Abstract
Species comparisons of personality structure (i.e. how many personality dimensions and the characteristics of those dimensions) can facilitate questions about the adaptive function of personality in nonhuman primates. Here we investigate personality structure in the brown capuchin monkey (Sapajus apella), a New World primate species, and compare this structure to those of chimpanzees (Pan troglodytes), orangutans (Pongo spp.), and rhesus macaques (Macaca mulatta). Brown capuchins evolved behavioral and cognitive traits that are qualitatively similar to those of great apes, and individual differences in behavior and cognition are closely associated with differences in personality. Thus, we hypothesized that brown capuchin personality structure would overlap more with great apes than with rhesus macaques. We obtained personality ratings from seven sites on 127 brown capuchin monkeys. Principal-components analysis identified five personality dimensions (Assertiveness, Openness, Neuroticism, Sociability, and Attentiveness), which were reliable across raters and, in a subset of subjects, significantly correlated with relevant behaviors up to a year later. Comparisons between species revealed that brown capuchins and great apes overlapped in personality structure, particularly chimpanzees in the case of Neuroticism. However, in some respects (i.e. capuchin Sociability and Openness) the similarities between capuchins and great apes were not significantly greater than those between capuchins and rhesus macaques. We discuss the relevance of our results to brown capuchin behavior, and the evolution of personality structure in primates.
Keywords: temperament, animal, Conscientiousness, phylogeny, New World Monkey, platyrrhine
Personality, defined here as consistent individual differences in behavior (Carere & Eens, 2005), has been studied in many animals, including mammals, birds, fish, reptiles, amphibians, and invertebrates (Gosling, 2001). Personality in nonhuman species has sometimes been labeled as “noise” deviating from behavioral norms. However, research shows that such traits are heritable (Adams, King, & Weiss, 2012; van Oers, de Jong, van Noordwijk, Kempenaers, & Drent, 2005), stable over time and across contexts (Bell, Hankison, & Laskowski, 2009; Capitanio, 1999; Stevenson-Hinde, Stillwell-Barnes, & Zunz, 1980; Uher, Asendorpf, & Call, 2008; Weiss, Adams, Widdig, & Gerald, 2011), and predict life history patterns (e.g., metabolic rate, reproduction, health, and longevity; Capitanio, 2011; Careau, Bininda-Emonds, Thomas, Reale, & Humphries, 2009; Cavigelli, Bennett, Michael, & Klein, 2008; Réale, Martin, Coltman, Poissant, & Festa-Bianchet, 2009; Weiss, Gartner, Gold, & Stoinski, 2012).
Personality traits (e.g. curious, fearful, and aggressive) tend to cluster into one or more broader dimensions. An individual’s score on a given dimension corresponds to their position along a particular behavioral continuum (e.g., the shy-bold axis; Wilson, Clark, Coleman, & Dearstyne, 1994). When assessed using standardized methods, comparisons of personality structure across species may help researchers address questions about the phylogeny and evolution of personality (Gosling & Graybeal, 2007). In the case of nonhuman primates (hereafter ‘primates’), King and Figueredo (1997) reported that chimpanzee (Pan troglodytes) personality is comprised of a species-specific dimension, Dominance, and five dimensions similar to those found in many human personality studies – Extraversion, Conscientiousness, Agreeableness, Neuroticism, and Openness (e.g., Digman, 1990; Eysenck, 1970; Lee, Ogunfowora, & Ashton, 2005). A study of orangutans (Pongo spp.) using the same scale found dimensions resembling chimpanzee Neuroticism, Extraversion, Agreeableness, and Dominance (Weiss, King, & Perkins, 2006). However, instead of the distinct Conscientiousness and Openness dimensions found in chimpanzees, orangutans have a dimension, Intellect, comprised of traits associated with both. More recently, a study of rhesus macaques (Macaca mulatta) using a comparable instrument found dimensions similar to chimpanzee Openness and chimpanzee/orangutan Dominance (Weiss, et al., 2011). However, unlike chimpanzees and orangutans, rhesus macaques do not have dimensions resembling Extraversion or Neuroticism; rather, traits shared with these dimensions are classified under dimensions labeled Activity (e.g. innovative and playful), Friendliness (e.g. affectionate, sociable, sensitive), Confidence (e.g. stable, dominant, cool), and Anxiety (e.g. impulsive, anxious, erratic). These findings suggest that some personality dimensions may be phylogenetically old and shared across species (e.g. Dominance-like dimensions), while others may have evolved more recently (e.g. Conscientiousness and Intellect).
To date, research on personality has predominantly been limited to catarrhines, that is apes, including humans, and Old World monkeys (Freeman & Gosling 2010). Platyrrhines (New World monkeys) are only distantly related to catarrhine species, sharing a common ancestor about 43 million years ago (Steiper & Young, 2006). However, some New World species exhibit behavioral and cognitive similarities to catarrhine species, particularly capuchin monkeys (Cebus and Sapajus spp.) and spider monkeys (Ateles spp.) (Amici, Aureli, & Call, 2008; Deaner, van Schaik, & Johnson, 2006; Fragaszy, Visalberghi, & Fedigan, 2004). Thus, comparative studies of personality in Old and New World species may help identify variables within the natural and social world of primates that contribute to personality evolution.
In this study, we examined personality structure in a New World primate, the brown capuchin monkey (Sapajus apella, formerly Cebus apella; Alfaro, Silva, & Rylands, 2012). We first derived personality structure in brown capuchins using observer ratings, and examined its association with systematically recorded behaviors. We then compared personality structure in brown capuchins to those reported in chimpanzees, orangutans, and rhesus macaques – all of which were rated using the same or similar scale. Ratings of brown capuchin monkeys on individual personality traits have been associated with cortisol reactivity (Byrne & Suomi, 2002). Thus, human observer ratings capture biologically-meaningful information about this taxon. However, it is unknown how individual traits cluster into personality dimensions in brown capuchins, and how the structure of these dimensions compares to those of other primates. Our study was conducted contemporaneously with another study which derived personality structure in white-faced capuchins (Cebus capucinus; Manson & Perry, in press).
Brown capuchins, chimpanzees, and orangutans have large brains relative to their body size, are extractive foragers, are very tolerant of non-kin, rely on social learning, and have “cultural” traditions (Fragaszy, Visalberghi, & Fedigan, 2004). Moreover, brown capuchins exhibit delayed gratification tolerances more like those of great apes than more closely related platyrrhines such as marmosets (Callithrix jacchus) and tamarins (Saguinus oedipus) (Addessi, Paglieri, & Focaroli, 2011). Unlike semi-solitary orangutans (Rodman, 1984), however, capuchins and chimpanzees are considerably more social and have many of the same basic properties of sociality as other group-living primates, including rhesus macaques. For example, they live in multimale-multifemale groups, have social hierarchies, provide coalitionary support to others, display post-conflict reconciliations, and frequently engage in social grooming (Fragaszy, et al., 2004; Maestripieri & Hoffman, 2012; Stanford, 1998). Thus, collectively, the behavior and cognitive traits of brown capuchins are qualitatively more like those of chimpanzees than other primate species.
Individual differences in behavioral and cognitive traits are closely associated with differences in personality (reviewed in Carere & Locurto, 2011). Thus, given the behavioral and cognitive similarities between brown capuchins and great apes, we hypothesized that the personality structure of brown capuchins would overlap more with great apes – and in particular, chimpanzees – than with rhesus macaques.
Methods
Subjects
Subjects were 127 captive brown capuchin monkeys that were at least 1 year old, belonging to 15 social groups from 5 sites in the United States, 1 site in the UK, and 1 site in France (see Table 1 and Supplementary Materials). Across all sites there were 60 males and 67 females. Age ranged from 1 to 40 years and the mean age was 11.0 years (SD = 8.9). This study was non-invasive, approved by local ethics committees, and complied with the 2012 regulations of the Association for the Study of Animal Behaviour.
Table 1.
Age, Sex, and Number of Study Subjects at Each Research Site
| Location | N | Groups | Age (mean years ± SD) | Sex Ratio (M:F) |
|---|---|---|---|---|
| Bucknell University | 13 | 1 | 8.77 ± 6.18 | 4:9 |
| CNRS Institut Pluridisciplinaire Hubert Curien | 18 | 1 | 13.67 ± 7.84 | 6:12 |
| Georgia State University Language Research Center | 12 | 2 | 9.67 ± 5.65 | 7:5 |
| Living Links, Edinburgh Zooa | 19 | 2 | 10.32 ± 10.99 | 12:7 |
| Living Links, USA | 29 | 2 | 14.90 ± 11.06 | 11:18 |
| National Institutes of Health | 26 | 6 | 8.39 ± 7.33 | 16:10 |
| Yale University | 10 | 1 | 7.9 ± 5.28 | 4:6 |
Note.
Due to the age-related death of one male, behavioral data were collected on only 18 subjects.
Ratings
Each subject was rated by one to seven raters (M = 3.24, SD = 1.61). Raters were 25 researchers and 3 care staff, and had at least one year of experience working with their subjects.
Ratings were made on the Hominoid Personality Questionnaire (Weiss et al., 2009) 1. The HPQ instructs raters to not discuss their ratings with others, and to answer each of 54 items on a seven point scale with ‘1’ indicating “Displays either total absence or negligible amounts of the trait” and ‘7’ indicating “Displays extremely large amounts of the trait”. The layout of the items is similar to the well-known Madingley Questionnaire (Stevenson-Hinde & Hinde, 2011; Stevenson-Hinde & Zunz, 1978). Each item consists of an adjective paired with one to three sentences defining it within the context of primate behavior. For example, fearful was defined as “Subject reacts excessively to real or imagined threats by displaying behaviors such as screaming, grimacing, running away or other signs of anxiety or distress”.
The HPQ is an expanded version of a 48 item questionnaire used to assess orangutan personality (Weiss, et al., 2006), which was an expanded version of a 43 item questionnaire used to assess chimpanzee personality (King & Figueredo, 1997). Of the 43 items, 40 were sampled from Goldberg’s (1990) Big Five taxonomy and 3 were devised by King and Figueredo. While the accompanying sentences were created for the purpose of setting adjectives in the context of chimpanzee behavior, they were of a general enough nature so as to be applicable to many primate species, including capuchin monkeys.
Of the 411 questionnaires turned in, 77 were missing between 1 and 28 items (median = 5). In cases where an item was missing, we replaced the missing value with the mean for that item. Excluding questionnaires with missing data for 10 or more items, i.e. those that exceeded the upper end of the 95% confidence interval for amount of missing data, did not yield a different personality structure. We thus did not exclude any questionnaires.
Behavioral Measures
Two types of behavioral measures were available for the 18 capuchins housed at the Living Links to Human Evolution Research Centre, Edinburgh Zoo, United Kingdom (Macdonald & Whiten, 2011). These data were collected as part of a separate study, and were used here to validate interpretations of personality components derived from ratings. Raters did not collect behavioral data.
The first type of behavioral data was collected one year after subjects were rated for personality. Fifty-four hours of focal observations were recorded over a 4-month sampling period, averaging 3 hours per monkey. Following Martin and Bateson (2007), behaviors (see Table 2) between the focal and other individuals were recorded at 1-min intervals for 10 minutes per day. In addition, in each minute the number of group members within a 2 m radius from the focal monkey was recorded. If no monkey was within 2 m, the focal was described as “solitary”. Subjects were sampled evenly across all periods of the day, usually from 9:00 until 17:30. Incidences of aggression initiated by each monkey were summed across sampling periods; all other behaviors are represented as the percentage of time individuals spent engaged in each behavior when in view.
Table 2.
Behaviors Recorded During Focal Sampling at Living Links, Edinburgh Zoo
| Behavior | Definition |
|---|---|
| Play | Wrestling, gymnastics, hitting, or chasing without intended aggression. |
| Feed | Searching for, or ingesting food. |
| Rest | Lying down or sitting, not exhibiting any other behavior. |
| Move | Locomoting from one point to another. |
| Alert | Visually scanning surroundings, head and body erect/tense. |
| Vigilant | Monitoring the activities of particular individual(s) (e.g. humans or other monkeys), usually from a high or exposed vantage point. |
| Groom | Picking through hair of another individual. Actor and recipient noted. |
| Aggression | Open-mouth threats, vocal threats, lunging, chasing, hitting, and/or biting. Actor and recipient noted. |
| Solitary | No monkey within 2 body lengths away from the focal. |
Note. Behavioral sampling took place one year after monkeys were rated for personality; none of the raters took part in collecting behavioral data; behavioral observations were made within the groups’ indoor/outdoor enclosures.
The second type of behavioral data was recorded four to seven months after subjects were rated for personality. Fourteen monkeys were filmed and scored on attention span during free-participation cognitive testing. At Edinburgh Zoo, capuchins can choose to engage in on-going non-invasive cognitive research (Macdonald & Whiten, 2011). Monkeys’ average attention span was scored across three problem-solving tasks (see Table 3) for 24 to 85 randomly selected trials (M = 71.4, SD = 23.9). The number of trials selected for each monkey depended on how often they participated during testing (Morton, Lee, & Buchanan-Smith, under review). Attention was assessed on a 3-point scale according to whether they exhibited high (“3”), medium (“2”), or low (“1”) attention. Monkeys were scored once during a trial, and once again after the trial ended. An overall average was calculated for each monkey across trials. High attention was defined as whenever the monkey’s head, body, and eyes were directly focused on the task during/between a trial. Medium attention was defined as when a monkey looked away from the task apparatus on one or two occasions during/between a trial. Low attention was defined as when a monkey looked away from the task apparatus on three or more occasions during/between a trial. Inter-observer reliability tests were conducted using a sub-sample of these data, whereby 120 trials from 5 monkeys were independently scored by two observers, during and after each trial. Cronbach’s alpha for each of the five monkeys ranged from .70 to .90, indicating that each observer’s scores were satisfactorily concordant. Regular participants were considered to be more comfortable and motivated to engage in testing (Morton, et al., under review). Thus, in our analysis, we distinguish between attention span scores calculated from all participants (i.e. > 0% participation), and scores calculated from regular participants (i.e. > 80% participation).
Table 3.
Description of Tasks Administered to Brown Capuchins at Living Links, Edinburgh Zoo
| Task | Description |
|---|---|
| 1 | During each trial a food reward was placed in front of one of two compartments. The location of the food reward (left or right compartment) was randomly selected for each new trial. The goal was for the monkey to learn that by walking and sitting in the compartment that had the food directly in front of it, the researcher would hand them the food. If the monkey failed to do this, no food was delivered, and the trial was ended. Monkeys received a maximum of 12 trials per session, with each trial separated by 5–7 seconds. |
| 2 | During each trial two white-opaque cups were placed in front of one of the two compartments. The position of each cup (left or right compartment) was randomly selected for each new trial. The two cups differed in size, with one cup twice as tall (height: 19cm, diameter: 6.4cm) as the other cup (height: 9.5cm, diameter: 6.4cm). For this task, the goal was for the monkey to learn that by moving and sitting in the compartment facing the larger cup, they would receive a food reward that was hidden inside the cup. The larger cup was always the ‘winner’, the smaller cup was always the ‘loser’. If the monkey failed a trial, no food was delivered, and the trial was ended. Monkeys received a maximum of 12 trials per session, with each trial separated by 5–7 seconds. |
| 3 | During each trial, two opaque cups were placed in front of the monkey. Each cup was placed directly in front of one of two compartments. Compartments were separated by a transparent door that was half-way open, and monkeys could walk freely between them. The position of each cup (left or right compartment) was randomly selected for each new trial. Both cups were the same size, shape, and color, and only differed by a symbol that was labelled on the front of each cup: the first cup had a large “X” on it, the second cup had a large “O” on it. For this task, the goal was for the monkey to learn that by moving and sitting in the compartment facing the “X” cup, they would receive a food reward that was located inside it. The “X” cup was always the ‘winner’ and the “O” cup was always the ‘loser’. If the monkey failed a trail, no food was rewarded, and the trial was ended. Monkeys received a maximum of 12 trials per session, with each trial separated by 5–7 seconds. |
Statistical Analyses
Statistical analyses were conducted using R, version 2.15.2 (R Development Core Team, 2012). Significance tests were two-tailed with critical values of .05.
Inter-rater reliabilities of items
Of the sample, 121 capuchins were rated by 2 to 7 raters (M = 3.35; SD = 1.57). We calculated two intraclass correlations (Shrout & Fleiss, 1979) to determine inter-rater reliabilities for subjects rated by at least two raters. The first, ICC(3,1), indicates the reliability of individual ratings. The second, ICC(3,k), indicates the reliability of the mean of k ratings.
Data reduction
We computed the means across raters for each reliable item and then determined the number of components by examining the scree plot and conducting a parallel analysis (Horn, 1965) using the paran function (Dinno, 2008). We then entered these means into a principal-components analysis (PCA) using the principal function (Revelle, 2011). Simulation studies show that comparable designs yield stable structures (Guadagnoli & Velicer, 1988). A recent simulation study found that stable personality structures can also be derived even when sample sizes are considerably smaller (de Winter, Dodou, & Wieringa, 2009). Likewise, a study of Barbary macaques (Macaca sylvanus; Konečná, Weiss, Lhota, & Wallner, 2012) demonstrated that the personality structure obtained via a PCA of 26 subjects was highly similar to that derived via regularized exploratory factor analysis, a factor extraction method devised specifically for cases in which the sample size is very small (Jung & Lee, 2011; Jung & Takane, 2008).
We rotated components using both varimax and promax procedures, and defined loadings ≥ |.4| as salient. For the purpose of creating unit-weighted component scores (Gorsuch, 1983), if multiple components had salient loadings on an item, we assigned the item to the component with the highest absolute loading.
Component interpretation and validation
We interpreted components based on the items onto which they loaded and their association with recorded behaviors. Behavioral correlations also served to validate components. We used Pearson correlations to test for the associations between components and recorded behaviors.
Inter-rater reliabilities and internal consistencies of components
Similar to our item-level analysis, for the 121 subjects rated by at least 2 raters we assessed the inter-rater reliability of components using Shrout and Fleiss’s (1979) ICC(3,1) and ICC(3,k). To determine the internal consistencies, we calculated Cronbach’s alpha for each component in the total sample using the alpha function (Revelle, 2011).
Cross-species comparisons
To compare capuchin components to those of other primate species, as in previous studies (Konečná, Weiss, Lhota, & Wallner, 2012; Weiss, et al., 2011), we computed unit-weighted scores based on the structures of the HPQ and related questionnaires in other species. One structure was obtained in a study where Japanese raters used a Japanese translation of the 54-item HPQ to rate 146 chimpanzees in Japan (see Table II in Weiss, et al., 2009). In another study, raters used the English language version of the original 43-item questionnaire to rate 100 chimpanzees in the U.S. (see Table 1 in King & Figueredo, 1997). In a third study, raters used a 48-item version of the questionnaire to rate 152 orangutans (see Table 3 in Weiss, et al., 2006). In the last of these studies, raters used the HPQ to rate 124 rhesus macaques (see Table 1 in Weiss, et al., 2011). We then obtained correlations between the unit-weighted capuchin personality scores and those of the other species. The highest correlation between each capuchin personality dimension and a personality dimension in the chimpanzee sample from Japan was then compared with the highest correlation between capuchin personality and each of the other primate samples. To compare correlations, we used the r.test function (Revelle, 2011) to conduct Williams’s tests.
Results
Item inter-rater Reliabilities
The inter-rater reliabilities of all 54 items are presented in Table 4. The mean ICC(3,1) and ICC(3,k), respectively, of these items were .36 (SD = .14) and .63 (SD = .14).
Table 4.
Inter-rater Reliabilities of Items
| Adjective | ICC(3,1) | ICC(3,k) |
|---|---|---|
| Playful | .75 | .91 |
| Submissive | .61 | .84 |
| Aggressive | .60 | .83 |
| Vulnerable | .58 | .82 |
| Dominant | .57 | .82 |
| Bullying | .56 | .81 |
| Timid | .55 | .81 |
| Anxious | .51 | .77 |
| Active | .50 | .77 |
| Inventive | .49 | .76 |
| Innovative | .46 | .74 |
| Gentle | .46 | .74 |
| Solitary | .45 | .73 |
| Stingy/Greedy | .45 | .73 |
| Autistic | .44 | .73 |
| Inquisitive | .44 | .73 |
| Intelligent | .42 | .71 |
| Sociable | .42 | .71 |
| Defiant | .41 | .70 |
| Fearful | .41 | .70 |
| Lazy | .41 | .70 |
| Cautious | .40 | .69 |
| Item | ICC(3,1) | ICC(3,k) |
|---|---|---|
| Imitative | .39 | .68 |
| Jealous | .38 | .68 |
| Dependent/Follower | .38 | .67 |
| Conventional | .37 | .66 |
| Impulsive | .34 | .63 |
| Irritable | .33 | .62 |
| Clumsy | .33 | .62 |
| Persistent | .32 | .61 |
| Curious | .31 | .60 |
| Distractible | .31 | .60 |
| Affectionate | .30 | .59 |
| Erratic | .29 | .58 |
| Excitable | .29 | .57 |
| Manipulative | .28 | .57 |
| Depressed | .28 | .57 |
| Protective | .28 | .57 |
| Reckless | .28 | .56 |
| Friendly | .26 | .55 |
| Disorganized | .25 | .53 |
| Sympathetic | .23 | .50 |
| Predictable | .23 | .50 |
| Thoughtless | .22 | .49 |
| Independent | .22 | .49 |
| Decisive | .21 | .47 |
| Helpful | .19 | .45 |
| Individualistic | .19 | .45 |
| Stable | .19 | .44 |
| Cool | .17 | .41 |
| Unemotional | .17 | .40 |
| Quitting | .14 | .36 |
| Sensitive | .13 | .33 |
| Unperceptive | .12 | .32 |
Note. Estimates based on 121 capuchin monkeys, each rated by an average of 3.35 raters.
ICC(3,1) = Reliability of individual ratings. ICC(3,k) = Reliability of mean ratings.
Data reduction
The scree plot suggested that six components should be extracted. Parallel analysis indicated that the eigenvalues of the first six components (14.16, 9.18, 6.80, 3.07, 2.67, and 2.18) were greater than expected under chance at the 95% confidence level. However, the last component, which loaded on persistent, curious, decisive, and stable, only had three loadings greater than or equal to |.6|, indicating that the component may not be replicable (Guadagnoli & Velicer, 1988).
To determine whether to retain the sixth component, we conducted an Everett test (Everett, 1988). This involved extracting five and six components in samples that excluded one of the sample sites. These five and six component solutions were then compared to the five and six component solutions derived using the full sample by means of targeted orthogonal Procrustes rotations (McCrae, Zonderman, Bond, Costa, & Paunonen, 1996). If all six components replicated when dropping individual sites, we could conclude that the last component is robust and should be retained. If all six components did not replicate, and all five components of the five component solution replicate, we would extract only five components. When comparing the six component solutions (see Table 5), the last component did not replicate in a sample that did not include subjects from Living Links, USA. On the other hand, when comparing the five component solutions, all five components consistently replicated. We thus extracted five components.2
Table 5.
Everett test of the Robustness of the Six and Five Component Solutions
| Six Component Solution | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | Congruence |
|---|---|---|---|---|---|---|---|
| Bucknell University | 1.00 | .98 | .98 | .96 | .95 | .98 | .98 |
| Living Links, USA | .98 | .97 | .96 | .96 | .99 | .48 | .93 |
| Georgia State University Language Research Center | 1.00 | 1.00 | 1.00 | .99 | 1.00 | 1.00 | 1.00 |
| Living Links, Edinburgh Zoo | 1.00 | 1.00 | .99 | .99 | .98 | .99 | .99 |
| National Institutes of Health | 1.00 | 1.00 | 1.00 | .99 | 1.00 | 1.00 | 1.00 |
| Yale University | 1.00 | 1.00 | 1.00 | 1.00 | .99 | 1.00 | 1.00 |
| CNRS Institut Pluridisciplinaire Hubert Curien | 1.00 | 1.00 | 1.00 | 1.00 | .99 | 1.00 | 1.00 |
| Five Component Solution
|
|||||||
| Bucknell University | .99 | .97 | .94 | .98 | .95 | --- | .97 |
| Living Links, USA | .99 | .99 | .96 | .95 | .95 | --- | .97 |
| Georgia State University Language Research Center | 1.00 | 1.00 | 1.00 | 1.00 | .99 | --- | 1.00 |
| Living Links, Edinburgh Zoo | 1.00 | .99 | .99 | .98 | .98 | --- | .99 |
| National Institutes of Health | 1.00 | 1.00 | .99 | 1.00 | 1.00 | --- | 1.00 |
| Yale University | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | --- | 1.00 |
| CNRS Institut Pluridisciplinaire Hubert Curien | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | --- | 1.00 |
Note. The site name in each row indicates the site that was left out to generate the loading matrix. The target matrix was either the six or five component varimax-rotated structure based on data from all sites. Components that did not replicate are indicated in boldface. PC = Principal component.
The varimax- and promax-rotated components were virtually identical (see Table 6). Targeted orthogonal Procrustes rotation (McCrae, et al., 1996) confirmed this similarity as all congruence coefficients were greater than .96. Correlations among the promax rotated components were modest (see Table 7). We therefore interpreted the varimax-rotated components, and used these definitions for all remaining analyses.
Table 6.
Structure Matrix of Varimax- and Promax-Rotated Component Loadings
| Adjective | Varimax Rotated Components
|
Promax Rotated Components
|
h2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Asst | Open | Neura | Socb | Attna | Assta | Open | Neura | Socb | Attna | ||
|
|
|
|
|||||||||
| Bullying | .92 | −.01 | .14 | .00 | −.03 | .98 | −.17 | .12 | −.03 | .02 | .87 |
| Aggressive | .91 | .04 | .17 | −.02 | −.04 | .96 | −.11 | .15 | −.05 | .02 | .86 |
| Submissive | −.89 | −.06 | .10 | −.31 | −.03 | −.92 | .12 | .11 | −.30 | .03 | .90 |
| Stingy/Greedy | .88 | .08 | .05 | .04 | −.03 | .90 | −.06 | .02 | .01 | −.02 | .78 |
| Dominant | .83 | −.08 | −.31 | −.05 | −.02 | .86 | −.21 | −.35 | −.05 | −.07 | .78 |
| Jealous | .82 | .24 | .08 | .02 | −.02 | .81 | .12 | .06 | −.03 | −.00 | .74 |
| Gentle | −.81 | −.06 | −.41 | .06 | .09 | −.87 | .07 | −.40 | .08 | −.02 | .84 |
| Vulnerable | −.75 | −.02 | .14 | −.34 | −.18 | −.79 | .15 | .12 | −.30 | −.12 | .73 |
| Timid | −.68 | −.40 | .19 | −.39 | −.12 | −.60 | −.28 | .19 | −.34 | −.01 | .81 |
| Irritable | .67 | .02 | .27 | −.32 | −.02 | .71 | −.06 | .24 | −.38 | .11 | .62 |
| Cautious | −.67 | −.37 | −.01 | −.33 | −.09 | −.62 | −.25 | −.01 | −.28 | −.04 | .70 |
| Dependent/Follower | −.63 | .03 | .41 | .23 | −.21 | −.63 | .11 | .43 | .30 | −.19 | .66 |
| Independent | .61 | .14 | −.42 | −.09 | .01 | .57 | .07 | −.46 | −.11 | −.07 | .57 |
| Manipulative | .59 | .29 | .15 | .39 | .09 | .56 | .17 | .17 | .35 | .06 | .61 |
| Fearful | −.57 | −.26 | .29 | −.38 | −.29 | −.52 | −.14 | .27 | −.31 | −.19 | .71 |
| Reckless | .53 | .49 | .14 | .06 | −.46 | .44 | .45 | .06 | .12 | −.50 | .76 |
| Protective | .37 | −.04 | −.22 | .35 | .30 | .40 | −.15 | −.18 | .30 | .23 | .41 |
| Inventive | .11 | .86 | −.06 | .18 | .09 | −.10 | .90 | −.08 | .09 | .02 | .80 |
| Innovative | .06 | .85 | −.03 | .17 | .15 | −.15 | .89 | −.03 | .07 | .10 | .78 |
| Inquisitive | .18 | .83 | .02 | .33 | −.03 | −.01 | .84 | .00 | .28 | −.12 | .83 |
| Playful | .05 | .76 | .23 | .35 | −.08 | −.11 | .78 | .22 | .32 | −.12 | .77 |
| Conventional | −.13 | −.73 | −.31 | −.03 | .19 | .03 | −.77 | −.28 | .00 | .17 | .68 |
| Active | .03 | .72 | .45 | .31 | .17 | −.11 | .73 | .49 | .20 | .21 | .85 |
| Curious | .11 | .70 | −.21 | .00 | −.08 | −.09 | .75 | −.26 | −.04 | −.16 | .56 |
| Lazy | −.05 | −.64 | −.39 | −.22 | −.37 | .07 | −.64 | −.45 | −.07 | −.44 | .74 |
| Imitative | −.05 | .63 | .13 | .44 | .07 | −.20 | .64 | .15 | .39 | .01 | .62 |
| Persistent | .35 | .55 | −.31 | −.21 | −.11 | .19 | .58 | −.38 | −.24 | −.17 | .57 |
| Defiant | .48 | .55 | .18 | .02 | −.21 | .38 | .51 | .13 | .00 | −.21 | .61 |
| Quitting | −.01 | −.50 | .17 | .04 | −.34 | .13 | −.54 | .16 | .17 | −.32 | .40 |
| Cool | .13 | .07 | −.76 | .26 | .24 | .07 | .03 | −.76 | .23 | .04 | .73 |
| Stable | .00 | .10 | −.71 | .01 | .14 | −.08 | .12 | −.73 | −.01 | −.02 | .53 |
| Excitable | .04 | .10 | .64 | −.10 | −.48 | .06 | .11 | .60 | −.02 | −.37 | .67 |
| Predictable | −.10 | −.39 | −.61 | .08 | −.01 | −.06 | −.40 | −.63 | .15 | −.15 | .55 |
| Unemotional | −.05 | −.17 | −.60 | .32 | −.00 | −.04 | −.20 | −.61 | .38 | −.19 | .50 |
| Decisive | .39 | .30 | −.59 | −.04 | .24 | .29 | .27 | −.61 | −.12 | .13 | .65 |
| Impulsive | .06 | .40 | .59 | −.13 | −.45 | .00 | .44 | .54 | −.08 | −.35 | .73 |
| Sympathetic | −.39 | .05 | −.45 | .38 | .24 | −.45 | .08 | −.42 | .37 | .09 | .57 |
| Sociable | .15 | .25 | −.16 | .82 | −.05 | .10 | .15 | −.14 | .87 | −.24 | .78 |
| Affectionate | −.22 | .11 | −.27 | .74 | −.00 | −.26 | .07 | −.24 | .80 | −.20 | .68 |
| Solitary | −.35 | −.34 | .01 | −.71 | −.05 | −.29 | −.23 | −.01 | −.71 | .08 | .74 |
| Depressed | −.38 | −.30 | .02 | −.68 | −.29 | −.34 | −.18 | −.03 | −.62 | −.19 | .79 |
| Friendly | −.37 | .22 | −.21 | .65 | .22 | −.45 | .22 | −.16 | .64 | .07 | .70 |
| Anxious | −.49 | −.32 | .25 | −.55 | −.27 | −.43 | −.20 | .22 | −.49 | −.14 | .78 |
| Autistic | −.38 | −.18 | .03 | −.49 | −.23 | −.37 | −.07 | −.01 | −.44 | −.16 | .47 |
| Disorganized | −.19 | .01 | .20 | −.21 | −.78 | −.19 | .08 | .10 | −.03 | −.78 | .72 |
| Unperceptive | .06 | −.08 | .17 | −.06 | −.77 | .09 | −.08 | .07 | .12 | −.80 | .63 |
| Thoughtless | −.05 | .13 | .04 | −.26 | −.77 | −.10 | .20 | −.08 | −.09 | −.81 | .68 |
| Clumsy | −.18 | −.26 | −.01 | .11 | −.67 | −.12 | −.26 | −.09 | .32 | −.76 | .56 |
| Distractible | .06 | −.10 | .43 | .33 | −.64 | .13 | −.16 | .39 | .51 | −.66 | .72 |
| Erratic | .14 | .23 | .54 | −.18 | −.56 | .13 | .25 | .47 | −.10 | −.47 | .71 |
| Helpful | −.25 | .26 | −.37 | .25 | .41 | −.35 | .29 | −.32 | .17 | .31 | .50 |
| Intelligent | .07 | .35 | −.23 | .13 | .37 | −.03 | .35 | −.20 | .02 | .32 | .33 |
| Sensitive | −.33 | −.05 | −.30 | .26 | .35 | −.35 | −.04 | −.24 | .22 | .27 | .39 |
| Individualistic | −.06 | .27 | −.08 | −.24 | −.29 | −.15 | .34 | −.14 | −.21 | −.31 | .23 |
| Proportion of variance | .20 | .15 | .11 | .11 | .10 | .20 | .15 | .11 | .11 | .09 | |
Note. Salient loadings are in boldface. As each component has at least four loadings greater than |.6|, the structure is likely to be stable (Guadagnoli & Velicer, 1988). Asst = Assertiveness, Open = Openness, Neur = Neuroticism, Socb = Sociability, Attn = Attentiveness. h2 = communality.
Component was reflected by multiplying loadings by −1.
Table 7.
Correlations Between Promax Rotated Components
| Component | Assertiveness | Openness | Neuroticism | Sociability |
|---|---|---|---|---|
| Openness | −.38 | |||
| Neuroticism | −.04 | .00 | ||
| Sociability | −.06 | .21 | .12 | |
| Attentiveness | .01 | −.10 | −.34 | −.40 |
Note. Correlations derived via prior to reflecting Assertiveness, Neuroticism, and Attentiveness
Component Interpretation and Validation
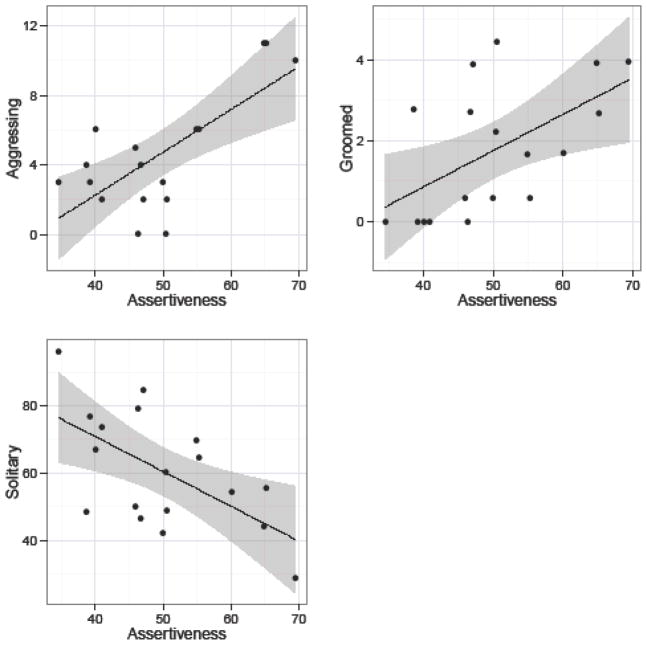
The first component, PC1 (see Table 6) was characterized by loadings on items describing high/low aggressive and despotic tendencies (e.g., bullying, aggressive, gentle). PC1 also negatively loaded onto items describing anxiety (e.g., fearful, cautious). In this respect, PC1 was broadly similar to dimensions termed Dominance in chimpanzees (King & Figueredo, 1997; Weiss, et al., 2009) but resembled rhesus macaque Dominance to a lesser degree (Weiss, et al., 2011). While the aggressive and despotic tendencies describing this component were shared with the orangutan Dominance dimension (Weiss, et al., 2006), the loadings related to anxiety were not. PC1 was positively correlated with the number of occasions monkeys were observed aggressing against others, being groomed by others3, and negatively correlated with the amount of time monkeys spent being solitary (see Table 8 and Figure 1). Given these loadings and behavioral correlations, we labeled PC1 “Assertiveness”.
Table 8.
Correlations between personality component scores and behavioral observations in the Living Links (Edinburgh Zoo) sample
| Assertiveness | Openness | Neuroticism | Sociability | Attentiveness | |
|---|---|---|---|---|---|
|
|
|||||
| Movea | −.12 (−.55, .37) | −.02 (−.48, .45) | .44 (−.03, .75) | −.45 (−.76, .02) | −.06 (−.51, .42) |
| Playa | −.21 (−.61, .29) | .62*** (.22, .84) | .41 (−.07, .74) | .31 (−.18, .68) | −.47** (−.77, −.01) |
| Alerta | −.16 (−.58, .33) | −.63** (−.85, −.24) | −.01 (−.47, .46) | −.58** (−.82, −.15) | .19 (−.30, .60) |
| Aggressionb | .72*** (.36, .89) | −.60*** (−.84, −.17) | −.43 (−.75, .06) | −.04 (−.51, .45) | .17 (−.34, .60) |
| Vigilanta | .09 (−.40, .53) | −.68*** (−.87, −.31) | −.58** (−.82, −.16) | −.17 (−.59, .33) | .58** (.15, .82) |
| Solitarya | −.60*** (−.83, −.19) | .27 (−.23, .65) | .41 (−.07, .74) | −.49** (−.78, −.04) | .06 (−.42, .51) |
| Attention (all participants)c | .12 (−.44, .61) | .13 (−.43, .62) | −.56** (−.84, −.04) | .30 (−.28, .71) | .44 (−.12, .79) |
| Attention (regular participants)d | −.16 (−.72, .52) | −.19 (−.73, .50) | −.58 (−.89, .08) | .25 (−.45, .76) | .70** (.12, .92) |
| Groomeda | .56** (.12, .81) | .17 (−.33, .59) | .04 (−.44, .49) | .19 (−.31, .60) | −.50** (−.79, −.05) |
Note. 95% confidence intervals are in parentheses.
n = 18.
n = 17.
n = 14.
n = 10.
p < .05.
p < .01.
Figure 1.
Relationship between Assertiveness and the amount of time monkeys spent aggressing others, being groomed, and solitary. Assertiveness scores have been scaled as T-scores (M = 50, SD = 10). Grooming and solitary behaviors are expressed as the percent of time focal individuals were engaged in each behavior. Incidences of aggression initiated by focal individuals are expressed as the total number of events summed across sampling periods.
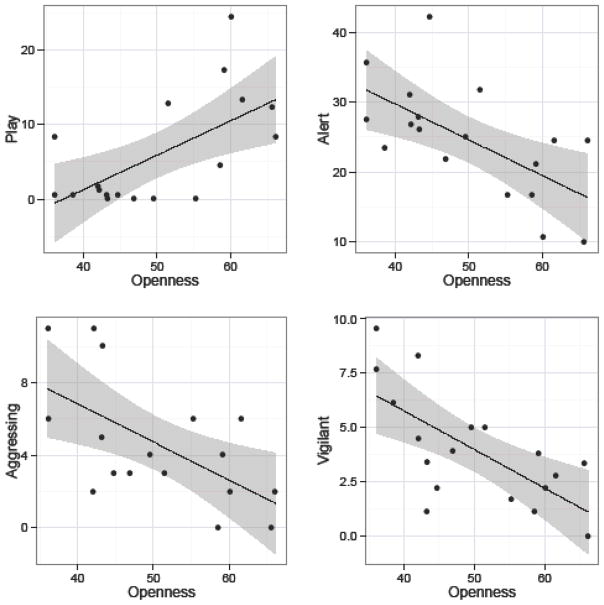
The second component, PC2, (see Table 6) loaded positively onto items describing exploratory and investigative behavior (e.g., inquisitive, curious), and items associated with creativity and originality (e.g., inventive, innovative). PC2 also loaded positively and negatively onto items describing high and low energy expenditure, respectively (e.g., active, playful, lazy), and items reflecting a tendency to persevere (e.g. quitting, persistent). PC2 was similar to dimensions labeled Openness in chimpanzees (King & Figueredo, 1997; Weiss, et al., 2009), and Openness/Activity in rhesus macaques (Weiss, et al., 2011). On the other hand, in orangutans, similar sets of items were associated with those related to Extraversion (Weiss, et al., 2006). PC2 was positively correlated with the time monkeys spent playing and negatively with time spent being alert, being vigilant, and the number of occasions monkeys were aggressive towards others (see Table 8 and Figure 2). None of the other recorded behavioral categories were significantly related to this component. The item loadings and behavioral correlates of PC2 led us to label it “Openness”.
Figure 2.
Relationship between Openness and the amount of time monkeys spent playing, alert, aggressing others, and vigilant. Openness scores have been scaled as T-scores (M = 50, SD = 10). Play, alert, and vigilant behaviors are expressed as the percent of time focal individuals were engaged in each behavior. Incidences of aggression initiated by focal individuals are expressed as the total number of events summed across sampling periods.
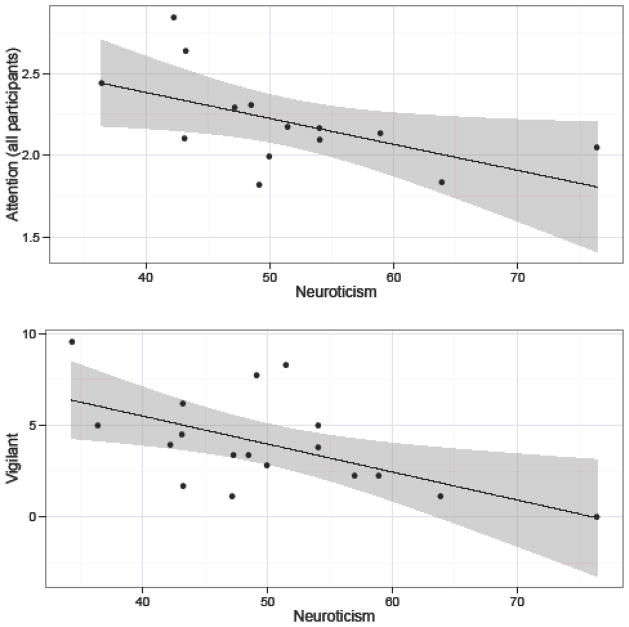
After reflecting the third component, PC3, by multiplying its loadings by −1 (see Table 6), it predominantly loaded positively on items describing an impulsive and volatile disposition (e.g., excitable, impulsive, erratic). This component also negatively loaded on items indicating a calmer disposition (e.g., cool, stable, predictable, unemotional). The clustering of traits associated with PC3 most closely resembled dimensions labeled Emotionality or Neuroticism in chimpanzees (King & Figueredo, 1997; Weiss, et al., 2009). To a lesser extent, PC3 also resembled rhesus macaque Anxiety and orangutan Neuroticism; however, compared to these latter dimensions, PC3 contained traits more characteristic of arousal and calmness (Weiss, et al., 2006; Weiss, et al., 2011). PC3 was negatively associated with time spent being vigilant, and, among monkeys that participated in cognitive testing, attention span (see Table 8 and Figure 3). No other observed behavior was significantly related to this component. Given the item loadings and associations with behaviors, we labeled the component “Neuroticism”.
Figure 3.
Relationship between Neuroticism and average attention span (all participants) and time spent being vigilant. Neuroticism scores have been scaled as T-scores (M = 50, SD = 10). Vigilant behavior is expressed as the percent of time focal individuals were engaged in this behavior. Scores on attention span were calculated during cognitive testing sessions according to whether monkeys exhibited high (“3”), medium (“2”), or low (“1”) attention during trials; scores were averaged across trials for each individual (see Methods).
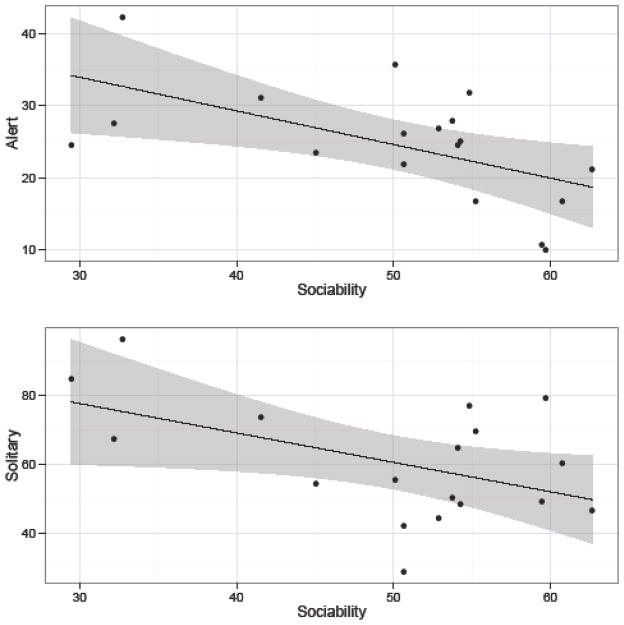
The fourth component, PC4, (see Table 6) loaded positively and negatively onto items indicative of overall social embeddedness (e.g., sociable, solitary). It also loaded positively on items describing positive social interactions (e.g., friendly, affectionate), and negatively on items describing negative affect (e.g., anxious, depressed). As such, PC4 shared common elements of social receptivity with orangutan Agreeableness and chimpanzee/orangutan Extraversion (King & Figueredo, 1997; Weiss, et al., 2009; Weiss, et al., 2006), as well as rhesus macaque Friendliness (Weiss, et al., 2011). PC4 was negatively associated with the amount of time monkeys spent being alert within their main enclosure, as well as time spent alone (see Table 8 and Figure 4). Given the trait loadings and behavioral associations, we labeled this component “Sociability”.
Figure 4.
Relationship between Sociability and the amount of time monkeys spent alert and solitary. Sociability scores have been scaled as T-scores (M = 50, SD = 10). Alert and solitary behaviors are expressed as the percent of time focal individuals were engaged in each behavior.
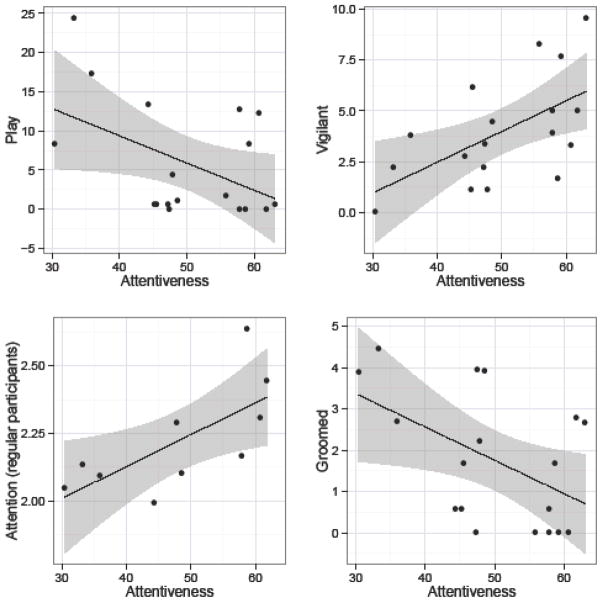
After reflecting the fifth component, PC5, (see Table 6) it loaded negatively on items indicating a lack of focus and meticulousness (e.g., thoughtless, distractible). This component also loaded positively and negatively, respectively, on items indicating pro-social tendencies and an ability to be discerning (e.g., helpful, unperceptive). The item content of PC5 did not overlap much with any of the orangutan (Weiss, et al., 2006) or rhesus macaque (Weiss, et al., 2011) dimensions. This component most closely resembled that of the Conscientiousness dimension found in the chimpanzee sample from Japan, which was also rated on the full HPQ (Weiss, et al., 2009). Scores on PC5 were negatively related to the amount of time monkeys spent playing and being groomed, and positively with time spent being vigilant. Additionally, among monkeys that participated in over 80% of sessions during cognitive testing, scores on PC5 were positively correlated with attention span (see Table 8 and Figure 5). The item content and behavioral correlations suggests that this component captures facets of the ability to focus one’s attention, and we therefore labeled this component “Attentiveness”.
Figure 5.
Relationship between scores on Attentiveness and average attention span (regular participants) and the amount of time monkeys spent playing, vigilant, being groomed. Attentiveness scores have been scaled as T-scores (M = 50, SD = 10). Play, vigilant, and grooming behaviors are expressed as the percent of time focal individuals were engaged in each behavior. Scores on attention span were calculated for monkeys that participated on > 80% of cognitive testing sessions, and were scored according to whether participants exhibited high (“3”), medium (“2”), or low (“1”) attention during trials; scores were averaged across trials for each individual (see Methods).
Inter-rater reliabilities and internal consistencies of components
The inter-rater reliabilities of components were highest for Assertiveness, Openness, and Sociability; and lowest, though still acceptable, for Attentiveness (see Table 9). The Cronbach’s alpha for Assertiveness, Openness, Neuroticism, Sociability, and Attentiveness were .95, .92, .85, .89, and .84, respectively.
Table 9.
Inter-rater Reliabilities and Capuchin Personality Components
| Component | ICC(3,1) | ICC(3,k) |
|---|---|---|
| Assertiveness | .71 | .89 |
| Openness | .70 | .89 |
| Neuroticism | .40 | .69 |
| Sociability | .58 | .82 |
| Attentiveness | .37 | .67 |
Note. Estimates based on 121 capuchin monkeys, each rated by an average of 3.35 raters.
ICC(3,1) = Reliability of individual ratings. ICC(3,k) = Reliability of mean ratings.
Cross-species Comparisons
Along with the examination of each component’s item content, comparing structures via cross-species correlations also highlighted similarities and differences between the capuchin personality dimensions and those of other species (see Table 10). Capuchin Assertiveness was significantly more similar to chimpanzee and orangutan Dominance than to rhesus macaque Dominance, William’s t = 4.27, p < .0001. Capuchin Openness was not significantly more similar to chimpanzee Openness than to rhesus macaque Openness, t = 1.80, p = .075. Capuchin Neuroticism was significantly more similar to chimpanzee Neuroticism than rhesus macaque Anxiety, t = 3.37, p < .01. Capuchin Sociability was significantly more similar to chimpanzee Extraversion than to orangutan Agreeableness, t = 2.86, p < .01. The degree of similarity between capuchin Attentiveness and Conscientiousness in the chimpanzee sample from Japan was comparable to that between capuchin Attentiveness and orangutan Intellect, t = 1.60, p = .113. However, this similarity was greater than that between capuchin Attentiveness and either Agreeableness in the sample of U.S. chimpanzees, t = 4.28, p < .0001, or rhesus macaque Anxiety, t = 3.00, p < .01.
Table 10.
Correlations Between Unit-Weighted Component Scores Based on Brown Capuchin, Chimpanzee, Orangutan, and Rhesus Macaque Structures
| Source of Structure Definition | Brown Capuchin Components
|
||||
|---|---|---|---|---|---|
| Asst | Open | Neur | Socb | Attn | |
| Chimpanzees in Japan | |||||
| Dominance | .96 | .41 | −.24 | .54 | .24 |
| Extraversion | .27 | .77 | −.04 | .86 | .22 |
| Conscientiousness | −.47 | −.29 | −.65 | .08 | .77 |
| Agreeableness | −.33 | −.26 | −.65 | .25 | .56 |
| Neuroticism | −.21 | −.06 | .91 | −.52 | −.57 |
| Openness | .33 | .95 | .00 | .52 | .14 |
|
| |||||
| U.S. Chimpanzees | |||||
| Dominance | .96 | .38 | −.23 | .47 | .21 |
| Extraversion | .27 | .80 | −.03 | .85 | .20 |
| Conscientiousness | −.65 | −.47 | −.59 | −.03 | .49 |
| Agreeableness | −.30 | −.04 | −.61 | .35 | .56 |
| Neuroticism | −.01 | .10 | .92 | −.29 | −.53 |
| Openness | .36 | .94 | .03 | .55 | .11 |
|
| |||||
| Orangutans | |||||
| Extraversion | .35 | .96 | .18 | .62 | .09 |
| Dominance | .96 | .37 | .13 | .31 | −.08 |
| Neuroticism | −.59 | −.23 | .73 | −.64 | −.58 |
| Agreeableness | .00 | .22 | −.52 | .75 | .46 |
| Intellect | .59 | .26 | −.51 | .35 | .68 |
|
| |||||
| Rhesus macaques | |||||
| Confidence | .83 | .36 | −.42 | .58 | .47 |
| Openness | .27 | .93 | .23 | .40 | −.14 |
| Dominance | .92 | .35 | .24 | .20 | −.20 |
| Friendliness | .29 | .46 | −.50 | .87 | .48 |
| Activity | .26 | .89 | .33 | .40 | .11 |
| Anxiety | .01 | −.08 | .84 | −.51 | −.63 |
Note. Boldfaced coefficients represent the highest correlation between components based on the capuchin structure and that of the other species. Boldface and underlined coefficients represent significantly different absolute correlations. Asst = Assertiveness, Open = Openness, Neur = Neuroticism, Socb = Sociability, Attn = Attentiveness.
Discussion
Our goals were to investigate personality structure in brown capuchin monkeys, and to compare the findings to personality structures reported in chimpanzees, orangutans, and rhesus macaques. The inter-rater reliabilities of items were comparable to those in other animal personality studies (e.g., Gosling, 2001; Weiss et al., 2006; Weiss et al., 2011; Uher & Asendorpf, 2008). They were also comparable to those reported in studies of items or lower-order facets of human personality scales (e.g., Kenrick & Stringfield, 1980; Costa & McCrae, 1992). The inter-rater reliabilities of components were comparable to those in studies of primates and other animals (Freeman & Gosling, 2010; Gosling, 2001) and comparable to, if not greater than, those found in studies of human personality (Connolly, Kavanagh, & Viswesvaran, 2007; Gomà-i-Freixanet, 1997; Gomà-i-Freixanet, Wismeijer, & Valero, 2005). Five robust and behaviorally validated components emerged from a PCA – Assertiveness, Openness, Neuroticism, Sociability, and Attentiveness.
The PCA results are unlikely to reflect anthropomorphic projection. For example, Weiss, Inoue-Murayama, King, Adams, and Matsuzawa (2012) found that after statistically adjusting for rater effects, rater biases on trait ratings did not influence the personality structures of chimpanzees and orangutans. Additionally, although a different scale was used, Manson and Perry’s (in press) study of wild white-faced capuchins found four personality dimensions which were similar to those found in the present study. Lastly, within the Edinburgh Zoo sample, scores on each component were linked to behaviors recorded up to one year after personality was assessed, suggesting that some element of behavioral consistency (i.e. personality) has been measured among our study subjects. More importantly, these latter findings demonstrate that the ratings do not merely reflect raters’ implicit understanding of how personality traits should correlate, and instead are representative of real-world behavioral trends among individual monkeys.
Collectively, the components derived for brown capuchins closely resembled personality dimensions of great apes, and particularly chimpanzees in the case of Neuroticism. However, in some respects (i.e. capuchin Openness and Sociability) these similarities were no more significant than those shared between capuchins and rhesus macaques. Thus, our results only partially support the hypothesis that personality structure in brown capuchins overlaps more with great apes than with rhesus macaques.
Behavioral Validation of Personality Structure in Brown Capuchins
Personality has been linked to social rank in various animals, such as fish (Colleter & Brown, 2011; Dahlbom, Lagman, Lundstedt-Enkel, Sundstrom, & Winberg, 2011), birds (David, Auclair, & Cezilly, 2011; Fox, Ladage, Roth, & Pravosudov, 2009), and primates (Anestis, 2005; Konečná, et al., 2012). Capuchin monkeys typically exhibit a social hierarchy, whereby dominant individuals (the alpha-male and alpha-female) win the majority of conflicts, have preferential access to socioecological resources (e.g. food, coalitions, and mates), and generally are “figures of attraction” for other group members (Fragaszy, et al., 2004; Izar et al., 2012; Janson, 1990a, 1990b; Robinson, 1981). Moreover, lower-ranking capuchins often receive considerably more aggression from other group members, compared to higher-ranking individuals (e.g., Ferreira, Izar, & Lee, 2006; Perry, 1996). In our study, Assertiveness reflects some of these “dominant” behaviors in brown capuchins. For instance, within our study population, Assertiveness is characterized by positive loadings on items such as aggressive, manipulative, and bullying. Additionally, individual scores on Assertiveness were positively related to the amount of time individuals spent being groomed, aggressing others, and time spent in close proximity (< 2 m) to others.
Scores on Openness were positively related to the amount of time monkeys spent playing, but negatively related to the amount of time monkeys were vigilant, alert, and aggressive towards others. Capuchins are unusual among primates due to their high tolerance of other group members, especially juveniles (Perry, 2011). Thus, the negative relationship between Openness and alert/vigilant behaviors could suggest that more open individuals are more socially tolerant and/or less concerned about the activities of other group members. In support of this suggestion, Byrne and Suomi (2002) found that individual brown capuchins with higher scores on curious (a trait that positively loaded onto Openness in this study) had lower levels of cortisol, a hormone associated with arousal and managing stress.
Studies in humans have found that neurotic traits are negatively related to individual differences in attention span – that is, individuals that score high on these traits are unable to focus as well as individuals that score low on these traits (Bredemeier, Berenbaum, Most, & Simons, 2011). Similarly, we found a negative relationship between scores on capuchin Neuroticism and average attention span during cognitive testing. Indeed, during testing, capuchins high in Neuroticism appeared to be easily distracted by on-going activities within the group’s main enclosure (e.g., frequently turning to smell or listen for sounds at the cubicle door leading to the group’s main enclosure). Scores on Neuroticism were also negatively related to the amount of time monkeys spent being vigilant. Although this latter finding seems counterintuitive, more neurotic individuals may engage in less vigilant behavior due to their increased agitation/restlessness. Indeed, Neuroticism was characterized by traits such as active, excitable, and erratic. Additionally, individuals high on Neuroticism appeared to become more restless when in the presence of other group members, frequently moving out of the way of others and/or continuously circling the main enclosure when others were nearby (FBM, pers. obs.).
Scores on Sociability were negatively related to the amount of time individuals spent alone. Additionally, Sociability was negatively related to the amount of time monkeys spent alert, i.e. scanning their surroundings. Lastly, Sociability was characterized by positive loadings on items such as friendly and affectionate, and negative loadings on items such as anxious and depressed. Individuals high on Sociability likely occupy central positions within their group’s social network (Krause, Lusseau, & James, 2009), and as a result, may have been less anxious about the activities and/or whereabouts of other group members. Indeed, Byrne and Suomi (2002) found that several traits similar to those describing low Sociability (e.g. apprehensive, tense, insecure) were positively related to baseline and peak cortisol levels in brown capuchins.
Scores on Attentiveness were negatively related to the amount of time individuals spent playing. Such findings mirror those reported by Weinstein and Capitanio (2008), who found a negative relationship between scores on the dimension “Equable” (e.g. calm and easy-going) and play behavior in rhesus macaques. Scores on Attentiveness were also positively related to vigilant behavior. Thus, highly Attentive individuals may have a different status, or role, within the group (e.g., social monitoring or “policing”; see Flack, Girvan, de Waal, & Krakauer, 2006), which is reflected by playing less and being more vigilant. Future studies should examine the association between individual differences in Attentiveness and pro-social behavior.
Attentiveness was also negatively related to the amount of time monkeys were groomed by others. Among capuchins, this behavior may alleviate stress and strengthen relationships within the group (Tiddi, Aureli, di Sorrentino, Janson, & Schino, 2011). Indeed, traits such as erratic, excited, and [not] helpful are characteristic of low Attentiveness. Thus, considering the social function of grooming, less Attentive individuals may be groomed more by other members of the group in order to strengthen relationships between them.
Cross-Species Comparisons of Personality Structure
Capuchin Assertiveness closely resembled Dominance in chimpanzees and orangutans (King & Figueredo, 1997; Weiss, et al., 2006), and to a lesser extent, Dominance in rhesus macaques (Weiss, et al., 2011). Personality dimensions similar to Dominance and Assertiveness are a common characteristic of primate personality structure (Freeman & Gosling, 2010), such as Dominance in western lowland gorillas, Gorilla gorilla gorilla (e.g., aggressive, irritable, strong; Gold & Maple, 1994), Confidence in Hanuman langurs, Semnopithecus entellus (e.g., aggressive, bullying, dominant; Konečná et al., 2008), and Extraversion in white-faced capuchins (e.g., domineering, assertive, aggressive; Manson & Perry, in press). Thus, our findings are consistent with the possibility that such dimensions are phylogenetically old among primates.
It is unclear why capuchin Assertiveness was significantly more like chimpanzee/orangutan Dominance than rhesus macaque Dominance. One possibility, however, may be that such differences reflect how assertive behaviors are expressed in macaques, compared to the other species. For example, as is typical of tool-using species, capuchins, chimpanzees, and orangutans are generally more tolerant of conspecifics than are rhesus macaques (van Schaik, Deaner, & Merrill, 1999).
Capuchin Openness closely resembled chimpanzee and macaque Openness (King & Figueredo, 1997; Weiss, et al., 2011) and orangutan Extraversion (Weiss, et al., 2006). As orangutans do not have a distinct Openness-like dimension, while the other three species do, such a dimension may have evolved multiple times during primate evolution. It remains unclear, however, why Openness-like dimensions would evolve in some species but not others. Moreover, why Openness traits would be subsumed under a different dimension in orangutans, i.e. Extraversion, remains unknown. Further comparative data are needed on other primate species to clarify this issue.
It is unclear why capuchin Neuroticism was significantly more like chimpanzee Neuroticism compared to dimensions in the other species. One possibility, however, could be that these findings reflect differences in the social organization of each species. For example, orangutans live in loose communities monopolized only by a single dominant male (Rodman, 1985). In rhesus macaques, all individuals are ranked according to a linear hierarchy, and such relationships are generally clear-cut (Maestripieri & Hoffman, 2012). In contrast, social rank among brown capuchins and chimpanzees is relatively more relaxed, and coalitionary support occurs among both sexes (de Waal, 1984; Fragaszy, et al., 2004). Thus, there may be more daily uncertainty in the social lives of capuchins and chimpanzees, compared to orangutans and rhesus macaques.
Capuchin Sociability closely resembled dimensions found in the other three species; however, it was significantly more like chimpanzee Extraversion and rhesus macaque Friendliness compared to orangutan Agreeableness. Such findings may simply reflect differences in sociality between orangutans and the other more sociable species (Berman, Rasmussen, & Suomi, 1997; Fragaszy, et al., 2004; Rodman, 1984; Stanford, 1998).
Out of the five dimensions derived in brown capuchins, Attentiveness least resembled those seen in the other species. However, capuchin Attentiveness was significantly more like chimpanzee Conscientiousness and orangutan Intellect than any other dimension. As discussed in the introduction, chimpanzees, orangutans, and capuchins share key traits associated with behavior, cognition, and learning (van Schaik, et al., 1999; Whiten & van Schaik, 2007). In chimpanzees (and humans), Conscientiousness comprises tendencies to be thorough, organized, reliable, goal-directed, and able to delay gratification (Costa & McCrae, 1992; King & Figueredo, 1997). In orangutans, Intellect is characterized bytraits that load on chimpanzee Conscientiousness and capuchin Attentiveness (e.g. decisive, [not] clumsy, [not] disorganized) (Weiss, et al., 2006). Lastly, capuchin Attentiveness is positively associated with individual differences in subjects’ ability to focus during cognitive tasks – which may facilitate learning through demonstration, i.e. social learning. Thus, do Conscientiousness-like dimensions evolve in large-brained species that rely on social learning, use tools, and have “cultural” traditions? If so, Conscientiousness-like dimensions may also exist in other large-brained tool-using species such as bottlenose dolphins (Tursiops truncatus) (Amici, et al., 2008; Connor, 2007; Gruber, Clay, & Zuberbuhler, 2010; Herrmann, Hare, Call, & Tomasello, 2010; Patterson & Mann, 2011; Tsai & Mann, 2012).
There were subtle differences between species with respect to three items related to pro-social behaviors. First, similar to gorillas (Gold & Maple, 1994), orangutans (Weiss, et al., 2006), and rhesus macaques (Weiss, et al., 2011), traits associated with irritability were associated with a capuchin dimension related to competitive nature, i.e. Assertiveness. By contrast, the same trait was a marker of low chimpanzee Conscientiousness (King & Figueredo, 1997). Second, being gentle was a marker of lower Assertiveness and higher Neuroticism in capuchins. While the same trait was related to lower Dominance in rhesus macaques and orangutans, it was also associated with higher Friendliness and Agreeableness, respectively, in these two species (Weiss, et al., 2011; Weiss, et al., 2006). In chimpanzees, behaviors captured by the trait gentle were only associated with higher Agreeableness (King & Figueredo, 1997). Third, being helpful was associated with higher Attentiveness in capuchins, but with higher Friendliness in rhesus macaques (Weiss, et al., 2011), and Agreeableness in chimpanzees and orangutans (King & Figueredo, 1997; Weiss, et al., 2006). Such differences may suggest that pro-social behaviors are important factors in personality evolution.
Brown capuchins are phylogenetically as distantly related to rhesus macaques as they are to orangutans or chimpanzees (Perelman et al. 2011). If common descent was the main driving force behind the evolution of personality structure in brown capuchins, we would expect this structure to overlap both with that of rhesus macaques and the great ape species. In many respects, brown capuchins appear to have evolved convergent properties of personality structure closely resembling those found in great apes, while diverging from those seen in rhesus macaques. Understanding the evolutionary history of primate personality will require a larger and more diverse sample across the various taxa, which can then be clearly rooted in a comparative analysis.
Future Directions
The present study is not without limitations. For one, the correlations between personality dimensions and behaviors were based on a small sample. Future research should validate our findings using larger sample sizes and a multitrait-multimethod approach (Campbell & Fiske, 1959).
Another limitation is that the majority of the capuchins in the present study regularly participate in cognitive studies. Thus, their personalities may partly reflect what they learned in these tasks, or the behaviors that were observed while they took part in these tasks. For example, this may underlie the clustering of traits found in the sixth component of the PCA (see results in “Data reduction”, and Table S1). We therefore encourage future studies to compare personality structure in wild and zoo-housed brown capuchin monkeys (that do not participate in cognitive research) using the HPQ scale and that used by Manson and Perry(in press).
To date, the majority of studies on primate personality structure have been conducted on great apes and Old World monkeys. Our study provides further insights into primate personality phylogeny. Further comparative work is still needed, however, to clarify the extent to which primate taxa differ in personality structure. Specifically, data are needed on a broader range of species exhibiting different phylogenetic proximities, relative brain sizes, levels of social tolerance, and behavioral/cognitive complexity. Such comparative analyses may reveal more about the adaptive function of personality among primates, including ourselves.
Supplementary Material
Acknowledgments
We are very grateful to the people who completed personality questionnaires: M. Baranay, A. Bates, S. Bower, D. Bryson, S. Calcutt, N. Cladiere, C. Fruteau, J. Griffey, B. Herman, H. Kuroshima, G. Long, T. McKenney, E. Messer, N. Moratscheck, C. Morin, C. Nisbitt, A. Parrish, D. Proctor, M. Pelé, D. Rice, T. Rubin, S. Steelandt, and C. Talbot. F.B.M. personally thanks Prof. Andrew Whiten, Director of Living Links at Edinburgh Zoo, for permission to conduct research there, and the staff/students who kindly provided support and assistance. We thank E. O’Sullivan for scoring monkeys’ attention span, which we used for the inter-observer reliability tests. We also thank K. Howie for statistical advice, and the BERG (Stirling University) for insightful comments. F.B.M gratefully acknowledges the Charles A. Lockwood Memorial Fund and The University of Stirling for funding.
Footnotes
Varimax and promax rotated six component solutions are available in Table S1. Correlations among the six promax-rotated components are available in Table S2.
This measure represents time engaged in grooming behavior, regardless of participant, and should therefore not be misconstrued as an indication of individuals grooming “up the hierarchy”.
Contributor Information
F. Blake Morton, Behaviour and Evolution Research Group and Scottish Primate Research Group, Psychology, School of Natural Sciences, University of Stirling, Stirling FK9 4LA, United Kingdom.
Phyllis C. Lee, Behaviour and Evolution Research Group and Scottish Primate Research Group, Psychology, School of Natural Sciences, University of Stirling, Stirling, United Kingdom
Hannah M. Buchanan-Smith, Behaviour and Evolution Research Group and Scottish Primate Research Group, Psychology, School of Natural Sciences, University of Stirling, Stirling, United Kingdom
Sarah F. Brosnan, Department of Psychology and Language Research Center, Georgia State University, Atlanta, GA, USA
Bernard Thierry, Département Ecologie, Physiologie and Ethologie, IPHC, Centre National de la Recherche Scientifique, Université de Strasbourg, France.
Annika Paukner, Laboratory of Comparative Ethology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, Poolesville, MD, USA.
Frans B. M. de Waal, Living Links, Yerkes National Primate Research Center, Emory University, Atlanta, GA, USA
Jane Widness, Comparative Cognition Laboratory, Department of Psychology, Yale University, New Haven, CT, USA.
Jennifer L. Essler, Department of Animal Behavior, Bucknell University, Lewisburg, PA, USA
Alexander Weiss, Scottish Primate Research Group, School of Philosophy, Psychology and Language Sciences, Department of Psychology, The University of Edinburgh, United Kingdom.
References
- Adams MJ, King JE, Weiss A. The majority of genetic variation in orangutan personality and subjective-well being is nonadditive. Behavior Genetics. 2012;42:675–686. doi: 10.1007/s10519-012-9537-y. [DOI] [PubMed] [Google Scholar]
- Addessi E, Paglieri F, Focaroli V. The ecological rationality of delay tolerance: Insights from capuchin monkeys. Cognition. 2011;119:142–147. doi: 10.1016/j.cognition.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Alfaro JW, Silva JD, Jr, Rylands AB. How different are robust and gracile capuchin monkeys? An argument for the use of Sapajus and Cebus. American Journal of Primatology. 2012;74:273–286. doi: 10.1002/ajp.22007. [DOI] [PubMed] [Google Scholar]
- Amici F, Aureli F, Call J. Fission-fusion dynamics, behavioral flexibility, and inhibitory control in primates. Current Biology. 2008;18:1415–1419. doi: 10.1016/j.cub.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Anestis SF. Behavioral style, dominance rank, and urinary cortisol in young chimpanzees (Pan troglodytes) Behaviour. 2005;142:1245–1268. doi: 10.1163/156853905774539418. [DOI] [Google Scholar]
- Bell AM, Hankison SJ, Laskowski KL. The repeatability of behaviour: a meta-analysis. Animal Behaviour. 2009;77:771–783. doi: 10.1016/j.anbehav.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman CM, Rasmussen KLR, Suomi SJ. Group size, infant development and social networks in free-ranging rhesus monkeys. Animal Behaviour. 1997;53:405–421. doi: 10.1006/anbe.1996.0321. [DOI] [Google Scholar]
- Bredemeier K, Berenbaum H, Most SB, Simons DJ. Links between neuroticism, emotional distress, and disengaging attention: Evidence from a single-target RSVP task. Cognition and Emotion. 2011;25:1510–1519. doi: 10.1080/02699931.2010.549460. [DOI] [PubMed] [Google Scholar]
- Byrne G, Suomi SJ. Cortisol reactivity and its relation to homecage behavior and personality ratings in tufted capuchin (Cebus apella) juveniles from birth to six years of age. Psychoneuroendocrinology. 2002;27:139–154. doi: 10.1016/S0306-4530(01)00041-5. [DOI] [PubMed] [Google Scholar]
- Campbell DT, Fiske DW. Convergent and discriminant validation by the multitrait-multimethod matrix. Psychological Bulletin. 1959;56:81–105. doi: 10.1037/h0046016. [DOI] [PubMed] [Google Scholar]
- Capitanio JP. Personality dimensions in adult male rhesus macaques: Prediction of behaviors across time and situation. American Journal of Primatology. 1999;47:299–320. doi: 10.1002/(SICI)1098-2345(1999)47:4<299::AID-AJP3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Capitanio JP. Nonhuman primate personality and immunity: Mechanisms of health and disease. In: Weiss A, King JE, Murray LE, editors. Personality, temperament, and behavioral syndromes in nonhuman primates. New York: Springer; 2011. pp. 233–255. [Google Scholar]
- Careau V, Bininda-Emonds ORP, Thomas DW, Reale D, Humphries MM. Exploration strategies map along fast-slow metabolic and life-history continua in muroid rodents. Functional Ecology. 2009;23:150–156. doi: 10.1111/j.1365-2435.2008.01468.x. [DOI] [Google Scholar]
- Carere C, Eens M. Unravelling animal personalities: how and why individuals consistently differ. Behaviour. 2005;142:1149–1157. doi: 10.1163/156853905774539436. [DOI] [Google Scholar]
- Carere C, Locurto C. Interaction between animal personality and animal cognition. Current Zoology. 2011;57:491–498. [Google Scholar]
- Cavigelli SA, Bennett JM, Michael KC, Klein LC. Female temperament, tumor development and life span: Relation to glucocorticoid and tumor necrosis factor alpha levels in rats. Brain Behavior and Immunity. 2008;22:727–735. doi: 10.1016/j.bbi.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colleter M, Brown C. Personality traits predict hierarchy rank in male rainbowfish social groups. Animal Behaviour. 2011;81:1231–1237. doi: 10.1016/j.anbehav.2011.03.011. [DOI] [Google Scholar]
- Connolly JJ, Kavanagh EJ, Viswesvaran C. The convergent validity between self and observer ratings of personality: A meta-analytic review. International Journal of Selection and Assessment. 2007;15:110–117. doi: 10.1111/j.1468-2389.2007.00371.x. [DOI] [Google Scholar]
- Connor RC. Dolphin social intelligence: Complex alliance relationships in bottlenose dolphins and a consideration of selective environments for extreme brain size evolution in mammals. Philosophical Transactions of the Royal Society B Biological Sciences. 2007;362:587–602. doi: 10.1098/rstb.2006.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Dahlbom SJ, Lagman D, Lundstedt-Enkel K, Sundstrom LF, Winberg S. Boldness predicts social status in zebrafish (Danio rerio) PLoS ONE. 2011;6:e23565. doi: 10.1371/journal.pone.0023565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M, Auclair Y, Cezilly F. Personality predicts social dominance in female zebra finches, Taeniopygia guttata, in a feeding context. Animal Behaviour. 2011;81:219–224. doi: 10.1016/j.anbehav.2010.10.008. [DOI] [Google Scholar]
- de Waal FBM. Sex differences in the formation of coalitions among chimpanzees. Ethology and Sociobiology. 1984;5:239–255. doi: 10.1016/0162-3095(84)90004-9. [DOI] [Google Scholar]
- de Winter JCF, Dodou D, Wieringa PA. Exploratory factor analysis with small sample sizes. Multivariate Behavioral Research. 2009;44:147–181. doi: 10.1080/00273170902794206. [DOI] [PubMed] [Google Scholar]
- Deaner RO, van Schaik CP, Johnson VE. Do some taxa have better domain-general cognition than others? A meta-analysis of nonhuman primate studies. Evolutionary Psychology. 2006;4:149–196. [Google Scholar]
- Digman JM. Personality structure: Emergence of the Five-Factor Model. Annual Review of Psychology. 1990;41:417–440. doi: 10.1146/annurev.ps.41.020190.002221. [DOI] [Google Scholar]
- Dinno A. paran: Horn’s test of principal components/factors. 2008 Retrieved from http://cran.r-project.org/web/packages/paran/index.html.
- Everett JD. Factor comparability as a means of determining the number of factors and their rotation. Multivariate Behavioral Research. 1988;18:197–218. doi: 10.1207/s15327906mbr1802_5. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. The biological basis of personality. 2. Springfield, IL: Charles C. Thomas; 1970. [Google Scholar]
- Ferreira RG, Izar P, Lee PC. Exchange, affiliation, and protective interventions in semifree-ranging brown capuchin monkeys (Cebus apella) American Journal of Primatology. 2006;68:765–776. doi: 10.1002/Ajp.20277. [DOI] [PubMed] [Google Scholar]
- Flack JC, Girvan M, de Waal FBM, Krakauer DC. Policing stabilizes construction of social niches in primates. Nature. 2006;439:426–429. doi: 10.1038/nature04326. [DOI] [PubMed] [Google Scholar]
- Fox RA, Ladage LD, Roth TC, II, Pravosudov VV. Behavioural profile predicts dominance status in mountain chickadees, Poecile gambeli. Animal Behaviour. 2009;2009:1441–1448. doi: 10.1016/j.anbehav.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragaszy DM, Visalberghi E, Fedigan LM. The complete capuchin: The biology of the genus Cebus. New York: Cambridge University Press; 2004. [Google Scholar]
- Freeman HD, Gosling SD. Personality in nonhuman primates: A review and evaluation of past research. American Journal of Primatology. 2010;72:653–671. doi: 10.1002/ajp.20833. [DOI] [PubMed] [Google Scholar]
- Gold KC, Maple TL. Personality assessment in the gorilla and its utility as a management tool. Zoo Biology. 1994;13:509–522. [Google Scholar]
- Goldberg LR. An alternative “description of personality”: the Big-Five factor structure. Journal of Personality and Social Psychology. 1990;59:1216–1229. doi: 10.1037/0022-3514.59.6.1216. [DOI] [PubMed] [Google Scholar]
- Gomà-i-Freixanet M. Consensual validity of the EPQ: Self-reports and spouse-reports. European Journal of Psychological Assessment. 1997;13:179–185. doi: 10.1027/1015-5759.13.3.179. [DOI] [Google Scholar]
- Gomà-i-Freixanet M, Wismeijer AAJ, Valero S. Consensual validity parameters of the Zuckerman-Kuhlman Personality Questionnaire: Evidence From self-reports and spouse reports. Journal of Personality Assessment. 2005;84:279–286. doi: 10.1207/s15327752jpa8403_07. [DOI] [PubMed] [Google Scholar]
- Gorsuch RL. Factor analysis. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1983. [Google Scholar]
- Gosling SD. From mice to men: What can we learn about personality from animal research? Psychological Bulletin. 2001;127:45–86. doi: 10.1037/0033-2909.127.1.45. [DOI] [PubMed] [Google Scholar]
- Gosling SD, Graybeal A. Tree thinking: A new paradigm for integrating comparative data in psychology. The Journal of General Psychology. 2007;134:259–277. doi: 10.3200/GENP.134.2.259-278. [DOI] [PubMed] [Google Scholar]
- Gruber T, Clay Z, Zuberbuhler K. A comparison of bonobo and chimpanzee tool use: evidence for a female bias in the Pan lineage. Animal Behaviour. 2010;80:1023–1033. doi: 10.1016/j.anbehav.2010.09.005. [DOI] [Google Scholar]
- Guadagnoli E, Velicer WF. Relation of sample size to the stability of component patterns. Psychological Bulletin. 1988;103:265–275. doi: 10.1037/0033-2909.103.2.265. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Hare B, Call J, Tomasello M. Differences in the cognitive skills of bonobos and chimpanzees. PLoS ONE. 2010;5:e12438. doi: 10.1371/journal.pone.0012438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965;30:179–185. doi: 10.1007/BF02289447. [DOI] [PubMed] [Google Scholar]
- Izar P, Verderane MP, Peternelli-Dos-Santos L, Mendonca-Furtado O, Presotto A, Tokuda M, Fragaszy D. Flexible and conservative features of social systems in tufted capuchin monkeys: comparing the socioecology of Sapajus libidinosus and Sapajus nigritus. American Journal of Primatology. 2012;74:315–331. doi: 10.1002/Ajp.20968. [DOI] [PubMed] [Google Scholar]
- Janson CH. Ecological consequences of individual spatial choice in foraging groups of brown capuchin monkeys, Cebus apella. Animal Behaviour. 1990a;40:922–934. doi: 10.1016/S0003-3472(05)80994-7. [DOI] [Google Scholar]
- Janson CH. Social correlates of individual spatial choice in foraging groups of brown capuchin monkeys, Cebus apella. Animal Behaviour. 1990b;40:910–921. doi: 10.1016/S0003-3472(05)80993-5. [DOI] [Google Scholar]
- Jung S, Lee S. Exploratory factor analysis for small samples. Behavioral Research Methods. 2011;43:701–709. doi: 10.3758/s13428-011-0077-9. [DOI] [PubMed] [Google Scholar]
- Jung S, Takane Y. Regularized common factor analysis. In: Shigemasu K, Okada A, Imaizumi T, Hoshino T, editors. New trends in psychometrics. Tokyo: University Academic Press; 2008. pp. 141–149. [Google Scholar]
- Kenrick DT, Stringfield DO. Personality traits and the eye of the beholder: Crossing some traditional philosophical boundaries in the search for consistency in all of the people. Psychological Review. 1980;87:88–104. doi: 10.1037/0033-295X.87.1.88. [DOI] [Google Scholar]
- King JE, Figueredo AJ. The Five-Factor Model plus Dominance in chimpanzee personality. Journal of Research in Personality. 1997;31:257–271. doi: 10.1006/jrpe.1997.2179. [DOI] [Google Scholar]
- Konečná M, Lhota S, Weiss A, Urbánek T, Adamová T, Pluháček J. Personality in free-ranging Hanuman langur (Semnopithecus entellus) males: Subjective ratings and recorded behavior. Journal of Comparative Psychology. 2008;122:379–389. doi: 10.1037/a0012625. [DOI] [PubMed] [Google Scholar]
- Konečná M, Weiss A, Lhota S, Wallner B. Personality in Barbary macaques (Macaca sylvanus): Temporal stability and social rank. Journal of Research in Personality. 2012;46:581–590. doi: 10.1016/j.jrp.2012.06.004. [DOI] [Google Scholar]
- Krause J, Lusseau D, James R. Animal social networks: an introduction. Behavioral Ecology and Sociobiology. 2009;63:967–973. [Google Scholar]
- Lee K, Ogunfowora B, Ashton MC. Personality traits beyond the Big Five: Are they within the HEXACO space? Journal of Personality. 2005;73:1437–1463. doi: 10.1111/j.1467-6494.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- Macdonald C, Whiten A. The ‘Living Links to Human Evolution’ Research Centre in Edinburgh Zoo: A new endeavour in collaboration. International Zoo Yearbook. 2011;45:7–17. doi: 10.1111/j.1748-1090.2010.00120.x. [DOI] [Google Scholar]
- Maestripieri D, Hoffman CL. Behavior and social dynamics of rhesus macaques on Cayo Santiago. In: Wang Q, editor. Bones, genetics, and behavior of rhesus macaques. New York: Springer; 2012. pp. 247–262. [Google Scholar]
- Manson JH, Perry S. Personality structure, sex differences, and temporal change and stability in wild white-faced capuchins, Cebus capucinus. Journal of Comparative Psychology. doi: 10.1037/a0031316. (in press) [DOI] [PubMed] [Google Scholar]
- Martin P, Bateson P. Measuring behaviour: An introductory guide. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- McCrae RR, Zonderman AB, Bond MH, Costa PT, Jr, Paunonen SV. Evaluating replicability of factors in the Revised NEO Personality Inventory: Confirmatory factor analysis versus Procrustes rotation. Journal of Personality and Social Psychology. 1996;70:552–566. doi: 10.1037/0022-3514.70.3.552. [DOI] [Google Scholar]
- Patterson EM, Mann J. The ecological conditions that favor tool use and innovation in wild bottlenose dolphins (Tursiops sp.) PLoS ONE. 2011;6:e22243. doi: 10.1371/journal.pone.0022243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. Female-female social relationships in wild white-faced capuchin monkeys, Cebus capucinus. American Journal of Primatology. 1996;40:167–182. doi: 10.1002/(SICI)1098-2345(1996)40:2<167::AID-AJP4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Perry S. Social traditions and social learning in capuchin monkeys (Cebus) Philosophical Transactions of the Royal Society B-Biological Sciences. 2011;366:988–996. doi: 10.1098/rstb.2010.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. Retrieved from http://www.R-project.org. [Google Scholar]
- Réale D, Martin J, Coltman DW, Poissant J, Festa-Bianchet M. Male personality, life-history strategies and reproductive success in a promiscuous mammal. Journal of Evolutionary Biology. 2009;22:1599–1607. doi: 10.1111/j.1420-9101.2009.01781.x. [DOI] [PubMed] [Google Scholar]
- Revelle W. psych: Procedures for personality and psychological research. Evanston, IL: Northwestern University; 2011. Retrieved from http://personality-project.org/r/psych.manual.pdf. [Google Scholar]
- Robinson JG. Spatial structure in foraging groups of wedge-capped capuchin monkeys Cebus Nigrivittatus. Animal Behaviour. 1981;29:1036–1056. doi: 10.1016/S0003-3472(81)80057-7. [DOI] [Google Scholar]
- Rodman PS. Foraging and social systems of orangutans. In: Hamburg DA, McCown ER, editors. The Great Apes. Menlo Park, CA: Benjamin/Cummings; 1984. [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037/0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Stanford CB. The social behavior of chimpanzees and bonobos: Empirical evidence and shifting assumptions. Current Anthropology. 1998;39:399–420. doi: 10.1086/204757. [DOI] [Google Scholar]
- Steiper ME, Young NM. Primate molecular divergence dates. Molecular Phylogenetics and Evolution. 2006;41:384–394. doi: 10.1016/j.ympev.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Stevenson-Hinde J, Hinde CA. Individual characteristics: Weaving psychological and ethological approaches. In: Weiss A, King JE, Murray L, editors. Personality and temperament in nonhuman primates. New York: Springer; 2011. pp. 3–14. [Google Scholar]
- Stevenson-Hinde J, Stillwell-Barnes R, Zunz M. Individual differences in young rhesus monkeys: Consistency and change. Primates. 1980;21:498–509. doi: 10.1007/BF02373838. [DOI] [Google Scholar]
- Stevenson-Hinde J, Zunz M. Subjective assessment of individual rhesus monkeys. Primates. 1978;19:473–482. doi: 10.1007/BF02373309. [DOI] [Google Scholar]
- Tiddi B, Aureli F, di Sorrentino EP, Janson CH, Schino G. Grooming for tolerance? Two mechanisms of exchange in wild tufted capuchin monkeys. Behavioral Ecology. 2011;22:663–669. doi: 10.1093/beheco/arr028. [DOI] [Google Scholar]
- Tsai Y-JJ, Mann J. Dispersal, philopatry, and the role of fission-fusion dynamics in bottlenose dolphins. Marine Mammal Science. 2012 doi: 10.1111/j.1748-7692.2011.00559.x. [DOI] [Google Scholar]
- Uher J, Asendorpf JB, Call J. Personality in the behaviour of great apes: Temporal stability, cross-situational consistency and coherence in response. Animal Behaviour. 2008;75:99–112. doi: 10.1016/j.anbehav.2007.04.018. [DOI] [Google Scholar]
- van Oers K, de Jong G, van Noordwijk AJ, Kempenaers B, Drent PJ. Contribution of genetics to animal personalities: A review of case studies. Behaviour. 2005;142:1191–1212. doi: 10.1163/156853905774539364. [DOI] [Google Scholar]
- van Schaik CP, Deaner RO, Merrill MY. The conditions for tool use in primates: Implications for the evolution of material culture. Journal of Human Evolution. 1999;36:719–741. doi: 10.1006/jhev.1999.0304. [DOI] [PubMed] [Google Scholar]
- Weinstein TAR, Capitanio JP. Individual differences in infant temperament predict social relationships of yearling rhesus monkeys, Macaca mulatta. Animal Behaviour. 2008;76:455–465. doi: 10.1016/j.anbehav.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Adams MJ, Widdig A, Gerald MS. Rhesus macaques (Macaca mulatta) as living fossils of hominoid personality and subjective well-being. Journal of Comparative Psychology. 2011;125:72–83. doi: 10.1037/a0021187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Gartner MC, Gold KC, Stoinski TS. Extraversion predicts longer survival in gorillas: An 18-year longitudinal study. Proceedings of the Royal Society B-Biological Sciences. 2012;20122231 doi: 10.1098/rspb.2012.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Inoue-Murayama M, Hong K-W, Inoue E, Udono S, Ochiai T, King JE. Assessing chimpanzee personality and subjective well-being in Japan. American Journal of Primatology. 2009;71:283–292. doi: 10.1002/ajp.20649. [DOI] [PubMed] [Google Scholar]
- Weiss A, Inoue-Murayama M, King JE, Adams MJ, Matsuzawa T. All too human? Chimpanzee and orang-utan personalities are not anthropomorphic projections. Animal Behaviour. 2012;83:1355–1365. doi: 10.1016/j.anbehav.2012.02.024. [DOI] [Google Scholar]
- Weiss A, King JE, Hopkins WD. A cross-setting study of chimpanzee (Pan troglodytes) personality structure and development: Zoological parks and Yerkes National Primate Research Center. American Journal of Primatology. 2007;69:1264–1277. doi: 10.1002/ajp.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, King JE, Perkins L. Personality and subjective well-being in orangutans (Pongo pygmaeus and Pongo abelii) Journal of Personality and Social Psychology. 2006;90:501–511. doi: 10.1037/0022-3514.90.3.501. [DOI] [PubMed] [Google Scholar]
- Whiten A, van Schaik CP. The evolution of animal ‘cultures’ and social intelligence. Philosophical Transactions of the Royal Society B-Biological Sciences. 2007;362:603–620. doi: 10.1098/rstb.2006.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DS, Clark AB, Coleman K, Dearstyne T. Shyness and boldness in humans and other animals. Trends in Ecology and Evolution. 1994;9:442–446. doi: 10.1016/0169-5347(94)90134-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.