Abstract
Objective
To investigate the eardrum mobility difference between acute otitis media (AOM) and experimental otitis media with effusion (OME).
Animal models
Thirty-three Hartley guinea pigs were included in this study. The AOM and OME were created by transbullar injection of Streptococcus pneumoniae and lipopolysaccharide into the middle ear, respectively.
Main Outcome Measures
Three days post inoculation, the morphological changes of the middle ear were assessed with otoscopy and histological sections. Vibrations of the tympanic membrane (TM) at umbo in response to pure tone sound were measured using laser Doppler vibrometry.
Results
The purulent effusion, ossicular adhesion, and thickened TM and middle ear mucosa were observed in the AOM ears, and the OME ears had serous effusion and less thickened TM and mucosa in the middle ear. The displacement of TM in AOM was lower than that in OME ears, especially at 0.2–4 kHz.
Conclusion
The TM mobility difference between the AOM and OME ears were mainly caused by the middle ear ossicular structure changes during the bacterial infection in AOM.
Keywords: acute otitis media, otitis media with effusion, middle ear, eardrum mobility
OBJECTIVE
Otitis media with effusion (OME) and acute otitis media (AOM) are two main types of otitis media (OM). OME describes the symptoms of middle ear effusion (MEE) without infection, and AOM is an acute infection of the middle ear and caused by bacteria in about 70% of cases (1). According to the guidelines of American Academy of Pediatrics, treatments for these two OMs are different. Antibiotic therapy is usually required for patients with AOM, but not recommended for OME.
Although the two OMs in certain cases can be identified by otoscopic examination, distinguishing between AOM and OME is still challenging (2). Treating OME as AOM may lead to inappropriate use of antibiotics. Since the movement of the tympanic membrane (TM) at umbo in response to sound is very sensitive to middle ear condition or structure (3, 4), the pathological changes in OM ears may result in different TM mobility or TM displacement over the frequency range. Therefore, the TM mobility measurement may provide insight for differentiation of AOM and OME, and improve the diagnostic accuracy.
In our previous studies, we reported the TM movement changes in human temporal bones caused by MEE (4) and in animal OME models (5–7). In this study, we created AOM and OME in guinea pigs concurrently and measured the TM mobility in both OM models. The goal is to compare the middle ear morphological and TM mobility changes in these two OMs and provide the correlation between the middle ear structural variation and biomechanical function changes in AOM and OME.
ANIMAL MODELS
Thirty-three Hartley guinea pigs, weighing between 260–480 g, were included in this study. The study protocol was approved by the IACUC of the University of Oklahoma and met the guideline of the National Institute of Health. The animals were free from middle ear disease as evaluated by otoscopic examination and were randomly divided into three groups: 7 controls – without any treatment in both ears, 15 AOMs – left ear was inoculated with Streptococcus pneumoniae type 3 (0.1 ml at 1.3–2.4 × 106 CFU/ml, ATTC 6303), and 11 OMEs – left ear was inoculated with lipopolysaccharide (LPS, 0.1 ml, 100 μg/ml, of Klebsiella pneumonia, Sigma). In our preliminary study, several right ears showed mild cross-infection after bacteria inoculated in the left ear. Thus, the right ears of the inoculated animals were not used as the control ears. It is noted that clinical OME may occur spontaneously because of poor Eustachian tube function or as a late result of AOM. The OME in the present study was induced by LPS, which might represent a different entity. However, it has been reported that LPS-induced experimental OME had symptoms similar to clinical OME (8, 9).
Three days post inoculation, two animals from each group were used for histology study. The ear was examined under otoscopy to determine the color of the TM as well as the presence of fluid in the middle ear. Measurements of tympanometry and laser vibrometry for umbo vibration in response to pure tones at 0.2–40 kHz were performed bilaterally in control guinea pigs (10 ears) and unilaterally in AOM (left only, 13 ears) and OME (left only, 9 ears) groups after the animal was anesthetized. Detailed methods of histology and laser vibrometry measurement can be found in Dai and Gan (5). At the end of the experiment the bulla was opened to expose the middle ear cavity for further examination of effusion volume and middle ear ossicles.
RESULTS
The microscopic observations of the TM and middle ear in control, OME, and AOM ears are shown in Fig. 1. Figs. 1A–C display the typical otoscopic images in control, OME, and AOM ears. In normal ear (Fig. 1A), the TM was translucent and no fluid was visible behind the TM. In OME ear (Fig. 1B), serous effusion was present behind the TM. In AOM ear (Fig. 1C), purulent effusion was observed in the middle ear and the TM showed distinct redness resulting from dilation of blood vessels.
Figure 1.
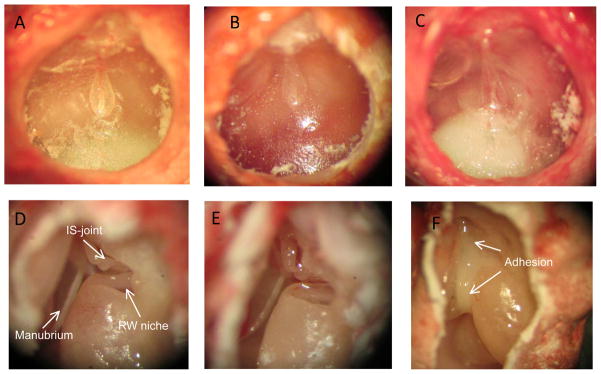
Observations of tympanic membrane (TM) and ossicles in control and diseased guinea pig ears three days post inoculation. Upper panel: otoscopic photographs of TM in control (A), OME (B), and AOM ear (C). Lower panel: microscopic photographs of middle ear ossicles in control (D), OME (E), and AOM ear (F).
Figs. 1D–F display the typical ossicular chain and the round window niche in normal, OME, and AOM ears. Note that the photomicrographs of the middle ear cavity in the diseased ears (Figs. 1E and 1F) were obtained after the drainage of the MEE. In control ear (Fig. 1D), there were no signs of middle ear inflammation, and the middle ear ossicles were clearly identified (Fig. 1D). In OME ear (Fig. 1E), the ossicular chain appeared normal. In AOM ear (Fig. 1F), the purulent adhesions were formed between the ossicles and the middle ear bony wall and commonly observed between the manubrium and promontory, and around the oval window and round window niche.
Fig. 2 shows the histological sections of the TM near the umbo at the posterior side in control, OME, and AOM ears. Thickness of the TM in the diseased ears (Fig. 2B and Fig. 2C) was increased compared with the control (Fig. 2A). In AOM ear, the TM was thicker than the OME ear, and the thickness was increased mainly in the epithelial layers caused by edema. Fig. 3 shows the middle ear mucosa on the roof of middle ear cavity in control, OME, and AOM ears. In OME ear, swelling of the subepithelial layer and infiltration of polymorphonuclear leukocytes (PMNs) were observed (Fig. 3B). In AOM ear, ciliated columnar epithelium, marked submucosal edema, infiltrations of PMNs and hyperemia were observed (Fig. 3C).
Figure 2.
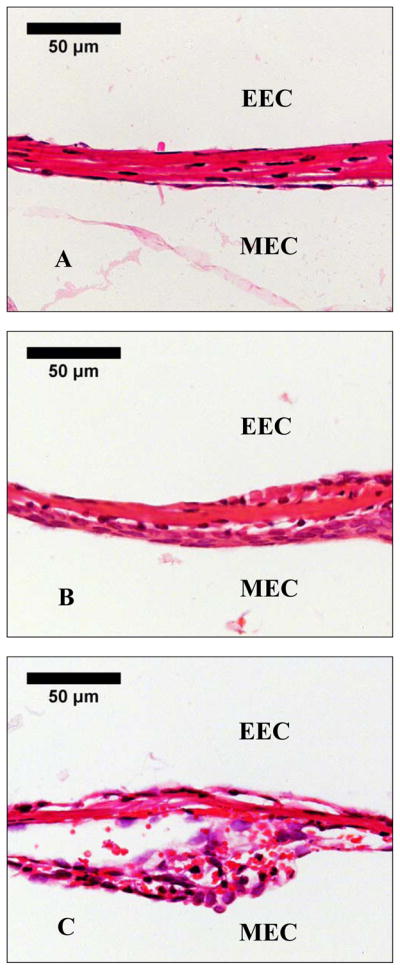
Histological photographs of the TM of (A) control ear, (B) OME ear, and (C) AOM ear 3 days after inoculation. EEC represents external ear canal and MEC represents middle ear cavity.
Figure 3.
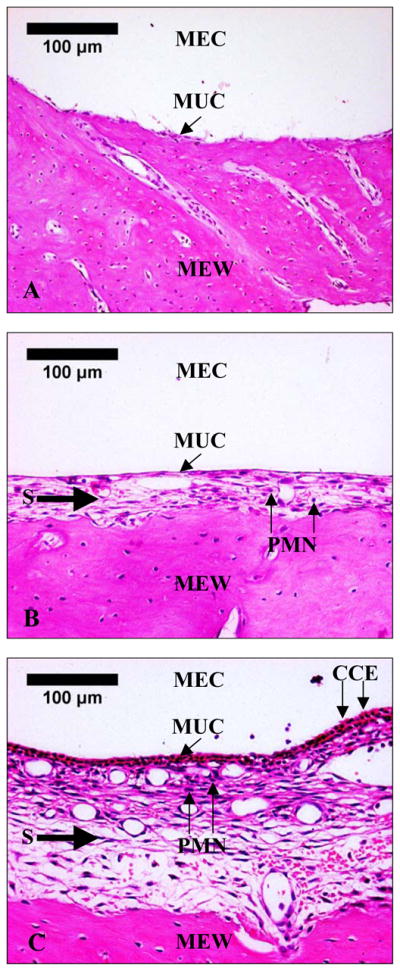
Histological photographs of middle ear roof mucosa of (A) control ear, (B) OME ear, and (C) AOM ear 3 days after inoculation. MEC, middle ear cavity; MUC, middle ear mucosa; MEW, middle ear bony wall; S, submucosal layer; PMN, polymorphonuclear leukocyte; CCE, ciliated columnar epithelium.
The volume of MEE in OME and AOM ears was about 0.1–0.13 ml and 0.09–0.15 ml, respectively. The tympanometry measurements indicated that the mean value ± SD of middle ear pressure (MEP) in OME and AOM ears was −180 ± 20 and −208 ± 40 daPa, respectively. No statistical difference was found in MEE volume or MEP between the two groups.
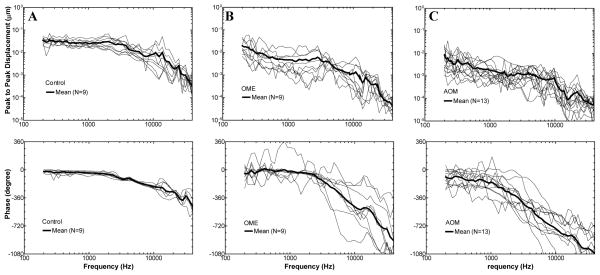
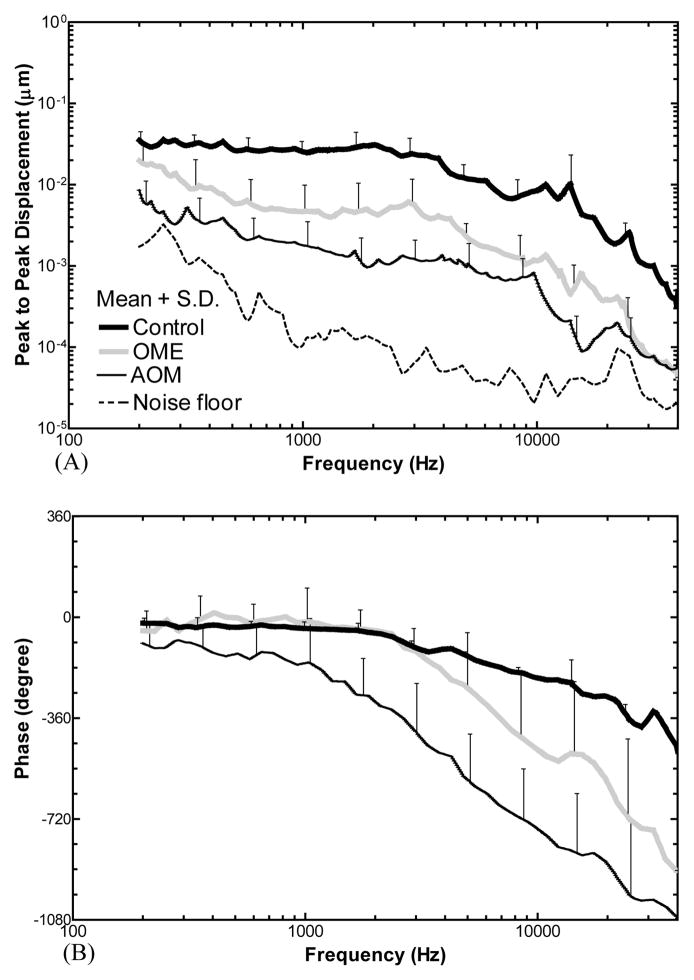
The displacement of the TM at the umbo in response to 80 dB SPL sound in the ear canal was measured in control, OME, and AOM ears. Fig. 4 shows the individual and the mean curves of the displacement magnitude and phase in each group. The mean curves were plotted in Fig. 5 with SD error bars. Unpaired t-tests (two tails) were performed on the TM magnitude data between OME and AOM. As shown in Fig. 5A, the mean displacement of control plateaued at f < 2 kHz, then decreased at higher frequencies. The displacement of OME ears decreased from 0.2 to 0.5 kHz, plateaued at 0.5–3 kHz, and further decreased over 3–40 kHz. The TM displacement of AOM ears was decreasing continuously from 0.2 to 40 kHz. The displacement of AOM was significantly lower than that of OME with a value of 6–14 dB at low frequencies (0.2–4 kHz, p < 0.05). The difference became less than 5 dB at 5–40 kHz except 16–18 kHz.
Figure 4.
Peak-to-peak displacement magnitude (upper panel) and phase (lower panel) of the TM at the umbo in response to 80 dB SPL sound input at the ear canal measured in (A) control, (B) OME, and (C) AOM ears. Individual results are presented by thin dashed lines and mean results are presented by bold solid lines. One control ear was excluded from this study due to its extraordinary high displacement.
Figure 5.
Mean peak-to-peak TM displacement curves at umbo in control (N=9, thick black line), OME (N=9, gray line), and AOM (N=13, thin black line) ears in response to 80 dB SPL sound input at the ear canal. The error bars indicate standard deviations. (A) Magnitude, (B) phase angle.
As displayed in Fig. 5B, the mean phase of the TM displacement in OME ears showed a plateau at 0.2–2 kHz, which overlapped with control, and decreased over 2 to 40 kHz. The phase of AOM decreased regularly over 0.2–40 kHz.
DISCUSSION
It is generally accepted that the TM vibration is affected by mass and stiffness of the TM and ossicular chain (3, 4). The MEE adds mass to the TM and decreases the TM movement mainly at high frequencies. However, the amount of effusion in the AOM ears was not much different from the OME ears, and the TM mobility of the AOM ears was not statistically different from that of the OME ears at high frequencies. Thus, the increase of TM mass, or the middle ear fluid, may not be the main reason to explain the difference of TM mobility between AOM and OME.
It has been reported that the response of TM movement at low frequencies is dominated by the middle ear stiffness and the stiffer TM leads to lower TM displacement below 2 kHz in human ears (4). Three factors may increase the middle ear stiffness: MEP (4), reduced middle ear air space (3), and ossicular fixation (10). In this study, the MEP and the residual air space in AOM ears was not much different from those in OME ears. However, all the AOM ears had purulent adhesions formed on the ossicles and between the manubrium and cochlear promontory. Those structural changes induced by the infection were not observed in the OME ears. The purulent adhesions in AOM ears fixed the manubrium and ossicular chain at certain degree and increased the TM stiffness as well as the middle ear ossicles’ stiffness (11, 12). Thus, the TM mobility difference between the AOM and OME ears was mainly caused by the purulent adhesions formed on the TM and ossicles, which increased the middle ear stiffness in AOM ears.
CONCLUSION
The TM mobility in AOM ears was lower than that in experimental OME ears at low frequencies, and the difference was mainly caused by the middle ear ossicular structure changes during the bacterial infection in AOM. These findings may help understanding the biomechanical difference for sound transmission between AOM and OME ears.
Acknowledgments
Supported by Oklahoma Center for the Advancement of Science and Technology HR09-033 and NIH R01DC011585
Contributor Information
Xiying Guan, University of Oklahoma, Norman, Oklahoma.
Wei Li, Hough Ear Institute, Oklahoma City, Oklahoma
Rong Z. Gan, University of Oklahoma, Norman, Oklahoma
References
- 1.Gould JM, Matz PS. Otitis media. Pediatrics In Review. 2010;31:102–110. doi: 10.1542/pir.31-3-102. [DOI] [PubMed] [Google Scholar]
- 2.Shaikh N, Hoberman A, Kaleida PH, et al. Otoscopic signs of otitis media. Pediatr Infect Dis J. 2011;30:822–826. doi: 10.1097/INF.0b013e31822e6637. [DOI] [PubMed] [Google Scholar]
- 3.Ravicz ME, Rosowski JJ, Merchant SN. Mechanisms of hearing loss resulting from middle-ear fluid. Hear Res. 2004;195:103–130. doi: 10.1016/j.heares.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Gan RZ, Dai C, Wood MW. Laser interferometry measurements of middle ear fluid and pressure effects on sound transmission. J Acoust Soc Am. 2006;120:3799–3810. doi: 10.1121/1.2372454. [DOI] [PubMed] [Google Scholar]
- 5.Dai C, Gan RZ. Change of middle ear transfer function in otitis media with effusion model of guinea pigs. Hear Res. 2008;24:78–86. doi: 10.1016/j.heares.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai C, Gan RZ. Change in cochlear response in an animal model of otitis media with effusion. Audiol Neurootol. 2010;15:155–167. doi: 10.1159/000241096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan X, Gan RZ. Effect of middle ear fluid on sound transmission and auditory brainstem response in guinea pigs. Hear Res. 2011;277:96–106. doi: 10.1016/j.heares.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dattaray P, Pudrith C, Nyc MA, et al. Effect of ciprofloxacin/dexamethasone versus ciprofloxacin/hydrocortisone on lipopolysaccharide-induced experimental otitis media. Otolaryngol Head Neck Surg. 2011;145(2):288–294. doi: 10.1177/0194599811403868. [DOI] [PubMed] [Google Scholar]
- 9.Pudrith C, Martin D, Kim YH, et al. Glucocorticoids reduce nitric oxide concentration in middle ear effusion from lipopolysaccharide induced otitis media. Int J Pediatr Otorhinolaryngol. 2010;74(4):384–386. doi: 10.1016/j.ijporl.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Rosowski JJ, Nakajima HH, Merchant SN. Clinical utility of laser-Doppler vibrometer measurements in live normal and pathologic human ears. Ear Hear. 2008;29:3–19. doi: 10.1097/AUD.0b013e31815d63a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cayé-Thomasen P, Hermansson A, Tos M, et al. Pathogenesis of middle ear adhesions. Laryngoscope. 1996;106:463–469. doi: 10.1097/00005537-199604000-00013. [DOI] [PubMed] [Google Scholar]
- 12.von Unge M, Decraemer WF, Bagger-Sjoback D, et al. Tympanic membrane changes in experimental purulent otitis media. Hear Res. 1997;106:123–36. doi: 10.1016/s0378-5955(97)00008-7. [DOI] [PubMed] [Google Scholar]