Abstract
Event-related potentials (ERPs), derived from electroencephalographic (EEG) recordings, can index electrocortical activity related to cognitive operations. The fronto-central P3a ERP is involved in involuntary processing of novel auditory information, whereas the parietal P3b indexes controlled attention processing. The amplitude of the auditory P3b has been found to be decreased in major depressive disorder (MDD). However, few studies have examined the relationship between the P3b, the related P3a, and antidepressant treatment response. We tested 53 unmedicated individuals (25 females) with MDD as well as 43 non-depressed controls (23 females) on the novelty oddball task, wherein infrequent deviant (target) and frequent standard (non-target) tones were presented, along with infrequent novel (non-target/distractor) sounds. The P3a and P3b ERPs were assessed to the novel and target sounds, respectively, as were accompanying behavioural performance measures. Depression ratings and antidepressant response status were assessed following 12 weeks of pharmacotherapy with three different regimens. Antidepressant treatment non-responders had smaller baseline P3a/b amplitudes than responders and healthy controls. Baseline P3b amplitude also weakly predicted the extent of depression rating changes by week 12. Females exhibited larger P3a/b amplitudes than males. With respect to task performance, controls had more target hits than treatment non-responders. ERP measures correlated with clinical changes in males and with behavioural measures in females. These results suggest that greater (or control-like) baseline P3a/b amplitudes are associated with a positive antidepressant response, and that gender differences characterize the P3 and, hence, basic attentive processes.
Keywords: P3a, P3b, major depressive disorder (MDD), antidepressant drugs, prediction, sex
Introduction
Major depressive disorder (MDD) is a common psychiatric illness that is one of the leading causes of disability worldwide (World Health Organization, 2012). Though MDD is characterized by decreased affect, it tends to be associated with cognitive dysfunction, specifically impaired attention (El Massioui and Lesevre, 1988; Giedke et al., 1981). Electrocortical indices of brain activity, by way of electroencephalographic (EEG)-derived event-related potentials (ERPs), can provide insight into brain activity (at millisecond resolution) underlying basic cognitive impairments in MDD. Additionally, emerging evidence indicates that certain ERPs, such as the auditory P3a/b, may hold potential in aiding with treatment selection and predicting response. This is particularly noteworthy in light of relatively high failure rates to initial antidepressant interventions.
Simple attentive processes can be probed using paradigms such as the classic auditory oddball task, wherein subjects respond to infrequent deviant stimuli (targets) couched within frequently presented standard stimuli. This deviant elicits the posterior P3b - a positive ERP peaking at ~ 300–500ms, which is thought to reflect working memory updating and attention allocation to expected target stimuli (Polich and Kok, 1995). It increases with task difficulty and decreased deviant probability; larger P3b amplitudes are thought to index greater cognitive function and attention resource allocation (Polich, 2004). P3b latency is associated with stimulus evaluation time, reflecting perceptual processing efficiency (Kutas et al., 1977). A shorter P3b indexes faster cortical processing speed/more efficient information processing.
A variant of the classic oddball introduces infrequent, novel non-target distractors (e.g., dog bark). This ‘novelty oddball task’ elicits a midline fronto-central P3a to the novel stimulus, which peaks at ~200–300ms (Squires et al., 1975). The P3a is thought to reflect involuntary orienting to unexpected stimuli (Iv et al., 2010; Bruder et al., 2012), and increases with the novelty of the distractor (Polich, 2007); its latency is related to cortical processing speed.
Though exceptions exist (Giedke et al., 1981; El Massioui and Lesevre, 1998), previous studies indicate that MDD patients exhibit smaller auditory oddball-elicited P3a/b amplitudes than controls (Bruder et al., 2009, 2012; Gangadhar et al., 1993; Kayser et al., 2003; Sara et al., 1994; Vandoolacghe et al., 1998), suggesting deficient stimulus evaluation/attention allocation and short-term memory updating in MDD (Diner et al., 1984). P3a amplitude reductions imply deficits in early involuntary attention shifts/allocation to novel stimuli in the disorder (Cavanagh and Geisler, 2006; El Massioui and Lesevre, 1988; Polich, 2007). These experimental observations may be related to problems with sustained attention/concentration and memory in depression (Marazziti et al., 2010). Most studies report no P3b latency differences between MDD patients and controls (Diner et al., 1984; Gangadhar et al., 1993; Giedke et al., 1981; Kawasaki et al., 2004; Sara et al., 1994), though some have found longer P3b latencies in MDD, suggesting slower stimulus processing and/or decreased cortical processing efficiency (Bange and Bathien, 1998; Cavanagh and Geisler, 2006; Karaaslan et al., 2003; Mehmet et al., 2012; Zhu et al., 2009).
Although larger P3s have been documented in females versus males (Hoffman and Polich, 1999) others have noted no such gender differences (Yagi et al., 1999). Given that MDD is more common in females, and that the genders are characterized by somewhat different depression symptom profiles (Smith et al., 2008; van Noorden et al., 2010), a more thorough exploration of putative P3 gender differences in the context of MDD is warranted.
The P3 is typically assessed at midline sites, although some groups have probed hemispheric effects on the P3. Given that the right hemisphere is implicated in sound localization, the right versus left P3 tends to be greater (Bruder et al., 1991; Kayser et al., 1998); this asymmetry increases with enhanced attention demands (Baranov-Kylov et al., 2007). Interestingly, MDD patients tend to exhibit attenuated or absent right-favouring P3 asymmetry (Bruder et al., 1998, 2012; Iv et al., 2010), possibly indicating that right hemisphere mediated attentional mechanisms are under-recruited in depression (Bruder et al., 1998, Baranov-Krylov et al., 2007).
Assessments of behavioural data from the auditory oddball indicate that MDD patients have longer reaction times (RTs) to targets and exhibit more errors than controls (Bange and Bathien, 1998; Diner et al., 1984; El Massioui and Lesevre, 1988, Giedke et al., 1981; Sara et al., 1994). However, others have found no group behavioural differences despite documented P3 differences (Bruder et al., 1998; Hoffman and Polich, 1999), indicating that subtle electrocortical alterations do not necessarily translate to behavioural changes.
Several studies have probed the effects of antidepressant interventions on the auditory P3. Following acute electroconvulsive therapy (ECT; Ancy et al., 1996) and chronic antidepressant pharmacotherapy (Bruder et al., 2012; Kalayam and Alexopoulos, 1999; Karaaslan et al., 2003; Mehmet et al., 2012; Yanai et al., 1997), P3b amplitude increases and/or latency decreases have been noted in depressed patients. Other work suggests that such changes only emerge in individuals with a favourable antidepressant response, as depressed patients who failed to respond to antidepressants exhibited unchanged or similar P3 characteristics as at baseline (Ancy et al., 1996; Mehmet et al., 2012; Kalayan & Alexopoulos, 1999).
A handful of studies have examined the association between baseline P3 characteristics and eventual antidepressant response. For instance, rapid ECT responders exhibited similar P3b amplitudes as controls at baseline, indicating that a “normal” P3 may index a positive treatment response (Ancy et al., 1996). Mehmet et al. (2012) found that non-responders to a selective serotonin reuptake inhibitor (SSRI) exhibited longer P3b latencies than responders and controls pre- and post-treatment; a similar finding was noted for non-responders to a tricyclic antidepressant (Kalayan and Alexopoulos, 1999). Given the paucity of research, firm conclusions regarding the usefulness of the P3 in antidepressant response prediction are difficult to draw.
This study probed P3a/b characteristics and auditory oddball task performance in controls and depressed antidepressant responders and non-responders prior to treatment (retrospective group assignment). Treatment (12 weeks) consisted of: the norepinephrine and dopamine reuptake inhibitor (NDRI) bupropion (BUP), the SSRI escitalopram (ESC) and ESC+BUP. The latter was used due to reported enhanced antidepressant effects with dual drug treatments (Segrave and Nathan, 2005; Zisook et al., 2011). We expected that antidepressant nonresponders would exhibit smallest baseline P3a/b amplitudes and longest latencies, and respond slowest to deviant targets. We hypothesized that baseline P3a/b amplitudes would predict depression score changes with treatment. Finally, larger P3a/b amplitudes and shorter latencies were expected in females.
Experimental Procedures
Participants
As outlined elsewhere (Jaworska et al., 2012a/b), 53 adults with a primary diagnosis of MDD (Table 1) were assessed. Diagnosis was established by psychiatrists using the Structured Clinical Interview for DSM IV-TR Diagnoses, Axis I, Patient Version (SCID-IV-I/P; First et al., 1997). The Hamilton Rating Scale for Depression (HAMD17; Hamilton, 1960) and Montgomery-Åsberg Depression Rating Scale (MADRS; Montgomery & Åsberg, 1979) were administered. All patients had MADRS scores ≥22 at enrolment. Exclusion criteria were: history of seizures, bipolar disorder, psychosis or anorexia/bulimia, current (<6 months) drug/alcohol abuse/dependence, unstable (≥3 months) medical condition and significant suicide risk. Individuals with significant hearing loss (unable to hear 60dB SPL, 1000Hz) were excluded. At time of testing, patients were not taking psychoactive drugs; appropriate drug washout periods were applied for previously medicated patients.
Table 1.
Responder, non-responder and control group characteristics & demographics (sexes collapsed)
| Control (N=43) |
Responder (N=26) |
Non-Responder (N=25) |
|
|---|---|---|---|
| Sex (Female/Male) | 23/20 | 14/12 | 14/11 |
| Age (M ± S.D.) | 36.51 ± 9.83 | 35.54 ± 10.95 | 45.48 ± 10.61 |
| Education Years (M ± S.D.) | 16.37 ± 1.92 | 15.50 ± 2.50 | 16.56 ± 2.40 |
| HAMD17 (M± S.D.) | - | 19.62 ± 5.88 | 21.60 ± 3.40 |
| MADRS (M ± S.D.) | - | 29.42 ± 4.49 | 31.72 ± 5.75 |
| BDI-II (M ± S.D.) | 3.71 ± 4.83 | - | - |
| Ethnicity | 39 Caucasian; 1 Asian; 2 | 24 Caucasian; 0 Asian; 0 | 22 Caucasian; 3 Asian; 1 |
| South Asian; 1 African | South Asian; 1 African | South Asian; 0 African |
HAMD17: Hamilton Rating Scale for Depression, 17-item version
MADRS: Montgomery- Åsberg Depression Rating Scale; BDI-II: Beck Depression Inventory-II
M: mean; S.D.: standard deviation
Patients were recruited from a clinical trial and tested before starting treatment [randomized (double-blind) to one of: ESC + placebo, BUP + placebo or ESC+BUP]. They were assessed weekly for the first four weeks and then bi-weekly. Dosing was raised if tolerated and remission (HAMD17 ≤7 over min. two consecutive visits) not reached [average week 12 dose (mg) for dual treatment: ESC=32.4, BUP=379.4; monotherapy: ESC=35.0, BUP=412.5]. Clinical measures of interest were: 1. MDD severity: HAMD17 and MADRS pre- and 12 weeks post-treatment and rating changes (baseline to 12 weeks). 2. Response: ≥50% MADRS score reduction by week 12 (or last session carried forward; ratings were not carried forward if dropout occurred <6 weeks).
Forty-three non-depressed, healthy controls were tested (Table 1). They had no psychiatric and substance abuse/dependence history [assessed with modified non-patient SCID (SCID-IV-I/NP)], history of seizures, brain trauma/lesion(s) or hearing loss. Controls were included if they scored ≤13 on the Beck Depression Inventory-II (BDI-II; Beck et al., 1996). Only controls with no psychiatric history in first-degree relatives were tested [Family Interview for Genetic Studies (FIGS)-assessed; Maxwell, 1992].
Before testing, all participants abstained for >3hr from caffeine and/or smoking/nicotine, as well as from alcohol/drugs beginning at midnight. Participants were compensated 30.00 CDN/session. This study was approved by the appropriate institutional ethics boards and informed consent was obtained from all participants.
Novelty Oddball Task
Eight hundred tones were presented in 4 blocks (200/block). Eighty percent were standard tones (160/block; 1000Hz, 70dB pure tones, 336ms), 10% were deviant target tones (20/block; 700Hz, all other parameters identical to standard tones) and 10% were novel non-target environmental sounds (e.g., baby cry; 169–399ms, 65–75dB; Kimbo et al., 2010). The inter-stimulus interval was 1000ms (Presentation Software, Neurobehavioral Systems, Albany, CA, USA). Participants were asked to press a button only to the deviant, low-pitched target tones. Hits (% correct responses to deviant targets), false alarms (FA; % responses to non-targets) and RTs to targets were recorded. Participants fixated on a cross ~1m in front of them during the task.
Electrophysiological Recordings & Data Reduction
EEG was recorded (500Hz) using a cap with 32 Ag/AgCl electrodes (EasyCap, Inning a. Ammersee, Germany) positioned according to the 10–10 system (Chatrian et al., 1985); an AFz electrode was the ground and averaged mastoids (TP9/10) were the reference. Electrooculographic activity was monitored with additional electrodes; impedance was maintained at ≤5KΩ (BrainVision Recorder, Gilching, Germany). Signals were filtered (0.1–30Hz) and ocular-corrected (Gratton et al., 1983). Data was segmented into 1100ms epochs (−100–1000ms post-stimulus). Artifact rejection followed, which excluded epochs of +/–75µV and with faulty channels/drift. Epochs were baseline corrected (mean activity 100ms pre-stimulus) and averaged for the deviant/target and novelty stimuli. Trials with FAs and target misses were excluded from averaged epochs in constructing the P3a and P3b, respectively. A minimum of >30 epochs/stimulus were required for statistical analyses; 4 MDD patients and 2 controls were excluded due to insufficient epochs or recording system failures (N=49 MDD; 41 controls; no group differences existed in the number of epochs).
P3a and P3b Extraction
P3a and P3b peak time windows were established from grand-averages (groups collapsed) for the novelty and deviant stimuli, respectively. The P3a (at Cz, where it was maximal) was the most positive peak at 200–450ms and P3b (at Pz, where it was maximal) was the most positive peak at 220–500ms post-stimulus. P3a/b amplitudes and latencies were measured at Fz, Cz, Pz, C3/4 and P3/4.
Statistical Analyses
Analyses of variances (ANOVAs) were carried out (SPSS Inc., Chicago, IL, USA) on demographic and clinical variables between groups. Behavioral data (hits, FA and RT) were separately assessed with univariate ANOVAs with group (antidepressant responders; non-responders; controls) and gender (male; female) as between-subject factors. P3a and P3b amplitudes and latencies were separately assessed with repeated-measures ANOVAs (rmANOVAs) with group (responders; non-responders; controls) and gender as between-subject factors, and hemisphere (C3/4 for P3a; P3/4 for P3b) as the within-subject factor. Spearman’s correlations were carried out between P3a (at Cz) and P3b (at Pz) amplitude/latency and clinical ratings at baseline (MADRS; HAMD17) and clinical rating changes from baseline to week 12 [(week 12 − baseline)*100/baseline)] for MDD males and females, separately. Correlations were also carried out between performance measures (hits, FA, RT) and clinical ratings at baseline, and percent change in clinical ratings from baseline to week 12 for MDD males and females, separately. Significance was set at p<.01 for correlations to adjust for multiple comparisons. All main effects and interactions were Greenhouse-Geisser corrected (p<.05). Exploratory linear regressions were carried out (regardless of whether correlations were significant) to probe if baseline P3b or P3a amplitude (i.e., predictor variable) predicted change in depression scores (MADRS and HAMD17) from baseline to week 12 (i.e., outcome measures). Multiple linear regressions were subsequently run using both P3b and P3a amplitudes as predictors.
Results
Patients
Two patients dropped out before week 6 and could not be classified as responders/nonresponders (final N=51; 25 non-responders/26 responders). Non-responders were older than responders and non-responders [F(2,91)=7.53, p<.001; pairwise comparisons: p<.001]; no other group differences existed. Clinical data are presented in Table 2.
Table 2.
Clinical ratings in eventual antidepressant treatment responders and non-responders at baseline and 12 weeks post-treatment as well as clinical ratings changes (mean ± standard deviation)
| Baseline | Week 12 | Rating change (%) from baseline to week 12 |
||||
|---|---|---|---|---|---|---|
| Clinical Measures |
Responder (N=26) |
Non- Responder (N=25) |
Responder (N=26) |
Non- Responder (N=25) |
Responder (N=26) |
Non- Responder (N=24) |
| HAMD17 | 19.6 ± 5.9 | 21.6 ± 3.5 | 4.4 ± 3.2 | 17.7 ± 6.5 | −75.8% ± 18.4 | −16.7% ± 28 |
| MADRS | 29.4 ± 4.5 | 31.9 ± 5.8 | 5.9 ± 4.9 | 26.5 ± 9.1 | −80.0% ± 16.6 | −16.9% ± 24.2 |
HAMD17: Hamilton Rating Scale for Depression, 17 item version
MADRS: Montgomery-Åsberg Depression Rating Scale
Behavioural Data
Behavioral data are presented in Table 3. A main effect of group (responders; non-responders; controls) existed for hits [F(5,87)=3.54, p=.033], with controls having more hits than non-responders (p=.02); a trend for more hits in controls versus responders also existed (p=.052). A trend for a group×sex interaction existed [F(5,87)=2.32, p=.10], with follow-up comparisons indicating more hits in male versus female non-responders (p=.023). A trend for a main effect of sex was noted for FAs [F(5,87)=3.20, p=.077], with a tendency for higher FAs in females versus males. Similarly, a trend for a main effect of sex existed for RTs [F(5,87)=3.83, p=.054], with a tendency for longer RTs in females.
Table 3.
Behavioral performance measures on the novelty auditory oddball task in antidepressant treatment responders, non-responders and controls (mean ± standard error)
| MDD Responders | MDD Non-Responders | Controls | ||||
|---|---|---|---|---|---|---|
| Performance Measures |
Female (N=14) |
Male (N=12) |
Female (N=13) |
Male (N=11) |
Female (N=23) |
Male (N=20) |
| Hits (%) | 98.0 ± 0.9 | 97.5 ± 1.0 | 95.6 ± 1.0 | 99.0 ± 1.1 | 99.5 ± 0.7 | 99.5 ± 0.8 |
| FA (%) | 5.6 ± 1.9 | 2.4 ± 2.1 | 5.9 ± 2.0 | 1.1 ± 2.2 | 1.3 ± 1.5 | 0.9 ± 1.6 |
| RT (ms) | 500.6 ± 23.5 | 470.5 ± 25.3 | 510.7 ± 24.3 | 463.5 ± 26.5 | 476.0 ± 18.3 | 442.7 ± 19.6 |
FA: false alarms; RT: reaction time
P3b Amplitude & Latency
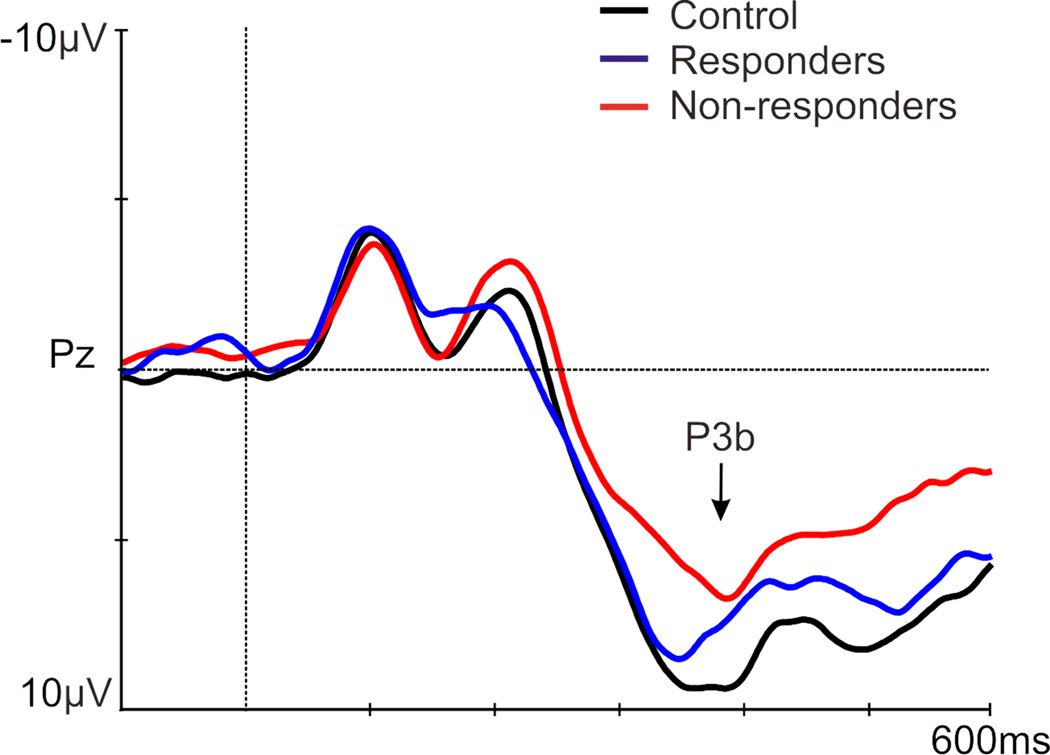
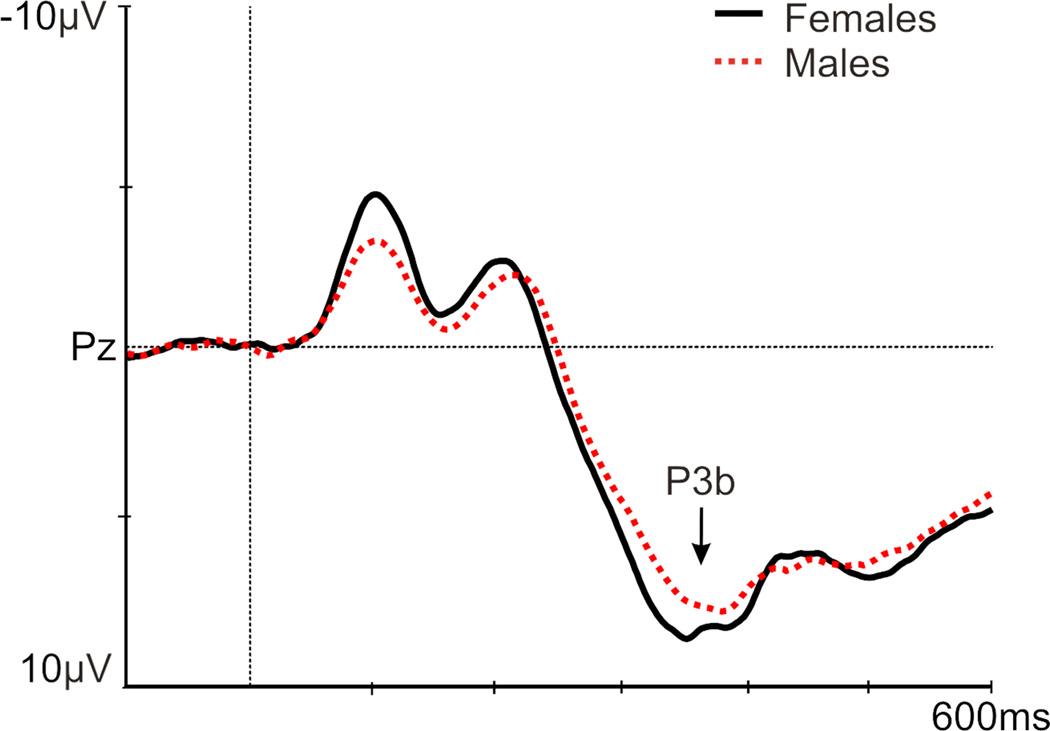
A main effect of group existed for P3b amplitude [F(1,83)=3.56, p=.033], with a smaller P3b in non-responders versus both responders (p=.019) and controls versus (p=.02; Figure 1; Table 4). A main effect of sex was found [F(1,83)=6.95, p=.010], with a larger P3b in females [p=.010; Figure 2: P3b is presented at Pz (where P3b is maximal), which was not included in the ANOVAs]. No significant results existed for P3b latency (Table 4).
Figure 1.
Grand average of the P3b (at Pz) to a novelty auditory oddball task, in controls (black), antidepressant treatment responders (blue), and non-responders (red), prior to treatment.
Table 4.
P3a/b measures on the novelty auditory oddball task in antidepressant treatment responders, non-responders and controls (mean ± standard error)
| ERP Measure | Controls (N=43) |
Responders (N=26) |
Non- Responders (N=25) |
Females (N=52) |
Males (N=44) |
|---|---|---|---|---|---|
| P3a Amplitude (µV) | 9.2 ± 0.6 | 8.71 ± 0.71 | 6.9 ± 0.7 | 9.2 ± 0.5 | 7.3 ± 0.6 |
| P3a Latency (ms) | 319.8 ± 6.3 | 332.4 ± 8.3 | 333.5 ± 8.3 | 325.7 ± 6.1 | 331.5 ± 6.4 |
| P3b Amplitude (µV) | 9.6 ± 0.7 | 10.0 ± 0.9 | 7.0 ± 0.9 | 10.2 ± 0.7 | 7.6 ± 0.7 |
| P3b Latency (ms) | 364.1 ± 6.4 | 355.2 ± 8.3 | 362.0 ± 8.3 | 356.7 ± 6.2 | 364.2 ± 6.5 |
Figure 2.
Grand average of the P3b (at Pz) in females (black) and males (red) to a novelty auditory oddball task.
Linear regressions indicated that P3b amplitude weakly predicted HAMD17 change scores from baseline to week 12 [adjusted R2=.069; F(1,45)=4.39, p=.042; HAMD17 change=−.30(P3b amplitude) + −2.39]. A similar finding existed for MADRS change scores [adjusted R2=.071; F(1,45)=4.52, p=.039; MADRS change=−.30(P3b amplitude) + −2.42].
P3a Amplitude & Latency
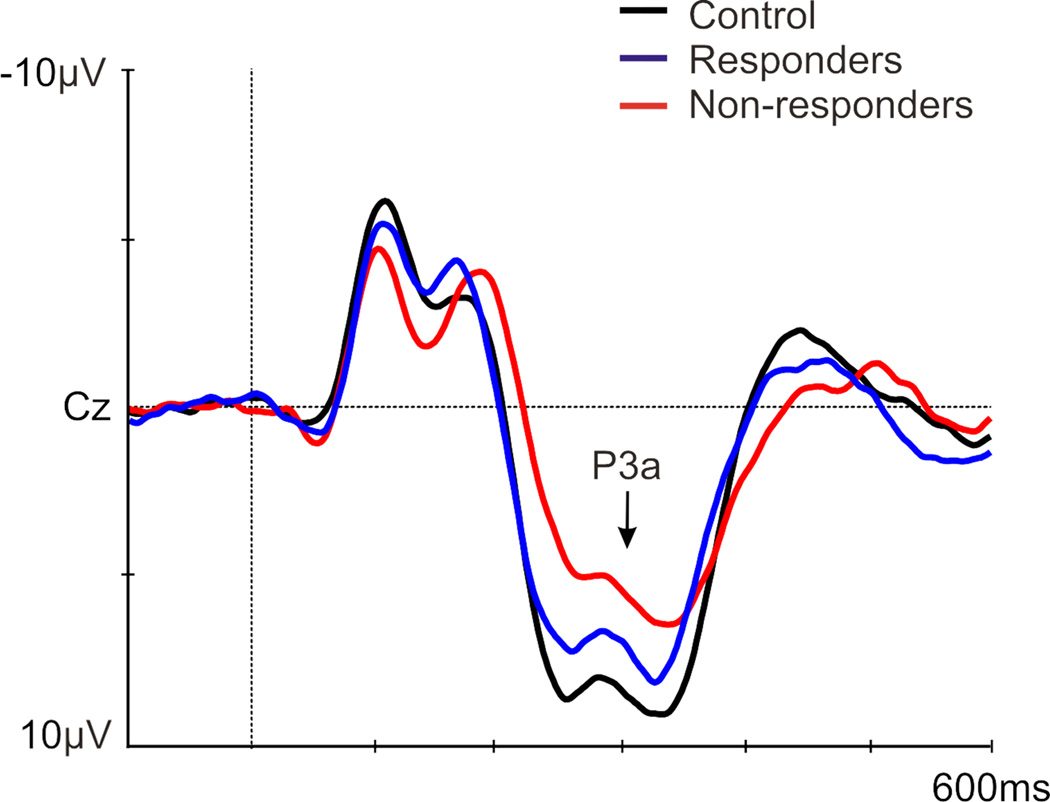
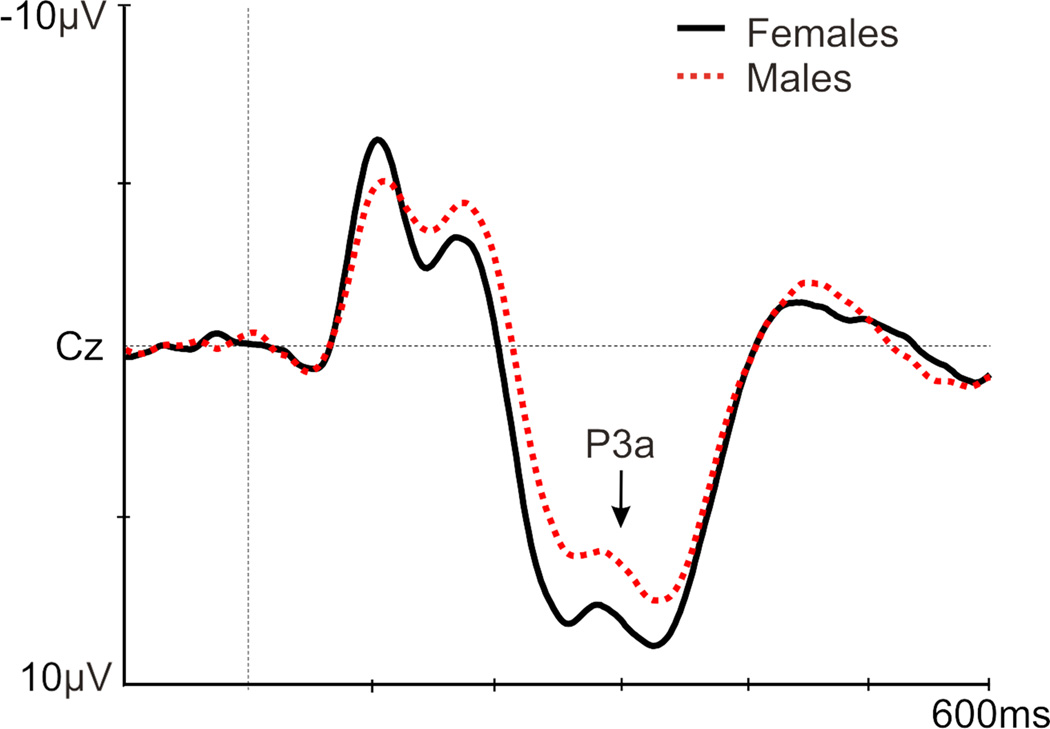
A main effect of group existed [F(1,83)=3.35, p=.040], with a larger P3a amplitude in controls versus non-responders (p=.01); a trend for a larger P3a existed in responders versus non-responders (p=.080; Figure 3; Table 4). A main effect of sex was found [F(1,83)=6.43, p=.013], with a greater P3a in females [p=.013; Figure 4: P3a is presented at Cz (where P3a is maximal), which was not included in the ANOVAs]. No significant results were noted for P3a latency (Table 4).
Figure 3.
Grand average of the P3a (at Cz) to a novelty auditory oddball task in controls (black), antidepressant treatment responders (blue), and non-responders (red) prior to treatment.
Figure 4.
Grand average of the P3a (at Cz) in females (black) and males (red) to a novelty auditory oddball task.
Linear regressions indicated that P3a amplitude did not predict clinical changes (HAMD17 and MADRS) from baseline to week 12. Including both P3a and P3b amplitudes as predictors into the regression did not improve the model’s strength in predicting clinical changes (insignificant p)
Correlations
When behavioural performance measures (RT, FA, hits) were correlated with ERP measures, no significant correlations emerged in males. In females, hits correlated positively with P3a amplitude [at Cz; r(48)=.38, p=.008]. FAs correlated negatively with P3a amplitude [r(48)= −.37, p=.009] and positively with P3b latency (at Pz) [r(48)=.39, p=.006].
When clinical measures were correlated with ERP measures, no significant correlations existed in females. In males, P3a latency correlated positively with baseline HAMD17 scores [r(24)=.62, p=.001]. P3b amplitude correlated negatively with HAMD17 score [r(21)=−.56, p=.008].
Discussion
This study examined if differences in the baseline auditory P3a/b, elicited by the novelty oddball task, and associated performance, exist in eventual antidepressant pharmacotherapy responders, non-responders and controls, as well as between the genders. Group differences were noted in P3a/b amplitude, with treatment non-responders exhibiting a smaller P3a/b than responders and controls. Larger P3a/b amplitudes were also found in females versus males. ERP measures correlated with clinical changes in males and with behavioural measures in females.
Consistent with predictions, treatment non-responders had smaller P3bs than responders and controls; responders did not differ from controls. Thus, a “normal” baseline P3b in MDD is associated with a positive antidepressant outcome while a decreased P3b is linked with the opposite. A comparable study by Ancy et al., (1996) found that slow responders to ECT had a smaller baseline P3b than controls and rapid ECT responders. Increased P3b latency was also associated with treatment non-response to pharmacotherapies (Kalayan and Alexopoulos, 1999; Mehmet et al., 2012). A decreased P3b may reflect impairments in context-specific memory updating and voluntary attention allocation; increased latency may reflect decreased cortical processing speed in treatment non-responders.
Similarly, treatment non-responders exhibited decreased P3a baseline amplitudes than responders and controls. To our knowledge, this study is the first to show that a normal/control-like P3a is associated with a positive antidepressant response. Thus, non-responders seem to also exhibit impairments in processing novel, unexpected stimuli and in involuntary attention orienting. This suggests dysfunction in fronto-cortical networks, which play a crucial role in attention allocation; previous neuroimaging research has implicated dysfunctional pre/frontal cortical function in MDD (Bremmer et al., 2004; Bruder et al., 2012; Drevets et al., 1997).
Exploratory linear regressions indicated that only baseline P3b amplitudes weakly predicted clinical changes pre- to post-treatment. Including both P3a and P3b amplitudes as predictor variables did not enhance the utility of the model in predicting clinical changes. Thus, the ERPs were not particularly useful in predicting the extent of depression symptom changes following intervention. Nevertheless, baseline P3a/b amplitudes demonstrated an association with treatment response (i.e., a categorical variable – responder/non-responder). Future work should aim to establish cut-off P3 amplitude measures, which could be used to determine the probability that a patient will exhibit a positive treatment effect. If, based on baseline P3 amplitude, the probability of a response is low it may be worthwhile to consider more aggressive antidepressant interventions at treatment initiation.
Neither P3b nor P3a latencies differed between groups, inconsistent with one study (Mehmet et al., 2012) and several electrophysiological studies reporting cognitive slowing in depression (Knott and Lapierre, 1987,1991, Knott et al., 1991a,b), though we did not directly compare depressed individuals (responders/non-responders collapsed) and controls. Of the studies that found P3 latency differences between MDD and control groups, most were found in P3s elicited by the more difficult visual oddball task (Bange and Bathien, 1998; Cavanagh and Geisler, 2006, but see Diner et al., 1984), thus, direct comparisons may not be prudent.
No differences existed between treatment responders, non-responders and controls on target RTs, inconsistent with several studies that have found longer RTs in MDD (El Massioui and Lesevre, 1988; Giedke et al., 1981; Schlegel et al., 1991). This discrepancy could be accounted for by age differences of the investigated sample and patient status (in- versus out-patient) (El Massioui and Lesevre, 1988; Giedke et al., 1981). A more difficult task may have also yielded group RT differences. Our findings suggest that compensatory brain mechanisms may have played a role in maintaining task performance.
As hypothesized, females exhibited larger P3b/a amplitudes than males. Greater P3s may be related to a larger corpus collosum in females (Weiss et al., 1988; Steinmetz et al., 1992) as P3 generation depends on intact inter-hemispheric processing of sensory information (Yamaguchi and Knight, 1991), which is mediated by the corpus callosum. Thus, corpus callosum size could affect inter-hemispheric communication and, consequently, P3 amplitude (Rogers et al., 1991; Mecklinger et al., 1998); however, this explanation is speculative.
No gender differences existed on performance measures, though males tended to perform better than females. FAs correlated positively with P3b latency in females suggesting that longer processing speed or cortical “inefficiency” was associated with more errors. It currently is unknown why this association existed only in females.
Previous studies have found that MDD patients exhibit reduced or absent right-hemispheric auditory P3 dominance (Bange and Bathien, 1998; Bruder et al., 1991, 1998). We found no main effects of hemisphere or hemisphere×group interactions. Bruder et al. (1998) found that depressed patients with higher anhedonic scores had decreased P3 asymmetry. As specific measures of anhedonia were not obtained in this study, it is difficult to state whether this, or other clinical or methodological factors, may have influenced our null P3 asymmetry findings.
Group differences were noted for correct hits, with controls exhibiting superior performance than treatment non-responders. While some have found that MDD patients perform less accurately on oddball tasks than controls (El Massioui et al., 1988; Sara et al., 1994), others have not (Bruder et al., 1989; Hoffman and Polich, 1999; Iv et al., 2010). Our performance results suggest that a smaller P3b in treatment non-responders, reflecting diminished attention allocation and working memory updating, translates to decreased response accuracy.
Correlational analyses between ERP measures and clinical data were only significant in males. P3a latency correlated positively with baseline HAMD17 scores in males suggesting that longer stimulus evaluation/processing is associated with more severe depressive symptoms. Greater baseline attention allocation and context updating (i.e., P3b amplitude) was inversely related to the extent of HAMD17 changes pre- to post-treatment (depression symptom reductions). This finding is inconsistent with precedent work (Iv et al., 2010; Marazziti et al., 2010), though not everyone has noted an association between ERP indices of specific aspects of attention (e.g. selective attention) and depression (Knott et al., 1991b).
Study Limitations and Conclusions
Although this is the first known study to assess both the P3a and P3b in antidepressant treatment responders and non-responders, limitations exist. Fatigue may have differentially affected the study groups and could have been more carefully controlled for, as it has been associated with smaller and longer P3s (Bruder et al., 1989; Polich and Kok, 1995). Factors such as caffeine, nicotine and heart rate have also been shown to affect P3 characteristics (Polich, 2004). Though we attempted to control for caffeine and nicotine exposure, compliance cannot be guaranteed. Additionally, treatment non-responders were older than non-responders and controls; adding age as a covariate did not alter the P3a results, but decreased the significance of the P3b results to statistical trends (p<.1). As such, age should be better controlled for in future work. Furthermore, how treatment responders/non-responders are defined warrants some thought as classifying them using the MADRS or HAMD17 may yield slightly different results. We defined our groups using the MADRS as it is more sensitive to detecting chronic treatment effects (Santen et al., 2009; Jiang and Ahmed, 2009). Lastly, future research should examine if the P3 is differentially modulated by depression subtype and how this relates to treatment outcome, since P3 reductions are most pronounced in melancholic depression (Ancy et al., 1996; Bruder et al., 1989, 1991; Gangadhar et al., 2003; Karaaslan et al., 2003). Indeed, a large proportion of our sample had a current MDD episode with melancholic features (N=23; N=16 atypical; N=12 neither melancholic, atypical or catatonic).
In sum, lowest P3a/b amplitudes existed in antidepressant treatment non-responders, while responders exhibited amplitudes comparable to controls. Baseline P3 amplitude weakly predicted improved symptoms with treatment. These results suggest diminished voluntary and involuntary attention allocation and memory updating in depressed treatment non-responders. As such, the P3, likely in conjunction with other physiological, neural and clinical measures, could potentially be used in predicting and tracking antidepressant response, thereby refining MDD treatment. However, future studies with large patient cohorts are necessary to better establish the reliability and sensitivity of such putative markers of response.
Acknowledgments
Funding: Patients were recruited from an NIH-funded clinical trial (5R01MH077285). NJ was funded through a graduate scholarship from the Canadian Institute of Health Research (CIHR).
Dr. Pierre Blier has been a speaker for, on the advisory boards of, and has received grants/honoraria from Biovail, Eli Lilly, Lundbeck, Organon, Pfizer, and Wyeth; and has a financial interest in Medical Multimedia Inc. None of these companies had any association with the work submitted in this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None of the other authors have any conflicts of interest to disclose.
References
- Ancy J, Gangadhar BN, Janakiramaiah N. 2018;Normal’ P300 amplitude predicts rapid response to ECT in melancholia. J Affect Disord. 1996;41:211–215. doi: 10.1016/s0165-0327(96)00090-0. [DOI] [PubMed] [Google Scholar]
- Bange F, Bathien N. Visual and cognitive dysfunction in depression: an event-related potential study. Electroencephalogr Clin Neurophysiol. 1998;108:472–481. doi: 10.1016/s0168-5597(98)00024-0. [DOI] [PubMed] [Google Scholar]
- Baranov-Krylov IN, Shuvaev VT, Kanunikov IE. Interhemisphere differences during tasks involving attention and selection of lateralized stimuli. Neurosci Behav Physiol. 2007;37(8):811–820. doi: 10.1007/s11055-007-0086-4. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Vaccarino V, Charney DS. Deficits in hippocampal and anterior cingulate functioning during verbal declarative memory encoding in midlife major depression. Am J Psychiatry. 2004;161:637–645. doi: 10.1176/appi.ajp.161.4.637. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Towey JP, Stewart JW, Friedman D, Tenke C, Quitkin FM. Event-relatd potentials in depression: influence of task, stimulus hemifield and clinical features on P3 latency. Biol Psychiatry. 1989;30:233–246. doi: 10.1016/0006-3223(91)90108-x. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Towey JP, Stewart JW, Friedman D, Tenke C, Quitkin FM. Event-related potentials in depression: influence of task, stimulus hemifield and clinical features on P3 latency. Biol Psychiatry. 1991;30:233–246. doi: 10.1016/0006-3223(91)90108-x. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Towey JP, Lette P, Fong R, Stewart JE, McGrath PJ, Quitkin FM. Brain ERPs of depressed patients to complex tones in an oddball task: Relation of reduced P3 asymmetry to physical anhedonia. Psychophysiology. 1998;35:54–63. [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, Tenke CE, McGrath PJ, Leite P, Bhattacharya N, Quitkin FM. Electroencephalographic and perceptual asymmetry differences between responders and nonresponders to an SSRI antidepressant. Biol Psychiatry. 2001;49:416–425. doi: 10.1016/s0006-3223(00)01016-7. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Kroppmann CJ, Kayser J, Stewart JW, McGrath PJ, Tenke CE. Reduced brain responses to novel sounds in depression: P3 findings in a novelty oddball task. Psychiatry Res. 2009;170:218–223. doi: 10.1016/j.psychres.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Kayser J, Tenke CE. The Oxford Handbook of Event-Related Potential Components. New York: Oxford Universiy Press; 2012. Event-related brain potentials in depression: clinical, cognitive and neurophysiologic implications; pp. 563–592. [Google Scholar]
- Cavanagh J, Geisler MW. Mood effects on the ERP processing of emotional intensity in faces: A P3 investigation with depressed students. Int J Psychophysiol. 2006;60:27–33. doi: 10.1016/j.ijpsycho.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Chatrian GE, Lettich E, Nelson PL. Ten percent electrode system for topographic studies of spontaneous and evoked EEG activity. Am J EEG Technol. 1985;25:83–92. [Google Scholar]
- Diner BC, Holcomb PJ, Dykman RA. P300 in major depressive disorder. Psychiatry Res. 1984;15:175–184. doi: 10.1016/0165-1781(85)90074-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalties in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- El Massioui F, Lesèvre N. Attention impairment and psychomotor retardation in depressed patients: an event-related potential study. Electroencephalogr Clin Neurophysiol. 1988;70:46–55. doi: 10.1016/0013-4694(88)90193-9. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) Washington, DC, USA: American Psychiatric Association; 1997. [Google Scholar]
- Gangadhar BN, Ancy J, Janakiramaiah N, Umapathy C. P300 amplitude in non-bipolar, melancholic depression. J Affect Disord. 1993;28:57–60. doi: 10.1016/0165-0327(93)90077-w. [DOI] [PubMed] [Google Scholar]
- Giedke H, Their P, Bolz J. The relationship between P3 latency and reaction time in depression. Biol Psychol. 1981;13:31–49. doi: 10.1016/0301-0511(81)90026-0. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman LD, Polich J. P300, handedness, and corpus callosal size: gender, modality, and task. Int J Psychophysiol. 1999;31:163–174. doi: 10.1016/s0167-8760(98)00050-6. [DOI] [PubMed] [Google Scholar]
- Iv J, Zhao L, Gong J, Chen C, Miao D. Event-related potential based evidence of cognitive dysfunction in patients during the first episode of depression using a novelty oddball task. Psychiatry Res: Neuroimaging. 2010;182:58–66. doi: 10.1016/j.pscychresns.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Jaworska N, Blier P, Fusee W, Knott V. Scalp- and sLORETA-derived loudness dependence of auditory evoked potentials (LDAEPs) in unmedicated depressed males and females and healthy controls. Clin Neurophysiol. 2012a doi: 10.1016/j.clinph.2012.02.076. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Jaworska N, Blier P, Fusee W, Knott V. The temporal electrocortical profile of emotive facial processing in depressed males and females and healthy controls. J Affect Disord. 2012b;136(3):1072–1081. doi: 10.1016/j.jad.2011.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Ahmed S. An analysis of correlations among four outcome scales employed in clinical trials of patients with major depressive disorder. Ann Gen Psychiatry. 2009;23(8):4. doi: 10.1186/1744-859X-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayam B, Alexopoulos GS. Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry. 1999;56:713–718. doi: 10.1001/archpsyc.56.8.713. [DOI] [PubMed] [Google Scholar]
- Karaaslan F, Gonul AS, Oguz A, Erdinc E, Esel E. P300 changes in major depressive disorders with and without psychotic features. J Affect Disord. 2003;73:283–287. doi: 10.1016/s0165-0327(01)00477-3. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Tanaka S, Wang J, Hokama H, Hiramatsu K. Abnormalities of P300 cortical current density in unmedicated depressed patients revealed by Loreta analysis of event-related potentials. Psychiatry Clin Neurosci. 2004;58:68–75. doi: 10.1111/j.1440-1819.2004.01195.x. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Bruder GE. Dissociation of brain ERP topographies for tonal and phonetic oddball tasks. Psychophysiology. 1998;35:576–590. doi: 10.1017/s0048577298970214. [DOI] [PubMed] [Google Scholar]
- Kayser J, Bruder GE, Tenke CE, Stewart JW, Quitkin FM. Event-related potentials (ERPs) to hemifield presentations of emotional stimuli: differences between depressed patients and healthy adults in P3 amplitude and asymmetry. Int J Psychophysiol. 2000;36:211–236. doi: 10.1016/s0167-8760(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Kimble MO, Fleming K, Bandy C, Zambetti A. Attention to novel and target stimuli in trauma survivors. Psychiatry Res. 2010;178(3):501–506. doi: 10.1016/j.psychres.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott V, Lapierre Y. Electrophysiological and behavioral correlates of psychomotor responsivity in depression. Biol Psychiatry. 1987;22:313–324. doi: 10.1016/0006-3223(87)90149-1. [DOI] [PubMed] [Google Scholar]
- Knott V, Lapierre Y. Temporal segmentation of response speed in depression: neuro-electrophysiological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:249–255. doi: 10.1016/0278-5846(91)90088-i. [DOI] [PubMed] [Google Scholar]
- Knott V, Lapierre Y, de Lugt D, Griffiths L, Bakish D, Browne M, Horn E. Preparatory brain potentials in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1991a;15:257–262. doi: 10.1016/0278-5846(91)90089-j. [DOI] [PubMed] [Google Scholar]
- Knott V, Lapierre Y, Griffiths L, de Lugt D, Bakish D. Event-related potentials and selective attention in major depressive illness. J Affect Disorders. 1991b;23:43–48. doi: 10.1016/0165-0327(91)90034-p. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An introduction to the Event-Related Potential Technique. Cambridge, Massachusetts: MIT Press; 2005. [Google Scholar]
- Marazziti D, Consoli G, Picchetti M, Carlini M, Faravelli L. Cognitive impairment in major depression. Eur J Pharmacol. 2010;626:83–86. doi: 10.1016/j.ejphar.2009.08.046. [DOI] [PubMed] [Google Scholar]
- Maxwell E. The Family Interview for Genetic Studies Manual. Washington, DC, USA: 1992. [Google Scholar]
- Mecklinger A, Maess B, Opitz B, Pfeifer E, Cheyne D, Weinberg H. A MEG analysis of the P300 in visual discrimination tasks. Electroencephalogr Clin Neurophysiol. 1998;108:45–56. doi: 10.1016/s0168-5597(97)00092-0. [DOI] [PubMed] [Google Scholar]
- Mehmet S, Mehmet AK, Murat E, Oguzhan OZ, Fuat O. Event-related potentials in major depressive disorder: the relationship between P300 and treatment response. Turk Psikiyatri Derg. 2012;23(1):33–39. [PubMed] [Google Scholar]
- Montgomery SA, Åsberg S. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Polich J, Kok A. Cognitive and biological determinants of P300: an integrative review. Biol Psychol. 1995;41:103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Polich J. Clinical application of the P300 event-related brain potential. Phys Med Rehabil Clin N Am. 2004;15:133–161. doi: 10.1016/s1047-9651(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: An integratie theory of P3a and P3b. Clin Neuropsychology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagone K, Fukuda M, Kato N. Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neurosci Res. 2004;50:1–11. doi: 10.1016/j.neures.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Santen G, Danhof M, Della Pasqua O. Sensitivity of the Montgomery Asberg Depression Rating Scale to response and its consequences for the assessment of efficacy. J Psychiatr Res. 2009;43(12):1049–1056. doi: 10.1016/j.jpsychires.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Santosh PJ, Malhotra S, Raghunathan M, Mehra YN. A study of P300 in melancholic depression – correlation with psychotic features. Biol Psychiatry. 1994;26:565–575. doi: 10.1016/0006-3223(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Sara G, Gordon E, Kraiuhin C, Coyle S, Howson A, Meares R. The P300 ERP component: an index of cognitive dysfunction in depression? J Affect Disord. 1994;31:29–38. doi: 10.1016/0165-0327(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Schlegel S, Nieber D, Hermann C, Bakauski E. Latencies of the P300 component of the auditory event-related potential in depression are related to the Bech-Rafaelsen Melancholia Scale but not the Hamilton Rating Scale for Depression. Acta Psychiatr Scand. 1991;83:438–440. doi: 10.1111/j.1600-0447.1991.tb05571.x. [DOI] [PubMed] [Google Scholar]
- Segrave R, Nathan P. Pindolol augmentation of selective serotonin reuptake inhibitors: accounting for the variability of results of placebo-controlled double-blind studies in patients with major depression. Hum Psychopharmacol. 2005;20:163–174. doi: 10.1002/hup.672. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Kyle S, Forty L, Cooper C, Walters J, Russell E, Caesar S, Farmer A, McGuffin P, Jones I, Jones L, Craddock N. Differences in depressive symptom profile between males and females. J Affect Disord. 2008;108:279–284. doi: 10.1016/j.jad.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroenceph Clin Neurophysiol. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Jancke L, Kleinschmidt A, Schlaug G, Volkmann J, Huang Y. Sex but no hand differences in the isthmus of the corpus callosum. Neurologo. 1992;42:749–752. doi: 10.1212/wnl.42.4.749. [DOI] [PubMed] [Google Scholar]
- Tang Y, Li Y, Wang N, Li H, Li H, Wang J. The altered cortical connectivity during spatial search for facial expression in major depressive disorder. Prog NeuroPsychopharmacol Biol Psychiatry. 2011;35:1891–1900. doi: 10.1016/j.pnpbp.2011.08.006. [DOI] [PubMed] [Google Scholar]
- van Noorden MS, Giltay EJ, den Hollander-Gijsman ME, van der Wee NJA, van Veen T, Zitman FG. Gender differences in clinical characteristics in a naturalistic sample of depressive outpatients: The leiden routine outcome monitoring study. J Affect Disord. 2010;125:116–123. doi: 10.1016/j.jad.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Vandoolaeghe E, van Hunsel F, Nuyten D, Maes M. Auditory event related potentials in major depression: prolonged P300 latency and increased P200 amplitude. J Affect Disord. 1998;48:105–113. doi: 10.1016/s0165-0327(97)00165-1. [DOI] [PubMed] [Google Scholar]
- Volpe U, Mucci A, Bucci P, Merlotti E, Galdersi S, Maj M. The cortical generators of P3a and P3b: A LORETA study. Brain Res Bull. 2007;73:220–230. doi: 10.1016/j.brainresbull.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Weiss S, Weber G, Wenger E, Kimbacher M. The human corpus callosum and the controversy about sexual dimorphism. Psychobiology. 1988;16:411–415. doi: 10.3109/00207458908987430. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Mental Health; Depression. [Accessed 21 August 2012];2012 [online] Available at:< http:/www.who.int/mental_health/management/depression/definition/en/index.html>.
- Yagi Y, Coburn KL, Estes KM, Arruda JE. Effects of aerobic exercise and gender on visual and auditory P300, reaction time, and accuracy. Eur J Appl Physiol. 1999;80:402–408. doi: 10.1007/s004210050611. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT. Anterior and posterior association cortex contributions to the somatosensory P300. J Neurosci. 1991;11:2039–2054. doi: 10.1523/JNEUROSCI.11-07-02039.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai I, Fujikawa T, Osada M, Yamawaki S, Touhouda Y. Changes in auditory P300 in patients with major depression and silent cerebral infarction. J Affect Disord. 1997;46:263–271. doi: 10.1016/s0165-0327(97)00100-6. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Hauser U, Conty C, Emrich HM, Dietrich DE. Familial risk for depression and P3b component as a possible neurocognitive vulnerability marker. Neuropsychobiology. 2007;55(1):14–20. doi: 10.1159/000103571. [DOI] [PubMed] [Google Scholar]
- Zhu CY, Zheng Z, Qiu CJ, Zou K, Nie XJ, Feng Y, Wu RZ, Zhang W. Brain evoked potential in patients with depression or anxiety. Journal of Sichuan University. 2009;40(4):708–711. [Article in Chinese]. [PubMed] [Google Scholar]
- Zisook S, Lesser IM, Lebowitz B, Rush AJ, Kallenberg G, Wisniewski, Nierenberg AA, Fava M, Luther JF, Morris DW, Trivedi MH. Effect of antidepressant medication treatment on suicidal ideation and behavior in a randomized trial: an exploratory report from the Combining Medications to Enhance Depression Outcomes Study. J Clin Psychiatry. 2011;72:1322–1332. doi: 10.4088/JCP.10m06724. [DOI] [PubMed] [Google Scholar]