Abstract
Background
We evaluated external ventricular drain placement for factors associated with placement accuracy. Data was acquired using an electronic health record data requisition tool.
Method
Medical records of all patients who underwent ventriculostomy from 2003–2010 were identified and evaluated. Patient demographics, diagnosis, type of guidance and number of catheter passes were searched for and recorded. Post-procedural hemorrhage and/or infection were identified. A grading scale was used to classify accuracy of catheter placements. A multiple logistic regression model was developed to assess features associated with accurate catheter placement.
Results
One hundred nine patients who underwent 111 ventriculostomies from 2003–2010 were identified. Patient diagnoses were classified into vascular (63%), tumor (21%), trauma (14%), and cyst (2%). Procedures were performed freehand in 90 (81%), with the Ghajar guide in 17 (15%), and with image guidance in 4 (4%) patients. Eighty-eight (79%) catheters were placed in the correct location. Trauma patients were more likely to have catheters misplaced (p=0.007) whereas patients in other diagnostic categories were not significantly associated with misplaced catheters. Post-procedural hemorrhage was noted in 2 (1.8%) patients on post-procedural imaging studies. Five (4.5%) definite and 6 (5.4%) suspected infections were identified.
Conclusions
External ventricular drain placement can be performed accurately in most patients. Patients with trauma are more likely to have catheters misplaced. Further development is required to identify and evaluate procedure outcomes using an electronic health record repository.
Keywords: Ventriculostomy, Cerebral ventricle, Frontal lobe, Intracranial hemorrhage, Bacterial meningitis, Electronic health record
INTRODUCTION
External ventricular drain (EVD) placement can be used for cerebrospinal fluid (CSF) diversion, to deliver medication, and/or to monitor intracranial pressure for patients who suffer from hydrocephalus, cerebrovascular events, infection, malignancy, or trauma. The procedure can be performed at the bedside or in the operating room (OR) often under difficult and time-sensitive conditions. This procedure, however, is not without risk and can result in complications.
To achieve the goal of external ventricular drainage, accurate catheter placement is essential with minimal manipulation. Accuracy rates using the freehand technique have been reported between 40 and 98 percent.[11, 9, 16, 23, 10, 7, 4, 27, 26, 12, 28] These studies differ in their reporting strategies, but a grading scale has been described to assess catheter placement accuracy. The implication of multiple catheter passes have not been extensively described.[9, 10, 7, 20, 25] However, if the catheter does not show free flow of CSF on the first pass, it is withdrawn and an additional attempt to cannulate the ventricle is performed. It has been postulated that multiple catheter passes may be a cause of post-EVD placement hemorrhage.[20]
Hemorrhage, a common complication of EVD placement, has been reported at rates ranging from 0.2 to 41 percent.[28, 25, 1, 15, 21, 22, 8] A recent meta-analysis showed the potential for significant hemorrhagic complication to be less than one percent of ventriculostomy patients.[3] The analysis, however, revealed differing opinions on how to define hemorrhage, and it reported that there were varying post-procedural imaging practices across the reporting institutions. In some institutions, all patients that receive an intracranial catheter have post-procedural radiographic imaging, while other institutions do not use this approach as a standard of practice.
Ventriculostomy-related infection, another common complication, has been reported to occur in approximately ten percent of patients but there are differing criteria for defining infection in each report.[12, 28, 25, 22, 1, 2, 14, 6, 18, 5, 19] In this article, we present an addition to the most commonly reported definitions of catheter-related infection.
This study evaluates the current practice for frontal EVD placement at a tertiary medical center with the goals of assessing complication rates and identifying factors associated with accurate catheter placement.
METHODS AND MATERIALS
We reviewed the clinical procedure history stored in a relational database extracted from the patient electronic health records (EHR) data repository to identify a cohort of patients for analyzing EVD practices and complications.
Study Setting and Population
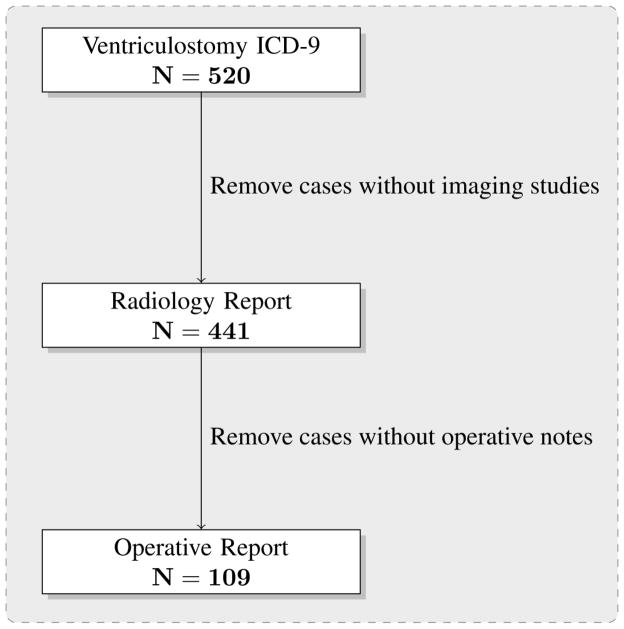
This retrospective Institutional Review Board (IRB)-approved and Health Insurance Portability and Accountability Act (HIPAA)-compliant study was performed in a metropolitan, tertiary care adult-only academic medical center. Eligible study patients were those 16 years of age or older who underwent ventriculostomy in the intensive care unit or in the operating room between 2003 and 2010. Medical records of all patients who had an International Classification of Diseases 9th Revision (ICD-9) procedure code of 02.2 (for ventriculostomy) as principal or secondary diagnosis were searched for in the Research Patient Data Repository (RPDR), a warehouse of medical records derived from the institution’s EHR, and analyzed using Structured Query Language (SQL). Information collected for further analysis included patient demographics, operative reports, post-procedural radiology reports for imaging studies completed within twenty-four hours of the procedure, microbiology reports for those examinations completed within four weeks of the ventriculostomy, and discharge summaries, if available. Only the first ventriculostomy a patient received at our institution was included in the study. Most procedure notes from the intensive care unit and emergency room were not available in the EHR because a paper chart was in use in those care settings. Figure 1 illustrates how the cohort was selected and how information was identified from multiple data sources (e.g. Radiology Information System, Hospital Information System, Laboratory Information System). Specifically, the figure demonstrates how a procedure ICD-9 code was the first step in patient identification and progressively how the lack of a radiology report and/or operative report decreased the size of the study cohort. Furthermore, we initially included patients identified through ICD-9 coded procedures with either handwritten bedside procedure notes or EHR operative procedure notes – that is, those patients without an operative procedure note were later excluded from the study.
Figure 1.
Patient cohort selection flowchart.
Study Variables
Study variables included patient age, sex, preoperative diagnosis, number of passes of the catheter, post-procedural catheter location, type of catheter guidance, and the presence of hemorrhage and/or infection. Age was analyzed as a continuous variable. All other variables were nominal.
A pre-existing grading scale for catheter placement accuracy modified by the addition of a grade (4) for failed procedures was used (Table 1).[11] Grade 1 was defined as a properly placed catheter in the ipsilateral frontal horn including the anterior portion of the third ventricle. Grade 2 catheter placement was identified if the catheter tip was in the contralateral frontal horn, corpus callosum or interhemispheric fissure. Catheters in other brain parenchyma or other fluid filled spaces were defined as Grade 3. The location of each catheter was determined by review of each patient’s post-procedural computed tomography (CT) imaging study. Those patients without post-procedural imaging were excluded as shown in Figure 1.
Table 1.
Ventricular catheter placement grading scale, adapted to include failed procedures.[11]
| Grade | Description |
|---|---|
| 1 | Appropriately placed catheter in the ipsilateral frontal horn including tip of the 3rd ventricle |
| 2 | Suboptimal placement in the contralateral frontal horn, corpus callosum or interhemispheric fissure |
| 3 | Suboptimal placement in other brain parenchyma or other fluid filled spaces |
| 4 | Failed procedure |
The number of passes of the ventriculostomy catheter was determined by review of operative notes. Number of passes was analyzed as a binary feature, “one pass” and “greater than one pass” of the catheter. Whenever the number of passes was not explicitly stated in the procedure note, the case was categorized as one pass.
Types of catheter guidance include use of Ghajar guide, image guidance, and no guidance (i.e. freehand). If there was no explicit documentation of Ghajar or image guidance the procedure was assigned to the no guidance category.
The presence of hemorrhage was defined as blood product along the catheter tract as observed on the CT image. Patients with trace and questionable hemorrhage were included in the no hemorrhage category. Questionable hemorrhage was defined as a subtle increase in density around the catheter on CT without identifiable collections of blood. All patients that we placed in the EVD-related hemorrhage group by review of their imaging studies were confirmed by review of the final radiology reports.
Definite EVD-related infection was defined by having a positive CSF culture or grossly purulent CSF followed by a course of antibiotics within four weeks of the ventriculostomy procedure. Patients with CSF pleocytosis, fever and/or change in mental status followed by antibiotics without an identified alternate source of infection were classified as suspected EVD related infections. All patients with infection were identified by review of microbiology reports and discharge summaries.
Statistical Analysis
Statistical analysis was performed using R Statistical Software (The R Foundation for Statistical Computing, Vienna, Austria). Multiple logistic regression was utilized to assess features associated with accurate catheter placement. Catheter placement grades 2 – 4 were grouped to form a category representative of inappropriately placed catheters, while catheter placement grade 1 was deemed a positive outcome, corresponding to appropriate catheter placement. The categories of “accurate” versus “inaccurate” determined by the method above were chosen despite drain functionality that occasionally occurred in the “inaccurate” group to evaluate factors associated with catheter placement accuracy using a multivariate logistic regression model. Independent variables included age, sex, pre-procedure diagnosis, number of passes and the types of guidance support. Univariate analysis was performed to select features to include in the model, excluding features with p>0.25. Using the remaining features, backward elimination was used to select variables for inclusion in the final model.
The model was evaluated using randomized ten-fold cross-validation. To test the ability of the model to distinguish between properly and improperly placed catheters, the area under the receiver operating characteristic (ROC) curve was calculated for each fold of cross-validation and the values were averaged. To test model calibration, the Hosmer-Lemeshow (H–L) statistic p-value was calculated.
RESULTS
One hundred nine patients that underwent 111 frontal EVD placements from 2003–2010 at our institution were identified in the RPDR. Two patients had bilateral EVD placements. The average patient age was 55 (range 16 – 96) years; 48% were female. Diagnoses were classified into four categories – vascular (63%), tumor (21%), trauma (14%), and cyst (2%) (Table 2).
Table 2.
Patient cohort
| N | 109 |
| Number of procedures | 111 (2 bilateral) |
| Age | mean 55 (16 – 96 years) |
| Sex | 52 % Male 48 % Female |
| Diagnosis | |
| Vascular | 63 % |
| Tumor | 21 % |
| Trauma | 14 % |
| Cyst | 2 % |
Procedures were performed freehand in 90 (81%) catheter placements. A Ghajar guide was used in 17 (15%) and image guidance was used in 4 (4%) placements. Post-procedural hemorrhage was noted on imaging study in 2 (1.8%) patients. Definite infection was noted in 5 (4.5%) patients. Suspected infection was found in 6 (5.4%) patients. There were 88 (79%) Grade 1, 16 (14%) Grade 2, 5 (5.0%) Grade 3, and 2 (2.0%) Grade 4 catheter placements. Multiple passes were attempted in 6 (5.4%) procedures (Table 3).
Table 3.
Patient outcome variables
| Variable | Percent |
|---|---|
| Type of Guidance | |
| Freehand | 81 |
| Ghajar | 15 |
| Image guidance | 4 |
| Complication | |
| Hemorrhage | 1.8 |
| Definitive Infection | 4.5 |
| Suspected Infection | 5.4 |
| Placement | |
| Grade 1 | 79 |
| Grade 2 | 14 |
| Grade 3 | 5 |
| Grade 4 | 2 |
| Multiple Passes | 5.4 |
Univariate Analysis
On univariate analysis, trauma-based diagnoses (p=0.007) and the number of catheter passes (p<0.001) were significantly associated with inaccurate catheter placement (Table 4). The diagnostic categories of vascular, tumor and cyst were not significantly associated with inaccurate placement. Patient demographics were not associated with inaccurate catheter placement.
Table 4.
Univariate analysis of variables to include in the multivariate guidance model
| Variable | P-value |
|---|---|
| Age | 0.37 |
| Male sex | 0.21 |
| Trauma | 0.007 |
| >1 pass | < 0.001 |
Multivariate Analysis
Patient age, sex, diagnosis and number of passes were included in the final model after backwards elimination. After randomized ten-fold cross-validation, trauma-based diagnoses were significantly associated with misplaced catheters (p=0.007) (Table 5). Multiple passes was not statistically associated with inaccurate placement after adjusting for other factors. Likewise, patient demographics were not associated with inaccurate catheter placement. The average area under the ROC was 0.75 ± 0.21 (95% CI 0.58 – 0.92). The model was adequately calibrated with an average H-L statistic p-value of 0.26.
Table 5.
Cross-validated model using catheter placement as an outcome.
| Variable | P-value | Odds Ratio |
|---|---|---|
| Age | 0.39 | 1.0 ± 0.007 |
| Male Sex | 0.077 | 0.32 ± 0.087 |
| Trauma | 0.007 | 14 ± 4.8 (95% CI 10–17) |
| >1 pass | 0.12 | 7.0 ± 3.3 |
Other Complications
There were two cases of hemorrhage. The first case was a 52 year-old male admitted for an elective sigmoid colectomy. The patient had respiratory arrest postoperatively and was resuscitated. Three days after admission, a head CT showed acute hemorrhage within bilateral cerebellar hemispheres with evidence of hemorrhage lying in the occipital horns of both lateral ventricles. The patient was taken to the OR for EVD placement and suboccipital craniotomy. The patient received a freehand right frontal grade 2 EVD on first pass. Hemorrhage was found postoperatively along the catheter tract. The patient expired four days after admission.
The second case was that of a 57 year-old male with untreated hypertension who was unresponsive on initial evaluation. CT of the head showed a left frontoparietal intracranial hemorrhage extending to the intraventricular spaces and diffuse subarachnoid hemorrhage with midline shift and brain edema. CT with contrast showed multiple venous and arterial structures consistent with either arteriovenous malformation or arteriovenous fistula. The patient received a freehand right frontal Grade 1 EVD on first pass. Hemorrhage was found post-procedurally along the catheter tract. The patient was taken to the OR multiple times for management of his primary problem: fistula repair and a post-procedural hematoma evacuation. An additional left EVD was placed at a later time. Upon EVD removal, acute meningitis was diagnosed and the patient had deterioration of his neurological status. There was purulent CSF on lumbar puncture, but no organisms were identified. An antibiotic regimen of ceftazidime and vancomycin was started with resolution of infection evident on CSF laboratory results within 1.5 weeks. The patient was discharged to rehabilitation 33 days after admission.
DISCUSSION
The ideal EHR would provide physicians with a centralized location to store and retrieve clinical information while improving the efficiency and quality of care. A typical inpatient hospital course requires a physician to document and locate clinical findings on one of many EHR software platforms, including information from laboratory information systems, radiology information systems, and other hospital legacy systems. These systems are not integrated at the level of clinical knowledge and are usually developed by different vendors. If a clinician poses a clinical question and requests data from the hospital’s warehouse, exhaustive database development is required to isolate the patient cohort and associated study variables. In this paper, we present the results of an example study where we use the electronic record as the sole data source.
This study evaluated EVD placement accuracy and assessed factors that were associated with catheter placement accuracy. EVD catheters were appropriately placed using a pre-defined grading scale in 79% of procedures. In prior studies at other institutions, accurate placement has been defined as the tip being located ipsilateral to the insertion site within the frontal horn of the lateral ventricle or tip of the third ventricle. These studies noted accurate placement occurring in between 40 and 98% of procedures.[11, 23, 10, 7, 26, 28] Other studies did not specifically provide definitions for appropriate placement but reported accurate catheter placement rates occurring between 79 and 94%.[9, 16, 4, 12] Findings from a study that followed pediatric shunt function suggest that the local environment of the catheter tip has an effect on shunt failure.[27] In that study, the authors defined the catheter as being located in the frontal horn, occipital horn, body of the lateral ventricle, third ventricle, embedded in brain, or unknown. Tip location was further described as surrounded by CSF, touching brain, or surrounded by brain parenchyma within the ventricle (slit ventricle). They determined shunt catheters in a pool of cerebrospinal fluid away from surrounding structures to be associated with lower rates of catheter malfunction[27]; shunt malfunctions necessitate adjustment or replacement of the catheter. Some studies have found a significant association between infection and EVD replacement.[2, 6] Our study used a grading scale that did not explicitly take catheter environment into account but described appropriate catheter location indicating accurate placement. Our study found no association between infection and catheter insertion accuracy. We did not specifically evaluate EVD malfunction and replacement because documentation was not readily available in the EHR.
Patients with a diagnosis of trauma were associated significantly with misplaced catheters. A previous study found trauma cases to be associated with increased rates of suboptimal EVD placement; the authors conjectured that distorted ventricular anatomy in trauma patients or small ventricles in younger patients, a population more prone to trauma, may contribute to catheter misplacement.[11] A significant number of patients who require EVD placement are trauma related.[16, 23, 15, 21, 22, 18, 12] On the other hand, trauma patients may not present with distorted ventricular anatomy.
Shifted and/or distorted anatomy may create technical difficulties for EVD placement. In these cases, it may be necessary to encourage the use of guidance technology. In our study, the usage of a guidance support system – Ghajar guide or an image guidance system – was not associated with either appropriate (Grade 1) or inappropriate (Grades 2–4) placement of EVD catheters. The Ghajar guide, in particular, is only useful in situations where ventricular anatomy is not distorted. Studies have shown that comfort levels with image guidance systems may take time to develop [13], and such systems are often too time-consuming to set up in an emergency setting.[17]
Multiple passes for EVD placement were found in only 5.4% of procedures, comparable with reported rates in other studies.[9, 10, 7, 25] Prospectively collected data may reveal an increased percentage of cases that require multiple attempts to accurately place an EVD catheter. Retrospective studies may suffer from data inadequacies such as unreported passes of the catheter.[20] None of the cases with multiple passes reported in the current study were complicated with hemorrhage. However, there were very few patients found to have received multiple catheter passes or have hemorrhagic complications in our cohort.
The rate of hemorrhagic complication was 1.8%. The presence of blood was noted along the catheter tract in both cases and was not a localized collection. Thus, the volume of blood could not be computed. None of the patients with catheter-related hemorrhage within the study time frame experienced neurologic deficit or required operative intervention for reasons that could be attributed to placement of an EVD. Although EVD-associated hemorrhage has been correlated by others with vascular diagnoses [15], we were unable to find such an association in our cohort despite a majority of cases resulting from vascular diagnoses (63%).
Prior studies from other institutions that discussed hemorrhage as immediate sequelae of EVD placement varied in their reporting strategies and presented rates ranging from 0.2 to 41%.[28, 25, 15, 21, 22, 8] Some did not explicitly provide guidelines for reviewing imaging studies, while others described categories of intraparenchymal hemorrhage volume with boundaries ranging from 0.1 to greater than 15 cm3. Variability in guidelines for reviewing post-procedural images may contribute to the wide range of EVD-related hemorrhage rates. The wide range may also stem from varying imaging practices in different hospitals. Not all practitioners routinely perform post-procedural imaging after placing intracranial catheters and this may cause selection bias. In a hospital where post-procedural imaging is not common practice, the clinician may request an imaging study when the patient has deterioration in mental status or to assess drain location after malfunction, thus increasing the apparent rate. Alternatively, subclinical hemorrhage may go unseen in those patients that do not receive post-procedural imaging and cause an artificially low reported rate. A meta-analysis that evaluated sixteen studies for EVD related hemorrhage found no significant difference between the numbers of patients reported to experience catheter related hemorrhage in hospitals that routinely perform post-procedural imaging versus those that do not.[3] In our study, we only included patients who underwent post-procedural imaging in the final cohort.
In our investigation of catheter-related infection we posited that standardizing the reporting of infection is relevant to the decision to treat. Patients with CSF infections generally are divided into categories of definite infection, suspected infection, colonization or contamination.[14] Most studies use a positive CSF culture as the definition of catheter-related infection and do not report on colonization and contaminants.[9, 23, 12, 1, 22, 2, 18, 19] Others expand the definition to include concomitant presence of clinical symptoms such as fever and decreased mental status for a more explicit definition.[16, 21, 6, 5, 24] Our definition for definite catheter-related infection was comparably broader than most studies because a positive culture was not required if purulent CSF was reported regardless of whether the cultures were positive. Our definition of suspected infection drew upon clinical and laboratory results in conjunction with the decision to clinically treat the infection. Craniotomy can cause additional risk for CSF infection and can confound isolation of the infectious source. However, patients that underwent EVD placement while undergoing craniotomy were not excluded from the cohort. Using our definition, infection rates in this study were in accordance with those reported in the literature, with 4.5% definite infection and 5.4% suspected infection.[14]
Limitations of Our Study
As exemplified in Figure 1, hospital information system database design, lack of integration between disparate systems, and inconsistent data sharing across multiple healthcare facilities limit the amount of data ultimately retrieved from multiple hospital data systems. This study was also limited by the unavailability of handwritten notes in the electronic health record. Handwritten notes for procedures performed in the intensive care unit or in the emergency room could provide a larger cohort for analysis and potentially identify additional cases with hemorrhagic or infectious complications. In addition, this study is based on the experience of a single institution and may, with difficulty, generalize to other institutions. Finally, we excluded patients that did not receive post-procedural radiographic imaging, introducing the potential for selection bias.
CONCLUSION
The development of more advanced data retrieval methods is necessary to correlate large amounts of clinical data from multiple hospital information systems and obtain a complete patient sample. In our study, accurate placement of EVD catheters occurred in nearly all patients. Patients with trauma were more likely to have catheters misplaced. The rate of EVD-related hemorrhage did not directly correlate with an increased number of attempts to place the drainage catheter. CSF infection was not associated with inaccurate EVD placement. Larger patient numbers would be required to better define factors associated with catheter placement accuracy.
Acknowledgments
Boston Informatics Research Training Program, National Library of Medicine, National Institutes of Health, Bethesda MD USA, T15 M007092-19, Alexa T McCray PhD
Center for Integration of Medicine and Innovative Technology, Boston MA USA
National Institutes of Health, Bethesda MD USA, R42 CA115112-03, Kirby G Vosburgh PhD
Footnotes
This manuscript is based on a thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in the Harvard-MIT Division of Health Science and Technology at the Massachusetts Institute of Technology in September 2011.
Conflict of Interest:
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Anderson RC, Kan P, Klimo P, Brockmeyer DL, Walker ML, Kestle JR. Complications of intracranial pressure monitoring in children with head trauma. J Neurosurg. 2004;101(1 Suppl):53–58. doi: 10.3171/ped.2004.101.2.0053. [DOI] [PubMed] [Google Scholar]
- 2.Arabi Y, Memish ZA, Balkhy HH, Francis C, Ferayan A, Al Shimemeri A, Almuneef MA. Ventriculostomy-associated infections: incidence and risk factors. Am J Infect Control. 2005;33(3):137–143. doi: 10.1016/j.ajic.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Bauer DF, Razdan SN, Bartolucci AA, Markert JM. Meta-Analysis of hemorrhagic complications from ventriculostomy placement by neurosurgeons. Neurosurgery. 2011;69(2):255–260. doi: 10.1227/NEU.0b013e31821a45ba. [DOI] [PubMed] [Google Scholar]
- 4.Bogdahn U, Lau W, Hassel W, Gunreben G, Mertens HG, Brawanski A. Continuous-pressure controlled, external ventricular drainage for treatment of acute hydrocephalus--evaluation of risk factors. Neurosurgery. 1992;31(5):898–903. doi: 10.1227/00006123-199211000-00011. discussion 903–904. [DOI] [PubMed] [Google Scholar]
- 5.Bota DP, Lefranc F, Vilallobos HR, Brimioulle S, Vincent J-L. Ventriculostomy-related infections in critically ill patients: a 6-year experience. J Neurosurg. 2005;103(3):468–472. doi: 10.3171/jns.2005.103.3.0468. [DOI] [PubMed] [Google Scholar]
- 6.Chi H, Chang K-Y, Chang H-C, Chiu N-C, Huang F-Y. Infections associated with indwelling ventriculostomy catheters in a teaching hospital. Int J Infect Dis. 2010;14(3):e216–219. doi: 10.1016/j.ijid.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Ehtisham A, Taylor S, Bayless L, Klein MW, Janzen JM. Placement of external ventricular drains and intracranial pressure monitors by neurointensivists. Neurocrit Care. 2009;10(2):241–247. doi: 10.1007/s12028-008-9097-4. [DOI] [PubMed] [Google Scholar]
- 8.Gardner PA, Engh J, Atteberry D, Moossy JJ. Hemorrhage rates after external ventricular drain placement. J Neurosurg. 2009;110(5):1021–1025. doi: 10.3171/2008.9.JNS17661. [DOI] [PubMed] [Google Scholar]
- 9.Hayat A, Rodrigues D, Crawford P, Mendelow D. External ventricular drains - Can morbidity be reduced? Pakistan Journal of Neuroscience. 2009;4(1):1–3. [Google Scholar]
- 10.Huyette DR, Turnbow BJ, Kaufman C, Vaslow DF, Whiting BB, Oh MY. Accuracy of the freehand pass technique for ventriculostomy catheter placement: retrospective assessment using computed tomography scans. J Neurosurg. 2008;108(1):88–91. doi: 10.3171/JNS/2008/108/01/0088. [DOI] [PubMed] [Google Scholar]
- 11.Kakarla UK, Kim LJ, Chang SW, Theodore N, Spetzler RF. Safety and accuracy of bedside external ventricular drain placement. Neurosurgery. 2008;63(Suppl 1):ONS162–167. doi: 10.1227/01.neu.0000335031.23521.d0. [DOI] [PubMed] [Google Scholar]
- 12.Khan SH, Kureshi IU, Mulgrew T, Ho SY, Onyiuke HC. Comparison of percutaneous ventriculostomies and intraparenchymal monitor: a retrospective evaluation of 156 patients. Acta Neurochir Suppl. 1998;71:50–52. doi: 10.1007/978-3-7091-6475-4_16. [DOI] [PubMed] [Google Scholar]
- 13.Lollis SS, Roberts DW. Robotic catheter ventriculostomy: feasibility, efficacy, and implications. J Neurosurg. 2008;108(2):269–274. doi: 10.3171/JNS/2008/108/2/0269. [DOI] [PubMed] [Google Scholar]
- 14.Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES., Jr Ventriculostomy-related infections: a critical review of the literature. Neurosurgery. 2008;62(Suppl 2):688–700. doi: 10.1227/01.neu.0000316273.35833.7c. [DOI] [PubMed] [Google Scholar]
- 15.Maniker AH, Vaynman AY, Karimi RJ, Sabit AO, Holland B. Hemorrhagic complications of external ventricular drainage. Neurosurgery. 2006;59(4 Suppl 2):ONS419–425. doi: 10.1227/01.NEU.0000222817.99752.E6. [DOI] [PubMed] [Google Scholar]
- 16.Ngo QN, Ranger A, Singh RN, Kornecki A, Seabrook JA, Fraser DD. External ventricular drains in pediatric patients. Pediatr Crit Care Med. 2009;10(3):346–351. doi: 10.1097/PCC.0b013e3181a320cd. [DOI] [PubMed] [Google Scholar]
- 17.O’Neill BR, Velez DA, Braxton EE, Whiting D, Oh MY. A survey of ventriculostomy and intracranial pressure monitor placement practices. Surg Neurol. 2008;70(3):268–273. doi: 10.1016/j.surneu.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Paramore CG, Turner DA. Relative risks of ventriculostomy infection and morbidity. Acta Neurochir (Wien) 1994;127(1–2):79–84. doi: 10.1007/BF01808552. [DOI] [PubMed] [Google Scholar]
- 19.Park P, Garton HJL, Kocan MJ, Thompson BG. Risk of infection with prolonged ventricular catheterization. Neurosurgery. 2004;55(3):594–601. doi: 10.1227/01.neu.0000134289.04500.ee. [DOI] [PubMed] [Google Scholar]
- 20.Roberts DW. Is good good enough? Neurocrit Care. 2009;10(2):155–156. doi: 10.1007/s12028-009-9194-z. [DOI] [PubMed] [Google Scholar]
- 21.Roitberg BZ, Khan N, Alp MS, Hersonskey T, Charbel FT, Ausman JI. Bedside external ventricular drain placement for the treatment of acute hydrocephalus. Br J Neurosurg. 2001;15(4):324–327. doi: 10.1080/02688690120072478. [DOI] [PubMed] [Google Scholar]
- 22.Rossi S, Buzzi F, Paparella A, Mainini P, Stocchetti N. Complications and safety associated with ICP monitoring: a study of 542 patients. Acta Neurochir Suppl. 1998;71:91–93. doi: 10.1007/978-3-7091-6475-4_28. [DOI] [PubMed] [Google Scholar]
- 23.Saladino A, White JB, Wijdicks EF, Lanzino G. Malplacement of ventricular catheters by neurosurgeons: a single institution experience. Neurocrit Care. 2009;10(2):248–252. doi: 10.1007/s12028-008-9154-z. [DOI] [PubMed] [Google Scholar]
- 24.Schade RP, Schinkel J, Visser LG, Van Dijk JMC, Voormolen JHC, Kuijper EJ. Bacterial meningitis caused by the use of ventricular or lumbar cerebrospinal fluid catheters. J Neurosurg. 2005;102(2):229–234. doi: 10.3171/jns.2005.102.2.0229. [DOI] [PubMed] [Google Scholar]
- 25.Stangl AP, Meyer B, Zentner J, Schramm J. Continuous external CSF drainage--a perpetual problem in neurosurgery. Surg Neurol. 1998;50(1):77–82. doi: 10.1016/s0090-3019(97)00301-7. [DOI] [PubMed] [Google Scholar]
- 26.Toma AK, Camp S, Watkins LD, Grieve J, Kitchen ND. External ventricular drain insertion accuracy: is there a need for change in practice? Neurosurgery. 2009;65(6):1197–1201. doi: 10.1227/01.NEU.0000356973.39913.0B. [DOI] [PubMed] [Google Scholar]
- 27.Tuli S, O’Hayon B, Drake J, Clarke M, Kestle J. Change in ventricular size and effect of ventricular catheter placement in pediatric patients with shunted hydrocephalus. Neurosurgery. 1999;45(6):1329–1335. doi: 10.1097/00006123-199912000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Woernle CM, Burkhardt J-K, Bellut D, Krayenbuehl N, Bertalanffy H. Do iatrogenic factors bias the placement of external ventricular catheters?--a single institute experience and review of the literature. Neurol Med Chir (Tokyo) 2011;51(3):180–186. doi: 10.2176/nmc.51.180. [DOI] [PubMed] [Google Scholar]