Abstract
Enzymes are remarkable catalysts that lie at the heart of biology, accelerating chemical reactions to an astounding extent with extraordinary specificity. Enormous progress in understanding the chemical basis of enzymatic transformations and the basic mechanisms underlying rate enhancements over the past decades is apparent. Nevertheless, it has been difficult to achieve a quantitative understanding of how the underlying mechanisms account for the energetics of catalysis, because of the complexity of enzyme systems and the absence of underlying energetic additivity. We review case studies from our own work that illustrate the power of precisely defined and clearly articulated questions when dealing with such complex and multi-faceted systems, and we also use this approach to evaluate our current ability to design enzymes. We close by highlighting a series of questions that help frame some of what remains to be understood, and we encourage the reader to define additional questions and directions that will deepen and broaden our understanding of enzymes and their catalysis.
Enzymes are amazing in their ability to accomplish enormous rate enhancements with extraordinary specificity for reactions carried out under mild conditions. And these chemical transformations are at the heart of biology because reactions, and just the right reactions, must be accelerated to outpace natural dissipative forces so that living systems can create and maintain their requisite order and organization. Competition between organisms, both within and across species, provides a further selective pressure to evolve faster (and more specific) enzymes that allow an organism to garner more nutrients, to grow and reproduce faster, to respond faster to changing conditions, and even to out-swim, out-crawl, or outrun a predator.
Given the centrality of enzymes in nearly all biological processes, and their prevalence as drug targets, it is no wonder that enzymes have been intensely scrutinized –conceptually, experimentally, and theoretically– over many decades. We have learned an enormous amount over these decades, including the centuries-old discovery of the existence of biological catalysis (1–3), the seminal finding that catalysis can occur outside of a living system (4), and the identification of proteins as the primary catalysts in biology (5).
The mid-20th century saw the rapid elaboration of the identity of the chemical transformations that are performed by enzymes, as well as the identification of coenzymes and cofactors that help facilitate these reactions (6). Practitioners of physical organic and bioorganic chemistry elucidated viable mechanisms for these chemical transformations outside of the enzyme environment (7, 8), and many clever kinetic and chemical tests were developed by enzymologists to indirectly (but powerfully) derive information about the transformations taking place within the active site (9–16). These tests provided information about reaction intermediates, covalently bound enzyme species, and the enzyme groups involved in forming these species and catalyzing these reactions. In the last two decades, structural studies have proliferated, providing a context for prior and ongoing functional and mechanistic studies, and even outstripping those studies to provide early information about the reaction environment from which mechanistic models and catalytic hypotheses can be generated.
So, where are we now? What do we understand about how enzymes work, and what is left to be understood? Remarkably, after taking a (good) course in enzymatic reaction mechanisms, a reasonably sophisticated undergraduate or beginning graduate student can, when presented with new biochemical reactions, propose plausible reaction mechanisms and identify coenzymes or cofactors that are likely to be utilized, and be correct a vast majority of the time. So, at the level of ‘arrow pushing’ or bioorganic chemistry our advances have been truly remarkable (17–19).1
But beyond understanding the reaction pathways of biochemical transformations in solution and within enzyme active sites, where are we with the parallel goal of understanding the enormous rate enhancements that enzymes achieve? And where are we with the ambitious goal of engineering new enzymes that catalyze new reactions? After a brief review of our current overall understanding, we present recent results that push the boundaries of our understanding and we discuss and evaluate attempts to engineer new enzymes. We close by presenting examples of remaining challenges.
General historical overview of understanding enzymatic rate enhancements
Very early ideas about how enzymes worked invoked complementarity between a reaction’s transition state and the binding surface of the enzyme –what we now refer to as the enzyme’s active site. Remarkably, these ideas predated knowledge of the atomic and molecular nature of enzymes (20–22). This complementarity can now be visualized from the myriad of enzyme x-ray structures, many with bound substrates, substrate analogs, or transition state analogs.
Functional work, much of it predating the structural determinations, supports the same overall picture that active sites are structurally optimized to discriminate even subtle differences between ground states and transition states. Modest changes of a substrate remote from the site of bond formation and cleavage, even the atomic-level change of an oxygen to a sulfur, can enormously alter the ability of an enzyme to utilize that substrate (e.g., (23–34)). Further, ligand binding can be strong when the shape and charge at a position is complementary to the active site shape and charge (i.e., positive/negative or hydrophobic/hydrophobic), but is weakened when these features are not matched. Indeed, the features of strong-binding ligands generally mimic those thought to be present in the transition state, and such ligands are often referred to as ‘transition state analogs’ (35, 36).
These structural, functional, and binding observations support a common picture in which active sites are complementary in shape and electrostatic properties to transition states of reactions (Figure 1). A formal description of this complementarity can be derived from transition state theory, which posits that the highest energy species on a reaction coordinate can be thought of as if it is in equilibrium with the ground state reactants, allowing a straightforward consideration and comparison of interactions in the ground state and ‘in’ the transition state, such that an enzyme ‘binds’ the transition state more strongly than the ground state (37–39).2
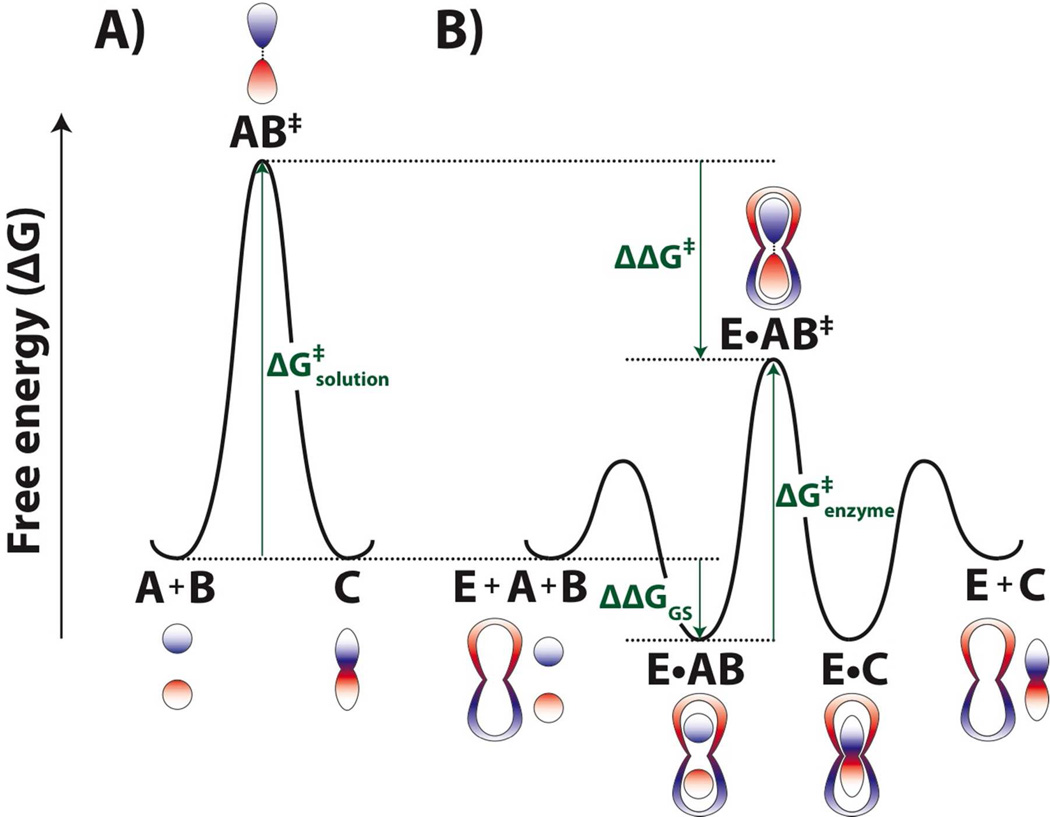
Figure 1. Hypothetical free energy profiles illustrating enzyme-transition state complementarity and selective stabilization of the transition state by an enzyme relative to the ground state.
A) Free energy profile for an uncatalyzed reaction. Changes in electrostatics and geometry as the reaction proceeds are shown with different colors and shapes. B) Free energy profile for the same reaction but now catalyzed by an enzyme. The enzyme positions the substrates with respect to another, and electrostatic and shape complementarity of the enzyme to the transition state, shown here schematically, renders interactions between the enzyme and the transition state more favorable than those between the enzyme and the ground state (ΔΔG‡ > ΔΔGGS). Thus, the barrier to reaction on the enzyme is smaller than in solution .
Insights from Selected Case Studies
Extreme Mutagenesis with Ketosteroid Isomerase
Most modern enzymology and functional research has focused on specific interactions within active sites and recognition regions, in part because of the ease and power of identifying interactions via x-ray crystallographic structures and of probing them via site-directed mutagenesis. We would like to be able to use site-directed mutagenesis to fully account for enzymatic catalysis from the contribution from each residue (or functional group), adding those contributions to fully account for the observed rate enhancement –i.e., the enzymatic reaction rate relative to the rate of the corresponding nonenzymatic reaction. However, this accounting is not possible, because the effects of individual groups on catalysis are neither additive nor independent: The sum of individual effects, from mutations, or deletion or truncation of substrate moieties, far exceeds the overall catalysis (40); the size of the effect from mutating a residue can depend on what other residues are present; and whether an effect is manifest in binding or catalysis can also depend on what other residues are present (40, 41).3
In this section we focus on the enzyme ketosteroid isomerase and its so-called ‘oxyanion hole.’ This and the following sections highlight the need for clearly defined questions and comparisons to make meaningful inroads toward understanding contributions to catalysis, and we present below a novel strategy that enables such one such comparison.
Ketosteroid Isomerase
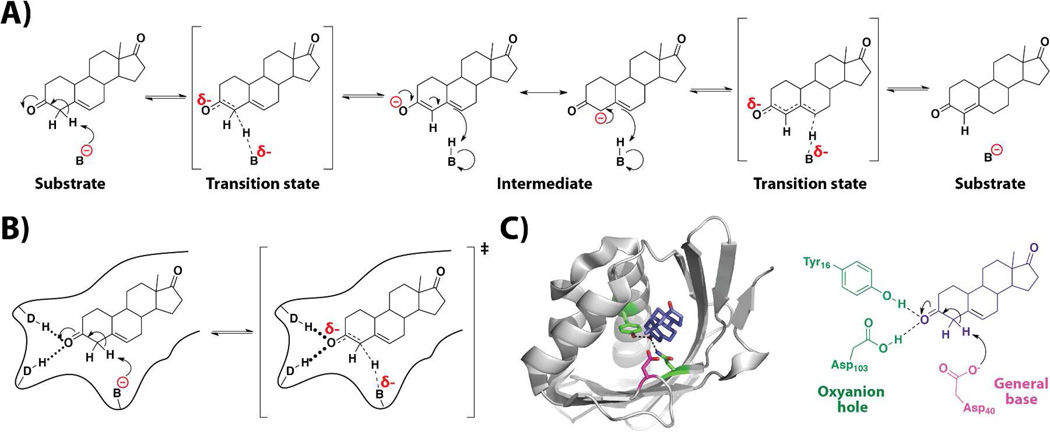
Bacterial ketosteroid isomerase (KSI) catalyzes a straightforward chemical transformation that is similar to many biological reactions (Figure 2A), and it falls into the common reaction classes for which reasonable mechanisms can be inferred from chemical intuition (42). A proton alpha to a carbonyl group is abstracted to give a carbanion equivalent that is stabilized by its ability to form an enolate intermediate; for KSI there is still further stabilization because a dienolate intermediate can form. Reprotonation, but at the other end of the conjugated system, as shown in Figure 2A, gives the isomerized product.
Figure 2. Chemical mechanism of the reaction catalyzed by ketosteroid isomerase (KSI).
A) Reaction scheme. B) Expected active site features of an enzyme that catalyzes this reaction, including a general base (B), hydrogen bond donors (D–H), and a binding pocket (black outline). C) Structure of KSI bound to a product (4-androstene-3,17-dione, purple) and a schematic representation with a general base Asp40 (magenta) and an oxyanion hole formed by the side chains of Tyr16 and Asp103 (green) (PDB ID = 3NHX).
Considering the expected transition states for this reaction, there is partial proton abstraction and charge accumulation on the incipient oxyanion (Figure 2A).4 Thus, even if we did not have years of functional work on this enzyme and numerous x-ray structures with bound transition state analogs, we would posit the following features: a general base to abstract the proton, positively charged groups or hydrogen bond donors situated around the incipient oxyanion, and a binding pocket that helps orient the substrate with respect to these catalytic features (Figure 2B). Indeed, NMR and x-ray structures ‘confirm’ these expectations, while also identifying the groups involved in these interactions and revealing their structural context (Figure 2C) (43–45).
The KSI oxyanion hole
Many enzymes have oxyanion intermediates and oxyanion-like transition states. The ‘oxyanion hole’ was first named in serine proteases (46, 47), and other enzymes that stabilize oxyanions provide hydrogen bonding or electrostatic stabilization that is analogous to the serine protease oxyanion hole (48–57). We use KSI to evaluate the energetics of these interactions because its oxyanion hole consists of two side chain hydrogen bond donors, Tyr16 and protonated Asp103 (Figure 2C), whereas protease oxyanion holes consist of at least one and usually two backbone amides and so limit the changes that can be introduced.
In principle, the simplest way to identify the energetic contribution from these (and other) hydrogen bonds would be if there were transferable energetic properties of hydrogen bonds. In other words, if the energetic contribution of a hydrogen bond were always the same from one system to another then we’d simply have to count hydrogen bonds (of each type) and add the results. Unfortunately, we know that this is not the case. This lack of transference is most strikingly seen in the ~40 kcal/mol difference in the energy of formation of the F− •HF hydrogen bond in the gas phase versus in aqueous solution (58–60). So the environment in which the hydrogen bond forms matters, and can matter a great deal. So does the degree of positioning of the interacting hydrogen bond partners, as we describe below.
The next simplest way to consider assigning energetic contributions to the oxyanion hydrogen bonds would be if there were empirical hydrogen bond energies that held in (all) active site environments. The observation that mutating away hydrogen bonding groups often gives similar energetic effects (48, 50, 52, 53, 61–65) might lead to optimism that this approach could work. Indeed, the oft-used phrase ‘the energy of the hydrogen bond’ implicitly implies that this is a transferable and additive property. But the overall energetics of catalysis cannot be described in terms of independent and additive components –the sum of energetic consequences of all mutations is greater than the observed catalysis in the systems. The same conclusion, and underlying concepts, hold for individual hydrogen bonding interactions. Consider an enzyme with the residues surrounding the oxyanion hole mutated so that the oxyanion hole residues are present but the oxyanion hole is not formed. In that case mutation of the oxyanion hole residues themselves will have no effect. In other words, so-called catalytic residues do not act alone; their energetics are synergistic with residues involved in forming the overall and local structure.3
The case of KSI below clearly illustrates the fundamental flaws in assuming the existence of an intrinsic or invariant free energy contribution from hydrogen bonds. This case study also illustrates the need for fully defining questions that can be answered by clear and explicit comparisons.
Consider first the simplest experiments, conservative mutagenesis of KSI’s oxyanion hole hydrogen bond donors. The effects of the Tyr16Phe and Asp103Ala/Leu mutations are large, 104 to 105 fold and 102 to 103 fold respectively, and mutants with both hydrogen bond donors removed gave very large deleterious effects on catalysis of 105–108 fold (Figure 3, exact values depend on the substrate used and enzyme source (66–69)). These results led to conclusions that the oxyanion hole provides enormous catalysis, and that its hydrogen bonds are particularly strong (44, 70, 71).
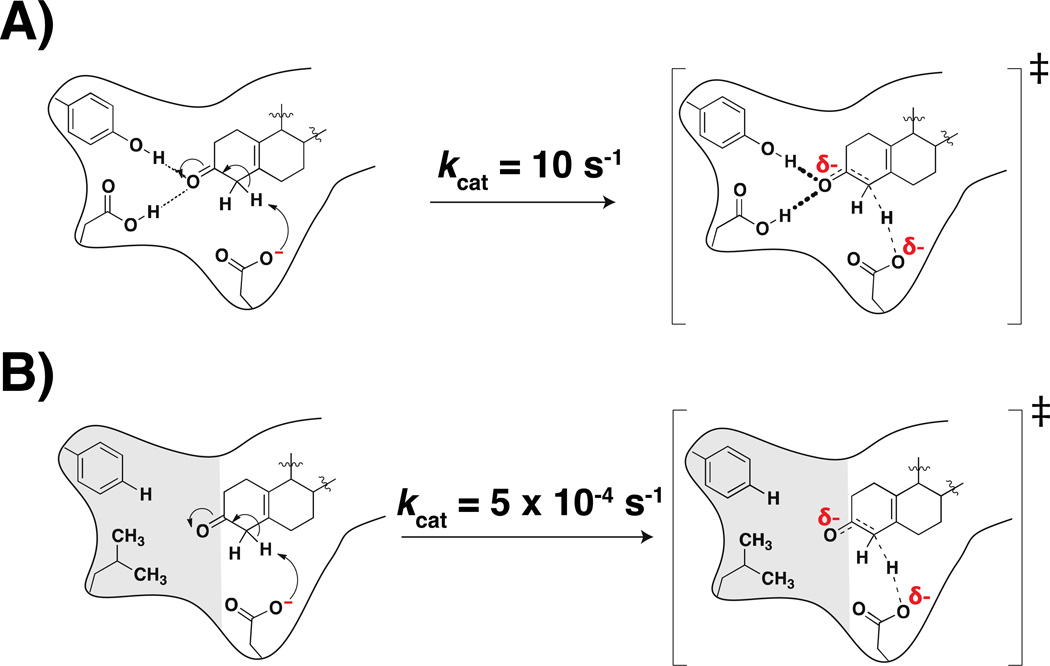
Figure 3. Schematic of the effect of conservative mutations to the oxyanion hole on KSI catalysis.
A) The wild-type KSI oxyanion hole has two hydrogen bond donors that stabilize the incipient oxyanion. B) Conservative mutations to the oxyanion hole replace the hydrogen bond donors with hydrophobic groups. Values are from (76).
But what do these rate effects really mean? What do the million-to-billion fold effects from these mutations tell us about catalytic mechanism and catalytic contributions? We must make explicit comparisons, and before that, ask clear questions. What question is asked when Tyr16 is mutated to Phe? In essence, we are asking about the ability to stabilize the incipient oxyanion with a neighboring hydrogen bond donor versus with a neighboring hydrophobic group. We know that anions are highly destabilized when removed from a hydrogen bonding solvent (water) and placed in a nonpolar solvent, and indeed, 19F NMR data provide strong evidence for hydrophobic character in this mutated site (72). So we should expect a large deleterious effect from mutations such as Tyr16Phe; indeed, any time a small effect is observed for such a mutation (provided that there is a genuine oxyanion interaction) it likely indicates an ability of the site to rearrange to make alternative interactions with other protein groups or with water that enters the mutated and rearranged site. Thus, two things are clear: i. These site-directed mutagenesis experiments do not reveal ‘intrinsic’ contributions to catalysis, and ii. The energetics involves many factors and is extremely complicated (73).
So what do the above results have to do with catalysis, other than to demonstrate the obvious –that generating an anion in a hydrophobic environment is not a good thing to do? How can we pose a question about catalysis that is both useful and answerable?
We first note that there are many such questions. There is no single question the answer to which will reveal all of an enzyme’s secrets. Claiming that one question is important while other questions are not has led to unnecessary and unproductive squabbles and has obscured advances in enzymology. Not clearly defining the question or questions being asked, has been still more obfuscatory.
We defined the following question about KSI’s oxyanion hole. Since enzymes’ substrates are (mostly) present in aqueous solution prior to binding and transformation, and these enzymes have evolved against a backdrop of aqueous reactivity, it is reasonable to ask the question, what is the rate enhancement for the enzymatic reaction relative to the corresponding reaction in aqueous solution? But even here we have to be more precise. What solution reaction are we using in our comparison? One comparison would be to pure water, say at pH 7. Questions using this comparison might be especially appropriate in considering questions evolving reactivity above certain background levels. But for mechanism one would minimally want to know if the aqueous reaction were acid or base catalyzed, and if so, something about the position and timing of the protonation or deprotonation events, in order to know how similar or dissimilar the solution process is to the process taking place in the enzyme’s active site.
For KSI, based on prior knowledge about the nonenzymatic and enzymatic reactions, we compare the enzymatic reaction, which uses an aspartate side chain as a general base in proton abstraction, to the solution reaction with acetate, which has essentially the same proton affinity and reactivity as the Asp side chain in water (Figure 4A&B). Thus, this comparison allows us to ask what is ‘special’ to the enzyme –i.e., we are looking at the same reaction (or nearly so)5 and asking how is it made more probable by the presence of the enzyme. We again emphasize that this is one of (many) possible useful questions. For example, we could have used as our reference state the analogous reaction in the gas phase. Indeed, gas phase reactivities are sometimes referred to as intrinsic reactivities (74). An advantage of the gas phase comparison is that, since our understanding of aqueous solution and solvation is limited, we can avoid consideration of these factors. A related advantage of the gas phase comparison is that, with fewer system components, higher-level theory and more thorough computation can be applied. Disadvantages of using gas phase comparison state include the experimental difficulty in measuring gas phase reactions, their tendency to follow distinct mechanisms, and the very large electrostatic energetic interactions that can dominate in the gas phase but are greatly dampened in solution and, very likely, in the enzyme environment. On a practical level, large gas phase energetic terms (and uncertainties in them) can make comparisons difficult.
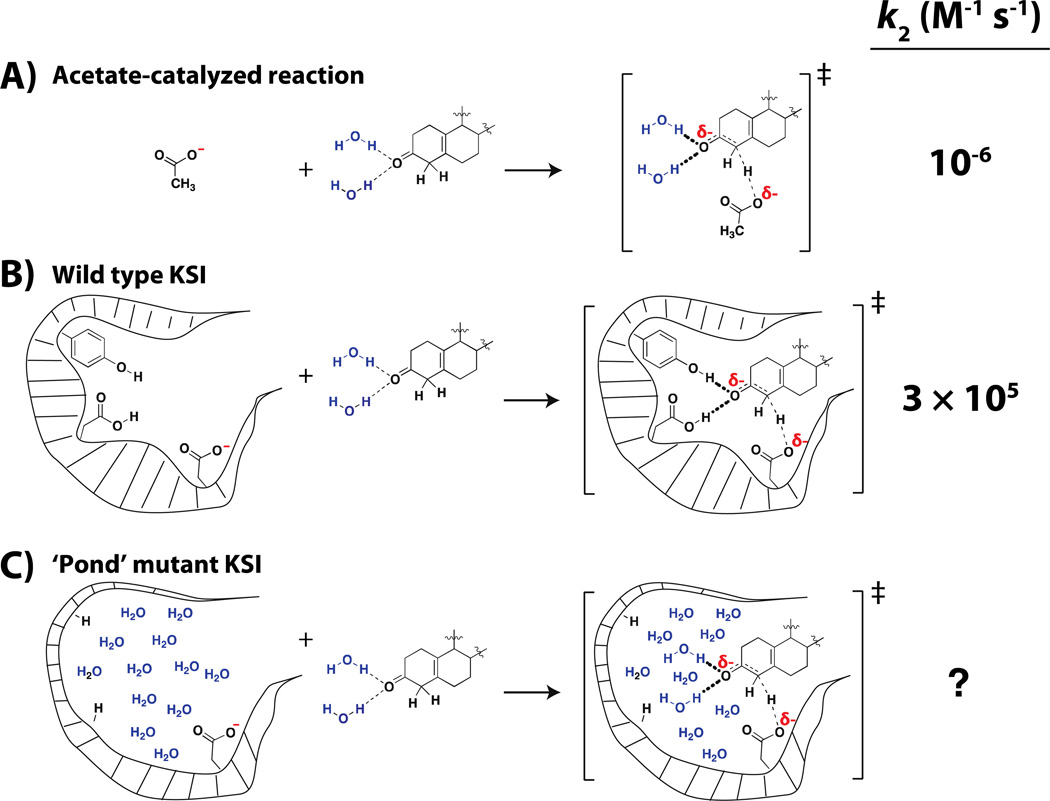
Figure 4. Evaluating the catalytic contribution from the KSI oxyanion hole.
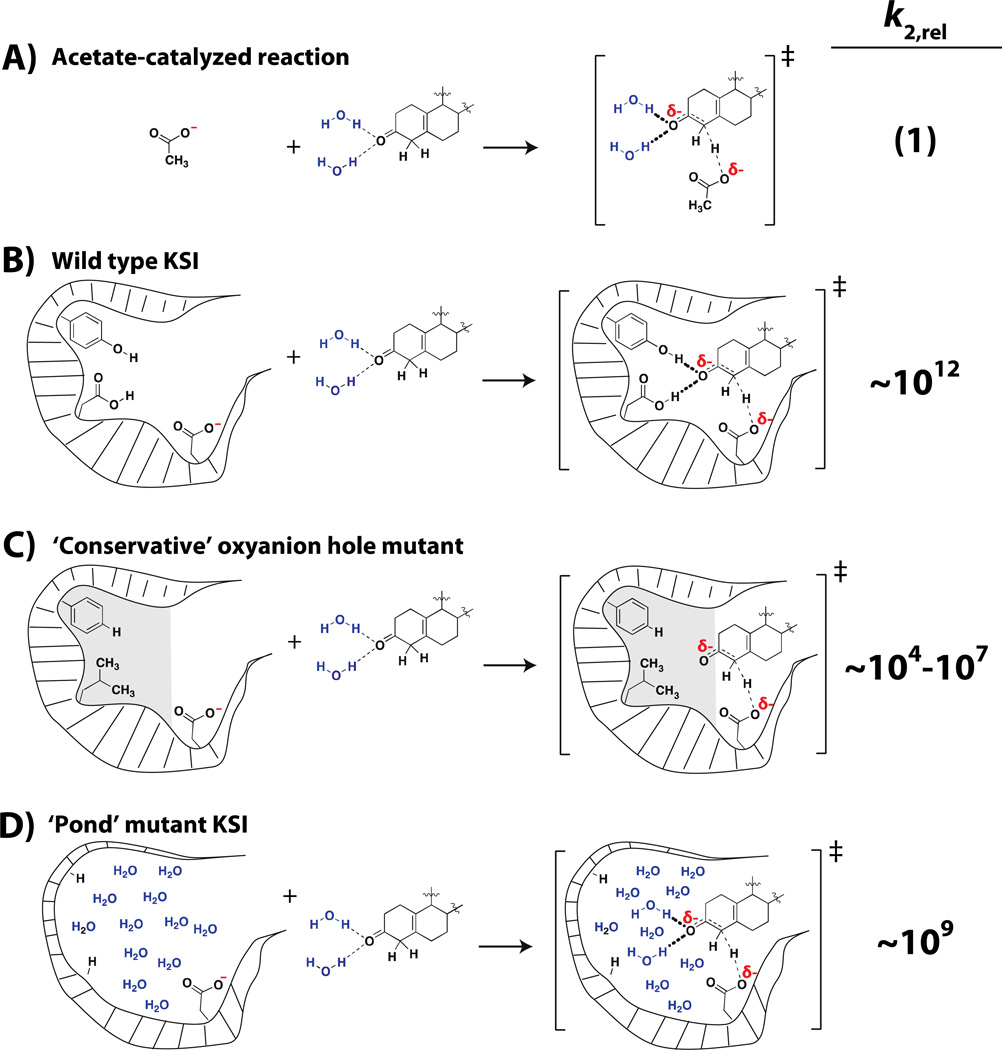
A) The second-order reaction between acetate and substrate in solution (75). B) The second-order reaction between wild-type KSI, which also uses a base with the same chemical reactivity as acetate, and substrate. Relative to the acetate-catalyzed reaction, wild-type KSI provides ~1012 fold catalysis (76). C) The second-order reaction between a ‘pond’ version of KSI, where the oxyanion hole has been replaced by an aqueous environment.
We have chosen, for now, to compare the KSI reaction to the corresponding solution reaction catalyzed by acetate (Figure 4A&B). The ratio of kcat/KM for the enzymatic reaction (for a substrate with a chemical rate-limiting step) to the second-order reaction with acetate ion, kOAc, is 1012 –i.e., by this comparison, KSI provides 1012 fold catalysis (75). We can now ask a defined and specific question: What is the contribution of the oxyanion hole to KSI catalysis relative to the analogous interactions in aqueous solution? Put into experimental terms: What is the catalysis of KSI relative to a version of this enzyme in which the oxyanion hole is replaced by an aqueous environment without compromising other catalytic features (Figure 4B&C)?
The above set of questions meets the criteria of being specific and of defining a comparison, rather than looking for an unattainable absolute energetic description. But that doesn’t mean the experiment can be done and the question answered. Indeed, mutations extensive enough to create a local aqueous environment will likely cause an enzyme to fall apart or greatly rearrange in most cases. For KSI, we appear to have been lucky, and there is even some evidence to suggest that the results obtained are reasonable and may be generalizable.
The comparison described above, reaction of wild type KSI versus the Tyr16Phe mutant (e.g., Figure 3) gave large deleterious effects. In most systems more extensive mutagenesis leads to greater rate effects, and this is typically attributed to secondary rearrangements that more broadly disrupt binding and/or catalysis. For KSI though, mutation of Tyr16 to Ala or several other small amino acids had a considerably smaller effect than the Phe mutation, and 19F NMR suggested a more, though not completely aqueous environment (72).
We decided to carry out extensive mutagenesis, to test whether a new group fortuitously provided partial rescue for the missing Tyr (and Asp) and to try to create a ‘pond’ where the oxyanion hole used to be –in other words, to remove sufficient bulk so as to allow access of the oxyanion to a large number of solvent molecules. Mutations were made to remove both hydrogen bond donors, to remove nearby Tyr residues to show that they could not substitute for Tyr16 or Asp103, and to remove surrounding residues to allow access by solvent molecules (Figure 5). The ability of KSI to maintain its overall catalytic structure was supported by x-ray structure of a mutant with both oxyanion hole hydrogen bond donors removed, by the negligible effects from truncation of more and more oxyanion hole side chains (to a total of nine side chains), and by the overall modest rate effects from this ablation (Figure 5; (76)).
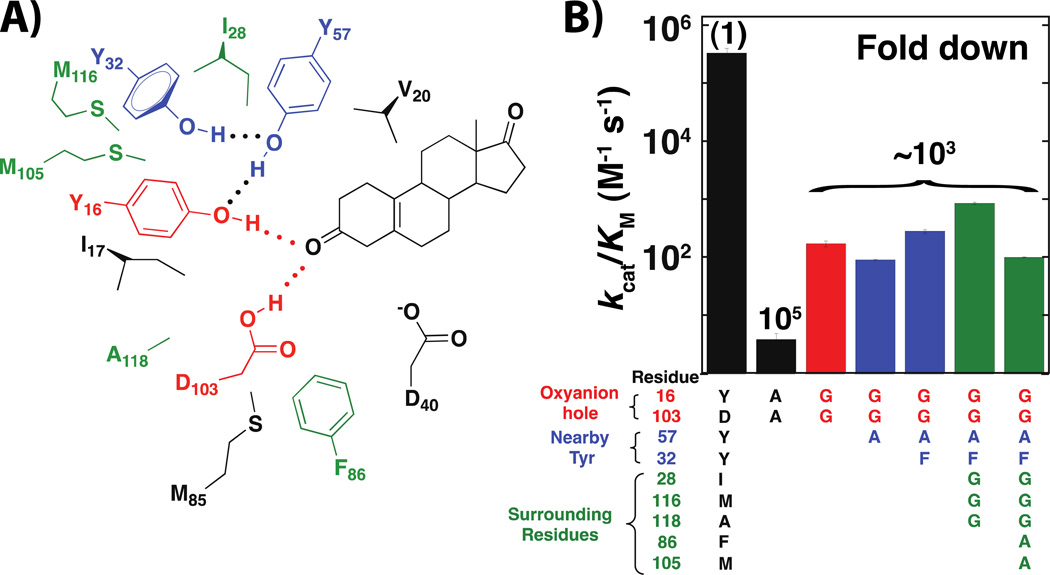
Figure 5. ‘Excavating’ the KSI oxyanion hole to evaluate its catalytic contribution.
A) Schematic of residues in and around the oxyanion hole, with the oxyanion hole residues shown in red, nearby tyrosine residues that could potentially replace the oxyanion hole donors shown in blue, and other residues surrounding the oxyanion hole shown in green. B) Rate effects from ‘excavating’ the oxyanion hole, with bars colored as in panel A from (76).
‘Pond’ mutants with space for ~20 water molecules resulted in only a ~103 fold rate decrease, far smaller than the deleterious effects from so-call ‘conservative’ mutations in either or both of the oxyanion hole hydrogen bond donors (Figure 5B; (76)). This means that KSI remains enormously efficient without its oxyanion hole, providing ~109 fold catalysis, relative to the nonenzymatic reaction with acetate ion (Figure 6). Had we assumed that all that was necessary to assess the catalytic contribution of the oxyanion hole was to ablate Tyr16 and Asp103 via the most conservative mutations, we would have concluded that the enzyme missing these residues provided only 104–107 fold catalysis (Figure 6), and that the oxyanion hole plays a far larger role in catalysis than it actually does.
Figure 6. Rate enhancement relative to the acetate-catalyzed reaction provided by different KSI variants.
A) The second-order reaction between acetate and substrate in solution. B) The second-order reaction between wild-type KSI and substrate. C) The second-order reaction between KSI with ‘conservative’ mutations to the oxyanion hole that replace the hydrogen bond donors with a hydrophobic environment. The rate enhancement provided depends on the particular mutant and substrate used (67, 69, 76). D) The second-order reaction between substrate and a version of KSI where the oxyanion hole has been replaced with an aqueous environment (76).
While it is possible that some of the contributions from binding and positioning and from general base catalysis are compromised due to the absence of the oxyanion hole or disruption of the internal electrostatics or dynamics of the enzyme, these would render the 103 fold effect an over-estimate. It is alternatively possible that removal of the oxyanion hole allows other interactions to be independently optimized and provide an increased contribution, a model that seems less probable but remains to be tested.
Thus, the KSI variant missing its oxyanion hole is a surprisingly effective catalyst, exhibiting a rate enhancement of 109 fold relative to the reaction with acetate in solution. We have shown that some of this rate enhancement arises from binding interactions that localize and position the substrate within the active site (75), and we have begun to dissect the KSI features that render it so effective in general base catalysis (77).
With subtilisin and related proteases, mutations of one of the two oxyanion hole hydrogen bond donors –the one coming from a side chain group rather than a backbone amide–to a wide range of amino acids give similar ~103 fold effects (48, 61, 62, 78). Given that this effect is not increased upon introduction of an anionic residue in the oxyanion hole (78) and that serine proteases bind substrates with their carbonyl facing away from the active site and into solution (79, 80), we suggest that the 103 fold effect for subtilisin may represent a similar complete loss of oxyanion hole interactions and replacement by an aqueous environment, although clearly additional tests and examples are called for.
Assuming that 103 represents a rough measure of the contribution of an oxyanion hole to KSI catalysis, how far does that take us toward understanding this contribution? Minimally, it defines an overall constraint –e.g., any model that invokes the oxyanion hole as the dominant catalytic factor or as necessary for effective general base catalysis is not correct. But these studies identify the size of an effect, not its origins. For example, the observed 103 fold effect represents the net of multiple differences that may include: the number of hydrogen bond donors (e.g., there may be three on average in solution but only two in the oxyanion hole); the partial positive charge on the hydrogen bond donor (hydrogen bond equilibria are dependent on partial charges, often empirically described by pKa and ΔpKa values; (81)); the degree to which these groups are restricted when their hydrogen bonds are formed in the transition state, relative to their freedom of motion in the respective ground states (i.e., prepositioning); effects of the extended environment (e.g., dipoles from the second shell and beyond); and whether the above factors affect the structural and energetic nature of the hydrogen bonds that are formed (e.g., (82, 83)). We have begun to address these questions (84–86), but much more work is needed.
Evaluation of Designed Enzymes
An ability to predict the behavior or properties of a system is valuable and often informative, but is not synonymous with understanding. Similarly, an ability to carry out design can provide an excellent test of one’s understanding, but doesn’t always, and the corollaries that ‘if you can design something you understand it’ and ‘if you can’t design it, you don’t understand it,’ are not correct. For example, a design can work that is not based on physical laws but rather on correlations derived from empirical observations or empirical computational algorithms (e.g., machine learning algorithms). Conversely, design can fail in the face of substantial understanding if a problem’s complexity exceeds computational capabilities. We consider two approaches to engineer or design new enzymes that have received the most attention and evaluate the lessons learned from these exercises.
Catalytic antibodies
Early efforts to ‘design’ enzymes involved the use of transition state analogs and the selection of antibodies that bound that analog, and the screening of the selected antibodies for catalytic activity (87, 88). Rate enhancements of up to 109 were observed, although more typical values ranged from 103–105, and there were presumably many antibodies with lower levels of activity that could not be distinguished from background (89–91). Given that naturally occurring enzymes typically provide much greater levels of catalysis, from ~1010 up to ~1029 fold (92, 93), ‘design’ efforts via catalytic antibodies have generally been viewed as limited in their success. What are the reasons for the limited catalytic capacity of these antibodies? Several models can be considered (see also Hilvert (91)):
The limited sequence space explored in antibody maturation (94) is likely less than typically explored in the optimization of new enzymes, and this exploration mainly via point mutations may not be as effective in developing new functions as the insertions and deletions that appear to be common in the evolutionary journeys between related enzymes (95).
The concentrations of antigen present as an antibody matures limits the affinity that can be selected for. For example, if the concentration is nanomolar, then there is little selective pressure for tighter than nanomolar binding. In addition, concentrations that are too low would result in antibody-antigen binding that is too slow (e.g., a concentration of 1 pM would give association halftimes of an hour or more, assuming typical association rate constants of 106 – 108 M−1 s−1). To put this affinity in context, formal application of transition state theory for enzymes with rate enhancements of 1015 and 1025 leads to apparent transition state affinities of ~10−17 and 10−27 M respectively.6
The antibody scaffold may be ill suited to catalysis. Certain structures have been used in biology to catalyze many different reactions –the eightfold beta-alpha or TIM barrel (TIM = triose phosphate isomerase) is most discussed, and is found in 21 superfamilies with many distinct catalytic functions (96). The antibody scaffold may be less amenable to the variations needed for a wide range of catalysts or, as noted above, there may be insufficient exploration of loop and domain additions during antibody maturation to create extensive binding and catalytic interactions needed for efficient catalysis.
Transition state analogs are imperfect mimics of actual transition states so that optimal catalytic groups are not selected for. The following two models represent variations on this model.
Because there is no counter selection against strong ground state binding, there may be too-strong binding of the (structurally related) ground states, such that saturation is too easily achieved and product release readily becomes rate limiting. However, this model could account only for low values of kcat, not low values of kcat/KM (39).
Catalytic antibodies may lack dynamics required for optimal catalysis. Such a limitation, if important, could arise from the static nature of the transition state analog used in selection and/or the nature of the antibody motif.
None of the above models is particularly profound. Yet they are rarely discussed together as a complete set. Of the above models, the suggestion of a need for dynamics is the most provocative, and indeed, it has sometimes been argued that the limited capabilities of catalytic antibodies provide evidence for the importance of dynamics in enzymatic catalysis. But this perspective ignores the other models and potential explanations, which are expected to hold to at least some degree.
Most generally, there is a need in enzymology and all areas of the biological and chemical sciences to go beyond ‘favorite models.’ There is a tendency in science to emphasize (and perhaps overstate) new or exotic mechanisms, as these seem most exciting and are likely to draw the most attention –and because we as investigators get most excited about the possibility of a novel explanation (e.g., (97)).7
To curb this temptation and to remain true to the scientific method we urge a two-step process: drawing out all possible models, as we have attempted to do above for the catalytic antibody case, and testing those models. Further, those tests need to be of a type that can readily falsify a model (98, 99); too often so-called ‘tests’ of models are in reality a quest for observations that are consistent with a particular favored model. As tests of the above models with catalytic antibodies have been limited, we turn to recently designed enzymes and mechanistic tests of the design features that were used.
Evaluation of a designed enzyme
There has been great excitement about the recent design of enzymes catalyzing retroaldol condensation, Kemp elimination, and other reactions (100–105). This work has been published in the highest profile journals, accompanied by perspectives (106, 107), and has been the subject of several news and review features (e.g., (108–112)).
It is remarkable that computational algorithms can be used to determine arrangements of amino acids that often lead to stable, folded proteins (113–115). And it is further remarkable that some of these folded proteins can be engineered to be catalysts. But it is also true that some proteins like bovine serum albumin (BSA), which did not evolve to be a catalyst, can catalyze certain reactions (116), and that enzymes can often catalyze reactions in addition to the reactions they were selected to carry out (117–119).8 Indeed, catalysis by these non-designed and ‘accidental’ enzymes is often similar to or greater than those of designed enzymes (111, 116).
Given this backdrop, how can we gain perspective on these new findings in the face of the associated excitement? We suggest that rational evaluation of designed enzymes is needed, first by determining the overall rate enhancement of the designed enzyme, and then by evaluating the individual designed catalytic features. We carried out such a study for one of the first designed retroaldolase enzymes, referred to as RA61, as outlined below (120).
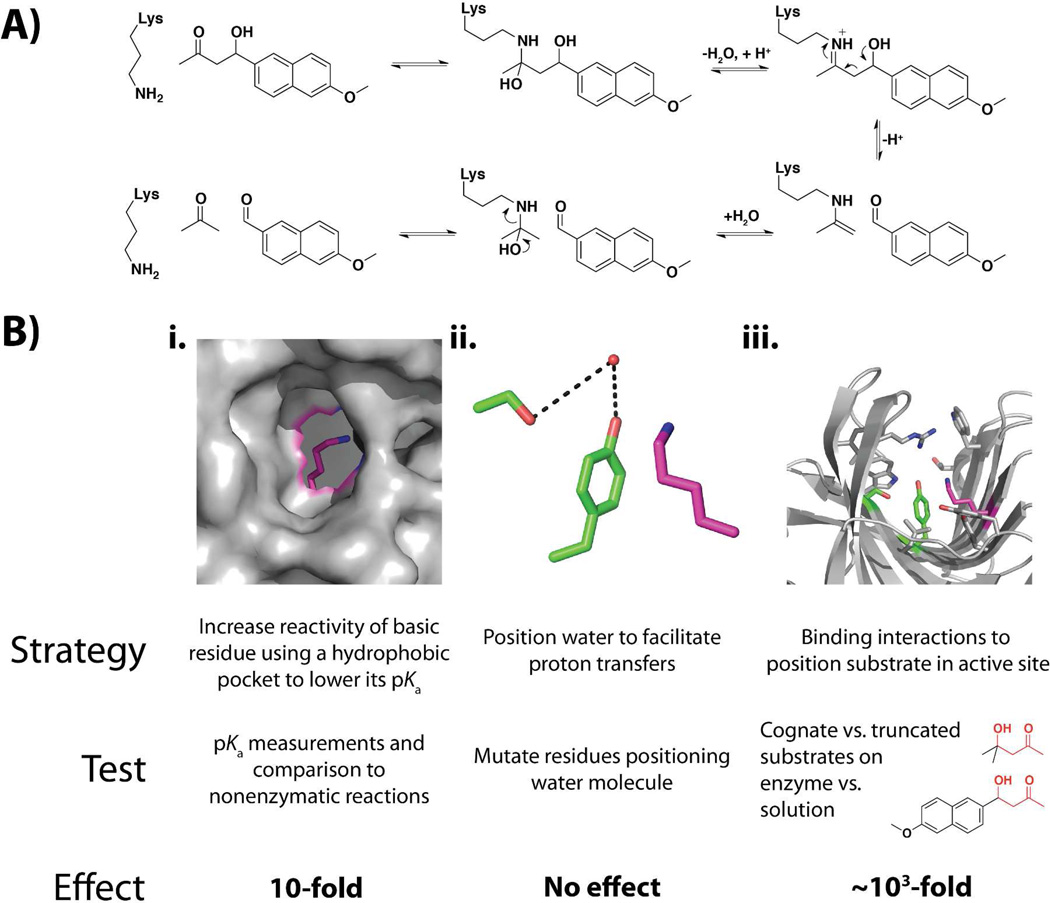
Figure 7A shows the retroaldolase reaction catalyzed by RA61. The substrate is not known in biology, and its leaving group is fluorescent, which simplifies activity assays. RA61 utilizes a primary amine of an active site Lys residue to form a Schiff base and act as an electron sink, as shown in the figure. Figure 7B shows the three design features of RA61: i. a catalytic Lys residue buried in a hydrophobic pocket to lower its pKa and thereby increase the amount of reactive neutral amine present at pH 7; ii. protein side chains to position a water molecule and thereby facilitate proton transfers; and iii. a hydrophobic pocket for binding and positioning the substrate.
Figure 7. Evaluating a computationally designed retroaldolase.
A) Reaction schematic with some proton transfers omitted for brevity. B) Strategies that were a part of the computational design and results from evaluating each of their catalytic contributions. The active site lysine is shown in magenta, the residues positioning water are shown in green, and residues in the binding pocket are shown in grey. Results are from (120).
We first determined the overall catalysis by this enzyme by comparing the second-order reaction with RA61 (kcat/KM) with the second-order nonenzymatic reaction with a simple analogous primary amine. The rate enhancement of 105-fold is very low relative to those for naturally occurring enzymes, which typically provide rate enhancements of >1010 fold and can provide rate enhancements of up to 1029-fold (92, 93). Low catalytic efficiency is a general observation for enzymes designed to date (111).
We next evaluated each of the catalytic strategies for their contribution to this overall rate enhancement (Figure 7B; (120)). The pKa of the reactive Lys was indeed lowered, by ~3 units to a pKa of ~7, as envisioned in the design. This lowering gives a higher fraction of the reactive neutral amine at pH 7 but is also expected to reduce its reactivity by rendering it a less strong base. Measuring the non-enzymatic reactivity of a series of primary amines of varying pKa allowed us to determine this reduction in reactivity, which partially counterbalances the increase in the fraction of neutral amine, and allowed us to estimate an overall catalytic effect of (only) 10-fold from this first catalytic strategy (Figure 7B, i). Mutation of the residues designed to facilitate proton transfers via a positioned water molecule gave no rate decrease, strongly suggesting that this catalytic design was unsuccessful (Figure 7B, ii).9 Finally we utilized a truncated substrate, missing most of the hydrophobic binding surface but maintaining the same reactive center and essentially the same nonenzymatic reactivity, to assess the contribution of binding interactions to catalysis (Figure 7B, iii). The rate enhancement for the truncated substrate was ~103 fold less than for the full substrate, and the full binding effect is likely larger since the truncated substrate retained two methyl groups that could interact in the binding pocket. In summary, of the overall 105-fold effect, 10-fold arises from lowering the Lys pKa and all or nearly all of the remaining catalysis arises from binding interactions that presumably localize substrates near to this Lys residue.10
As noted above, the overall catalysis by RA61, and other designed enzymes, is much lower than typical of evolved enzymes, and the following observations reveal additional properties typical of natural enzymes that are not reproduced by the design (120): i. The designed enzyme operates on both substrate enantiomers with equal or near equal efficiency; in other words, stereospecificity, a hallmark of naturally occurring enzymes, is lacking. ii. The aldehyde reaction product ‘slides’ over in the active site to form a Schiff base with the active-site Lys and strongly inhibit the reaction. Evolved enzymes rarely exhibit such promiscuous binding and typically are not subject to strong product inhibition.
The designed enzymes, like non-enzymatic, biomimetic catalysts that preceded them (121), use positioning, or co-localization, of reactive groups to facilitate reaction –in this case the Lys side chain and the substrate carbonyl group. But the results above suggest that this positioning is not sophisticated or optimized. Indeed, the designed enzymes can be improved by screening of mutant libraries for more active variants, and these results suggest limitations in packing and binding arrangements in the original design, consistent with the low specificity (111, 122, 123). In addition, the designs did not utilize hydrogen bonding for positioning, due to practical computational limitations (102), whereas natural enzymes typically use extensive networks of hydrogen bonding and hydrophobic packing to position groups, and hydrogen bonds generally provide greater precision in positioning than hydrophobic interactions (124).
Larger rate enhancements will be possible with designs utilizing more hydrophobic surface area for binding and incorporating highly reactive abiological active site cofactors, but such successes will not illuminate the mechanisms underlying the highly efficient and specific catalysis of naturally occurring enzymes. Are the low observed rates in designed enzymes because of suboptimal use of catalytic strategies or because not enough strategies or interactions were used? For the retroaldolase it seems likely that both play a role –non-optimized positioning of the reactive Lys residue and substrate and an absence of facilitating interactions with the reactant’s oxygen atoms. Additional complications are introduced by attempting to design a catalyst for a multi-step reaction such as retroaldol condensation, although rate enhancements for one-step reactions have been similar (111).
While we cannot rule out new or exotic mechanisms that are fundamental to enzymatic catalysis but currently beyond our ken, it seems likely that the predominant reasons underlying the engineering shortcomings are limitations in the selection process for catalytic antibodies and limitations in computational power and accuracy for designed enzymes. So it seems that design, while captivating and potentially transformative, for now represents an imperfect use of recognized catalytic strategies.11
Outlook and Future Questions
Overall, despite our limited practical capabilities in enzyme engineering and the lack of a complete and quantitative description of enzymatic catalysis, we have a good sense of the mechanisms employed by enzymes and fundamental principles that underlie their action, built up from many contributions and multiple perspectives over the past half-century. But there are also real limits to our understanding.
Enzymes are complex systems with non-additive energetics, so by definition simple, fully additive descriptions do not exist. Consider, for example, our dissection above of KSI’s oxyanion hole. We removed the oxyanion hole and replaced it with a ‘pond,’ thereby providing a meaningful comparison to the corresponding reaction in aqueous solution. But the Tyr and Asp residues when present in the oxyanion hole depend on neighboring residues and the overall enzyme structure and architecture to provide their stabilizing hydrogen bonds. And the energetics of these hydrogen bonds and the comparison to the hydrogen bonds present for the solution reaction are very complex. A current challenge might be to attempt to engineer or reengineer just a portion of an enzyme such as an oxyanion hole, and follow that with in-depth structural, dynamic, and energetic analyses.
A corollary of the above-noted non-additivity, and a complementary way to appreciate the inherent challenges in understanding the energetics of enzyme catalysis, is to recognize that there is no single description or comparison that will yield a full understanding. We introduced this important point in the section above on KSI, prior to comparing the enzymatic reaction to the reaction in aqueous solution following the same overall reaction mechanism, noting that other comparisons are possible and will be instructive.
Specific unresolved (and sometimes ill-defined) issues tend to be the focus of discussion. This is natural for the cutting-edge literature, but can also obscure our foundational understanding and substantial foundational agreement.12 We have emphasized above the need to clearly define unresolved questions and to clearly lay out comparisons to allow enzymologists to communicate effectively with one another and with those outside the field, and to allow enzymologists to identify important areas that are in need of further investigation.
Below we highlight some current questions that help elucidate outstanding challenges to push our understanding of enzyme catalysis to the next level. These questions will need to be further honed in the context of specific systems and in no way represent the full set of challenges and opportunities for enzymologists, but we hope that they stimulate discussion and new experiments to address these and other fascinating questions that lie ahead in enzymology.
Current Questions in Mechanistic Enzymology
How good are enzymes at positioning substrates and catalytic groups?
The statistical mechanical concept of conformational positioning through reduction in translational and rotational entropy is simple, as initially outlined for enzymologists by Page and Jencks (125), yet assessing these effects in complex solution and enzyme environments remains an unmet challenge (40, 126– 128).
NMR relaxation methods can provide information about dynamics over a wide range of timescales to assess the degree and types of motion that remains in enzyme-substrate and enzyme-transition state analog complexes, and there are exciting pioneering efforts to use NMR data to learn about conformational ensembles and even conformational entropy (129–131). In principle computational approaches can be used to dissect these effects, but computation of conformational ensembles is a highly challenging problem –and coupling of such computations to the quantum mechanical approaches required to obtain information about transition states is still more challenging.
Computation has the potential to ‘see’ events on the atomic scale, a scale not possible with current experimental approaches, which explains the popularity of such approaches in enzymology. Nevertheless, there is growing recognition that computational results must be directly tied to nontrivial experimental predictions in order to move this and other fields forward (e.g., (132–134)). Stated another way, computational results are models (based on approximate force fields and quantum mechanical treatments), and empirical (experimental) observation is our direct window to reality that we require in testing all models –computational and experimental. An enormous future challenge is to bring together conceptual, experimental, and computational approaches to frame and quantitatively address the question of substrate and catalytic group positioning.
What are the energetic consequences of carrying out reactions in the ‘special’ environment of the enzyme interior?
It is clear that the energetics of association, such as complex formation involving hydrogen bonds referred to above, is highly sensitive to the surrounding environment. It is also clear that an enzyme interior is different than an aqueous environment, the gas phase, or any continuous solvent. But it is much harder to understand that unique environment –in part because it is in direct physical contact with the reactants, unlike a homogeneous dielectric material that responds to electric fields through space and can be described by a dielectric constant, and in part because it is not homogeneous –instead it is an idiosyncratic, heterogeneous medium that responds to charge and charge rearrangement differently at different points within and it cannot be characterized by any single descriptor.
There have been many interesting proposals about the potential importance of the enzyme environment, beyond its role in positioning groups for reaction. Models include creating an environment where hydrogen bonds are stronger; positioning dipoles throughout the enzyme to ‘focus’ electrostatic stabilization of the transition state; and excluding water to eliminate its ability to dampen differential electrostatic effects between ground and transition states (e.g., (71, 135–138)).
Advances in vibrational and Stark spectroscopy with site-specific probes are providing new windows into local electrostatic fields within enzyme interiors and how these fields respond to ligand binding and charge rearrangements (139–144). Coupling such measurements to functional studies that correlate changes in electrostatic fields and effects on catalysis, binding of transition state analogs, and proton transfer equilibria provide novel probes of the energetic effects of the enzyme environment (84, 143, 145). Our probes of the energetics of KSI catalysis, including the work described above uncovering a smaller-than-expected contribution of its oxyanion hole, suggest that sculpting of the electrostatic environment of proteins beyond the interacting groups may contribute less than is often proposed (76, 84).
Another challenge arises because electrostatic and dynamic properties of the enzyme interior that contribute to and affect transition state interaction energies, are also connected to the ability of enzymes to position groups for reaction. This connection underscores the interrelatedness of catalytic features and mechanisms and the ongoing challenge to understand these fundamental catalytic contributors.
Are transition states changed on enzymes?
There are many examples of reaction pathways on enzymes that are distinct from those in solution due to the recruitment of cofactors and the placement of reactive groups in the enzyme active site. Here we consider reactions that, ostensibly, follow the same reaction mechanism in active sites and in solution, and ask the question of whether the transition states are similar or different in those different environments.
For group transfer reactions, such as glycosyl and phosphoryl transfer, the preponderance of evidence suggests that transition states on and off enzymes are generally similar in character – with oxocarbenium-like transition states for glycosyl transfer and loose transition states for phosphoryl from mono-substituted species (36, 146, 147). Nevertheless, extensive isotope effect data on glycosyl transfer reactions from Schramm and others has revealed differences in isotope effects for reactions of the same or similar substrates carried out in the active sites of different enzymes (36, 148–150). Thus, there are differences in these transition states off and on enzymes, though the scale of these changes may be modest.
There have been more extensive discussions of changes in reaction pathways for reactions that involve proton, hydrogen, and hydride transfer, based on extensive isotope effect studies and substantial modeling work (151–156). Indeed, primary and secondary isotope effects for KSI, our highlighted enzyme above, are different in the corresponding solution (acetate-catalyzed) and enzyme-catalyzed reactions and consistent with enhanced coupled motion of the substrate along the reaction coordinate in the enzymatic reaction (156). But it is difficult to ascertain whether the changes in reaction properties in this and other enzymatic reactions are causative for catalysis. Thus, the current challenge is to understand why these changes occur – both their physical origins and their significance for rate enhancement. Stated another way, are these changes selected for because they cause the reaction to be more efficient, or are they simply a consequence of placing reactants in an enzymatic environment or in different enzymatic environments?
Considering a more specific current model, it has been suggested that reactions of enzyme-bound species occur preferentially from transient conformers that are higher in energy than the most prominent conformational states but have the reactants more closely positioned (157, 158). An appealing aspect of this model is that tunneling probability (and thus reaction rates) is expected to be increased by transiently bringing reactants closer together and by matching their energy levels with those of the products, as tunneling probability is highly dependent on barrier width and matched energy states (158). Of course positioning is also an important catalytic factor in group transfer reactions, and in those reactions substrates must approach more closely than the sum of the van der Waals radii to allow a new bond to form, so predominant reaction from states other than the preferred ground state may hold in those cases as well. Questions that arise from these models include the following: Does the general tendency to restrict conformational freedom in active sites cause both rate enhancements and altered vibrational properties (and thus observed isotope effects)? Or are special vibrational modes that increase the probability of forming transient matched reactant/product energy states selected for?13
Regardless of the extent of direct catalytic relevance, isotope effects, because they are highly sensitive reaction probes, will likely continue to provide a window into the detailed properties of enzymatic reactions and a strong experimental constraint on computational models.
Recently, enzymes have been made heavy via isotopic substitution and potential promoting vibrations within enzymes have been explored (159–162). These exciting and provocative results will be subject to further experimental tests over time, and questions analogous to those above will be posed: As all reactions require vibrations to exchange energy and react, are promoting vibrations in enzymes specially selected for, or are the observed vibrational properties a consequence of being a molecular system (and especially one that is tightly packed)? And if selected for, what is the rate advantage contributed by this mechanism, distinct from other roles of residues and networks engaged in these vibrations?
Are there special dynamic properties of enzymes that contribute to catalysis?
Our understanding of a system can be only as good as the clarity of our fundamental definitions and descriptions of models, as we have emphasized herein. And there are so many facets of dynamics in an enzymatic reaction that it is often difficult to know what aspects are under discussion and what precise comparison is being made.14
Consider the question: “Are dynamics important for enzyme function?” If the enzyme is myosin, the answer is ‘of course,’ because motion is the function of the enzyme. Or if one is asking questions about the most basic process of bond making and bond breaking, then dynamics are ‘of course’ important because bond making and breaking is dynamic by definition. Although the answers to the questions above are both ‘yes,’ these answers are not illuminating because our questions are not clear and precise.
Consider then a different question: Does a loop closure event help catalysis? Now we are focused on a particular dynamic event. But we need to hone the question still further, and define comparison states. What if we compare the enzyme to one that is locked (hypothetically or via mutagenesis) with its loop in the open state: Does the loop closure help? In this comparison the loop closure helps because, say, it brings a general base to the substrate that otherwise would not be able to participate in the reaction. We have an answer, and although this answer alone doesn’t provide much enlightenment, it spurs us on to ask additional questions.
For example, let’s now make our comparison state the enzyme ‘locked in’ (or favoring) the closed state.15 We can ask a very simple question: Is the rate of the chemical step the same for the wild type and locked enzyme? We have a strong expectation that it will be –which helps refine the model of what loop dynamics are doing for the enzyme.
Following this expectation, let us now assume that the overall loop opening and closing event per se does not contribute to reaction probability within the closed ensemble of states, a quite reasonable supposition. We can now move to the next set of questions about why the loop and loop motion exist, and we focus on this question both because loop and domain closures are common in enzymes and because there has not always been clarity surrounding analysis of these phenomena. In the interest of brevity we list possible models:
Loop closure excludes solvent and thus may alter the electrostatic environment or exclude water that could engage in side hydrolysis reactions (163, 164). These mechanisms are often assumed in the literature and in textbooks, without consideration of other possible models.
There is no benefit from a flexible loop, relative to the same interactions arising from a fixed conformational element, but evolution took a pathway that introduced these interactions via insertion of a sequence coding for a loop. It is possible that loops are frequent in enzymes because they arise via an evolutionarily probable path.
Loop and domain closure events often create a bound state from which substrates and products cannot escape –and conversely, states that would be inaccessible for substrates to bind to. So, if the favored catalytic state is ‘closed,’ then an ‘open’ state is needed for substrate and product ingress and egress. This model requires coupling with (at least) one of the other models to account for why the closed state exists. A related model is that loop closure slows release of reactive intermediates that could have deleterious side reactions (165).
The simplest argument for the advantage of a closed state is that closed states allow an enzyme to maximize interactions with its substrates –forming interactions with all regions and surfaces of the substrates (35, 166). Maximizing interactions with substrate functional groups in turn can provide specificity for the correct substrates and can provide the highest catalysis via optimal positioning of substrates and active site groups.
Superficially, it would seem that increasing interactions with substrates would continually increase reaction specificity. But when binding is too strong, the favored substrate gets ‘sticky,’ such that every time it binds it goes on to react, so that further increases in the rate of conversion of bound species increases the reactivity for non-cognate substrates alone, thereby lowering rather than raising specificity (167, 168). Thus, having a conformational step that weakens binding can increase specificity. It can also increase KM values and thereby prevent premature saturation (167, 169).
The above are fascinating models that, through the process of laying them out, provide clarity and directions for current and future research.
In this Perspective we’ve focused mainly on the actual chemical reactions –i.e., the transformations once substrates are bound. Reactions are, by definition, dynamic processes. As introduced above, all reactions, enzymatic and nonenzymatic, require vibrations and energy exchange, so observation of these features alone does not indicate that these are catalytic properties that have been selected through evolution to enhance catalysis. Nevertheless, many enzymes have moving parts that are integral to performing work or assembling the active form of the enzyme/substrate complex, and all proteins are (much) more than the single static structure that we so clearly see in x-ray crystallography. But regardless of whether these dynamic properties are or are not selected aspects of the enzyme’s catalysis, we want to understand the dynamics in order to better understand the basic properties of enzymes and active sites and to derive better and more complete energetic models for enzymatic catalysis in terms of microstates of the enzyme/substrate complex, the probability of reaction from each microstate, and the dynamic properties of the reactions from each of those microstates. While an ability to provide such comprehensive and detailed descriptions remains a ways off, this vision helps us focus on a deep understanding of and an in-depth description of enzymatic reactions.
Closing Remarks
In closing, we are awed by the insights and advances in enzymology over the past decades and even centuries, arising from careful experimental observations, clever analyses, and deep consideration of conceptual issues. We believe that there is much more excitement ahead for enzymology. This excitement builds on a strong foundation and a newly emerging ability to apply multiple experimental and computational approaches focused on ‘well-described’ systems with well-honed questions, and this will lead us to a deeper and more comprehensive understanding of enzymes and how they act. There are many other exciting areas where enzymology will leave its mark that have not been emphasized herein. One important area to note as we traverse the post-genomic era and move from identifying genes to the processes and interactions that they are involved in, is that the tools and approaches of enzymology have an enormous amount to offer towards understanding the processes that underlie all of biology –how and why they occur as they do, and how they might be manipulated in engineering and medicine.
Acknowledgement
We thank John Richard, Jesse Zalatan, Paul Sigala, Jason Schwans and Max Greenfeld for comments on the manuscript, and members of the Herschlag lab for stimulating discussions.
This work was supported by grants from the National Institutes of Health (GM64798) and the National Science Foundation (MCB-1121778) to D.H. A.N. was supported in part by a Howard Hughes Medical Institute International Student Research Fellowship, a Natural Sciences and Engineering Research Council of Canada Postgraduate Scholarship, and a William and Sara Hart Kimball Stanford Graduate Fellowship.
Abbreviations
- EM
effective molarity
- GS
ground state
- KSI
ketosteroid isomerase
- TS
transition state
Footnotes
While this statement holds in general, there remain numerous frontiers in enzymatic reaction mechanism where the reaction intermediates and the basic reaction pathways have yet to be elucidated. Many of these remaining reactions involve radical and redox chemistries, which have been more difficult to develop non-enzymatic models for. There is much vibrant and exciting current research in this area.
E.g., if an interaction with an unreactive region of a substrate remains the same throughout the course of a reaction: E•S → E•P, then (in the simplest model) removal of that interaction would not affect the probability of reaction (i.e., the reaction rate). That interaction, though, is made upon binding (E+S → E•S) so that it can increase the probability of reaction from free E and S. One can then describe such an interaction as providing ‘uniform binding’ in the ground and transition states. Consideration of these interactions in the transition state complex as if they were equilibrium interactions is useful conceptually and, further, allows predictions about the catalytic consequences of changes in such interactions (see accompanying Perspective by Amyes and Richard).
The fundamental interdependence of contributions to catalysis –i.e., the non-additivity- can be seen most generally from a complete mutagenic cycle for a hypothetical enzyme, all the way to converting each and every residue to Ala (40). Mutations of the so-called catalytic residues reduce catalysis, as expected. But if all of the binding residues are gone (mutated) or all of the ‘structural’ residues are gone, then introducing these same ‘catalytic residues’ does not provide catalysis. Similarly, if the ‘binding’ and ‘catalytic’ residues are present but the ‘structural’ residues are removed, there is no significant catalysis. Now if we add back the ‘structural’ residues sequentially, we will cross some threshold at which catalysis becomes significant, and catalysis will continue to increase as additional ‘structural’ residues are added back, until a point is reached at which the structure is sufficiently robust and there is little or no additional catalytic advantage from re-introduction of the final residues. But again, had those residues been added earlier, then they would have been counted among residues that provide catalysis. Thus, ‘structural,’ ‘binding’ and ‘catalytic’ residues all contribute to catalysis. However, at least some of their contributions are masked in the types of experiments that are typically performed by us and accessible to us (40, 41, 170). The energetic description of the catalytic contributions of residues changes in accord with the suite of other residues that are present. Thus, there is no unique or complete description of enzymatic catalysis that can be made in terms of its chemical components; the system is not made up of additive and independent components.
As the dienolate is a transiently stable species, we expect two transition states, for formation from substrate and for formation of product (or, equivalently, for partitioning of the intermediate to substrate or product). These transition states are expected to have very similar properties and for simplicity we consider only one transition state. There are also geometrical changes that we also do not address here for simplicity. Some discussions of geometric complementarity can be found in the following references: (80, 85), and future work will address geometrical effects in the KSI reaction (unpublished results).
The details of the nonenzymatic and enzymatic reactions can, in principle, vary, due to different positioning and environmental effects that bias the nature and properties of the reactions’ transition states (see “Are transition states changed on enzymes?” and (36, 147)).
These apparent affinities are obtained by application of transition state theory, which postulates that chemical reactions proceed via the formation of a rare species, the transition state, that exists in equilibrium with the ground state reactants (with equilibrium constant K‡), and that upon formation, the transition state decomposes within the lifetime of a bond vibration to generate products (with a rate constant v). Within this framework, the second-order rate constant for an uncatalyzed reaction between water and a substrate is given by k2 = vK‡ = v[S‡]/([H2O][S]). Similarly, the apparent second-order rate constant for product formation in the presence of enzyme and substrate under sub-saturating conditions kcat/KM = v[ES‡]/([E][S]). If rate acceleration is defined as (kcat/KM)/k2, then by standard algebraic manipulation, the formal affinity of the enzyme for the transition state Kd (ES‡) = [E][S‡]/[ES‡] = [H2O](kcat/KM)/k2. See (37, 38) for details.
One of us recalls Bob Abeles saying the following: “If a result seems too good to be true, chances are it is. (But of course you should test it and find out.)” The same advice is often given about buying bridges, other financial investments, and advertising claims for weight loss, hair gain, and other miracle cures.
This property is referred to as ‘catalytic promiscuity’ and likely has been central in the evolution of new enzymatic activities (171, 172).
This result provides strong evidence that the desired design was not met. In contrast, an observation of a large rate decrease would not show that the designed mechanism was employed-rather that result would show that removal of a particular side chain and replacement by another has a deleterious effect on some aspect of catalysis. Further studies would then be required to address mechanistic origins of the observed effect.
We have not addressed the important question of non-additivity directly, but the Lys pKa is lowered for free RA61, suggesting that the 10-fold enhancement in amine reactivity at pH 7 is not interdependent with the binding interactions (in the context of the already folded protein).
Indeed, designs to-date have used theoretical templates, ‘theozymes,’ derived from reasonably sophisticated quantum mechanical models. But there is no indication that designs require this level of sophistication or have this level of discrimination. It would be interesting to carry out blind design trials with different assumed transition states and with crude models of transition state partial charges and geometries map conjured by knowledgeable organic chemists.
It is also important to recognize that even in the midst of general agreement about catalytic principles, the details of any individual system present real challenges. Even the best-studied enzymes are not fully understood. Ribonuclease A, often touted as the consummate example of an enzyme with an established mechanism, lacks comprehensive and unifying models to account for the detailed roles and the energetics of the active site His and Lys residues and for the effects of substituting fluorohistidine for one or both of the active site His residues (173–176). It is often uncertainty about these (important) details that obscures broader agreement.
As a simple model, consider two unrestricted reactants in solution. They will occasionally come close, with a rather flat distribution of probabilities versus approach distance (all other factors being ignored in this simple illustrative example). Now imagine, again in an over-simplified illustrative example, that the only thing an enzyme does is to restrict the conformational space explored by reactants. In essence the reactants are placed in a box –and the better the enzyme the smaller the box. With a sufficiently small box –or a sufficiently restrictive enzyme– one can imagine that the bias in the conformational landscape could lead to a higher fraction of time that the reactants are very close (even if these aren’t the most probable states) and thus preferential reaction from the subset of very close states. We don’t know if such restrictions might preferentially enhance reactions that can involve tunneling, how difficult (or likely) it is to evolve such a mechanism for rate enhancement, and whether such a mechanism is generally accessible or idiosyncratic to particular reactions and active sites. As a null model, one can imagine that the restricted space could alter the vibrational properties of the reactants and transition state and thereby alter the observed isotope effects (Think of our reactants coupled by a simple spring potential; restriction in a ‘box’ would not allow the spring to fully expand but would not hinder spring contraction or close approach of our reactants). We emphasize that this is a purposely oversimplified example to help illustrate general questions and the null model in which an enzyme evolved to enhance catalysis by positioning reactants could also, as a consequence, alter vibrational properties, reactant distances from which tunneling occurs, and observed isotope effects.
We addressed some aspects of dynamics under the preceding question, but dynamics has been so widely discussed in enzymology over the past decade that it warrants additional attention. Because transition state theory does not account for the dynamic events in chemical transformations, there has been a tendency to argue against the utility of this framework. Nevertheless, all models are approximations, and the utility of a model is dependent on the questions being asked. We hope that the both the usefulness of the transition state theory approach, its limitations, and when it can and cannot be productively invoked are apparent from this and the accompanying Perspectives.
This comparison currently needs to be to a hypothetical enzyme, but creating actual enzymes to serve as these and other comparison states are good goals for current selection and design efforts, as such enzymes would serve the double goal of serving as tools for understanding and providing good systems for evaluating and honing our ability to carry out design.
References
- 1.de Réaumur RAF. Sur la digestion des oiseaux, second mémoire: De la mannière dont elle se fait dans l'estomac des oiseaux de proie. Acad R Sci. (Paris) de l'Année 1752, Mém. Math. Phys. tirè des Regist. 1761;2:701–743. [Google Scholar]
- 2.Spallanzani L. Translated from the Italian of the Abbe Spallanzani. London: J. Murray; 1784. Dissertations relative to the natural history of animals and vegetables. [Google Scholar]
- 3.Friedmann HC. Enzymes: benchmark Papers in biochemistry. Stroudsburg: Hutchinson Ross Publishing Company; 1981. pp. 6–151. [Google Scholar]
- 4.Buchner E. Alkoholische gährung ohne hefezellen. Ber. Dt. Chem. Ges. 1897;30:117–124. [Google Scholar]
- 5.Sumner JB. The isolation and crystallization of the enzyme urease. J. Biol. Chem. 1926;69:435–441. [Google Scholar]
- 6.Bugg TDH. The development of mechanistic enzymology in the 20th century. Nat. Prod. Rep. 2001;18:465–493. doi: 10.1039/b009205n. [DOI] [PubMed] [Google Scholar]
- 7.Bruice TC, Benkovic SJ. Bioorganic Mechanisms. New York: W.A. Benjamin, Inc; 1966. [Google Scholar]
- 8.Jencks W. Catalysis in chemistry and enzymology. 2nd ed. New York: Dover; 1987. [Google Scholar]
- 9.Sørensen SPL. Enzyme studies, second report: On the measurement and significance of the hydrogen ion concentration in enzymatic processes. In: Friedmann HC, editor. Enzymes (Benchmark papers in Biochemistry 1981) Stroudsburg: Hutchinson Ross Publishing Company; 1909. pp. 272–283. [Google Scholar]
- 10.Stern KG. On the mechanism of enzyme action. A study of the decomposition of hydrogen peroxide by catalase and of an intermediate enzyme-substrate compound. J Biol Chem. 1936;114:473–494. [Google Scholar]
- 11.Fisher HF, Conn EE, Vennesland B, Westheimer FH. The enzymatic transfer of hydrogen. I. The reaction catalyzed by alcohol dehydrogenase. J. Biol. Chem. 1953;202:687–697. [PubMed] [Google Scholar]
- 12.Schaffer NK, May SC, Summerson WH. Serine phosphoric acid from diisopropylphosphoryl chymotrypsin. J. Biol. Chem. 1953;202:67–76. [PubMed] [Google Scholar]
- 13.Hartley BS, Kilby BA. The reaction of p-nitrophenyl esters with chymotrypsin and insulin. Biochem. J. 1954;56:288–297. doi: 10.1042/bj0560288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voet JG, Abeles RH. The mechanism of action of sucrose phosphorylase. Isolation and properties of a beta-linked covalent glucose-enzyme complex. J. Biol. Chem. 1970;245:1020–1031. [PubMed] [Google Scholar]
- 15.Rose IA, O'Connell EL, Litwin S. Determination of the rate of hexokinase-glucose dissociation by the isotope-trapping method. J. Biol. Chem. 1974;249:5163–5168. [PubMed] [Google Scholar]
- 16.Midelfort CF, Rose IA. A stereochemical method for detection of ATP terminal phosphate transfer in enzymatic reactions. Glutamine synthetase. J. Biol. Chem. 1976;251:5881–5887. [PubMed] [Google Scholar]
- 17.Walsh CT. Enzymatic reaction mechanisms. San Francisco: W.H. Freeman; 1979. [Google Scholar]
- 18.Silverman RB. The organic chemistry of enzyme-catalyzed reactions. San Diego: Academic Press; 2002. [Google Scholar]
- 19.Sinnott ML, editor. Comprehensive biological catalysis: a mechanistic reference. San Diego: Academic Press; 1998. [Google Scholar]
- 20.Polanyi M. On adsorption catalysis. Z. Elektrochem. 1921;27:142–150. [Google Scholar]
- 21.Haldane JBS. Enzymes. London: Longmans, Green and Co; 1930. [Google Scholar]
- 22.Pauling L. Molecular architecture and biological reactions. Chem. Eng. News. 1946;24:1375–1377. [Google Scholar]
- 23.Thompson RC, Blout ER. Dependence of the kinetic parameters for elastase-catalyzed amide hydrolysis on the length of peptide substrates. Biochemistry. 1973;12:57–65. doi: 10.1021/bi00725a011. [DOI] [PubMed] [Google Scholar]
- 24.Moore SA, Jencks WP. Formation of active site thiol esters of CoA transferase and the dependence of catalysis on specific binding interactions. J. Biol. Chem. 1982;257:10893–10907. [PubMed] [Google Scholar]
- 25.Whitty A, Fierke CA, Jencks WP. Role of binding energy with coenzyme A in catalysis by 3-oxoacid coenzyme A transferase. Biochemistry. 1995;34:11678–11689. doi: 10.1021/bi00037a005. [DOI] [PubMed] [Google Scholar]
- 26.Wells TN, Fersht AR. Use of binding energy in catalysis analyzed by mutagenesis of the tyrosyl-tRNA synthetase. Biochemistry. 1986;25:1881–1886. doi: 10.1021/bi00356a007. [DOI] [PubMed] [Google Scholar]
- 27.Namchuk MN, Withers SG. Mechanism of agrobacterium β-glucosidase: kinetic analysis of the role of non-covalent enzyme/substrate interactions. Biochemistry. 1995;34:16194–16202. doi: 10.1021/bi00049a035. [DOI] [PubMed] [Google Scholar]
- 28.McCarter JD, Adam MJ, Withers SG. Binding energy and catalysis. Fluorinated and deoxygenated glycosides as mechanistic probes of Escherichia coli (lacZ) beta-galactosidase. Biochem. J. 1992;286:721–727. doi: 10.1042/bj2860721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stubbe J, Abeles RH. Mechanism of action of enolase: effect of the β-hydroxy group on the rate of dissociation of the α-carbon-hydrogen bond. Biochemistry. 1980;19:5505–5512. doi: 10.1021/bi00565a007. [DOI] [PubMed] [Google Scholar]
- 30.Kati WM, Acheson SA, Wolfenden R. A transition state in pieces: major contributions of entropic effects to ligand binding by adenosine deaminase. Biochemistry. 1992;31:7356–7366. doi: 10.1021/bi00147a021. [DOI] [PubMed] [Google Scholar]
- 31.Amyes TL, O'Donoghu AC, Richard JP. Contribution of phosphate intrinsic binding energy to the enzymatic rate acceleration for triosephosphate isomerase. J. Am. Chem. Soc. 2001;123:11325–11326. doi: 10.1021/ja016754a. [DOI] [PubMed] [Google Scholar]
- 32.Amyes TL, Richard JP, Tait JJ. Activation of orotidine 5′-monophosphate decarboxylase by phosphite dianion: The whole substrate is the sum of two parts. J. Am. Chem. Soc. 2005;127:15708–15709. doi: 10.1021/ja055493s. [DOI] [PubMed] [Google Scholar]
- 33.Shostak K, Jones ME. Orotidylate decarboxylase: insights into the catalytic mechanism from substrate specificity studies. Biochemistry. 1992;31:12155–12161. doi: 10.1021/bi00163a026. [DOI] [PubMed] [Google Scholar]
- 34.Jencks WP. Binding energy, specificity, and enzymic catalysis: the circe effect. Adv. Enzymol. Relat. Areas Mol. Biol. 1975;43:219–410. doi: 10.1002/9780470122884.ch4. [DOI] [PubMed] [Google Scholar]
- 35.Wolfenden R. Transition state analog inhibitors and enzyme catalysis. Annu. Rev. Biophys. Bioeng. 1976;5:271–306. doi: 10.1146/annurev.bb.05.060176.001415. [DOI] [PubMed] [Google Scholar]
- 36.Schramm VL. Enzymatic transition states and transition state analog design. Annu. Rev. Biochem. 1998;67:693–720. doi: 10.1146/annurev.biochem.67.1.693. [DOI] [PubMed] [Google Scholar]
- 37.Lienhard GE. Enzymatic catalysis and transition-state theory. Science. 1973;180:149–154. doi: 10.1126/science.180.4082.149. [DOI] [PubMed] [Google Scholar]
- 38.Wolfenden R. Analog approaches to the structure of the transition state in enzyme reactions. Acc. Chem. Res. 1972;5:10–18. [Google Scholar]
- 39.Fersht AR. Structure and mechanism in protein science: a guide to enzyme catalysis and protein folding. New York: W.H. Freeman and Co; 1999. [Google Scholar]
- 40.Kraut DA, Carroll KS, Herschlag D. Challenges in enzyme mechanism and energetics. Annu. Rev. Biochem. 2003;72:517–571. doi: 10.1146/annurev.biochem.72.121801.161617. [DOI] [PubMed] [Google Scholar]
- 41.Narlikar GJ, Herschlag D. Direct demonstration of the catalytic role of binding interactions in an enzymatic reaction. Biochemistry. 1998;37:9902–9911. doi: 10.1021/bi980495t. [DOI] [PubMed] [Google Scholar]
- 42.Pollack RM. Enzymatic mechanisms for catalysis of enolization: ketosteroid isomerase. Bioorg. Chem. 2004;32:341–353. doi: 10.1016/j.bioorg.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Wu ZR, Ebrahimian S, Zawrotny ME, Thornburg LD, Perez-Alvarado GC, Brothers P, Pollack RM, Summers MF. Solution structure of 3-oxo-Δ5-steroid isomerase. Science. 1997;276:415–418. doi: 10.1126/science.276.5311.415. [DOI] [PubMed] [Google Scholar]
- 44.Kim SW, Cha S-S, Cho H-S, Kim J-S, Ha N-C, Cho M-J, Joo S, Kim KK, Choi KY, Oh B-H. High-resolution crystal structures of Δ5-3-ketosteroid isomerase with and without a reaction intermediate analogue. Biochemistry. 1997;36:14030–14036. doi: 10.1021/bi971546+. [DOI] [PubMed] [Google Scholar]
- 45.Cho HS, Ha NC, Choi G, Kim HJ, Lee D, Oh KS, Kim KS, Lee W, Choi KY, Oh BH. Crystal structure of Δ5-3-ketosteroid isomerase from Pseudomonas testosteroni in complex with equilenin settles the correct hydrogen bonding scheme for transition state stabilization. J Biol Chem. 1999;274:32863–32868. doi: 10.1074/jbc.274.46.32863. [DOI] [PubMed] [Google Scholar]
- 46.Robertus JD, Kraut J, Alden RA, Birktoft JJ. Subtilisin. Stereochemical mechanism involving transition-state stabilization. Biochemistry. 1972;11:4293–4303. doi: 10.1021/bi00773a016. [DOI] [PubMed] [Google Scholar]
- 47.Hedstrom L. Serine protease mechanism and specificity. Chem. Rev. 2002;102:4501–4524. doi: 10.1021/cr000033x. [DOI] [PubMed] [Google Scholar]
- 48.Menard R, Carriere J, Laflamme P, Plouffe C, Khouri HE, Vernet T, Tessier DC, Thomas DY, Storer AC. Contribution of the glutamine 19 side chain to transition-state stabilization in the oxyanion hole of papain. Biochemistry. 1991;30:8924–8928. doi: 10.1021/bi00101a002. [DOI] [PubMed] [Google Scholar]
- 49.Joerger RD, Haas MJ. Alteration of chain length selectivity of a Rhizopus delemar lipase through site-directed mutagenesis. Lipids. 1994;29:377–384. doi: 10.1007/BF02537305. [DOI] [PubMed] [Google Scholar]
- 50.Nicolas A, Egmond M, Verrips CT, de Vlieg J, Longhi S, Cambillau C, Martinez C. Contribution of cutinase serine 42 side chain to the stabilization of the oxyanion transition state. Biochemistry. 1996;35:398–410. doi: 10.1021/bi9515578. [DOI] [PubMed] [Google Scholar]
- 51.Jiang M, Chen X, Wu X-H, Chen M, Wu Y-D, Guo Z. Catalytic mechanism of SHCHC synthase in the menaquinone biosynthesis of Escherichia coli: Identification and mutational analysis of the active site residues. Biochemistry. 2009;48:6921–6931. doi: 10.1021/bi900897h. [DOI] [PubMed] [Google Scholar]
- 52.Carlos JL, Klenotic PA, Paetzel M, Strynadka NCJ, Dalbey RE. Mutational evidence of transition state stabilization by serine 88 in Escherichia coli type I signal peptidase. Biochemistry. 2000;39:7276–7283. doi: 10.1021/bi000301l. [DOI] [PubMed] [Google Scholar]
- 53.Larsen NA, Lin H, Wei R, Fischbach MA, Walsh CT. Structural characterization of enterobactin hydrolase IroE. Biochemistry. 2006;45:10184–10190. doi: 10.1021/bi060950i. [DOI] [PubMed] [Google Scholar]
- 54.de Jong RM, Dijkstra BW. Structure and mechanism of bacterial dehalogenases: different ways to cleave a carbon–halogen bond. Curr. Opin. Struct. Biol. 2003;13:722–730. doi: 10.1016/j.sbi.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 55.Klimacek M, Nidetzky B. The oxyanion hole of Pseudomonas fluorescens mannitol 2-dehydrogenase: a novel structural motif for electrostatic stabilization in alcohol dehydrogenase active sites. Biochem J. 2009;425:455–463. doi: 10.1042/BJ20091441. [DOI] [PubMed] [Google Scholar]
- 56.Chen M, Jiang M, Sun Y, Guo Z-F, Guo Z. Stabilization of the second oxyanion intermediate by 1,4-dihydroxy-2-naphthoyl-coenzyme A synthase of the menaquinone pathway: Spectroscopic evidence of the involvement of a conserved aspartic acid. Biochemistry. 2011;50:5893–5904. doi: 10.1021/bi200376x. [DOI] [PubMed] [Google Scholar]
- 57.Hamed RB, Batchelar ET, Clifton IJ, Schofield CJ. Mechanisms and structures of crotonase superfamily enzymes - How nature controls enolate and oxyanion reactivity. Cell. Mol. Life Sci. 2008;65:2507–2527. doi: 10.1007/s00018-008-8082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larson JW, McMahon TB. Gas-phase bifluoride ion. An ion cyclotron resonance determination of the hydrogen bond energy in fluoride ion (FHF-) from gasphase fluoride transfer equilibrium measurements. J. Am. Chem. Soc. 1982;104:5848–5849. [Google Scholar]
- 59.Wenthold PG, Squires RR. Bond Dissociation Energies of F2- and HF2-A Gas-Phase Experimental and G2 Theoretical Study. J. Phys. Chem. 1995;99:2002–2005. [Google Scholar]
- 60.Kresge AJ, Chiang Y. Solvent isotope effects on the ionization of hydrofluoric acid. J. Phys. Chem. 1973;77:822–825. [Google Scholar]
- 61.Bryan PP, Pantoliano MWM, Quill SGS, Hsiao HYH, Poulos TT. Site-directed mutagenesis and the role of the oxyanion hole in subtilisin. Proc. Natl. Acad. Sci. U.S. A. 1986;83:3743–3745. doi: 10.1073/pnas.83.11.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wells JA, Cunningham B, Graycar T, Estell D. Importance of hydrogen-bond formation in stabilizing the transition state of subtilisin. Philos T Roy Soc A. 1986;317:415–423. [Google Scholar]
- 63.Carter P, Wells JA. Functional interaction among catalytic residues in subtilisin BPN'. Proteins. 1990;7:335–342. doi: 10.1002/prot.340070405. [DOI] [PubMed] [Google Scholar]
- 64.Braxton S, Wells JA. The importance of a distal hydrogen bonding group in stabilizing the transition state in subtilisin BPN'. J. Biol. Chem. 1991;266:11797–11800. [PubMed] [Google Scholar]
- 65.Vazeux G, Iturrioz X, Corvol P, Llorens-Cortés C. A tyrosine residue essential for catalytic activity in aminopeptidase A. Biochem. J. 1997;327:883–889. doi: 10.1042/bj3270883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuliopulos A, Mildvan AS, Shortle D, Talalay P. Kinetic and ultraviolet spectroscopic studies of active-site mutants of Δ5-3-ketosteroid isomerase. Biochemistry. 1989;28:149–159. doi: 10.1021/bi00427a022. [DOI] [PubMed] [Google Scholar]
- 67.Choi G, Ha N-C, Kim SW, Kim D-H, Park S, Oh B-H, Choi KY. Asp-99 donates a hydrogen bond not to Tyr-14 but to the steroid directly in the catalytic mechanism of Δ5-3-ketosteroid isomerase from Pseudomonas putida biotype B. Biochemistry. 2000;39:903–909. doi: 10.1021/bi991579k. [DOI] [PubMed] [Google Scholar]
- 68.Thornburg LD, Hénot F, Bash DP, Hawkinson DC, Bartel SD, Pollack RM. Electrophilic assistance by Asp-99 of 3-Oxo-Δ5-steroid isomerase. Biochemistry. 1998;37:10499–10506. doi: 10.1021/bi980099a. [DOI] [PubMed] [Google Scholar]
- 69.Thornburg LD, Goldfeder YR, Wilde TC, Pollack RM. Selective catalysis of elementary steps by Asp-99 and Tyr-14 of 3-oxo-Δ5-steroid isomerase. J. Am. Chem. Soc. 2001;123:9912–9913. doi: 10.1021/ja016683f. [DOI] [PubMed] [Google Scholar]
- 70.Zhao Q, Abeygunawardana C, Talalay P, Mildvan AS. NMR evidence for the participation of a low-barrier hydrogen bond in the mechanism of Δ5-3-ketosteroid isomerase. Proc. Natl. Acad. Sci. U.S. A. 1996;93:8220–8224. doi: 10.1073/pnas.93.16.8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cleland WW, Frey PA, Gerlt JA. The low barrier hydrogen bond in enzymatic catalysis. J Biol Chem. 1998;273:25529–25532. doi: 10.1074/jbc.273.40.25529. [DOI] [PubMed] [Google Scholar]
- 72.Kraut DA, Sigala PA, Fenn TD, Herschlag D. Dissecting the paradoxical effects of hydrogen bond mutations in the ketosteroid isomerase oxyanion hole. Proc. Natl. Acad. Sci. U.S. A. 2010;107:1960–1965. doi: 10.1073/pnas.0911168107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fersht AR. Relationships between apparent binding energies measured in site-directed mutagenesis experiments and energetics of binding and catalysis. Biochemistry. 1988;27:1577–1580. doi: 10.1021/bi00405a027. [DOI] [PubMed] [Google Scholar]
- 74.Chabinyc ML, Craig SL, Regan CK, Brauman JI. Gas-phase ionic reactions: Dynamics and mechanism of nucleophilic displacements. Science. 1998;279:1882–1886. doi: 10.1126/science.279.5358.1882. [DOI] [PubMed] [Google Scholar]
- 75.Schwans JP, Kraut DA, Herschlag D. Determining the catalytic role of remote substrate binding interactions in ketosteroid isomerase. Proc. Natl. Acad. Sci. U.S. A. 2009;106:14271–14275. doi: 10.1073/pnas.0901032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwans JP, Sunden F, Gonzalez A, Tsai Y, Herschlag D. Evaluating the catalytic contribution from the oxyanion hole in ketosteroid isomerase. J. Am. Chem. Soc. 2011;133:20052–20055. doi: 10.1021/ja208050t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwans JP, Sunden F, Herschlag D. Unpublished results [Google Scholar]
- 78.Brenner C, Bevan A, Fuller RS. One-step site-directed mutagenesis of the Kex2 protease oxyanion hole. Curr Biol. 1993;3:498–506. doi: 10.1016/0960-9822(93)90040-u. [DOI] [PubMed] [Google Scholar]
- 79.Bobofchak KM. Energetic and structural consequences of perturbing Gly-193 in the oxyanion hole of serine proteases. J. Biol. Chem. 2005;280:25644–25650. doi: 10.1074/jbc.M503499200. [DOI] [PubMed] [Google Scholar]
- 80.Simón L, Goodman JM. Enzyme catalysis by hydrogen bonds: The balance between transition state binding and substrate binding in oxyanion Holes. J. Org. Chem. 2010;75:1831–1840. doi: 10.1021/jo901503d. [DOI] [PubMed] [Google Scholar]
- 81.Shan S-O, Herschlag D. Hydrogen bonding in enzymatic catalysis: analysis of energetic contributions. Meth. Enzymol. 1999;308:246–276. doi: 10.1016/s0076-6879(99)08013-1. [DOI] [PubMed] [Google Scholar]
- 82.Hibbert F, Emsley J. Hydrogen bonding and chemical reactivity. Adv. Phys. Org. Chem. 1991;26:255–379. [Google Scholar]
- 83.Perrin CL. Are Short, Low-Barrier Hydrogen Bonds Unusually Strong? Acc. Chem. Res. 2010;43:1550–1557. doi: 10.1021/ar100097j. [DOI] [PubMed] [Google Scholar]
- 84.Kraut DA, Sigala PA, Pybus B, Liu CW, Ringe D, Petsko GA, Herschlag D. Testing electrostatic complementarity in enzyme catalysis: Hydrogen bonding in the ketosteroid isomerase oxyanion hole. PLoS Biol. 2006;4:501–519. doi: 10.1371/journal.pbio.0040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sigala PA, Kraut DA, Caaveiro JMM, Pybus B, Ruben EA, Ringe D, Petsko GA, Herschlag D. Testing geometrical discrimination within an enzyme active site: Constrained hydrogen bonding in the ketosteroid isomerase oxyanion hole. J. Am. Chem. Soc. 2008;130:13696–13708. doi: 10.1021/ja803928m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sigala PA, Caaveiro JMM, Ringe D, Petsko GA, Herschlag D. Hydrogen bond coupling in the ketosteroid isomerase active site. Biochemistry. 2009;48:6932–6939. doi: 10.1021/bi900713j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tramontano A, Janda KD, Lerner RA. Catalytic antibodies. Science. 1986;234:1566–1570. doi: 10.1126/science.3787261. [DOI] [PubMed] [Google Scholar]
- 88.Pollack SJ, Jacobs JW, Schultz PG. Selective chemical catalysis by an antibody. Science. 1986;234:1570–1573. doi: 10.1126/science.3787262. [DOI] [PubMed] [Google Scholar]
- 89.Wagner J, Lerner RA, Barbas CF. Efficient aldolase catalytic antibodies that use the enamine mechanism of natural enzymes. Science. 1995;270:1797–1800. doi: 10.1126/science.270.5243.1797. [DOI] [PubMed] [Google Scholar]
- 90.Stewart JD, Benkovic SJ. Transition-state stabilization as a measure of the efficiency of antibody catalysis. Nature. 1995;375:388–391. doi: 10.1038/375388a0. [DOI] [PubMed] [Google Scholar]
- 91.Hilvert D. Critical analysis of antibody catalysis. Annu. Rev. Biochem. 2000;69:751–793. doi: 10.1146/annurev.biochem.69.1.751. [DOI] [PubMed] [Google Scholar]
- 92.Wolfenden R, Snider MJ. The depth of chemical time and the power of enzymes as catalysts. Acc. Chem. Res. 2001;34:938–945. doi: 10.1021/ar000058i. [DOI] [PubMed] [Google Scholar]
- 93.Edwards DR, Lohman DC, Wolfenden R. Catalytic proficiency: the extreme case of S–O cleaving sulfatases. J. Am. Chem. Soc. 2012;134:525–531. doi: 10.1021/ja208827q. [DOI] [PubMed] [Google Scholar]
- 94.Glanville J, Zhai W, Berka J, Telman D, Huerta G, Mehta GR, Ni I, Mei L, Sundar PD, Day GMR. Precise determination of the diversity of a combinatorial antibody library gives insight into the human immunoglobulin repertoire. Proc. Natl. Acad. Sci. U.S. A. 2009;106:20216–20221. doi: 10.1073/pnas.0909775106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stemmer WP. Rapid evolution of a protein in vitro by DNA shuffling. Nature. 1994;370:389–391. doi: 10.1038/370389a0. [DOI] [PubMed] [Google Scholar]
- 96.Nagano N, Orengo CA, Thornton JM. One fold with many functions: The evolutionary relationships between TIM barrel families based on their sequences, structures and functions. J. Mol. Biol. 2002;321:741–765. doi: 10.1016/s0022-2836(02)00649-6. [DOI] [PubMed] [Google Scholar]
- 97.Ioannidis JPA. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilson EB. An introduction to scientific research. 1st ed. New York: McGraw-Hill; 1952. [Google Scholar]
- 99.Popper K. The logic of scientific discovery. London: Hutchinson; 1959. [Google Scholar]
- 100.Bolon DN, Mayo SL. Enzyme-like proteins by computational design. Proc. Natl. Acad. Sci. U.S. A. 2001;98:14274–14279. doi: 10.1073/pnas.251555398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaplan J, DeGrado WF. De novo design of catalytic proteins. Proc. Natl. Acad. Sci. U.S. A. 2004;101:11566–11570. doi: 10.1073/pnas.0404387101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jiang L, Althoff EA, Clemente FR, Doyle L, Rothlisberger D, Zanghellini A, Gallaher JL, Betker JL, Tanaka F, Barbas CF, DD H, Houk KN, Stoddard BL, Baker D. De Novo Computational Design of Retro-Aldol Enzymes. Science. 2008;319:1387–1391. doi: 10.1126/science.1152692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Röthlisberger D, Khersonsky O, Wollacott AM, Jiang L, DeChancie J, Betker J, Gallaher JL, Althoff EA, Zanghellini A, Dym O, Albeck S, Houk KN, Tawfik DS, Baker D. Kemp elimination catalysts by computational enzyme design. Nature. 2008;453:190–195. doi: 10.1038/nature06879. [DOI] [PubMed] [Google Scholar]
- 104.Siegel JB, Zanghellini A, Lovick HM, Kiss G, Lambert AR, St Clair JL, Gallaher JL, DD H, Gelb MH, Stoddard BL, Houk KN, Michael FE, Baker D. Computational design of an enzyme catalyst for a stereoselective bimolecular Diels-Alder reaction. Science. 2010;329:309–313. doi: 10.1126/science.1190239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khare SD, Kipnis Y, Greisen PJ, Takeuchi R, Ashani Y, Goldsmith M, Song Y, Gallaher JL, Silman I, Leader H, Sussman JL, Stoddard BL, Tawfik DS, Baker D. Computational redesign of a mononuclear zinc metalloenzyme for organophosphate hydrolysis. Nat. Chem. Biol. 2012;8:294–300. doi: 10.1038/nchembio.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ghirlanda G. Computational biochemistry: Old enzymes, new tricks. Nature. 2008;453:164–166. doi: 10.1038/453164a. [DOI] [PubMed] [Google Scholar]
- 107.Lutz S. Reengineering enzymes. Science. 2010;329:285–287. doi: 10.1126/science.1192224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilson EK. Virtually created enzymes. Chem. Eng. News. 2008;86:13. [Google Scholar]
- 109.Nanda V. Do-it-yourself enzymes. Nat. Chem. Biol. 2008;4:273–275. doi: 10.1038/nchembio0508-273. [DOI] [PubMed] [Google Scholar]
- 110.Halford B. Build your own enzyme. Chem. Eng. News. 2010;88:5. [Google Scholar]
- 111.Baker D. An exciting but challenging road ahead for computational enzyme design. Protein Sci. 2010;19:1817–1819. doi: 10.1002/pro.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.F1000 Prime Recommendations, Dissents and Comments for. Röthlisberger D, et al. Nature. 2008;453(7192):190–195. doi: 10.1038/nature06879. F1000Prime.com/1103758. [DOI] [PubMed] [Google Scholar]
- 113.Harbury PB, Plecs JJ, Tidor B, Alber T, Kim PS. High-resolution protein design with backbone freedom. Science. 1998;282:1462–1467. doi: 10.1126/science.282.5393.1462. [DOI] [PubMed] [Google Scholar]
- 114.Dahiyat BI, Mayo SL. De novo protein design: Fully automated sequence selection. Science. 1997;278:82–87. doi: 10.1126/science.278.5335.82. [DOI] [PubMed] [Google Scholar]
- 115.Kuhlman B, Dantas G, Ireton GC, Varani G, Stoddard BL, Baker D. Design of a novel globular protein fold with atomic-level accuracy. Science. 2003;302:1364–1368. doi: 10.1126/science.1089427. [DOI] [PubMed] [Google Scholar]
- 116.Hollfelder F, Kirby AJ, Tawfik DS, Kikuchi K, Hilvert D. Characterization of proton-transfer catalysis by serum albumins. J. Am. Chem. Soc. 2000;122:1022–1029. [Google Scholar]
- 117.O'Brien PJ, Herschlag D. Functional interrelationships in the alkaline phosphatase superfamily: phosphodiesterase activity of Escherichia coli alkaline phosphatase. Biochemistry. 2001;40:5691–5699. doi: 10.1021/bi0028892. [DOI] [PubMed] [Google Scholar]
- 118.Schmidt DMZ, Mundorff EC, Dojka M, Bermudez E, Ness JE, Govindarajan S, Babbitt PC, Minshull J, Gerlt JA. Evolutionary potential of (β/α)8-barrels: functional promiscuity produced by single substitutions in the enolase superfamily. Biochemistry. 2003;42:8387–8393. doi: 10.1021/bi034769a. [DOI] [PubMed] [Google Scholar]
- 119.Zalatan JG, Fenn TD, Brunger AT, Herschlag D. Structural and functional comparisons of nucleotide pyrophosphatase/phosphodiesterase and alkaline phosphatase: implications for mechanism and evolution. Biochemistry. 2006;45:9788–9803. doi: 10.1021/bi060847t. [DOI] [PubMed] [Google Scholar]
- 120.Lassila JK, Baker D, Herschlag D. Origins of catalysis by computationally designed retroaldolase enzymes. Proc. Natl. Acad. Sci. USA. 2010;107:4937–4942. doi: 10.1073/pnas.0913638107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Breslow R. Biomimetic chemistry and artificial enzymes: catalysis by design. Acc. Chem. Res. 1995;28:146–153. [Google Scholar]
- 122.Khersonsky O, Röthlisberger D, Wollacott AM, Murphy P, Dym O, Albeck S, Kiss G, Houk KN, Baker D, Tawfik DS. Optimization of the in-silico-designed Kemp eliminase KE70 by computational design and directed evolution. J. Mol. Biol. 2011;407:391–412. doi: 10.1016/j.jmb.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Althoff EA, Wang L, Jiang L, Giger L, Lassila JK, Wang Z, Smith M, Hari S, Kast P, Herschlag D, Hilvert D, Baker D. Robust design and optimization of retro-aldol enzymes. Protein Sci. 2012;21:717–726. doi: 10.1002/pro.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fersht AR. The hydrogen bond in molecular recognition. Trends Biochem. Sci. 1987;12:301–304. [Google Scholar]
- 125.Page MI, Jencks WP. Entropic contributions to rate accelerations in enzymic and intramolecular reactions and the chelate effect. Proc. Natl. Acad. Sci. U.S. A. 1971;68:1678–1683. doi: 10.1073/pnas.68.8.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Blow D. So do we understand how enzymes work? Structure. 2000;8:R77–R81. doi: 10.1016/s0969-2126(00)00125-8. [DOI] [PubMed] [Google Scholar]
- 127.Jen-Jacobson L, Engler LE, Jacobson LA. Structural and thermodynamic strategies for site-specific DNA binding proteins. Structure. 2000;8:1015–1023. doi: 10.1016/s0969-2126(00)00501-3. [DOI] [PubMed] [Google Scholar]
- 128.Armstrong AA, Amzel LM. Role of entropy in increased rates of intramolecular reactions. J. Am. Chem. Soc. 2003;125:14596–14602. doi: 10.1021/ja0344359. [DOI] [PubMed] [Google Scholar]
- 129.Igumenova TI, Frederick KK, Wand AJ. Characterization of the fast dynamics of protein amino acid side chains using NMR relaxation in solution. Chem. Rev. 2006;106:1672–1699. doi: 10.1021/cr040422h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Frederick KK, Marlow MS, Valentine KG, Wand AJ. Conformational entropy in molecular recognition by proteins. Nature. 2007;448:325–329. doi: 10.1038/nature05959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mittermaier AK, Kay LE. Observing biological dynamics at atomic resolution using NMR. Trends Biochem. Sci. 2009;34:601–611. doi: 10.1016/j.tibs.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 132.Moult J, Pedersen JT, Judson R, Fidelis K. A large-scale experiment to assess protein structure prediction methods. Proteins: Structure, Function and Genetics. 1995;23:ii–iv. doi: 10.1002/prot.340230303. [DOI] [PubMed] [Google Scholar]
- 133.Nielsen JE, Gunner MR, García-Moreno EB. Nielsen JE, Gunner MR, editors. The pKa cooperative: A collaborative effort to advance structure-based calculations of pKa values and electrostatic effects in proteins. Proteins. 2011;79:3249–3259. doi: 10.1002/prot.23194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cruz JA, Blanchet MF, Boniecki M, Bujnicki JM, Chen SJ, Cao S, Das R, Ding F, Dokholyan NV, Flores SC, Huang L, Lavender CA, Lisi V, Major F, Mikolajczak K, Patel DJ, Philips A, Puton T, Santalucia J, Sijenyi F, Hermann T, Rother K, Rother M, Serganov A, Skorupski M, Soltysinski T, Sripakdeevong P, Tuszynska I, Weeks KM, Waldsich C, Wildauer M, Leontis NB, Westhof E. RNA-Puzzles: A CASP-like evaluation of RNA three-dimensional structure prediction. RNA. 2012;18:610–625. doi: 10.1261/rna.031054.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Warshel A. Electrostatic origin of the catalytic power of enzymes and the role of preorganized active sites. J. Biol. Chem. 1998;273:27035–27038. doi: 10.1074/jbc.273.42.27035. [DOI] [PubMed] [Google Scholar]
- 136.Perutz MF. Concluding Remarks. Proc. Roy. Soc. B: Biological Sciences. 1967;167:448–448. [Google Scholar]
- 137.Cleland WW, Kreevoy MM. Low-barrier hydrogen bonds and enzymic catalysis. Science. 1994;264:1887–1890. doi: 10.1126/science.8009219. [DOI] [PubMed] [Google Scholar]
- 138.Frey PA, Whitt SA, Tobin JB. A low-barrier hydrogen bond in the catalytic triad of serine proteases. Science. 1994;264:1927–1930. doi: 10.1126/science.7661899. [DOI] [PubMed] [Google Scholar]
- 139.Cheng H, Nikolic-Hughes I, Wang JH, Deng H, O’Brien PJ, Wu L, Zhang ZY, Herschlag D, Callender R. Environmental effects on phosphoryl group bonding probed by vibrational spectroscopy: Implications for understanding phosphoryl transfer and enzymatic catalysis. J. Am. Chem. Soc. 2002;124:11295–11306. doi: 10.1021/ja026481z. [DOI] [PubMed] [Google Scholar]
- 140.Carey PR, Tonge PJ. Unlocking the secrets of enzyme power using Raman spectroscopy. Acc. Chem. Res. 1995;28:8–13. [Google Scholar]
- 141.Kriegl JM, Nienhaus K, Deng P, Fuchs J, Nienhaus GU. Ligand dynamics in a protein internal cavity. Proc. Natl. Acad. Sci. U.S. A. 2003;100:7069–7074. doi: 10.1073/pnas.1231856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sigala PA, Fafarman AT, Bogard PE, Boxer SG, Herschlag D. Do ligand binding and solvent exclusion alter the electrostatic character within the oxyanion hole of an enzymatic active site? J. Am. Chem. Soc. 2007;129:12104–12105. doi: 10.1021/ja075605a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fafarman AT, Sigala PA, Schwans JP, Fenn TD, Herschlag D, Boxer SG. Quantitative, directional measurement of electric field heterogeneity in the active site of ketosteroid isomerase. Proc. Natl. Acad. Sci. USA. 2012;109:E299–E308. doi: 10.1073/pnas.1111566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Boxer SG. Stark Realities. J. Phys. Chem. B. 2009;113:2972–2983. doi: 10.1021/jp8067393. [DOI] [PubMed] [Google Scholar]
- 145.Jha SK, Ji M, Gaffney KJ, Boxer SG. Direct measurement of the protein response to an electrostatic perturbation that mimics the catalytic cycle in ketosteroid isomerase. Proc. Natl. Acad. Sci. USA. 2011;108:16612–16617. doi: 10.1073/pnas.1113874108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zechel D, Withers S. Glycosidase mechanisms: Anatomy of a finely tuned catalyst. Acc. Chem. Res. 2000;33:11–18. doi: 10.1021/ar970172+. [DOI] [PubMed] [Google Scholar]
- 147.Lassila JK, Zalatan JG, Herschlag D. Biological phosphoryl-transfer reactions: Understanding mechanism and catalysis. Annu. Rev. Biochem. 2011;80:669–702. doi: 10.1146/annurev-biochem-060409-092741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Berti PJ, Tanaka KSE. Transition state analysis using multiple kinetic isotope effects: Mechanisms of enzymatic and non-enzymatic Glycoside Hydrolysis and Transfer. Adv. Phys. Org. Chem. 2002;37:239–314. [Google Scholar]
- 149.Berti PJ, McCann JAB. Toward a detailed understanding of base excision repair enzymes: Transition state and mechanistic analyses of N-glycoside hydrolysis and N-glycoside transfer. Chem. Rev. 2006;106:506–555. doi: 10.1021/cr040461t. [DOI] [PubMed] [Google Scholar]
- 150.Schramm VL. Transition state variation in enzymatic reactions. Curr. Opin. Chem. Biol. 2001;5:556–563. doi: 10.1016/s1367-5931(00)00246-5. [DOI] [PubMed] [Google Scholar]
- 151.Bahnson BJ, Anderson VE. Crotonase-catalyzed β-elimination is concerted: a double isotope effect study. Biochemistry. 1991;30:5894–5906. doi: 10.1021/bi00238a013. [DOI] [PubMed] [Google Scholar]
- 152.Alston WC, II, Kanska M, Murray CJ. Secondary H/T and D/T isotope effects in enzymatic enolization reactions. Coupled motion and tunneling in the triosephosphate isomerase reaction. Biochemistry. 1996;35:12873–12881. doi: 10.1021/bi960831a. [DOI] [PubMed] [Google Scholar]
- 153.Doll KM, Bender BR, Finke RG. The first experimental test of the hypothesis that enzymes have evolved to enhance hydrogen tunneling. J. Am. Chem. Soc. 2003;125:10877–10884. doi: 10.1021/ja030120h. [DOI] [PubMed] [Google Scholar]
- 154.Valley MP, Fitzpatrick PF. Comparison of enzymatic and non-enzymatic nitroethane anion formation: Thermodynamics and contribution of tunneling. J. Am. Chem. Soc. 2004;126:6244–6245. doi: 10.1021/ja0484606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nagel ZD, Klinman JP. Tunneling and dynamics in enzymatic hydride transfer. Chem. Rev. 2006;106:3095–3118. doi: 10.1021/cr050301x. [DOI] [PubMed] [Google Scholar]
- 156.Wilde TC, Blotny G, Pollack RM. Experimental evidence for enzyme-enhanced coupled motion/quantum mechanical hydrogen tunneling by ketosteroid isomerase. J. Am. Chem. Soc. 2008;130:6577–6585. doi: 10.1021/ja0732330. [DOI] [PubMed] [Google Scholar]
- 157.Hammes-Schiffer S, Benkovic SJ. Relating protein motion to catalysis. Annu. Rev. Biochem. 2006;75:519–541. doi: 10.1146/annurev.biochem.75.103004.142800. [DOI] [PubMed] [Google Scholar]
- 158.Nagel ZD, Klinman JP. A 21st century revisionist's view at a turning point in enzymology. Nat. Chem. Biol. 2009;5:543–550. doi: 10.1038/nchembio.204. [DOI] [PubMed] [Google Scholar]
- 159.Silva RG, Murkin AS, Schramm VL. Femtosecond dynamics coupled to chemical barrier crossing in a Born-Oppenheimer enzyme. Proc. Natl. Acad. Sci. USA. 2011;108:18661–18665. doi: 10.1073/pnas.1114900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Antoniou D, Ge X, Schramm VL, Schwartz SD. Mass modulation of protein dynamics associated with barrier crossing in purine nucleoside phosphorylase. J. Phys. Chem. Lett. 2012;3:3538–3544. doi: 10.1021/jz301670s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pudney CR, Guerriero A, Baxter NJ, Johannissen LO, Waltho JP, Hay S, Scrutton NS. Fast protein motions are coupled to enzyme H-transfer reactions. J. Am. Chem. Soc. ASAP. 2013 doi: 10.1021/ja311277k. [DOI] [PubMed] [Google Scholar]
- 162.Toney MD, Castro JN, Addington TA. Heavy-enzyme kinetic isotope effects on proton transfer in alanine racemase. J. Am. Chem. Soc. ASAP. 2013 doi: 10.1021/ja3101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bennett WS, Steitz TA. Glucose-induced conformational change in yeast hexokinase. Proc. Natl. Acad. Sci. U.S. A. 1978;75:4848–4852. doi: 10.1073/pnas.75.10.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pai EF, Sachsenheimer W, Schirmer RH, Schulz GE. Substrate positions and induced fit in crystalline adenylate kinase. J. Mol. Biol. 1977;114:37–45. doi: 10.1016/0022-2836(77)90281-9. [DOI] [PubMed] [Google Scholar]
- 165.Pompliano DL, Peyman A, Knowles JR. Stabilization of a reaction intermediate as a catalytic device: definition of the functional role of the flexible loop in triosephosphate isomerase. Biochemistry. 1990;29:3186–3194. doi: 10.1021/bi00465a005. [DOI] [PubMed] [Google Scholar]
- 166.Wolfenden R. Enzyme catalysis: conflicting requirements of substrate access and transition state affinity. Mol. Cell. Biochem. 1974;3:207–211. doi: 10.1007/BF01686645. [DOI] [PubMed] [Google Scholar]
- 167.Herschlag D. The role of induced fit and conformational changes of enzymes in specificity and catalysis. Bioorg. Chem. 1988;16:62–96. [Google Scholar]
- 168.Herschlag D. Implications of ribozyme kinetics for targeting the cleavage of specific RNA molecules in vivo: more isn't always better. Proc. Natl. Acad. Sci. U.S. A. 1991;88:6921–6925. doi: 10.1073/pnas.88.16.6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Alber TC, Davenport RC, Giammona DA, Lolis E, Petsko GA, Ringe D. Crystallography and site-directed mutagenesis of yeast triosephosphate isomerase: what can we learn about catalysis from a “simple” enzyme? Cold Spring Harbor Symposia on Quantitative Biology. 1987;52:603–613. doi: 10.1101/sqb.1987.052.01.069. [DOI] [PubMed] [Google Scholar]
- 170.Hertel KJ, Peracchi A, Uhlenbeck OC, Herschlag D. Use of intrinsic binding energy for catalysis by an RNA enzyme. Proc. Natl. Acad. Sci. U.S. A. 1997;94:8497–8502. doi: 10.1073/pnas.94.16.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.O'Brien PJ, Herschlag D. Catalytic promiscuity and the evolution of new enzymatic activities. Chem Biol. 1999;6:R91–R105. doi: 10.1016/S1074-5521(99)80033-7. [DOI] [PubMed] [Google Scholar]
- 172.Khersonsky O, Roodveldt C, Tawfik DS. Enzyme promiscuity: evolutionary and mechanistic aspects. Curr. Opin. Chem. Biol. 2006;10:498–508. doi: 10.1016/j.cbpa.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 173.Jackson DY, Burnier J, Quan C, Stanley M, Tom J, Wells JA. A designed peptide ligase for total synthesis of ribonuclease A with unnatural catalytic residues. Science. 1994;266:243–247. doi: 10.1126/science.7939659. [DOI] [PubMed] [Google Scholar]
- 174.Raines RT. Ribonuclease A. Chem. Rev. 1998;98:1045–1066. doi: 10.1021/cr960427h. [DOI] [PubMed] [Google Scholar]
- 175.Messmore JM, Fuchs DN, Raines RT. Ribonuclease A: Revealing structure-function relationships with semisynthesis. J. Am. Chem. Soc. 1995;117:8057–8060. doi: 10.1021/ja00136a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Messmore JM, Raines RT. Pentavalent organo-vanadates as transition state analogues for phosphoryl transfer reactions. J. Am. Chem. Soc. 2000;122:9911–9916. doi: 10.1021/ja0021058. [DOI] [PMC free article] [PubMed] [Google Scholar]