Abstract
Accurate tRNA selection by the ribosome is essential for the synthesis of functional proteins. Previous structural studies indicated that the ribosome distinguishes between cognate and near-cognate tRNAs by monitoring the geometry of the codon-anticodon helix in the decoding center using the universally conserved 16S rRNA bases G530, A1492 and A1493. These bases form hydrogen bonds with the 2’-hydroxyl groups of the codon-anticodon helix, which are expected to be disrupted with a near-cognate codon-anticodon helix. However, a recent structural study showed that G530, A1492 and A1493 form hydrogen bonds in an identical manner with both cognate and near-cognate codon-anticodon helices. To understand how the ribosome discriminates between cognate and near-cognate tRNAs, we made 2’-deoxynucleotide and 2’-fluoro substituted mRNAs, which disrupt the hydrogen bonds between the A site codon and G530, A1492 and A1493. Our results show that multiple 2’-deoxynucleotide substitutions in the mRNA substantially inhibit tRNA selection, whereas multiple 2’-fluoro substitutions in the mRNA have only modest effects on tRNA selection. Furthermore, the miscoding antibiotics paromomycin and streptomycin rescue the defects in tRNA selection with the multiple 2’-deoxynucleotide substituted mRNA. These results suggest that steric complementarity in the decoding center is more important than the hydrogen bonds between the A site codon and G530, A1492 and A1493 for tRNA selection.
Keywords: protein synthesis, elongation factor Tu, kinetics, peptide bond, GTP hydrolysis
Introduction
During protein synthesis the ribosome selects aminoacyl tRNAs corresponding to the codons in the mRNA. The aminoacyl-tRNA binds as a ternary complex with elongation factor Tu (EF-Tu) and GTP to the aminoacyl site (A site) in the ribosome. The ribosome selects the cognate EF-Tu ternary complex with an error frequency of 1 × 10−3 to 1 × 10−4 1; 2. To achieve this level of accuracy in tRNA selection, the ribosome uses a kinetic proofreading mechanism consisting of two selection steps designated “initial selection” and ‘proofreading”, which are irreversibly separated by GTP hydrolysis on EF-Tu 3; 4; 5; 6. More recent studies showed that the ribosome also uses an induced fit mechanism to increase the accuracy of tRNA selection 7; 8; 9. These studies showed that the ribosome accelerates the rates of GTP hydrolysis and tRNA accommodation when a cognate EF-Tu ternary complex binds. In contrast, when a non-cognate or near-cognate EF-Tu ternary complex binds to the ribosome, the rates of GTP hydrolysis and tRNA accommodation are inhibited. Thus, the accuracy of tRNA selection is greatly improved by accelerating two critical forward reactions (GTP hydrolysis and tRNA accommodation) when the cognate tRNA binds to the ribosome.
Insight into how the ribosome may use an induced fit mechanism to accelerate the rates of GTP hydrolysis and tRNA accommodation was provided by X-ray crystallography 10. Crystal structures showed that when a cognate tRNA binds to the A site, universally conserved 16S rRNA bases G530, A1492 and A1493 undergo a conformational change that stabilize their interactions with the minor groove of the codon-anticodon helix (Figure 1a). Discrimination is achieved by G530, A1492 and A1493 precisely recognizing the Watson-Crick base pair geometry of the codon-anticodon helix by forming hydrogen bonds with the N3 of purine base, the O2 of pyrimidine base and the ribose 2’-hydroxyl groups (Figure 1a) 10. In addition, G530, A1492 and A1493 also interact by steric complementarity with the first and second base pairs of the codon-anticodon helix 10.
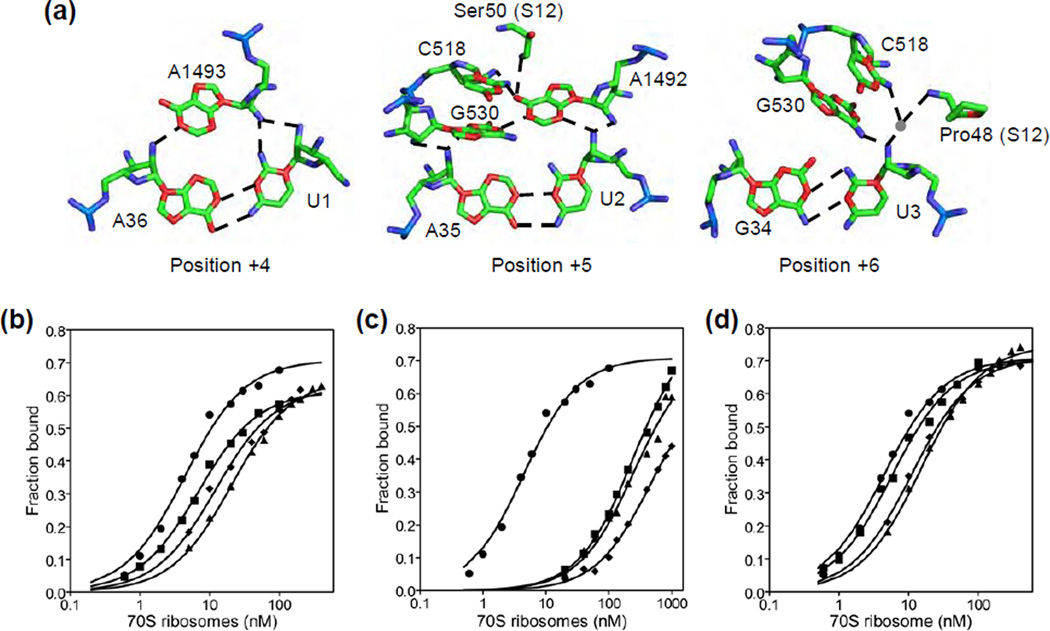
Figure 1. Interaction of the A site codon with the ribosome and equilibrium binding of EF-Tu ternary complex to the ribosome.
(a) Interaction of the mRNA at positions +4 to +6 with the decoding center (PDB ID: 1IBM). Shown are mRNA bases (U1, U2 and U3), tRNA bases (A36, A35 and G34), 16S rRNA bases (A1493, A1492, G530 and C518) and residues from ribosomal protein S12 (Ser50 and Pro48). Hydrogen bonds are represented by the dotted lines. (b to d) Representative equilibrium binding curves for EF-Tu (H84A)•Phe-tRNAPhe•GTP ternary complex binding to the ribosomal A site. Data are shown for the control mRNA (λ) and mRNAs with a single 2’-deoxynucleotide substitution at positions +4 (ν), +5 (σ), and +6 (υ). The data were fit to a one-site binding hyperbolic equation to determine KD. (c) Representative binding curves for the control mRNA (λ), mRNAs with two 2’-deoxynucleotide substitutions at positions +4 and +5 (ν) or +5 and +6 (σ) and an mRNA with three 2’-deoxynucleotide substitutions at positions +4, +5 and +6 (υ). (d) Representative binding curves for the control mRNA (λ), an mRNA with a single 2’-fluoro substitution at position +5 (ν), an mRNA with two 2’-fluoro substitutions at positions +5 and +6 (σ), and an mRNA with three 2’-fluoro substitutions at positions +4, +5 and +6 (υ).
Importantly, the binding of the cognate tRNA to the A site trigger a change in the structure of the 30S subunit from an open to a closed conformation (designated “domain closure”) 11. Domain closure involves the rotation of the 30S subunit head toward the shoulder and the subunit interface and of the shoulder toward the inter-subunit space and the platform region 11. Domain closure was proposed to accelerate GTP hydrolysis on EF-Tu and the accommodation of the cognate tRNA into the peptidyl transferase center 11; 12. By contrast, the anticodon of a non-cognate or near-cognate tRNA forms mismatches with the codon, which distorts the geometry of the codon-anticodon helix and precludes stable interactions with G530, A1492 and A1493 11. This in turn may inhibit domain closure and decrease the rates of GTP hydrolysis and tRNA accommodation.
However, recent crystal structures showed that G530, A1492, and A1493 form hydrogen bonds in an identical manner with cognate and near-cognate tRNAs in the A site 13; 14. Furthermore, near-cognate tRNAs also induced domain closure to a similar extent as the cognate tRNA 13; 14. Discrimination against near-cognate tRNAs was proposed to occur because domain closure forced the codon-anticodon helix with unpaired bases at the first or second position to adopt the geometry of Watson-Crick base pairs 14. Since this is energetically unfavorable, the near-cognate tRNA dissociates from the ribosome.
To determine the contribution of the hydrogen bonds between the codon and G530, A1492, and A1493 in tRNA selection, we synthesized a library of mRNAs with single or multiple 2’-deoxynucleotide and 2’-fluoro substitutions in the A site codon, which systematically disrupt these hydrogen bonds. We used equilibrium binding and pre-steady state kinetic methods to analyze the effects of the mRNA modifications on tRNA selection. Our results show that triple 2’-deoxynucleotide substitutions in the A site codon inhibits the rate of GTP hydrolysis by 30-fold and the rate of peptide bond formation by >4,400-fold. In contrast, triple 2’-fluoro substitutions in the A site codon inhibits the rate of GTP hydrolysis by 12-fold and the rate of peptide bond formation by 4-fold only. The >1000-fold increase in the rate of peptide bond formation with the triple 2’-fluoro substituted codon compared to the triple 2’-deoxynucleotide codon indicates that steric complementarity is more important than the hydrogen bonds between the codon and G530, A1492 and A1493 for tRNA selection by the ribosome. Furthermore, we show that paromomycin and streptomycin can rescue the defects in peptide bond formation caused by the triple 2’-deoxynucleotide substitutions in the codon by 2,700fold. Since streptomycin induces domain closure without stabilizing the interaction of A1492 and A1493 with the codon-anticodon helix 15, our results suggest that domain closure is important for tRNA accommodation on the ribosome.
Results
Binding of EF-Tu ternary complex to the ribosome is inhibited by multiple 2’-deoxynucleotide substitutions in the A site codon
To analyze the role of the hydrogen bonds formed by the 2’-hydroxyl groups in the A site codon in tRNA selection, we synthesized mRNAs with single, double or triple 2’-deoxynucleotide substitutions in the A site codon (positions +4 to +6). Previous studies have shown that 2’-deoxynucleotide substitutions in the A site codon inhibit the binding of aminoacylated and deacylated tRNA to the ribosomal A site 16; 17;18; 19. However, these binding studies only examined the affinity of the tRNA in the fully accommodated A/A state on the ribosome. To analyze the effects of the 2’- deoxynucleotide substitutions in the A site codon during initial selection, it is important to measure the binding affinity of the ternary complex in the A/T state on the ribosome. We, therefore, analyzed the binding of EF-Tu ternary complex in the A/T state to the ribosome. We used a mutant EF-Tu with the catalytic histidine 84 changed to alanine to inhibit GTP hydrolysis 20; 21. Since EF-Tu (His84Ala) ternary complex cannot induce GTP hydrolysis in the time frame of the filter-binding assay, the ternary complex is in the A/T state on the ribosome. Ternary complex was formed with EF-Tu (His84Ala), GTP and Phe-[32P]-tRNAPhe and filter-binding experiments were carried out with varying concentrations of ribosome complex. The equilibrium dissociation constant (KD) with the unmodified control mRNA was 4.5 ± 0.3 nM, whereas the KD with mRNAs having a single 2’-deoxynucleotide substitution in the A site codon was increased by 1.6- to 4.2-fold (Table 1, and Figure 1b). The KD with mRNAs having two and three 2’-deoxynucleotide substitutions in the A site codon were increased by 63-fold and 110-fold, respectively (Figure 1c). The binding data show that single 2’-deoxynucloeotide substitutions in the A site codon only modestly inhibit the binding of EF-Tu ternary complex to the ribosome whereas, multiple 2’-deoxynucleotide substitutions in the A site codon are not thermodynamically additive but have a more drastic effect.
Table 1.
Binding and the rate of peptide bond for the different mRNAs
| mRNA | KD (nM) | kpep (s−1) | Fp |
|---|---|---|---|
| Control | 4.5 + 0.3 | 8.8 + 1.4 | 0.94 + 0.09 |
| +4D | 7.3 + 0.8 | 6.5 + 1.4 | 0.92 + 0.10 |
| +5D | 18.9 + 3.7 | 5.2 + 1.0 | 0.85 + 0.11 |
| +6D | 12.9 + 1.5 | 5.9 + 0.1 | 0.86 + 0.13 |
| +4D+5D | 274 + 30 | 3.4 + 0.3 | 0.51 +0.05 |
| +5D+6D | 278 + 35 | 1.4 + 0.2 | 0.35 + 0.04 |
| +4D+5D+6D | 445 + 33 | 0.002 + 0.001 | <0.02 |
| +5F | 5.8 + 0.5 | 10.5 + 0.2 | 0.96 + 0.02 |
| +5F+6F | 14.4 + 2.0 | 4.2 + 0.6 | 0.97 + 0.03 |
| +4F+5F+6F | 11.0 + 1.3 | 2.0 + 0.1 | 0.55 + 0.03 |
Fp, fraction of fMet-Phe dipeptide formed.
Multiple 2’-fluoro substitutions in the A site codon have small effects on EF-Tu ternary complex binding to the ribosome
The incorporation of a 2'-deoxynucleotide not only abolishes hydrogen bonding but also may disrupt the precise A-minor interactions in the decoding center because of the removal of the oxygen atom from the ribose sugar. Studies have shown that 2'-fluoro substitutions are less disruptive than 2'-deoxynucleotide substitutions in structured RNA 22. The 2’-fluoro substitution prefers the c3’-endo sugar pucker found in RNA 23; 24 and the fluoro atom may participate as a weak hydrogen bond acceptor 22; 25; 26; 27; 28; 29;30. In the decoding center, the 2’-hydroxyl groups of the A site codon participate mainly as hydrogen bond donors (Figure 1a). Therefore, the 2’-fluoro substitutions in the A site codon should disrupt the hydrogen bonds formed with G530, A1492 and A1493 without significantly interfering with steric complementarity.
To distinguish between theses possibilities, we synthesized mRNAs with single (+5F), double (+5F+6F) or triple (+4F+5F+6F) 2’-fluoro substitutions in the A site codon. Filter-binding experiments showed that the KD with mRNA+5F was similar to the control mRNA (Table 1 and Figure 1d). The KD with mRNAs having two or three 2’-fluoro substitutions in the A site codon were increased by 2.5 to 3-fold compared to the control mRNA. Thus, ribosomes programmed with mRNAs having multiple 2’-fluoro substitutions in the A site codon showed dramatically improved binding affinity for EF-Tu ternary complex compared to mRNAs having multiple 2’-deoxynucleotide substitutions (Table 1). The rescue by the 2’-fluoro analogs indicate that the favorable steric complementarity of the A site codon is more important for binding EF-Tu ternary complex to the ribosome than the ability of the 2’-hydroxyl groups to form hydrogen bonds.
GTP hydrolysis by EF-Tu is inhibited by 2’-deoxynucleotide and 2’-fluoro substitutions in the A site codon
According to the kinetic model for tRNA selection, codon recognition by the cognate EF-Tu ternary complex triggers GTP hydrolysis on EF-Tu; whereas, non-cognate ternary complex fails to trigger GTP hydrolysis and dissociate from the ribosome 7; 9. To determine whether disrupting the interactions between the codon and the ribosome affects GTP hydrolysis, we measured the pre-steady state rate of GTP hydrolysis on EF-Tu with ribosomes having 2’-deoxynucleotide substitutions in the A site codon. GTP hydrolysis experiments were performed with a limiting concentration of EF-Tu ternary complex (0.1 µM final conc.) and a large excess of ribosomal complex (1.25 µM final conc.) to saturate the binding of the EF-Tu ternary complex to the ribosome (Figure S1) 31. The rate of GTP hydrolysis was reduced by 2- to 4-fold with the single 2’-deoxynucleotide substituted mRNAs (Figure 2a) (kobs = 20.2 ± 1.2 s−1, 10.0 ± 2.7 s−1, 5.2 ± 0.9 s−1, and 7.2 ± 1.5 s−1 for control mRNA, mRNA+4D, mRNA+5D, and mRNA+6D, respectively). The rate of GTP hydrolysis was reduced by 16-, 22-, and 50-fold with mRNA+4D+5D (kobs = 1.3 ± 0.1 s−1), mRNA+5D+6D (kobs = 0.9 ± 0.3 s−1), and mRNA+4D+5D+6D (kobs = 0.4 ± 0.2 s−1), respectively (Figure 2b and Figure S2). In contrast to the rate of GTP hydrolysis, the extent of GTP hydrolyzed by EF-Tu was similar in all cases. These results show that multiple 2’-deoxynucleotide substitutions in the A site codon inhibit GTP hydrolysis on EF-Tu possibly due to unfavorable interactions between the codon and the ribosome.
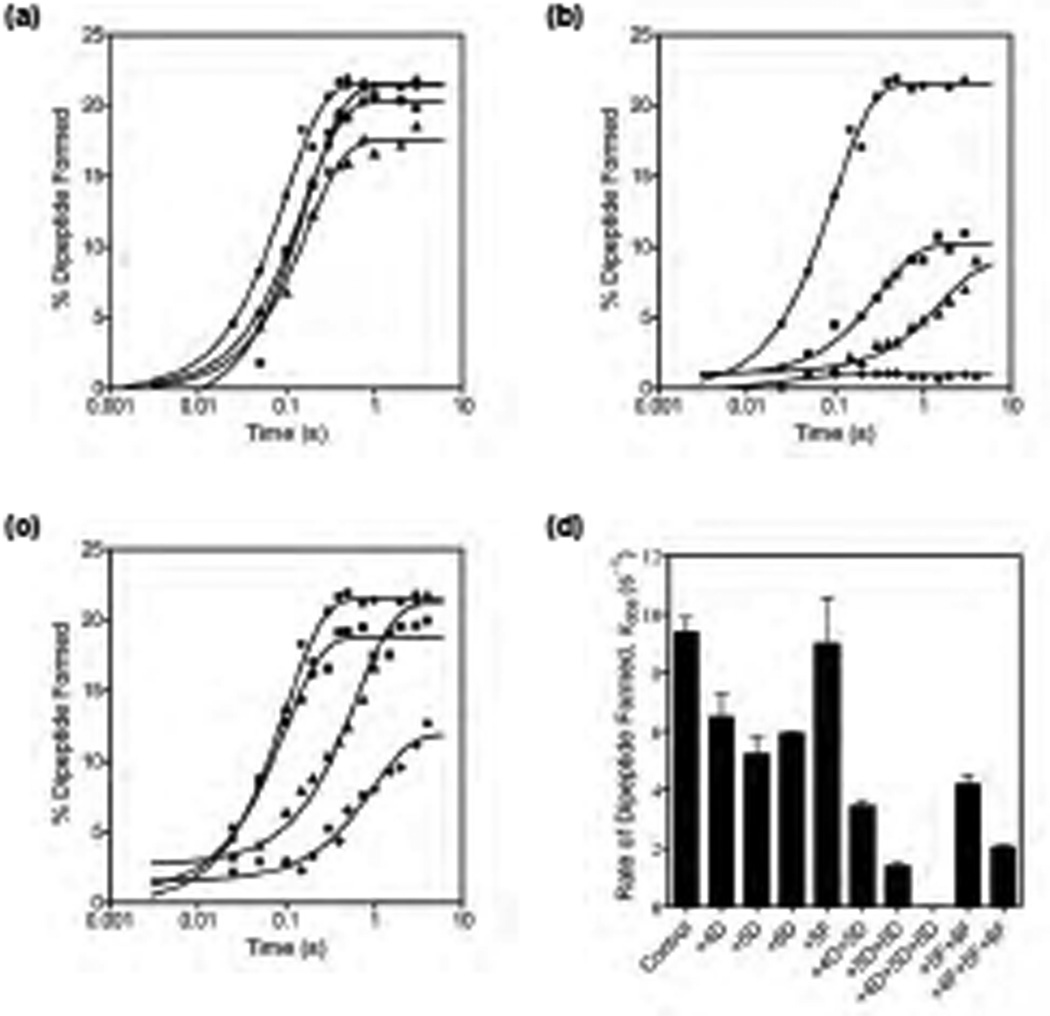
Figure 2. Rate of GTP hydrolysis by EF-Tu ternary complex.
(a) Representative time course for ribosome-dependent GTP hydrolysis by EF-Tu ternary complex. Data are shown for the control mRNA (λ) and mRNAs with a single 2’-deoxynucleotide substitution at positions +4 (ν), +5 (σ), and +6 (υ). The data were fit to a single exponential equation to obtain the rate of GTP hydrolysis. (b) Representative GTP hydrolysis data for the control mRNA (λ), mRNAs with two 2’-deoxynucleotide substitutions at positions +4 and +5 (ν) or +5 and +6 (σ) and an mRNA with three 2’-deoxynucleotide substitutions at positions +4, +5 and +6 (υ). (c) Representative GTP hydrolysis data for the control mRNA (λ), an mRNA with a single 2’-fluoro substitution at position +5 (ν), an mRNA with two 2’-fluoro substitutions at positions +5 and +6 (σ), and an mRNA with three 2’-fluoro substitutions at positions +4, +5 and +6 (υ). (d) Graph showing the rate of GTP hydrolysis at varying concentration of ribosomes for the control mRNA (λ), an mRNA with two 2’-deoxynucleotide substitutions at positions +5 and +6 (σ), an mRNA with three 2’-deoxynucleotide substitutions at positions +4, +5 and +6 (ν), and an mRNA with three 2’-fluoro substitutions at positions +4, +5 and +6 (υ).
To find out whether the 2’-fluoro substitutions are better tolerated by the ribosome, we determined the rate of GTP hydrolysis on EF-Tu with ribosomes programmed with mRNAs having the 2’-fluoro substitutions in the A site codon (Figure S3). The rate of GTP hydrolysis was reduced by 3- to 7-fold with mRNA+5F (kobs = 6.4 ± 1.9 s−1), mRNA+5F+6F (kobs = 4.1 ± 0.3 s−1), and mRNA+4F+5F+6F (kobs = 3.1 ± 0.6 s−1) indicating that the 2’-fluoro substitutions are less disruptive than the 2’-deoxynucleotide substitutions in the A site codon (Figure 2c). To estimate the saturation rate of GTP hydrolysis (kGTP), we measured the rate of GTP hydrolysis at varying concentrations of ribosome complex and fit the data to a hyperbolic equation. Since large amounts of ribosomes, tRNAs, mRNAs, etc. are required for this, we determined kGTP only for the following representative mRNAs: control mRNA, mRNA+5D+6D, mRNA+4D+5D+6D, and mRNA+4F+5F+6F (Figure 2d). The kGTP was 54 s−1 for the control mRNA, 4 s−1 for mRNA+5D+6D, 2 s−1 for mRNA+4D+5D+6D, and 4 s−1 for mRNA+4F+5F+6F. The kGTP is substantially reduced with the modified mRNAs even at ribosome concentrations that are higher than the KD for ternary complex binding to the ribosome. It is possible that the “induced fit” required for accelerating GTP hydrolysis on EF-Tu is compromised with these modified mRNAs bound to the ribosome 7; 8; 9.
Multiple 2’-deoxynucleotide substitutions in the A site codon drastically inhibit peptide bond formation
Following GTP hydrolysis on EF-Tu, the aminoacyl tRNA is accommodated into the 50S subunit A site and participates in peptide bond formation. To determine whether the hydrogen bonds formed by the 2’-hydroxyl groups in the A site codon with G530, A1492 and A1493 are important for tRNA accommodation, we measured the rate of peptide bond formation with mRNAs having 2’-deoxynucleotide or 2’ fluoro substitutions (Figure S4) 31. Again, we used a limiting concentration of EF-Tu ternary complex (0.25 µM final conc.) and a large excess of the ribosome complex (1.25 µM final conc.) to overcome the binding defects observed with the 2’-deoxynucleotide substituted mRNAs. The maximum extent of dipeptide formed was expected to be 20% because we used 5-fold lower concentration of EF-Tu ternary complex relative to the ribosome complex (see Materials and Methods). The rate of peptide bond formation with the control mRNA was 8.8 ± 1.4 s−1, which is similar to the saturation rate (kpep) reported previously at 20 °C 9; 32; 33. The rates of peptide bond formation with single 2’-deoxynucleotide substituted mRNAs were similar to the control mRNA (Table 1 and Figure 3a). In contrast, mRNAs with double 2’-deoxynucleotide substitutions showed a 2.6 to 6-fold reduced rate of peptide bond formation (Figure 3b and Figure S5). Additionally, the extent of dipeptide formed was reduced by 50% to 65% compared to the control mRNA (Table 1). This indicated that after GTP hydrolysis on EF-Tu ≈50% of the A site tRNA dissociated from the ribosome during the accommodation step. Finally, the extent of dipeptide formed was less than 2% in 10 seconds with the triple 2’- deoxynucleotide substituted mRNA (Figure 3b). Therefore, we manually measured the rate of peptide bond formation for the triple 2’-deoxynucleotide substituted mRNA (Figure S5 e–f). Our conservative estimation is that mRNA with the triple 2’-deoxynucleotide substitutions is at least 4,400-fold defective in the rate of peptide bond formation and is consistent with a previous report 32. Furthermore, the previous study showed that with the triple 2’-deoxynucleotide substituted mRNA the rate of peptide bond formation saturates at 0.5 µM ribosomes 32. Thus, 2’-deoxynucleotide substitutions at all three positions in the A site codon drastically inhibited the accommodation of the tRNA into the A site in the 50S subunit and the tRNAs were largely rejected by the ribosome.
Figure 3. Rate of peptide bond formation.
(a) Representative time course showing the kinetics of dipeptide formation. Ribosomes contained f[35S]Met-tRNAfMet in the P site and were reacted with EF-Tu•Phe-tRNAPhe•GTP ternary complex. Data are shown for the control mRNA (λ) and mRNAs with a single 2’-deoxynucleotide substitution at positions +4 (ν), +5 (σ), and +6 (λ). The data were fit to a single exponential equation to obtain the rate of peptide bond formation. (b) Representative peptide bond formation data for the control mRNA (λ), mRNAs with two 2’-deoxynucleotide substitutions at positions +4 and +5 (ν) or +5 and +6 (σ) and an mRNA with three 2’-deoxynucleotide substitutions at positions +4, +5 and +6 (υ). (c) Representative peptide bond formation data for the control mRNA (λ), an mRNA with a single 2’-fluoro substitution at position +5 (ν), an mRNA with two 2’-fluoro substitutions at positions +5 and +6 (σ), and an mRNA with three 2’-fluoro substitutions at positions +4, +5 and +6 (υ). (d) Bar graph showing the rate of peptide bond formation with the 2′-deoxynucleotide and the 2′-fluoro substituted mRNAs. Error bars represent s.d. from two experiments.
Multiple 2’-fluoro substitutions in the A site codon moderately inhibit peptide bond formation
To determine whether the 2’-fluoro substitutions in the A site codon affect tRNA accommodation, we measured the rate of peptide bond formation with the 2’-fluoro substituted mRNAs (Figure S6). The rate of peptide bond formation with a single 2’-fluoro substitution at position +5 in the mRNA was similar to the control mRNA (Table 1 and Figure 3c). mRNA with the two 2’-fluoro substitutions at positions +5 and +6 showed a 2-fold reduced rate of peptide bond formation (Figure 3c). Surprisingly, the mRNA with the three 2’-fluoro substitutions at positions +4, +5 and +6 showed only a 4-fold reduced rate of peptide bond formation and the extent of dipeptide formed was reduced by 45% compared to the control mRNA (Figure 3c). This is at least a 1000-fold increase in the rate of peptide bond formation relative to the mRNA with the three 2’-deoxynucleotide substitutions in the A site codon (Figure 3d). This remarkable recovery both in the rate of peptide bond formation and in the extent of dipeptides formed with mRNA+4F+5F+6F indicate that steric complementarity of G530, A1492 and A1493 with the codon-anticodon helix are critical for the accommodation of the tRNA into the A site and for peptide bond formation.
Paromomycin and streptomycin restores the rates of GTP hydrolysis by EF-Tu and peptide bond formation
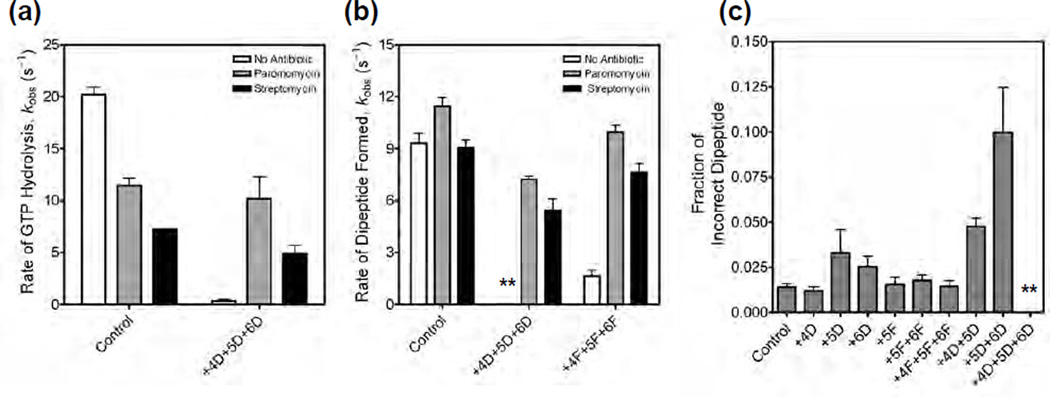
We wondered to what extent could miscoding antibiotics compensate for the loss of favorable interactions with the 2’-deoxynucleotide substituted A site codons. Antibiotics such as paromomycin, neomycin, and streptomycin are known to reduce the fidelity of translation 34. In addition, some of these miscoding antibiotics have been shown to stimulate the translation of single-stranded DNA 35; 36, potentially by stabilizing codon-anticodon interactions in the A site. Paromomycin binds to helix 44 in the decoding center and displaces A1492 and A1493 toward the codon-anticodon helix in the A site 10; 15. Paromomycin, therefore, makes the interaction of A1492 and A1493 with the codon-anticodon helix of the near-cognate tRNA energetically more favorable and also induces domain closure explaining the reduced fidelity of translation 10. Consistent with this proposal, kinetic studies showed that paromomycin accelerates the rates of GTP hydrolysis and accommodation of near-cognate tRNAs 37. We determined the rate of GTP hydrolysis in the presence of paromomycin with the control mRNA and the triple 2’-deoxynucleotide substituted mRNA (Figure 4a and Figure S7). In the presence of paromomycin the rate of GTP hydrolysis with the triple 2’-deoxynucleotide substituted mRNA (kobs = 10.2 ± 3 s−1) was similar to the control mRNA (kobs = 11.5 ± 1 s−1). We also tested streptomycin, another miscoding antibiotic that binds to a site that is distinct from the paromomycin-binding site on the ribosome 15. Streptomycin is proposed to reduce the fidelity of translation by inducing “domain closure” with near-cognate tRNAs 15; 38. We measured the rate of GTP hydrolysis in the presence of streptomycin (Figure S8). Streptomycin also increased the rate of GTP hydrolysis with the triple 2’-deoxynucleotide substituted mRNA (kobs = 4.9 ± 1 s−1) to almost the same rate as with the control mRNA (kobs = 7.3 ± 0.1 s−1) (Figure 4a). We next tested the rate of peptide bond formation in the presence of paromomycin and streptomycin (Figure S9 and Figure S10). Both miscoding antibiotics substantially improved the rate and the extent of peptide formation with the triple 2’-deoxynucleotide and the triple 2’-fluoro substituted mRNAs (Table 2 and Figure 4b). These results showed that paromomycin and streptomycin can compensate for the unfavorable interactions that the triple 2’-deoxynucleotide and 2’-fluoro substituted mRNAs make with the decoding center.
Figure 4. Effect of paromomycin and streptomycin on the rates of GTP hydrolysis and peptide bond formation.
(a) Bar graph showing the rates of GTP hydrolysis by EF-Tu ternary complex with the control mRNA and with the mRNA having three 2’-deoxynucleotide substitutions at positions +4, +5 and +6. Experiments were done in the absence of antibiotics (white bar), in the presence of paromomycin (grey bar) or in the presence of streptomycin (black bar). (b) Bar graph showing the rates of peptide bond formation with the control mRNA, with the mRNA having three 2’-deoxynucleotide substitutions at positions +4, +5 and +6, and with the mRNA having three 2’-fluoro substitutions at positions +4, +5 and +6. Labels for the bar graph are as indicated above. The asterisks are used to indicate that in the absence of antibiotics the rate of peptide bond formation was very slow with the triple 2’-deoxynucleotide substituted mRNA. (c) Bar graph showing the error frequency with 2’ -deoxynucleotide and 2’-fluoro substituted mRNAs. The asterisks are used to indicate that only negligible amounts of dipeptides were formed with the triple 2’-deoxynucleotide substituted mRNA. In all cases, the error bars represent s.d. from at least two experiments.
Table 2.
Rate of peptide bond formation with antibiotics
| mRNA | kpep (s−1) | Fp |
|---|---|---|
| Control (Par) | 11.4 + 0.7 | 0.85 + 0.13 |
| Control (Str) | 9.0 + 0.6 | 0.95 + 0.28 |
| +4D+5D+6D (Par) | 7.2 + 0.3 | 0.84 + 0.01 |
| +4D+5D+6D (Str) | 5.4 + 0.9 | 0.78 + 0.03 |
| +4F+5F+6F (Par) | 9.9 + 0.6 | 0.95 + 0.09 |
| +4F+5F+6F (Str) | 7.7 + 0.7 | 1.07 + 0.03 |
Fp, fraction of fMet-Phe dipeptide formed. Paromomycin (Par). Streptomycin (Str).
Fidelity of protein synthesis is decreased by 2’-deoxynucleotide substitutions in the A site codon
To determine whether the loss of the hydrogen bonds and steric complementarity between the A site codon and the ribosome lowers the fidelity of protein synthesis, we measured the error frequency of translation. Ribosomes programmed with the different mRNAs and with f[35S]Met-tRNAfMet in the P site were reacted with a mixture of aminoacylated tRNAs (Figure S11) 31. The error frequency was calculated from the ratio of fMet-Leu (incorrect dipeptide) to fMet-Phe (correct dipeptide) + fMet-Leu (incorrect dipeptide) formed. The error frequency with mRNA+4D, mRNA+5F, mRNA+5F+6F and mRNA+4F+5F+6F were similar to the control mRNA (Figure 4c). mRNA+5D and mRNA+6D showed about a 2-fold higher error frequency. Interestingly, mRNA+4D+5D and mRNA+5D+6D showed 3-fold and 7-fold higher error frequency, respectively, than the control mRNA. The increase in the error frequency with mRNA+4D+5D and mRNA+5D+6D is due to the reduced formation of the correct fMet-Phe dipeptide. We could not determine error frequency with mRNA+4D+5D+6D because the amount of correct fMet-Phe dipeptide formed was negligible. These results show that disrupting the interactions between the codon and the A site lowers the fidelity of protein synthesis by decreasing the efficiency with which the ribosome accepts the cognate tRNA.
Discussion
The mechanism of tRNA selection by the ribosome has been investigated for more than 30 years. Recent X-ray crystal structures provide a structural explanation for how the ribosome is able to discriminate between a cognate and a near-cognate tRNA. Crystal structures showed that the ribosome recognizes the geometry of the cognate codon-anticodon helix by using universally conserved bases G530, A1493 and A 1493 in the 16S rRNA (Figure 1a) 10; 39. The interaction of G530, A1492, and A1493 with the cognate codon-anticodon helix induces domain closure, GTP hydrolysis by EF-Tu, and the accommodation of the cognate tRNA into the ribosome. In contrast, G530, A1492, and A1493 fail to interact stably with near-cognate codon-anticodon helix and fail to induce domain closure causing the near-cognate tRNA to dissociate from the ribosome 11; 40. However, more recent crystal structures show that G530, A1492, and A1493 interact with the cognate and near-cognate codon-anticodon helix in an identical manner 13; 14. In addition, domain closure is induced even when a near-cognate tRNA binds to the A site, which forces the bases in the codon-anticodon helix to adopt the geometry of Watson-Crick base pairs. Since this is energetically unfavorable, it causes the near-cognate tRNA to dissociate from the ribosome. Thus, there are now two different structural models that explain the mechanism of tRNA selection by the ribosome.
To understand the mechanism of tRNA selection, we focused on the interactions between the 2’-hydroxyl groups of the A site codon and bases G530, A1492 and A1493. Previous studies showed that single 2’-deoxynucleotide substitutions in A site codon inhibited the binding of tRNA to the A site by 3- to 4-fold 17; 18, while 2’-deoxynucleotide substitution at all three positions in the A site codon reduced the tRNA binding affinity by 10-fold 16. Since the A site tRNA does not directly contact the 2’-hydroxyl groups in the codon, these results are consistent with the idea that the loss of the 2’-hydroxyl groups from the A site codon inhibit “domain closure” and weakens the interaction of the tRNA with the ribosomal A site 17. However, no studies have been carried out to systematically examine the role of these 2’-hydroxyl groups in the A site codon in individual steps of the tRNA selection pathway.
The first step in the tRNA selection pathway is the interaction of the EF-Tu ternary complex with the ribosome. We determined the binding affinity of EF-Tu ternary complex in the A/T state on the ribosome. Our studies showed that single 2’- deoxynucleotide substitutions in the A site codon inhibit the binding of EF-Tu ternary complex modestly, whereas double or triple 2’-deoxynucleotide substitutions drastically inhibit the binding of EF-Tu ternary complex to the ribosome (KD is 100-fold higher with the triple 2’-deoxynucleotide substituted mRNA compared to the control mRNA). Therefore, the tRNA bound to EF-Tu•GTP, in the A/T state on the ribosome, is more sensitive to 2’-deoxynucleotide substitutions in the A site codon compared to the previous tRNA binding studies, which analyzed the fully accommodated A/A state 16; 17; 18. A possible explanation for this difference is that the tRNA bound to EF-Tu•GTP in the A/T state on the ribosome is in a strained conformation compared to the fully accommodated tRNA in the A/A state (see below) 41. Interestingly, the KD of EF-Tu ternary complex binding to the ribosome is increased by only 2-fold with the triple 2’-fluoro substituted A site codon. This suggests that steric complementarity in the decoding center dominates the interaction of the EF-Tu ternary complex with the ribosome and is consistent with the hypothesis proposed by Potapov 42.
We next analyzed the importance of the 2’-hydroxyl groups in the A site codon for two key steps in tRNA selection: GTPase activation on EF-Tu and tRNA accommodation. Since GTPase activation is rate-limiting for GTP hydrolysis, and tRNA accommodation is rate-limiting for peptide bond formation, we measured these chemical steps using well-established pre-steady state kinetic assays 7; 9. Our studies showed that single 2’-deoxynucleotide or 2’-fluoro substitutions in the A site codon only modestly inhibit the rates of GTP hydrolysis and peptide bond formation. However, triple 2’-deoxynucleotide substitutions in the A site codon inhibited the rates of GTP hydrolysis and peptide bond formation by 30-fold and >4,400-fold, respectively. In contrast, triple 2’-fluoro substitutions in the A site codon inhibited the rates of GTP hydrolysis and peptide bond formation by 12-fold and 4-fold, respectively. The significant recovery with the triple 2’-fluoro substituted A site codon suggests that the hydrogen bonds formed by the 2’-hydroxyl groups in the A site codon with G530, A1492 and A1493 are not the major determinant for GTP hydrolysis and peptide bond formation. Rather, tRNA selection mainly depends on G530, A1492 and A1493 recognizing the geometry of the codon-anticodon helix by steric complementarity (using favorable van der Waals interaction, hydrophobic contacts and base stacking). Indeed, previous studies have shown that the A-minor interactions critically depend on steric complementarity between adenine and the minor groove, which provide optimal van der Waals contact, hydrogen bonding, and a hydrophobic environment creating a highly energetically favorable interaction43; 44; 45; 46; 47
Interestingly, the extent of GTP hydrolyzed is similar with all the mRNAs tested whereas, the extent of dipeptide formed is severely reduced especially with the triple 2’-deoxynucleotide substituted A site codon (< 2% fMet-Phe dipeptide formed) (Figure 3b). This shows that the aminoacyl tRNA is rejected after GTP hydrolysis during the accommodation of the tRNA. Surprisingly, in the presence of paromomycin or streptomycin, both the extent and the rates of GTP hydrolysis and peptide bond formation were similar with the control mRNA and the triple 2’-deoxynucleotide substituted mRNA (Table 2). The complete recovery in the extent of fMet-Phe formed and the ≈3000-fold improvement in the rate of peptide bond formation with the triple 2’-deoxynucleotide substituted mRNA in the presence of paromomycin or streptomycin provide new insights into the mechanism of tRNA selection. Paromomycin stabilizes the flipped out conformation of A1492 and A1493 10. However, streptomycin does not stabilize the flipped out conformation of A1492 and A1493 15. On the other hand, both paromomycin (with a tRNA in the A site) and streptomycin (even without a tRNA in the A site) induce domain closure 10; 11; 15. Since streptomycin in effect uncouples codon-anticodon recognition from domain closure, it suggests that the improvement in the rates of GTP hydrolysis and peptide bond formation with the miscoding antibiotics are due to the stabilization of the domain closed state of the ribosome. Thus, domain closure is critical for preventing the rejection of the tRNA with the triple 2’-deoxynucleotide substituted A site codon during the proofreading step.
Our results show that steric complementarity by G530, A1492, and A1493 with the cognate codon-anticodon complex and domain closure play an active role in tRNA selection and are consistent with the model proposed by Ramakrishnan and co-workers 10; 11. We speculate that the increased rate of rejection for the near-cognate ternary complex during proofreading is largely because of the failure to induce domain closure. Both cognate and near cognate tRNAs are in a strained configuration when bound to EF-Tu•GTP on the ribosome (in the A/T state) 40; 41; 48; 49; 50. Nonetheless, domain closure occurs only with the cognate tRNA and it causes a tightening of the decoding center around the anticodon arm of the tRNA 11; 40. GTP hydrolysis on EF-Tu and the dissociation of EF-Tu•GDP from the ribosome allows the acceptor arm of the cognate tRNA to swing into the A site on the 50S subunit, which relaxes the tRNA into its canonical structure 48. In contrast, due to the lack of steric complementarity in the decoding center and the absence of domain closure, the interaction of the near-cognate tRNA with the 30S subunit is weakened. This may allow the near-cognate tRNA to relax from the A/T state without coupling it to the movement of the acceptor arm into the A site on the 50S subunit resulting in the rejection of the near-cognate tRNA from the ribosome.
Materials and Methods
Preparation of ribosomes, mRNAs, tRNAs and EF-Tu
Tight-couple ribosomes were purified from E. coli MRE600 and washed in high salt buffer as described 31. Synthetic mRNAs with the following sequence: 5’-AAGGAGGUAAAAAUGUUUGCU-3’, where the underlined nucleotides correspond to the A site codon (positions +4 to +6) were purchased from Dharmacon. 2′-deoxynucleotide or 2′-fluoro substitutions were incorporated during synthesis at positions +4 to +6 in the mRNAs. E. coli tRNAPhe was purified as described previously 31. EF-Tu, EF-Tu (H84A) and nucleotide-free EF-Tu were purified using the IMPACT-CN system according to the supplier's protocol (New England Biolabs) and as described 31. Aminoacylation of tRNAfMet and tRNAPhe were performed using purified E. coli histidine-tagged synthetase, essentially as described 51. Formylation of initiator tRNAfMet was performed as described 51. The aminoacylated tRNAs were purified by HPLC on a C18 reverse phase column 51. The extent of aminoacylation was verified by acid gel electrophoresis, and the level of aminoacylation was greater than 95%. f[35S]Met-tRNAfMet was prepared as described 52.
Equilibrium binding of EF-Tu ternary complex to the ribosomal A site
E. coli tRNAPhe was 32P-labeled at the 3’ end using [α-32P] ATP and tRNA nucleotidyl transferase as described 53. Equilibrium binding of EF-Tu ternary complex to the A site was determined as described previously 54. Filter binding experiments were performed in buffer A (50 mM Tris-HCl pH 7.5, 70 mM NH4Cl, 30 mM KCl, 15 mM MgCl2, 0.5 mM spermidine, 8 mM putrescine and 2 mM DTT) so that the endpoints for binding reached 70% to 80% with the 2’-deoxynucleotide substituted mRNAs 55. Briefly, initiation complexes were prepared by incubating activated 70S ribosomes, mRNA (10-fold excess over ribosome) and tRNAfMet (5-fold excess over ribosome) at 37 °C for 30 min. The initiation complex was diluted to give a range of concentrations (0.1 to 1000 nM as shown in Figure 1) using buffer A. 15 µl of each initiation complex dilution was transferred to a 96 well conical bottom plate (Nunc). EF-Tu (H84A) ternary complex was prepared by combining EF-Tu (H84A) (2 nM), GTP (1 mM), phosphoenol pyruvate (3 mM) and pyruvate kinase (0.25 µg/µl) in buffer A and incubating at 37 °C for 30 min and then mixed with 3’ 32P-labeled Phe-tRNAPhe (0.2 nM). The ternary complex reaction mix was incubated at 37 °C for 5 min and then placed on ice. 15 µl of ternary complex was added to the initiation complexes present in the 96 well plate (final volume 30 µl), mixed and incubated at room temperature for 1 min. 25 µl of the final reaction mix was filtered through a modified 96 well filtration apparatus having a nitrocellulose membrane (Osmonics 0.45 µm) at the top and a nylon membrane (Amersham 0.45 µm) at the bottom 56. The filters were washed three times with 100 µl of buffer A per well. The membranes were dried and quantified with a phosphorimager (BioRad). All binding experiments were independently repeated more than three times. The equilibrium dissociation constant (KD) was determined by fitting the binding data to a one-site binding hyperbolic equation (GraphPad Prism).
Kinetics of GTP hydrolysis by EF-Tu ternary complex
GTP hydrolysis experiments were performed in high-fidelity buffer B (50 mM Tris-HCl pH 7.5, 70 mM NH4Cl, 30 mM KCl, 3.5 mM MgCl2, 0.5 mM spermine, 8 mM putrescine, and 2 mM DTT), essentially as described previously 31. In a typical experiment, initiation complexes were prepared in buffer B by incubating activated 70S ribosomes (2.5 µM), mRNA (5 µM) and tRNAfMet (3.75 µM) at 37 °C for 10 min. Ternary complex was prepared by mixing nucleotide-free EF-Tu (1 µM), [γ-32P] GTP (0.2 µM) and HPLC purified Phe-tRNAPhe (1 µM) and incubating at 37 °C for 5 min. To determine the rate of GTP hydrolysis 15 µl of 70S initiation complex (2.5 µM) was rapidly mixed with 15 µl of EF-Tu•GTP•Phe-tRNAPhe ternary complex (0.20 µM) and quenched with 15 µl of 40% formic acid in a quench-flow instrument (µQFM-400, BioLogic). The concentration of ribosome and ternary complexes were 1.25 µM and 100 nM, respectively, after mixing. To determine the saturation rate of GTP hydrolysis, the concentration of the ribosome was varied as indicated in the figure. Experiments with streptomycin (Sigma) and paromomycin (Sigma) were performed by adding 400 µM antibiotics to the initiation complex and incubating for 10 minutes at room temperature before performing quench flow experiments. Free phosphate was separated by PEI-cellulose TLC in 0.5 M potassium phosphate (pH 3.5). The extent of hydrolysis was quantified with a phosphorimager (Bio-Rad). All experiments were repeated independently at least two times. The GTP hydrolysis rate was determined by fitting the data to a single exponential equation (GraphPad Prism).
Rate of peptide bond formation
The 70S initiation complex and the EF-Tu•GTP•Phe-tRNAPhe ternary complex were prepared as described previously 31. To determine the rate of dipeptide formation, 15 µl of 70S initiation complex (2.5 µM) was rapidly mixed with 15 µl of the reaction mix containing EF-Tu•GTP•Phe-tRNAPhe (0.5 µM) and quenched with 15 µl of 1M KOH in a quench-flow instrument (µQFM-400, BioLogic). In the case of the triple 2’-deoxynucleotide substituted mRNA, the reaction was very slow and the rate of dipeptide formation was determined manually. Experiments with streptomycin and paromomycin were performed by adding 400 µM antibiotics to the initiation complex and incubating for 10 min at room temperature before loading on to the quench-flow instrument. The dipeptide was resolved by electrophoresis on cellulose TLC plates and quantified using a phosphorimager (Bio-Rad). All experiments were repeated independently at least two times. The rate of peptide bond formation was determined by fitting the data to a single exponential equation (GraphPad Prism). Because we used 70S complex having f[35S]Met-tRNAfMet in the P site at 5-fold excess over the EF-Tu•GTP•Phe-tRNAPhe ternary complex, we expect the maximum extent of dipeptide formed to be 20%. Therefore, the fraction dipeptide formed (Fp) = % Dipeptide Formed/20.
Determination of error frequency
Fidelity experiments were done as described earlier 31. The dipeptides f[35S]Met-Phe and f[35S]Met-Leu were resolved by electrophoresis on cellulose TLC plates and quantified using a phosphorimager (Bio-Rad). The extent of misincorporation was estimated by calculating the ratio of f[35S]Met-Leu/f[35S]Met-Phe + f[35S]Met-Leu.
Supplementary Material
Highlights.
Mechanism of tRNA selection analyzed using modified mRNAs.
2’-deoxynucleotide substitutions in the mRNA inhibited key steps in tRNA selection.
2’-fluoro substitutions in the mRNA have only modest effects on tRNA selection.
Shape complementarity in the decoding center is critical for tRNA selection.
Acknowledgements
We thank Jack Kyte and Dan Herschlag for useful discussions and Ulrich Muller for comments on the manuscript. This work was supported by NIH Grant GM065265 to S.J.
Abbreviations used
- rRNA
ribosomal RNA
- EF-Tu
elongation factor Tu
- GTPase
guanosine triphosphatase
Biographies



Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kurland CG, Ehrenberg M. Optimization of translation accuracy. Prog Nucleic Acid Res Mol Biol. 1984;31:191–219. doi: 10.1016/s0079-6603(08)60378-5. [DOI] [PubMed] [Google Scholar]
- 2.Parker J. Errors and alternatives in reading the universal genetic code. Microbiol Rev. 1989;53:273–298. doi: 10.1128/mr.53.3.273-298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hopfield JJ. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc Natl Acad Sci U S A. 1974;71:4135–4139. doi: 10.1073/pnas.71.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ninio J. Kinetic amplification of enzyme discrimination. Biochimie. 1975;57:587–595. doi: 10.1016/s0300-9084(75)80139-8. [DOI] [PubMed] [Google Scholar]
- 5.Thompson RC, Stone PJ. Proofreading of the codon-anticodon interaction on ribosomes. Proc Natl Acad Sci U S A. 1977;74:198–202. doi: 10.1073/pnas.74.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson RC. EFTu provides an internal kinetic standard for translational accuracy. Trends Biochem Sci. 1988;13:91–93. doi: 10.1016/0968-0004(88)90047-3. [DOI] [PubMed] [Google Scholar]
- 7.Pape T, Wintermeyer W, Rodnina MV. Complete kinetic mechanism of elongation factor Tu-dependent binding of aminoacyl-tRNA to the A site of the E. coli ribosome. EMBO J. 1998;17:7490–7497. doi: 10.1093/emboj/17.24.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pape T, Wintermeyer W, Rodnina M. Induced fit in initial selection and proofreading of aminoacyl-tRNA on the ribosome. Embo J. 1999;18:3800–3807. doi: 10.1093/emboj/18.13.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gromadski KB, Rodnina MV. Kinetic determinants of high-fidelity tRNA discrimination on the ribosome. Mol Cell. 2004;13:191–200. doi: 10.1016/s1097-2765(04)00005-x. [DOI] [PubMed] [Google Scholar]
- 10.Ogle JM, Brodersen DE, Clemons WM, Jr, Tarry MJ, Carter AP, Ramakrishnan V. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science. 2001;292:897–892. doi: 10.1126/science.1060612. [DOI] [PubMed] [Google Scholar]
- 11.Ogle JM, Murphy FV, Tarry MJ, Ramakrishnan V. Selection of tRNA by the ribosome requires a transition from an open to a closed form. Cell. 2002;111:721–732. doi: 10.1016/s0092-8674(02)01086-3. [DOI] [PubMed] [Google Scholar]
- 12.Ogle JM, Carter AP, Ramakrishnan V. Insights into the decoding mechanism from recent ribosome structures. Trends Biochem Sci. 2003;28:259–266. doi: 10.1016/S0968-0004(03)00066-5. [DOI] [PubMed] [Google Scholar]
- 13.Jenner L, Demeshkina N, Yusupova G, Yusupov M. Structural rearrangements of the ribosome at the tRNA proofreading step. Nat Struct Mol Biol. 2010;17:1072–1078. doi: 10.1038/nsmb.1880. [DOI] [PubMed] [Google Scholar]
- 14.Demeshkina N, Jenner L, Westhof E, Yusupov M, Yusupova G. A new understanding of the decoding principle on the ribosome. Nature. 2012;484:256–259. doi: 10.1038/nature10913. [DOI] [PubMed] [Google Scholar]
- 15.Carter AP, Clemons WM, Brodersen DE, Morgan-Warren RJ, Wimberly BT, Ramakrishnan V. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature. 2000;407:340–348. doi: 10.1038/35030019. [DOI] [PubMed] [Google Scholar]
- 16.Potapov AP, Triana AF, Nierhaus KH. Ribosomal decoding processes at codons in the A or P sites depend differently on 2'-OH groups. J Biol Chem. 1995;270:17680–17684. doi: 10.1074/jbc.270.30.17680. [DOI] [PubMed] [Google Scholar]
- 17.Fahlman RP, Olejniczak M, Uhlenbeck OC. Quantitative analysis of deoxynucleotide substitutions in the codon-anticodon helix. J Mol Biol. 2006;355:887–892. doi: 10.1016/j.jmb.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Dale T, Fahlman RP, Olejniczak M, Uhlenbeck OC. Specificity of the ribosomal A site for aminoacyl-tRNAs. Nucleic Acids Res. 2009;37:1202–1210. doi: 10.1093/nar/gkn1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khade PK, Joseph S. Messenger RNA interactions in the decoding center control the rate of translocation. Nat Struct Mol Biol. 2011;18:1300–1302. doi: 10.1038/nsmb.2140. [DOI] [PubMed] [Google Scholar]
- 20.Scarano G, Krab IM, Bocchini V, Parmeggiani A. Relevance of histidine-84 in the elongation factor Tu GTPase activity and in poly(Phe) synthesis: its substitution by glutamine and alanine. FEBS Lett. 1995;365:214–218. doi: 10.1016/0014-5793(95)00469-p. [DOI] [PubMed] [Google Scholar]
- 21.Daviter T, Wieden HJ, Rodnina MV. Essential role of histidine 84 in elongation factor Tu for the chemical step of GTP hydrolysis on the ribosome. J Mol Biol. 2003;332:689–699. doi: 10.1016/s0022-2836(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 22.Forconi M, Schwans JP, Porecha RH, Sengupta RN, Piccirilli JA, Herschlag D. 2'-Fluoro substituents can mimic native 2'-hydroxyls within structured RNA. Chem Biol. 2011;18:949–954. doi: 10.1016/j.chembiol.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guschlbauer W, Jankowski K. Nucleoside conformation is determined by the electronegativity of the sugar substituent. Nucleic Acids Res. 1980;8:1421–1433. doi: 10.1093/nar/8.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uesugi S, Miki H, Ikehara M, Iwahashi H, Kyogoku Y. Linear Relationship between Electronegativity of 2'-Substituents and Conformation of Adenine Nucleosides. Tetrahedron Letters. 1979:4073–4076. [Google Scholar]
- 25.Moran S, Ren RX, Kool ET. A thymidine triphosphate shape analog lacking Watson-Crick pairing ability is replicated with high sequence selectivity. Proc Natl Acad Sci U S A. 1997;1994:10506–10511. doi: 10.1073/pnas.94.20.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parsch J, Engels JW. C-F center dot center dot center dot H-C hydrogen bonds in ribonucleic acids. Journal of the American Chemical Society. 2002;124:5664–5672. doi: 10.1021/ja012116g. [DOI] [PubMed] [Google Scholar]
- 27.Dunitz JD, Taylor R. Organic fluorine hardly ever accepts hydrogen bonds. Chemistry-a European Journal. 1997;3:89–98. [Google Scholar]
- 28.Barbarich TJ, Rithner CD, Miller SM, Anderson OP, Strauss SH. Significant inter- and intramolecular O–H center dot center dot center dot FC hydrogen bonding. Journal of the American Chemical Society. 1999;121:4280–4281. [Google Scholar]
- 29.Evans TA, Seddon KR. Hydrogen bonding in DNA - a return to the status quo. Chemical Communications. 1997:2023–2024. [Google Scholar]
- 30.Brady K, Wei AZ, Ringe D, Abeles RH. Structure of Chymotrypsin Trifluoromethyl Ketone Inhibitor Complexes - Comparison of Slowly and Rapidly Equilibrating Inhibitors. Biochemistry. 1990;1929:7600–7607. doi: 10.1021/bi00485a009. [DOI] [PubMed] [Google Scholar]
- 31.Hetrick B, Khade PK, Lee K, Stephen J, Thomas A, Joseph S. Polyamines accelerate codon recognition by transfer RNAs on the ribosome. Biochemistry. 2010;49:7179–7189. doi: 10.1021/bi1009776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Youngman EM, He SL, Nikstad LJ, Green R. Stop codon recognition by release factors induces structural rearrangement of the ribosomal decoding center that is productive for peptide release. Mol Cell. 2007;28:533–543. doi: 10.1016/j.molcel.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Wohlgemuth I, Pohl C, Rodnina MV. Optimization of speed and accuracy of decoding in translation. Embo J. 2010;29:3701–3709. doi: 10.1038/emboj.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies J, Davis BD. Misreading of ribonucleic acid code words induced by aminoglycoside antibiotics. The effect of drug concentration. J Biol Chem. 1968;243:3312–3316. [PubMed] [Google Scholar]
- 35.Holland JJ, McCarthy BJ. Stimulation of Protein Synthesis in Vitro by Denatured DNA. Proc Natl Acad Sci U S A. 1964;52:1554–1561. doi: 10.1073/pnas.52.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan AR, Wells RD, Khorana HG. Studies on polynucleotides. LXXIV. Direct translation in vitro of single-stranded DNA-like polymers with repeating nucleotide sequences in the presence of neomycin B. J Mol Biol. 1967;26:477–497. doi: 10.1016/0022-2836(67)90316-6. [DOI] [PubMed] [Google Scholar]
- 37.Pape T, Wintermeyer W, Rodnina MV. Conformational switch in the decoding region of 16S rRNA during aminoacyl-tRNA selection on the ribosome. Nat Struct Biol. 2000;7:104–107. doi: 10.1038/72364. [DOI] [PubMed] [Google Scholar]
- 38.Gromadski KB, Rodnina MV. Streptomycin interferes with conformational coupling between codon recognition and GTPase activation on the ribosome. Nat Struct Mol Biol. 2004;11:316–322. doi: 10.1038/nsmb742. [DOI] [PubMed] [Google Scholar]
- 39.Selmer M, Dunham CM, Murphy FVt, Weixlbaumer A, Petry S, Kelley AC, Weir JR, Ramakrishnan V. Structure of the 70S ribosome complexed with mRNA and tRNA. Science. 2006;313:1935–1942. doi: 10.1126/science.1131127. [DOI] [PubMed] [Google Scholar]
- 40.Agirrezabala X, Schreiner E, Trabuco LG, Lei J, Ortiz-Meoz RF, Schulten K, Green R, Frank J. Structural insights into cognate versus near-cognate discrimination during decoding. Embo J. 2011;30:1497–1507. doi: 10.1038/emboj.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmeing TM, Voorhees RM, Kelley AC, Gao YG, Murphy FVt, Weir JR, Ramakrishnan V. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science. 2009;326:688–694. doi: 10.1126/science.1179700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potapov AP. A stereospecific mechanism for the aminoacyl-tRNA selection at the ribosome. FEBS Lett. 1982;146:5–8. doi: 10.1016/0014-5793(82)80693-5. [DOI] [PubMed] [Google Scholar]
- 43.Nissen P, Ippolito JA, Ban N, Moore PB, Steitz TA. RNA tertiary interactions in the large ribosomal subunit: The A-minor motif. PNAS. 2001;98:4899–4903. doi: 10.1073/pnas.081082398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silverman SK, Cech TR. Energetics and cooperativity of tertiary hydrogen bonds in RNA structure. Biochemistry. 1999;38:8691–8702. doi: 10.1021/bi9906118. [DOI] [PubMed] [Google Scholar]
- 45.Doherty EA, Batey RT, Masquida B, Doudna JA. A universal mode of helix packing in RNA. Nat Struct Biol. 2001;8:339–343. doi: 10.1038/86221. [DOI] [PubMed] [Google Scholar]
- 46.Battle DJ, Doudna JA. Specificity of RNA-RNA helix recognition. Proc Natl Acad Sci U S A. 2002;99:11676–11681. doi: 10.1073/pnas.182221799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abramovitz DL, Friedman RA, Pyle AM. Catalytic role of 2'-hydroxyl groups within a group II intron active site. Science. 1996;271:1410–1413. doi: 10.1126/science.271.5254.1410. [DOI] [PubMed] [Google Scholar]
- 48.Frank J, Sengupta J, Gao H, Li W, Valle M, Zavialov A, Ehrenberg M. The role of tRNA as a molecular spring in decoding, accommodation, and peptidyl transfer. FEBS Lett. 2005;579:959–962. doi: 10.1016/j.febslet.2004.10.105. [DOI] [PubMed] [Google Scholar]
- 49.Mittelstaet J, Konevega AL, Rodnina MV. Distortion of tRNA upon near-cognate codon recognition on the ribosome. J Biol Chem. 2011;286:8158–8164. doi: 10.1074/jbc.M110.210021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yarus M, Valle M, Frank J. A twisted tRNA intermediate sets the threshold for decoding. Rna. 2003;9:384–385. doi: 10.1261/rna.2184703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feinberg JS, Joseph S. Ribose 2'-hydroxyl groups in the 5' strand of the acceptor arm of P-site tRNA are not essential for EF-G catalyzed translocation. RNA. 2006;12:580–588. doi: 10.1261/rna.2290706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Ortega L, Stephen J, Joseph S. Precise alignment of peptidyl tRNA by the decoding center is essential for EF-G-dependent translocation. Mol Cell. 2008;32:292–299. doi: 10.1016/j.molcel.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 53.Ledoux S, Uhlenbeck OC. [3'-32P]-labeling tRNA with nucleotidyltransferase for assaying aminoacylation and peptide bond formation. Methods. 2008;44:74–80. doi: 10.1016/j.ymeth.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ledoux S, Uhlenbeck OC. Different aa-tRNAs are selected uniformly on the ribosome. Mol Cell. 2008;31:114–23. doi: 10.1016/j.molcel.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cochella L, Green R. An active role for tRNA in decoding beyond codon:anticodon pairing. Science. 2005;308:1178–1180. doi: 10.1126/science.1111408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong I, Lohman TM. A double-filter method for nitrocellulose-filter binding: application to protein-nucleic acid interactions. Proc Natl Acad Sci U S A. 1993;90:5428–5432. doi: 10.1073/pnas.90.12.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.