powered to determine a difference in early onset sepsis (<2%) between the groups. Apgar scores ≤ 3 at 5 minutes were more commonly seen in infants with subacute necrotizing funisitis (31%) compared with infants without evidence of placental inflammation (9%; p=0.007). Approximately 11% of placentas with intact membranes and absence of clinical chorioamnionitis had features consistent with FIR. In the comparative analysis between FIR and no antenatal steroids/incomplete course of antenatal steroids, no biologic interaction for severe NDI/death was found (Table VI; available at www.jpeds.com).
Discussion
This study demonstrates that prolonged/severe fetal inflammatory response characterized by subacute necrotizing funisitis and severe chorionic plate vasculitis with thrombosis are associated with higher mortality and NDI at 18 to 22 months of age in extremely preterm infants, even after adjustment for potential confounders. We found that mild funisitis with any chorionic plate vasculitis was associated with higher NDI/death when compared with mild funisitis without any chorionic plate vasculitis, signifying the importance of chorionic plate vasculitis as an indicator of greater intensity of fetal inflammation, and as a predictor of NDI/death. Our study also confirms the strong dose-dependent relationship between chorionic plate vasculitis and increasing duration of umbilical cord inflammation.19
The exposure rates found in this single center study might differ from those reported in population-based studies, as our center is a regional perinatal center with a high-risk obstetric service (referral bias). This type of bias could explain the wide discrepancy in the association between histologically identified chorioamnionitis and NDI reported in previous studies, particularly for the direction of effect (protective vs. risk factor) which seems to be highly dependent on the often underreported severity of FIR.3,5 One of the limitations of our study is that the cause of preterm delivery (i.e. spontaneous versus indicated) was not recorded in our database. Higher rates of spontaneous preterm delivery may be associated with more placental inflammation. Another limitation is that that the rates of exposure in those who survive and are followed-up may differ from those who are not evaluated due to death or loss to follow-up.29 In order to reduce the likelihood of survival bias and to include the worst outcomes, deaths were included in the primary outcome. Defining NDI based on a list of heterogeneous long-term outcomes is another limitation of our study; however, most clinical studies that measure long-term outcomes in preterm infants use similar operational definitions despite these limitations. Finally, our classification is limited to characteristics of inflammation in the placenta. Other non-inflammatory placental features such as those inherent in multiple gestation (e.g. mono-chorionic vs. di-chorionic placentas) and other vascular changes in the placenta may also be associated with poor neurologic outcomes.30
The association between prolonged/severity of fetal inflammation and NDI/death identified in our study is unlikely to be explained by chance and is independent of birth weight. The strengths of our study include minimization of information bias for the exposure status using standardized histopathologic methods for diagnosis of severity of fetal inflammatory response compatible with previous definitions6,20,21,31 that have high level of intra- and inter-observer agreement, strong external validity, use of adjusted risk ratios instead of odds ratios to avoid overestimation of risk, and sufficient sample size. The use of BSID III, a conservative estimator of NDI for assessment of neurodevelopment, is a further strength of this study.26
There is biological plausibility for the association between fetal inflammation and NDI, possibly due to alterations in fetal cytokine concentrations that may cause brain injury. 7–11, 14, 18, 32 Cytokine concentrations have a linear relationship with increasing severity of fetal inflammation.31 Cytokine concentrations in cord blood are elevated in presence of chorionic vasculitis.19 Although several observational studies have reported higher risk of motor impairment in infants with higher cytokine concentrations;14,18,32 others have failed to detect such associations.
The majority of studies that evaluated the association between any FIR and NDI found they were associated with lower cognitive scores7,15 and CP9,14 but other studies have not.3, 13 The lack of consistency in reporting severity of FIR makes comparisons between studies difficult. In general, only severe FIR is associated with white matter injury, lower cognitive scores, and CP, especially when chorionic plate vasculitis is present.7, 14, 20
Histologically identified chorioamnionitis is a risk factor for early mortality, particularly for infants born to mothers presenting with clinical signs of chorioamnionitis.16 Studies comparing any histologically identified chorioamnionitis with the absence of placental inflammation reported higher survival rates associated with histologically identified chorioamnionitis.3,5 Studies that compared mortality between infants with histologic chorioamnionitis alone (maternal response only) and those with histologic chorioamnionitis and FIR (maternal and fetal response) found a higher mortality in those preterm infants with FIR.3, 25 These findings underscore the importance of reporting well-defined features of increasing duration and severity of placental inflammation. A time-dependent dual effect of inflammation on neurodevelopmental outcomes is plausible.11,14 Brief exposure to mild inflammation may induce neuroprotection via immune processes11,18 and amplification of repair mechanisms in the developing brain.3, 5, 11
The absence of significant differences on cord pH values based on increasing prolongation/severity of FIR observed in our study suggests that neonatal injury in presence of fetal inflammation is independent of metabolic acidosis at birth. Finally, the lack of interaction between antenatal steroids, placental/fetal inflammation, and NDI/death suggests that antenatal steroids improve neurodevelopmental outcomes and reduce mortality regardless of the stage or degree of placental inflammation.2, 12, 27
Our study indicates that early prediction of outcomes in preterm infants may be improved by inclusion of subacute necrotizing funisitis and severe chorionic plate vasculitis as a categorical variable in regression models. Similarly, the use of detailed placental pathology for early detection of infants with FIR may be useful for individualized postnatal care and stratification in clinical trials evaluating neurodevelopmental impairment and death in this vulnerable population.
Supplementary Material
Abbreviations
- BSID
Bayley Scales of Infant Development
- CP
Cerebral palsy
- FIR
Fetal inflammatory response
- IVH
Intraventricular hemorrhage
- NICU
neonatal intensive care unit
- PVL
Periventricular leukomalacia
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee BH, Stoll BJ, McDonald SA, Higgins RD National Institute of Child H, Human Development Neonatal Research N. Neurodevelopmental outcomes of extremely low birth weight infants exposed prenatally to dexamethasone versus betamethasone. Pediatrics. 2008;121:289–96. doi: 10.1542/peds.2007-1103. [DOI] [PubMed] [Google Scholar]
- 3.Hendson L, Russell L, Robertson CM, Liang Y, Chen Y, Abdalla A, et al. Neonatal and neurodevelopmental outcomes of very low birth weight infants with histologic chorioamnionitis. J Pediatr. 2011;158:397–402. doi: 10.1016/j.jpeds.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Ambalavanan N, Baibergenova A, Carlo WA, Saigal S, Schmidt B, Thorpe KE, et al. Early prediction of poor outcome in extremely low birth weight infants by classification tree analysis. J Pediatr. 2006;148:438–44. doi: 10.1016/j.jpeds.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 5.Lahra MM, Jeffery HE. A fetal response to chorioamnionitis is associated with early survival after preterm birth. Am J Obstet Gynecol. 2004;190:147–51. doi: 10.1016/j.ajog.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Dammann O, Allred EN, Leviton A, Shen-Schwarz S, Heller D, Genest DR, et al. Fetal vasculitis in preterm newborns: interrelationships, modifiers, and antecedents. Placenta. 2004;25:788–96. doi: 10.1016/j.placenta.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Redline RW, Wilson-Costello D, Borawski E, Fanaroff AA, Hack M. The relationship between placental and other perinatal risk factors for neurologic impairment in very low birth weight children. Pediatr Res. 2000;47:721–6. doi: 10.1203/00006450-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatr Res. 1999;46:566–75. doi: 10.1203/00006450-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–81. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 10.Dammann O, Leviton A. Inflammatory brain damage in preterm newborns--dry numbers, wet lab, and causal inferences. Early Hum Dev. 2004;79:1–15. doi: 10.1016/j.earlhumdev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Malaeb S, Dammann O. Fetal inflammatory response and brain injury in the preterm newborn. J Child Neurol. 2009;24:1119–26. doi: 10.1177/0883073809338066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kent A, Lomas F, Hurrion E, Dahlstrom JE. Antenatal steroids may reduce adverse neurological outcome following chorioamnionitis: neurodevelopmental outcome and chorioamnionitis in premature infants. J Paediatr Child Health. 2005;41:186–90. doi: 10.1111/j.1440-1754.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- 13.Andrews WW, Cliver SP, Biasini F, Peralta-Carcelen AM, Rector R, Alriksson-Schmidt AI, et al. Early preterm birth: association between in utero exposure to acute inflammation and severe neurodevelopmental disability at 6 years of age. Am J Obstet Gynecol. 2008;198:466, e1–e11. doi: 10.1016/j.ajog.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leviton A, Allred EN, Kuban KC, Hecht JL, Onderdonk AB, O’Shea TM, et al. Microbiologic and histologic characteristics of the extremely preterm infant’s placenta predict white matter damage and later cerebral palsy. the ELGAN study. Pediatr Res. 2010;67:95–101. doi: 10.1203/PDR.0b013e3181bf5fab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rovira N, Alarcon A, Iriondo M, Ibanez M, Poo P, Cusi V, et al. Impact of histological chorioamnionitis, funisitis and clinical chorioamnionitis on neurodevelopmental outcome of preterm infants. Early Hum Dev. 2011;87:253–7. doi: 10.1016/j.earlhumdev.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Pappas A Shankaran S, Stoll BJ, Kendrick DE, Bell EF, Laptook AR, et al. Histological choriamnionitis and early childhood outcomes among preterm infants < 27 weeks GA. E-PAS20113836.381. [Google Scholar]
- 17.Redline RW. Infections and other inflammatory conditions. Semin Diagn Pathol. 2007;24:5–13. doi: 10.1053/j.semdp.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Dammann O, O’Shea TM. Cytokines and perinatal brain damage. Clin Perinatol. 2008;35:643–63. doi: 10.1016/j.clp.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 20.Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med. 2006;11:296–301. doi: 10.1016/j.siny.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Faye-Petersen OM, Heller DS, Joshi VV. Handbook of placental pathology. 2. Andover, UK: Taylor & Francis; 2006. p. xiii.p. 239. [Google Scholar]
- 22.Machin G. Funisitis and chorionic vasculitis: relation to chorioamnionitis, timing and scoring. Fetal Pediatr Pathol. 2011;30:414–30. doi: 10.3109/15513815.2011.618872. [DOI] [PubMed] [Google Scholar]
- 23.Hecht JL, Allred EN, Kliman HJ, Zambrano E, Doss BJ, Husain A, et al. Histological characteristics of singleton placentas delivered before the 28th week of gestation. Pathology. 2008;40:372–6. doi: 10.1080/00313020802035865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen AR, Collins MH, Genest D, Heller D, Shen-Schwarz S, Banagon P, et al. Very low birthweight placenta: clustering of morphologic characteristics. Pediatr Dev Pathol. 2000;3:431–8. doi: 10.1007/s100249910044. [DOI] [PubMed] [Google Scholar]
- 25.Lau J, Magee F, Qiu Z, Hoube J, Von Dadelszen P, Lee SK. Chorioamnionitis with a fetal inflammatory response is associated with higher neonatal mortality, morbidity, and resource use than chorioamnionitis displaying a maternal inflammatory response only. Am J Obstet Gynecol. 2005;193:708–13. doi: 10.1016/j.ajog.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Vohr BR, Stephens BE, Higgins RD, Bann CM, Hintz SR, Das A, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J Pediatr. 2012;161:222–8. doi: 10.1016/j.jpeds.2012.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlo WA, McDonald SA, Fanaroff AA, Vohr BR, Stoll BJ, Ehrenkranz RA, et al. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks’ gestation. JAMA. 2011;306:2348–58. doi: 10.1001/jama.2011.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 29.Castro L, Yolton K, Haberman B, Roberto N, Hansen NI, Ambalavanan N, et al. Bias in reported neurodevelopmental outcomes among extremely low birth weight survivors. Pediatrics. 2004;114:404–10. doi: 10.1542/peds.114.2.404. [DOI] [PubMed] [Google Scholar]
- 30.Helderman JB, O’Shea TM, Kuban KC, Allred EN, Hecht JL, Dammann O, et al. Antenatal antecedents of cognitive impairment at 24 months in extremely low gestational age newborns. Pediatrics. 2012;129:494–502. doi: 10.1542/peds.2011-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hecht JL, Fichorova RN, Tang VF, Allred EN, McElrath TF, Leviton A, et al. Relationship between neonatal blood protein concentrations and placenta histologic characteristics in extremely low GA newborns. Pediatr Res. 2011;69:68–73. doi: 10.1203/PDR.0b013e3181fed334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlo WA, McDonald SA, Tyson JE, Stoll BJ, Ehrenkranz RA, Shankaran S, et al. Cytokines and neurodevelopmental outcomes in extremely low birth weight infants. J Pediatr. 2011;159:919–25. e3. doi: 10.1016/j.jpeds.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.