Abstract
Objectives
To analyze the postoperative complications associated with cochlear implant (CI) surgery in a large consecutive case series of older adults (≥ 60 years)
Study Design
Retrospective case review
Setting
Tertiary referral center
Patients
445 individuals ≥60 who received a first CI between1999–2011
Interventions
Cochlear implantation
Main Outcome Measure(s)
Postoperative complications classified as major (meningitis, immediate postoperative facial weakness, device failure, flap dehiscence, surgical removal) and minor (surgical site infection, balance problems, delayed postoperative facial weakness, facial nerve stimulation)
Results
The mean age at implantation was 72.7 years (60–94.9) and the median duration of follow-up was 4.8 years (0.1–12.5). There were 42 minor complications in 41 patients (9.2%) and 36 major complications in 21 patients (4.7%). Seventeen patients (3.8%) required surgical device removal, 15 of whom underwent reimplantation. A Kaplan-Meier analysis of rates of device explantation demonstrated that at 5 and 10 years after CI, respectively, 95.4% and 93.1% of patients retained their original CI. When comparing complications between patients aged 60–74 years and those aged 75 years and older, there was a higher prevalence of balance problems lasting more than 1 month in the older group (9.5% vs. 4.9%, p = .05).
Conclusions
Our results indicate that the safety profile of cochlear implantation in an older population is comparable to that of younger adults and children. We suggest that concerns for increased postoperative complications in patients of advanced age do not need to be a primary consideration when determining CI candidacy.
INTRODUCTION
Hearing impairment affects approximately 70% of older adults in the United States, and its prevalence nearly doubles with every decade of age.1–3 Recent epidemiologic studies have demonstrated that hearing loss is independently associated with poorer cognitive function, rates of cognitive decline,4,5 and the risk of incident dementia.6,7 These associations may be mediated through social isolation and/or cognitive load,8 raising the possibility that hearing rehabilitative interventions could potentially mitigate these outcomes.9 Amplification through hearing aids is a mainstay of hearing rehabilitative therapy in the elderly. However, for those with severe-to-profound hearing loss, comprising approximately 1% of all adults over age 70, hearing aids cannot provide sufficient amplification.10 For these individuals, cochlear implants (CIs) can serve as a useful means of hearing rehabilitation.11
Although the gains conferred by CIs are comparable between older and young patients,12,13 the rate of CI use in older adults who meet candidacy criteria is less than 5%.14 The reasons for low rates of CI use in older adults are likely multifactorial, and include lack of awareness, poor access to CI centers and concern for an increased likelihood of complications. While CI surgery has become a routine outpatient surgery, both major (e.g. severe infection, flap dehiscence, device failure) and minor complications (e.g. transient facial palsy, balance problems) remain possible.15 The safety of CI surgery has been well-documented in children and young adults,15–23 but there have been fewer reports in older adults19–23 (i.e. those older than 60 years at implantation) and no studies to date focusing solely on the latter population. The aim of the current study is to investigate the safety of CI surgery in a consecutive series of patients aged 60 and older who received their first CI at the Johns Hopkins Hospital from 1999–2011.
MATERIALS AND METHODS
STUDY COHORT
The Johns Hopkins Listening Center is one of the largest providers of cochlear implants in North America and maintains a prospective database of all patients receiving cochlear implants. We queried this database to ascertain all individuals ≥60 years who underwent a first CI from 1999–2011. Although there is no standard definition for what constitutes an older CI candidate, we chose 60 years as our cutoff based on the United Nations agreed definition for older adults.24 We then performed a retrospective chart review of the electronic patient record (EPR) system at Johns Hopkins up to August 2011 to abstract data on postoperative follow-up and complications. Exclusion criteria were: age less than 60 at date of CI, patients who received a previous CI, and/or patients who had a CI placed before 1999. We excluded anyone who received a CI before 1999 because of inconsistent use of the EPR system for storing medical records prior to that date. Our protocol was approved by the Johns Hopkins institutional review board.
DATA ABSTRACTION
Complications were categorized as being major or minor consistent with prior studies.15,21–23 Major complications included meningitis, immediate postoperative facial weakness, hard and soft device failure, flap dehiscence, and surgical explantation. Minor complications included surgical site infections responding to conservative local wound care and/or antibiotics, balance problems lasting greater than one month, delayed and transient postoperative facial weakness, and facial nerve stimulation with electrode activation. Hard device failures were validated by device interrogation and telemetry, whereas soft device failures were based on patient report despite the presence of normal telemetry and testing. We defined balance problems as being self-reported disequilibrium, imbalance, or vertigo lasting more than one month after the implantation surgery. Data were abstracted through a comprehensive review of all electronic medical records, and questions regarding data specification were adjudicated in conferences with the senior author (F.L.). Quality checks of the abstracted data and data validation were performed by randomly sampling 15% of the sample for repeat data abstraction by other study personnel.
STATISTICAL METHODOLOGY
Fisher’s exact test was used to compare the rate of cochlear implantation complications in patients < 75 years versus ≥ 75 years. We used a Kaplan-Meier survival curve to estimate the time (in years) until device explantation. Statistical significance was accepted at a p < 0.05 level. All statistical analyses were conducted using STATA 12 (StataCorp, College Station, TX).
RESULTS
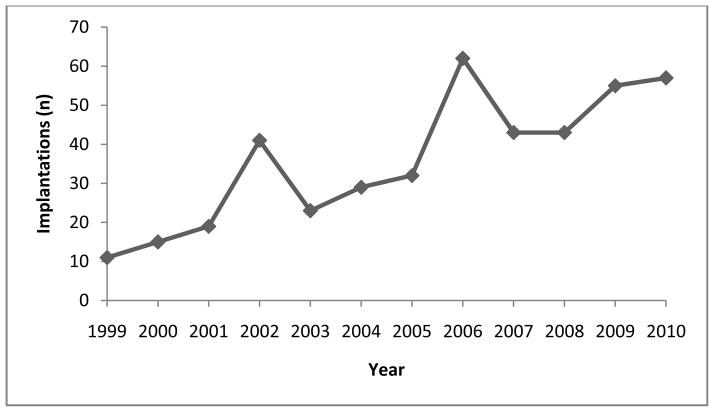
There were 445 older adult patients who received a first CI at the Johns Hopkins Hospital between 1999 and 2011. Eight individuals underwent a subsequent contralateral implantation. The patients ranged from 60 to 94.9 years (mean [± SD] 72.7 ± 7.69) at the time of implantation. Five patients had congenital hearing loss, 60 with hearing loss of pediatric onset, and 339 with adult-onset hearing loss. Onset of hearing loss was unknown for 41 patients. The mean duration of follow-up was 4.8 years (range 0.1–12.5). Demographic data are summarized in Table 1 and the number of cochlear implants per year during the study period is shown in Figure 1.
Table 1.
Cohort demographics and cochlear implantation (CI) characteristics of adults ≥ 60 years old receiving their first cochlear implant at Johns Hopkins, 1999–2011
| Characteristic | n (%) |
|---|---|
| Sex | |
| Male | 224 (49.7) |
| Female | 221 (50.3) |
| Age at initial CI, yr | |
| 60–69 | 184 (41.4) |
| 70–79 | 175 (39.3) |
| 80–89 | 82 (18.4) |
| 90–100 | 4 (0.9) |
| Mean age, yr (SD) | 72.7 (7.69) |
| [Range] | [60.0–94.9] |
| Onset of Hearing Loss* | |
| Congenital | 5 (1.1) |
| < 18 yrs | 60 (13.5) |
| >18 yrs | 339 (76.2) |
| First Side Implanted | |
| Right ear | 238 (53.5) |
| Left ear | 207 (46.5) |
| Subsequent Contralateral Implantation† | 8 (1.8) |
| Duration of follow-up | |
| Median, yr | 4.8 |
| [Range], yr | [0.1–12.5] |
41 (9.2%) subjects had missing HL age of onset
8 patients subsequently received a contralateral implant
Figure 1.
Number of cochlear implants per year, 1999–2010
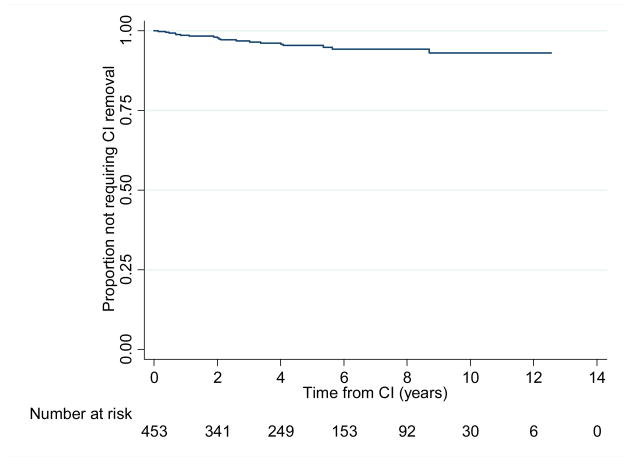
There were a total of 42 minor complications (surgical site infection, balance problems, delayed transient facial weakness, facial nerve stimulation) in 41 patients (9.2%) and 36 major complications (device failure, skin flap dehiscence, surgical device removal) in 21 patients (4.7%)(Table 2). There were no cases of meningitis or postoperative facial paralysis. Surgical explantation and device failures were the most commonserious complications. There were 14 device failures, and the average time to device failure was 4.8 years [SD = 3.3, range = 0.09 to 13.2yrs] (Figure 2). Of all complications, balance problems were the most common and observed in 30 patients (6.6%).
Table 2.
Major and minor complications after a first cochlear implantation
| Complications | n (%) |
|---|---|
| MAJOR | |
| Meningitis | 0 (0) |
| Immediate postoperative facial weakness | 0 (0) |
| Device failure (hard) | 6 (1.3) |
| Device failure (soft/unspecified) | 8 (1.8) |
| Flap dehiscence | 5 (1.1) |
| Surgical removals | 17 (3.8) |
| MINOR | |
| Surgical site infection | 7 (1.6) |
| Balance problems > 1 month | 30 (6.6) |
| Delayed postoperative facial weakness* | 2 (0.4) |
| Facial nerve stimulation | 3 (0.7) |
Transient facial weakness with no lasting sequelae
Figure 2.
Time to surgical removal of CI*
*Due to surgical site infection, device failure, non-use, flap dehiscence, or large vestibular schwannoma found post-op
Seventeen patients (3.8%) required surgical device removal. Of these 17 patients, 15 underwent reimplantation surgery (88.2%). Reasons for surgical device removal included surgical site infections, device failures (hard, soft, unspecified), non-use, flap dehiscence, and in one case, a large vestibular schwannoma which was discovered postoperatively (Table 3). Of the 15 patients who underwent reimplantation, there were six complications in four patients, consisting of one device failure (6.7%), one case of flap dehiscence (6.7%), two surgical removals (13.3%), and two cases of balance problems (13.3%). In one case, skin breakdown occurred over the site of reimplantation leading to device exposure and ultimately device removal. This was attributed in the record to an atypical reaction to Silastic from the device. In a second case, the reimplanted device underwent soft device failure and was subsequently removed. In the other two cases, both patients experienced persistent balance problems that lasted for longer than two months without any diagnosis. We did not find any common characteristics among these four patients which may have been related to their complications.
Table 3.
Reasons for Surgical Removal (n=17)
| Complications | n (%) | Time to Removal in Years |
|---|---|---|
| Surgical site infection | 2 (11.8) | 0.4, 3.1 |
| Device failure (Unspecified) | 2 (11.8) | 0.2, 4.2 |
| Device failure (Hard) | 4 (23.5) | 0.8, 1.1, 2.0, 4.6 |
| Device failure (Soft) | 5 (29.4) | 2.3, 2.5, 2.7, 5.5, 5.9 |
| Non-use | 1 (5.9) | 7.3 |
| Flap dehiscence | 2 (11.8) | 0.4, 1.9 |
| Large vestibular schwannoma found post-op | 1 (5.9) | 0.7 |
The duration of time to device explantation was shortest for flap dehiscence and surgical site infections, and longest for soft device failure and non-use, with 90% of explantations occurring within six years of implantation (Table 3). A Kaplan-Meier analysis of time to device explantation demonstrates that the expected rate of retaining a first CI is 95.4% at 5 years, 93.1% at 10 years, and 93.1% at 12.5 years (Figure 2).
To investigate the possible association of age with CI surgery complications, we compared complication rates between individuals ages 60 to 75 years (n = 284) and versus 75 years and older (n = 169) at time of implantation. Complication rates were not different between the 2 groups except for balance problems, which were more prevalent in the older cohort (9.5% vs. 4.9%, p = .05) (Table 4).
Table 4.
Comparison of complication rates after CI in patients aged <75 years versus ≥ 75 years
| Complications | < 75 years old n= 284 n(%) |
≥ 75 years old n=169 n(%) |
P-value |
|---|---|---|---|
| Surgical site infection | 5 (1.8) | 2 (1.2) | 0.48 |
| Balance problems > 1 month | 14 (4.9) | 16 (9.5) | 0.05 |
| Facial weakness | 1 (0.4) | 1 (0.6) | 0.61 |
| Facial nerve stimulation | 3 (1.1) | 0 (0) | 0.25 |
| Device failure (hard) | 3 (1.1) | 3 (1.8) | 0.40 |
| Device failure (soft) | 5 (1.8) | 3 (1.8) | 0.63 |
| Flap dehiscence | 4 (1.4) | 1 (0.6) | 0.38 |
| Number of surgical removals | 11 (4.2) | 6 (3.6) | 0.54 |
DISCUSSION
Our results are based on the largest consecutive case series of older adult CI recipients to date and demonstrate a major complication rate of 4.7% and a minor complication rate of 9.2%. When comparing complication rates between patients aged 60–74 years and those aged 75 years and older, we found no significant differences except for a higher prevalence of balance problems lasting more than 1 month in the older group (9.5% vs. 4.9%, p = .05). At 5 and 10 years after CI, respectively, 95.4% and 93.1% of patients retained their original CI.
Our complication rates are within the range established by previous studies. Major and minor CI complication rates have ranged in the literature from 1.5%–10.2% and 0.7%–32%, respectively.15,17,19,20 The most frequent types of postoperative complications vary by report, although device failure, infection and vertigo have been demonstrated in several studies to be the most common. The average length of follow-up has ranged in previous studies from 1.4 to 6.9 years, compared to 4.8 years in the present study.15,19 Our study has a relatively long follow-up period compared to previous reports, few of which include subjects greater than 60 years of age.15–23 Table 5 summarizes findings from other studies on CI complications.
Table 5.
Cochlear Implantation complications: findings from other studies
| Study | Year | Study Design | Implantations | Mean Age at Implantation (Range) | Length of Follow- up Time in Years Mean (Range) | Major Complications n (%) | Minor Complications n (%) |
|---|---|---|---|---|---|---|---|
| Brito et al.21 | 2012 | Retrospective | 550 | Not Provided | 3.9 (Range Not Provided) | 49 (8.9) | 43 (7.8) |
| Ciorba et al.20 | 2011 | Retrospective | 438 | 18 (0.6 – 86.0) | 3.8 (0.8 – 7.0) | 15 (3.4) | 25 (5.7) |
| Qiu et al.19 | 2011 | Retrospective | 416 | 6.0 (0.9 – 53) | 2.6 (0.2 – 10.0) | 6 (1.5) | 23 (5.5) |
| Loundon et al.17 | 2010 | Retrospective | 434 | 4.7 (0.6–16.0) | 5.5 (0.1 – 17.0) | 24 (5.5) | 19 (4.4) |
| McJunkin& Jeyakumar16 | 2010 | Retrospective | 136 | Not Provided | 3.0 (Range Not Provided) | 8 (5.9) | 1(0.7) |
| Ovesen& Johansen22 | 2009 | Retrospective | 313 | A(median)=50 (18 – 79) P(median)=2.8 (0.5 – 17) |
A(median)=2.7 (0.4 – 9.5) P(median)=3.7 (0.4 – 9.6) |
14 (4.5) | 35(11.2) |
| Venail et al.18 | 2008 | Retrospective | 500 | 21.5 (0.8 – 80.8) | 6.9 (0.8 –17.8) | 51 (10.2) | 28 (5.6) |
| Postelmans et al.14 | 2007 | Retrospective | 112 | A=53.4(14.7 – 82.0) P=4.3(1.6 – 11.4) |
A=2.2 (0.1 – 4.0) P=1.4 (0.1 –4.0) |
4 (3.6) | 36 (32.0) |
| Bhatia et al.15 | 2004 | Prospective | 300 | 5.1 (1.3 – 16.9) | 4.0 (0.1 – 14.0) | 7 (2.3) | 29 (9.7) |
P=Pediatric cases A=Adult cases
Device failure was the most common major complication in our study, and has been reported in past studies to be a frequent complication of implantation.17,19,21,22. Our Kaplan-Meier analysis of time to device removal is similar to results obtained by Venail et al,19 and suggests that well over 90% of patients retain their original device at 12 years following implantation. Acute issues such as surgical site infection and flap dehiscence had the shortest time to explantation (less than two years), while issues such as device failure and non-use tended to have longer times to explantation. Of the seventeen patients who underwent surgical explantation, 15 opted for a reimplant procedure.
We found that transient postoperative balance problems were the most common complication overall and were significantly more frequent in patients ≥ 75 years versus 60–74 years old. This observation may be due to a higher prevalence of factors such as cognitive impairment, decreased peripheral proprioception, and/or muscle weakness in older subjects, all of which would be expected to contribute to subjective balance problems.
Our study has several potential limitations. First, our retrospective data may not account for patients who did not follow up after implantation or for complications that were not recorded in the electronic medical record system. This creates an ascertainment bias that may underestimate the true rate of complications. However, because more severe complications would most likely bring patients back to the clinic, and CI patients need to regularly report to clinic for programming of their device, we believe that our dataset captures the vast majority of post-CI complications, particularly those considered to be serious. Second, the criteria for major and minor complications differ across the literature, and some studies16,18,20 do not consider device failure to be a complication of surgery. The inclusion of hard and soft device failures in our analysis may thus overestimate our complication rates relative to other studies. Third, our results are based on data obtained at the Johns Hopkins Listening Center, which is a large tertiary referral center for CI. As a result, our findings may not be generalizable to rates at other CI centers. Finally, we did not investigate factors potentially associated with complication rates in our analyses. These data were unavailable in our present database, and it is unclear if we would have sufficient power to examine the association of factors such as surgical approach, surgeon or type of device with complication rates using a multivariate approach that would be necessary to draw any substantive conclusions.
Our results are based on the largest series of older adults CI recipients to date and demonstrate that the safety profile of CI in this older cohort is comparable to that of younger adults and children. We believe that concerns for increased postoperative complications in older adults undergoing CI do not need to be a primary consideration when determining CI candidacy. Future research is needed to better understand factors contributing to low rates of cochlear implantation in older adults and the impact of CIs on the social, cognitive, and physical functioning of older adults.7
Acknowledgments
Funding: This manuscript was supported in part by NIH K23DC011279, the Eleanor Schwartz Charitable Foundation, and a Triological Society/American College of Surgeons Clinician Scientist Award.
Footnotes
Disclosures: Dr. Lin reports being a consultant to Cochlear, Autifony, and Pfizer.
References
- 1.Lin FR, Niparko JK, Ferrucci L. Hearing loss prevalence in the United States. Arch Intern Med. 2011 Nov 14;171(20):1851–1852. doi: 10.1001/archinternmed.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins JG. Prevalence of selected chronic conditions: United States, 1990–1992. Vital Health Stat. 1997;10(194):1–89. [PubMed] [Google Scholar]
- 3.Sprinzl GM, Riechelmann H. Current trends in treating hearing loss in elderly people: a review of the technology and treatment options - a mini-review. Gerontology. 2010;56(3):351–358. doi: 10.1159/000275062. [DOI] [PubMed] [Google Scholar]
- 4.Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011 Nov;25(6):763–770. doi: 10.1037/a0024238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin FR. Hearing loss and cognition among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66(10):1131–1136. doi: 10.1093/gerona/glr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011 Feb;68(2):214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin FR. Hearing loss in older adults: who’s listening? JAMA. 2012 Mar 21;307(11):1147–1148. doi: 10.1001/jama.2012.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wingfield A, Tun PA, McCoy SL. Hearing loss in older adulthood - What it is and how it interacts with cognitive performance. Current directions in psychological science. 2005 Jun;14(3):144–148. [Google Scholar]
- 9.Mulrow CD, Aguilar C, Endicott JE, et al. Quality-of-life changes and hearing impairment. A randomized trial. Ann Intern Med. 1990;113(3):188–194. doi: 10.7326/0003-4819-113-3-188. [DOI] [PubMed] [Google Scholar]
- 10.Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011 May;66(5):582–590. doi: 10.1093/gerona/glr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labadie RF, Carrasco VN, Gilmer CH, Pillsbury HC., 3rd Cochlear implant performance in senior citizens. Otolaryngol Head Neck Surg. 2000 Oct;123(4):419–424. doi: 10.1067/mhn.2000.109759. [DOI] [PubMed] [Google Scholar]
- 12.Vermeire K, Brokx JP, Wuyts FL, Cochet E, Hofkens A, Van de Heyning PH. Quality-of-life benefit from cochlear implantation in the elderly. Otol Neurotol. 2005 Mar;26(2):188–195. doi: 10.1097/00129492-200503000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Shin YJ, Fraysse B, Deguine O, et al. Benefits of cochlear implantation in elderly patients. Otolaryngol Head Neck Surg. 2000 Apr;122(4):602–606. doi: 10.1067/mhn.2000.98317. [DOI] [PubMed] [Google Scholar]
- 14.Murray Brendan Cochlear Europe Limited. Personal Communication. Sep 22, 2012.
- 15.Postelmans JT, Cleffken B, Stokroos RJ. Post-operative complications of cochlear implantation in adults and children: five years’ experience in Maastricht. J Laryngol Otol. 2007 Apr;121(4):318–323. doi: 10.1017/S0022215106003471. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia K, Gibbin KP, Nikolopoulos TP, O’Donoghue GM. Surgical complications and their management in a series of 300 consecutive pediatric cochlear implantations. Otol Neurotol. 2004;25(5):730–739. doi: 10.1097/00129492-200409000-00015. [DOI] [PubMed] [Google Scholar]
- 17.McJunkin J, Jeyakumar A. Complications in pediatric cochlear implants. American journal of otolaryngology. 2010 Mar-Apr;31(2):110–113. doi: 10.1016/j.amjoto.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Loundon N, Blanchard M, Roger G, Denoyelle F, Garabedian EN. Medical and surgical complications in pediatric cochlear implantation. Arch Otolaryngol Head Neck Surg. 2010 Jan;136(1):12–15. doi: 10.1001/archoto.2009.187. [DOI] [PubMed] [Google Scholar]
- 19.Venail F, Sicard M, Piron JP, et al. Reliability and complications of 500 consecutive cochlear implantations. Arch Otolaryngol Head Neck Surg. 2008 Dec;134(12):1276–1281. doi: 10.1001/archoto.2008.504. [DOI] [PubMed] [Google Scholar]
- 20.Qiu J, Chen Y, Tan P, et al. Complications and clinical analysis of 416 consecutive cochlear implantations. Int J Pediatr Otorhinolaryngol. 2011 Sep;75(9):1143–1146. doi: 10.1016/j.ijporl.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Ciorba A, Bovo R, Trevisi P, et al. Postoperative complications in cochlear implants: a retrospective analysis of 438 consecutive cases. Eur Arch Otorhinolaryngol. 2012 Jun;269(6):1599–1603. doi: 10.1007/s00405-011-1818-1. [DOI] [PubMed] [Google Scholar]
- 22.Brito R, Monteiro TA, Leal AF, Tsuji RK, Pinna MH, Bento RF. Surgical complications in 550 consecutive cochlear implantation. Braz J Otorhinolaryngol. 2012 Jun;78(3):80–85. doi: 10.1590/S1808-86942012000300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ovesen T, Johansen LV. Post-operative problems and complications in 313 consecutive cochlear implantations. J Laryngol Otol. 2009 May;123(5):492–496. doi: 10.1017/S0022215108003691. [DOI] [PubMed] [Google Scholar]
- 24. [Accessed 19 Nov, 2012];Definition of an older or elderly person. http://www.who.int/healthinfo/survey/ageingdefnolder/en/index.html.