Abstract
Background
Nulliparity is associated with an increased risk of endometrial cancer. Less clear is whether nulliparity modifies the association between other established hormone-related risk factors. The proportion of nulliparous women has increased since the mid-1970s, but most individual studies are too small to test the hypothesis that endometrial cancer risk factors may be more strongly associated with risk among nulliparous women compared with parous women.
Methods
We aggregated data on 26,936 postmenopausal, Caucasian nulliparous women (360 endometrial cancers) and 146,583 postmenopausal Caucasian parous women (1,378 endometrial cancers) from four U.S. prospective studies (1979–2006). We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) in stratified analyses.
Results
As expected, endometrial cancer risk was higher among nulliparous women than among parous women (HR, nulliparous vs. parous = 1.42, 95% CI 1.26 to 1.60). Stratified associations between endometrial cancer and hormone-related risk factors did not differ among nulliparous vs. parous women: among both groups, oral contraceptives and earlier menopause were associated with reduced risk. The highest HRs were for obesity; body mass index ≥30 kg/m2 (vs. <25 kg/m2) increased endometrial cancer risk three-fold among nulliparous (HR= 3.04, 95% CI 2.34 to 3.94) and parous (HR= 2.88, 95% CI 2.52 to 3.29) women.
Conclusions
The results from this large, pooled analysis of data from four large prospective studies suggest that nulliparity does not modify endometrial cancer risks associated with established hormone-related risk factors.
Keywords: endometrial cancer, nulliparity, reproductive history, oral contraceptives, hormonal, obesity
Introduction
Parous women are 20% to 40% less likely than nulliparous women to develop endometrial cancer 1–3. The exact mechanism by which parity reduces risk is not known, but a number of hypotheses have been proposed. Elevated progesterone levels during pregnancy could inhibit estrogen-driven endometrial cell proliferation and promote differentiation and apoptosis of endometrial cells 4–6. Vaginal delivery itself or the postpartum involution of the uterus could facilitate the shedding of precancerous or cancerous cells in the endometrial lining of the uterus 7, 8. Certain infertility conditions, such as anovulatory disorders, that lead to nulliparity could also contribute to higher endometrial cancer risks among nulliparous women 9.
These mechanisms that are hypothesized to account for the protective effects of parity among parous women occur during the reproductive years, but most endometrial cancers are diagnosed many years later, after menopause 10. Parity could therefore shift the trajectory of endometrial cancer risk by modifying how other risk factors, such as oral contraceptive (OC) use, age at menopause, obesity, and menopausal hormone therapy (MHT), exert their risk-increasing or risk-decreasing effects on the uterus. We hypothesized that hormone-related endometrial cancer risk factors may be more strongly associated with risk among nulliparous women, who are not exposed to the protective effects of a full-term pregnancy. Even though the prevalence of nulliparity among U.S. women in their 40s has doubled in the last 30 years, from 10% in the mid-1970s to 18% in 2008 11, most individual studies have insufficient statistical power to formally evaluate potential risk-factor heterogeneity by parity. We therefore conducted a large, aggregated analysis of data from four prospective studies to quantify and compare the associations between established hormone-related risk factors for endometrial cancer among postmenopausal nulliparous women and parous women.
Materials and Methods
Selected studies
This aggregated analysis included four National Cancer Institute (NCI) prospective studies: the Breast Cancer Detection Demonstration Project (BCDDP) Follow-up 12 Study; NIHAARP Diet and Health Study (NIH-AARP) 13; Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) 14; and United States Radiologic Technologists Study 15. Each study used self-administered questionnaires or telephone-based interviews to collect information about hormonal, reproductive, and other exposures, as described previously 12–15. Each study obtained informed consent from participants and has been approved annually by the Special Studies Institutional Review Board of the NCI.
Inclusion criteria
Within each study, we restricted the analysis to postmenopausal Caucasian women, due to limited numbers of non-Caucasians in each of the four studies. Women who reported one or more live births or provided an age at first birth were classified as parous. Women who reported no live births and reported no age at first birth were classified as nulliparous. Women who had a hysterectomy before baseline, had unknown hysterectomy status at baseline, or had a personal history of cancer (other than non-melanoma skin cancer) were excluded. We included the approximately 11% of the BCDDP cohort participants whose personal history of cancer was unknown at baseline.
Ascertainment and classification of endometrial cancer
The four cohorts used self-report, retrieval of medical and pathology reports, and linkage with state cancer registries and the National Death Index to identify cancers diagnosed during follow-up 12–15. A summary of the study-specific methods was provided in a previous manuscript in which we aggregated these four studies 16. From each study, we identified incident first primary endometrial cancers based on International Classification of Diseases (ICD) 9 codes 182.0 and 182.9 and ICD-10 codes C54.0, C54.1, C54.2, C54.3, C54.8, C54.9, or C55.9. Uterine corpus cancers with histological classification of 8800 17 (uterine sarcomas) or higher were excluded.
Statistical methods
We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) by using multivariable Cox regression methods (Proc PHREG, SAS v. 9.1). Age was the underlying time metric. In NIH-AARP and PLCO, where all women were at least 50 years old at baseline and the majority was postmenopausal, follow-up for this analysis began at the age of the baseline questionnaires. In BCDDP and USRT, which included younger and premenopausal women, follow-up began at the first questionnaire (baseline or follow-up) on which a participant reported that she was postmenopausal. Person-time accrued until the diagnosis of endometrial or any cancer (excluding non-melanoma skin cancer), death, loss to follow-up, age 85, or the study end date (i.e., administrative censoring) 12–15, whichever occurred first. Analyses in BCDDP incorporated self-reported hysterectomy during follow-up as an additional censoring criterion. After Wald chi-square tests of study-specific HRs for hormone-related risk factors among parous women revealed no evidence of heterogeneity across studies, we aggregated data from all studies into a single dataset. We then fit separate models to nulliparous women and parous women to obtain parity-stratified HRs for hormone-related risk factors. From each stratum-specific model we obtained log hazard ratios and the covariance matrix of the estimates for each level of a given hormone-related factor (e.g., categories of age at menopause). Heterogeneity between estimates for nulliparous and parous women was assessed by a Wald type chi-square test based on properly chosen statistical contrasts between the parameter vectors of stratum-specific log-hazard ratio estimates standardized by their variance-covariances. Models assessing BMI were stratified by MHT, and vice versa, based on previously reported interactions between these exposures 18–21. To estimate the joint associations between nulliparity and BMI, OCs, and MHT, we fit models that included all women (nulliparous and parous) and used parous women in the lowest category of exposure (e.g., parous women with BMI <25 kg/m2) as the reference group.
Fully-adjusted models included ages at menarche (<13, ≥13) and menopause (<45, 45–49, 50–54, ≥55), baseline BMI, based on measured or self-reported height and weight (<25, 25– <30, ≥30 kg/m2), ever use of OCs (never, ever), cumulative duration of MHT use at baseline (never, <5 years, ≥5 years), diabetes (no, yes), smoking (never, former, current), study, and calendar years of birth (<1933, ≥1933) and study entry (<1995, ≥1995). Models with parous women also included number of births. All covariates were modeled categorically with a separate category for missing data. The hazard plots for all of these covariates revealed no violations of proportional hazards assumptions. Outlier values for age at menopause (<30 or >64 years) and BMI (<16 kg/m2) were considered missing. All significance tests were two-sided, with P <0.05 considered statistically significant.
The four individual cohorts (Table 1) collected baseline and follow-up data during a time period of substantial changes in how MHT was prescribed and used. By 1996 22 it became clear that unopposed estrogen should be used only by women who had a hysterectomy. As information about hormone formulation was not available from all baseline questionnaires, we used calendar year to classify baseline MHT use. Because all women in the present analysis had an intact uterus at the start of follow-up (see above), we assumed all current MHT use reported in 1996 or after was estrogen plus progestin (E + P). To minimize the potential influence of the markedly elevated endometrial cancer risks that persist after cessation of unopposed estrogen use12, we limited the analysis of MHT to include only a) NIH-AARP participants who reported never-use or current MHT use at baseline (1995–1996) and b) PLCO and USRT participants who entered those studies on or after 1/1/1996. In addition, analyses of risk associated with E + P were administratively censored at 6/30/2002, because none of the cohorts possessed enough subsequent follow-up data to characterize the unprecedented and widespread decline in overall MHT use after July 200223.
Table 1.
Study characteristics and risk of endometrial cancer among postmenopausal Caucasian nulliparous women compared with parous women
| Study | No. Women (no. cases) | Mean age, years, at baseline | Nulliparous vs. parous | ||
|---|---|---|---|---|---|
| Nulliparous | Parous | Nulliparous | Parous | Hazard ratios (95% confidence intervals) | |
| NIH-AARPa | 16,355 (211) | 81,034 (755) | 62.4 | 61.4 | 1.41 (1.21, 1.65)b |
| BCDDPc | 3,870 (52) | 21,648 (178) | 59.6 | 57.4 | 1.42 (1.04, 1.94)b |
| PLCOd | 4,217 (55) | 36,345 (362) | 62.8 | 62.9 | 1.31 (0.99, 1.75)b |
| USRTe | 2,494 (42) | 7,556 (83) | 58.1 | 56.0 | 1.67 (1.13, 2.47)b |
| Total | 26,936 (360) | 146,583 (1,378) | 61.0 | 61.5 | 1.42 (1.26, 1.60) f |
NIH-AARP: NIH-AARP Diet and Health Study
Study-specific estimates adjusted for birth year and entry year (BCDDP, PLCO, USRT), age at last menstrual period, age at menarche, body mass index, oral contraceptive use, menopausal hormone therapy use, diabetes, and smoking.
BCDDP: The Breast Cancer Detection Demonstration Project Follow-up Study
PLCO: The Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial
USRT: The US Radiologic Technologist Study;
Aggregated estimated adjusted for birth year, calendar year of entry, study, age at menopause, age at menarche, body mass index, oral contraceptive use, menopausal hormone therapy use, diabetes, and smoking.
Results
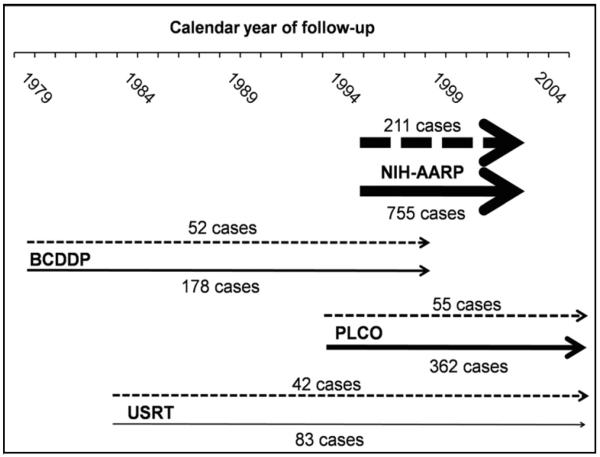
The pooled dataset included 360 incident endometrial cancers among 26,936 nulliparous women and 1,378 cancers among 146,583 parous women (Figure 1, Table 1). Histologic type was available for approximately half of the cases; most were endometrioid adenocarcinomas. The aggregate HR for nulliparity was 1.42 (95% CI 1.26 to 1.60), with study-specific HRs between 1.31 and 1.67 (Table 1).
Figure 1.
Calendar year of follow-up and endometrial cancer case numbers among postmenopausal Caucasian nulliparous and parous women in the four studies included in the aggregated analysis
Nulliparous women represented by dashed (- - - -) lines. Parous women represented by solid lines.
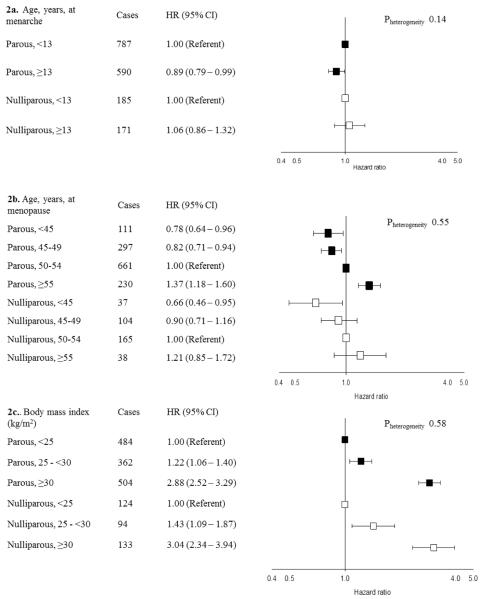
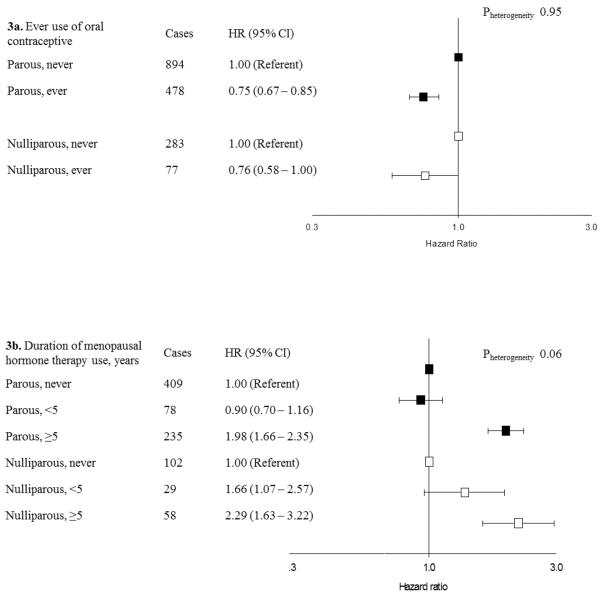
There was no evidence of statistically significant heterogeneity by parity for any of the hormone-related risk factors (Figures 2–3). Endometrial cancer rates were approximately 25% lower among women who reported a history of OC use (Figure 3a). Endometrial cancer risks increased with older age at menopause, increasing BMI, and longer-duration E + P use. The largest HRs, approximately 3-fold, were observed for BMI ≥30 kg/m2 compared with <25 kg/m2. For the risk factors shown in Figures 2–3, similar patterns were observed among parous women with 1–2 births and those with ≥3 births (data not shown).
Figure 2.
Ages at menarche and menopause and body mass index and endometrial cancer risk stratified by parity in the aggregated sample of postmenopausal Caucasian women
Hazard ratios (HRs) and 95% confidence intervals (CI) estimated using Cox proportional hazards models with age as the underlying time scale with separate models fit to nulliparous and parous women. Models include all variables presented in Figures 2 and 3 as well as study, birth year, calendar year of entry, diabetes, and smoking status. Parous models further adjusted for number of births. P-values are presented for the heterogeneity between parous and nulliparous women based on a Wald type chi-square test. HRs for nulliparous women represented by white boxes. HRs for parous women represented black boxes.
2a. In PLCO, age at menarche categorized as <14 and ≥14 years.
Figure 3.
Use of oral contraceptives and menopausal hormone therapy and risk of endometrial cancer stratified by parity in the aggregated sample of postmenopausal Caucasian women
Hazard ratios (HRs) and 95% confidence intervals (CI) estimated using Cox proportional hazards models with age as the underlying time scale with separate models fit to nulliparous and parous women. Models include all variables presented in Figures 2 and 3 as well as study, birth year, calendar year of entry, diabetes, and smoking status. Parous models further adjusted for number of births. P-values are presented for the heterogeneity between parous and nulliparous women based on a Wald type chi-square test. HRs for nulliparous women represented by white boxes. HRs for parous women represented black boxes.
3a. In NIH-AARP, never use includes <1 year
3b. In PLCO, duration of E + P use categorized as <6 years and ≥6 years. Analyses restricted to subset of women who reported never or current use from NIH-AARP or entry into PLCO and USRT after 1995. Follow-up censored at June 30, 2002.
As expected, the association between BMI and endometrial cancer was strongest among women who reported no history of E + P use at baseline (Table 2). In this group, obesity was associated with a more than 5-fold increased risk of endometrial cancer. Similarly, the association between E + P and endometrial cancer was strongest among the leanest women (data not shown). Consistent with the overall analyses, stratified analyses of BMI and E + P use revealed no significant heterogeneity between nulliparous and parous women (all p-values for heterogeneity ≥ 0.05).
Table 2.
Hazard Ratios and 95% Confidence Intervals for the association between body mass index and endometrial cancer risk in the aggregated sample of postmenopausal Caucasian women stratified by parity and menopausal hormone therapy use: subset of women who reported never or current menopausal hormone therapy use at entry into NIH-AARP or after 1995 in PLCO and USRT
| Nulliparous women | Parous women | ||||
|---|---|---|---|---|---|
| Cases | HRs (95% CIs)a | Cases | HRs (95% CIs)a,b | P heterogeneity c | |
| Body mass index among never users of menopausal hormone therapy | |||||
| <25 kg/m2 | 23 | 1.00 (Referent) | 86 | 1.00 (Referent) | |
| 25–<30 kg/m2 | 30 | 1.87 (1.09, 3.23) | 125 | 1.73 (1.31, 2.27) | |
| ≥30 kg/m2 | 80 | 5.59 (3.46, 9.01) | 315 | 5.48 (4.30, 7.00) | 0.96 |
| Body mass index among current users of menopausal hormone therapy | |||||
| <25 kg/m2 | 45 | 1.00 (Referent) | 200 | 1.00 (Referent) | |
| 25–<30 kg/m2 | 32 | 1.36 (0.86, 2.14) | 113 | 1.00 (0.79, 1.26) | |
| ≥30 kg/m2 | 24 | 1.72 (1.03, 2.89) | 67 | 1.25 (0.94, 1.65) | 0.39 |
HRs: Hazard Ratios; 95% Confidence Intervals; Separate models fit to nulliparous and parous women. Models adjusted for age at menarche, age at menopause, oral contraceptive use as well as birth year, study, calendar year of entry, diabetes, and smoking status.
Further adjusted for number of births.
P-value for the heterogeneity between parous and nulliparous women based on a Wald type chi-square test.
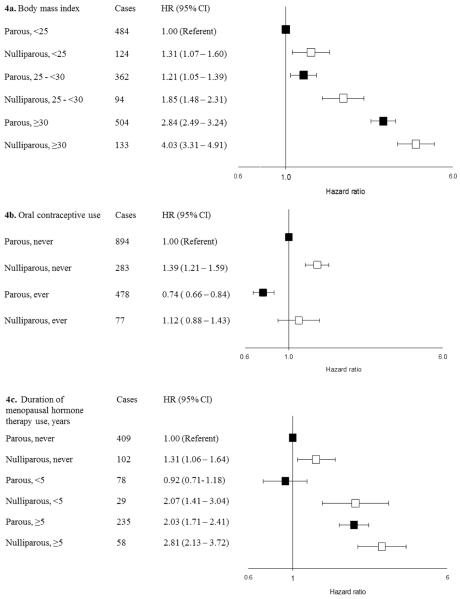
Models that included all women jointly quantified the associations between nulliparity and three modifiable risk factors: BMI, OC use, and cumulative duration of E + P use at baseline (Figure 4a–c). Compared with parous women with BMI <25 kg/m2, nulliparous women who were obese at baseline had a 4-fold increased risk of endometrial cancer (HR= 4.03; 95% CI 3.31 to 4.91) (Figure 4a). Nulliparous women who had used OCs had a small, nonsignificant risk of endometrial cancer compared with parous women who had never used OCs (HR = 1.12; 95% CI 0.88 to 1.43) (Figure 4b). The joint association between long-term E + P use at baseline and nulliparity was 2.81 (95% CI 2.13 to 3.72) (Figure 4c).
Figure 4.
Joint associations of body mass index, oral contraceptive use, and menopausal hormone therapy use with parity in the aggregated sample of postmenopausal Caucasian women.
Hazard ratios (HRs) and 95% confidence intervals (CI) estimated using Cox proportional hazards models with age as the underlying time scale with separate models fit to nulliparous and parous women. Models include all variables presented in Figures 2 and 3 as well as study, birth year, calendar year of entry, diabetes, and smoking status. HRs for nulliparous women represented by white boxes. HRs for parous women represented black boxes.
4b. In NIH-AARP, never use includes <1 year
4c. In PLCO, duration of E + P use categorized as <6 years and ≥6 years. Analyses restricted to the subset of women who reported never or current use from NIH-AARP or entry into PLCO and USRT after 1995. Follow-up censored at June 30, 2002.
Sensitivity analyses
The associations presented in Figures 2–3 were robust in sensitivity analyses, including the use of calendar-time as the underlying time metric, complete-case analyses, and censoring follow-up at age 80 years. Information about hysterectomy during follow-up was only available from BCDDP. In this cohort, censoring women at hysterectomy (“censoring”) or ignoring the hysterectomy (“delayed censoring”) did not markedly alter the results presented above.
Discussion
Our pooled analysis of 360 cases of endometrial cancer among 26,936 nulliparous women and 1,378 cases among 146,583 parous women indicates that associations between endometrial cancer and hormone-related risk factors do not differ between nulliparous and parous women. We found no statistically significant interactions between parity and age at menarche, oral contraceptive use, BMI, age at menopause, or MHT use, and there were no differences in associations among parous women with 1–2 vs. 3 or more births.
We had hypothesized that endometrial cancer risk factors could be more strongly associated with risk among women who did not experience the protective effects of a full term pregnancy. In the breast, it is thought that the unique hormonal milieu of pregnancy induces persistent structural changes, such as differentiation of terminal duct units, that leave that breast tissue less susceptible to carcinogenesis and parous women at reduced risk of developing breast cancer24. Because parous women are similarly less likely than nulliparous women to develop endometrial cancer, an analogous process could plausibly occur in the uterus. In addition, any parity-induced molecular or structural changes that affect long-term susceptibility to cancer might be even stronger in the uterus than in the breast, because the uterus may experience even more substantial changes during pregnancy, delivery, and postpartum than does the breast. Hypothesized explanations for the protective effect of parity on endometrial cancer include higher levels of progesterone during pregnancy4, “clearance” of premalignant lesions or cancerous cells during childbirth7 or postpartum involution of the uterus8. Our analysis did not directly test those hypotheses, but our results suggest that the potential effects of other hormone-related risk factors on endometrial cancer risk are not modified by parity status.
Previous studies that reported risk-factor differences between nulliparous and parous women reported inconsistent findings25–31, but most studies included fewer than 100 nulliparous cases. The low prevalence of nulliparous women, particularly in older studies, makes this question well-suited for data pooling. Our pooled dataset included a larger sample size and broader and more detailed risk-factor data, which together reduce the chances that spurious false-negative or false-positive findings arise. Consistent with our results, some of the earlier and smaller studies reported no evidence of interaction between parity and BMI19, 29, menopausal estrogen therapy use26, 28, and OC use27, 30, 31. Our results did not replicate a previously reported interaction, which was not statistically significant, between parity and OC use25.
In addition to our large sample size and detailed risk-factor data, our analysis was strengthened by including prospective studies that each utilized sound methods of data collection and follow-up. Important limitations also exist. Even with our large sample size, some exposure categories had small sample sizes. As most of the participants in these cohorts were Caucasian, we restricted the analyses to this group, which potentially limits the generalizability of our findings to other racial and ethnic groups32. We did not investigate whether the null results further differed among Type I vs. Type II cancers. Hormone-related exposures might be more important for Type I tumors than Type II tumors33, but the differences between these two groups of tumors do not indicate that hormone-related risk factors affect risk of only Type I tumors. We focused on multiplicative interactions between nulliparous and parous women, on the assumption that potential differences would be greatest between these groups. More subtle differences might exist by number of births among parous women, but our analyses revealed similar associations in analyses stratified by parity among parous women. Gravidity might also affect potential differences by parity, on the assumption that hormonal effects present in a full-term pregnancy might also be present but attenuated among incomplete pregnancies. Testing that hypothesis would require detailed data on the duration of incomplete pregnancies. Precise data on the duration of incomplete pregnancies is often not collected in epidemiologic studies, and that level of data was not available in the cohorts included in our pooled study. The extent of any such further modification of risk by hormonal or other exposures due to incomplete pregnancies is unknown in our analysis. We also lacked sufficient data to further stratify analyses by reason for nulliparity, such as infertility. Future studies with detailed infertility data are needed to better understand whether or how biologic factors related to infertility affect subsequent endometrial cancer risk, particularly among subgroups such as women who give birth after a previous period of infertility.
The changing patterns of MHT use during the years in which these four cohort studies enrolled participants and collected data made it challenging to reconcile and pool the divergent data collected in the four studies. We lacked sufficient detail to assess risks by specific formulation and regimen, which is particularly important for endometrial cancer. Endometrial cancer is strongly associated with unopposed estrogen, but increasing evidence points to moderately elevated risks among women who use estrogen plus progestin for long durations12, 18, 20, 34–37. Our aggregate analysis utilized calendar year to make assumptions about whether self-reported current MHT use was unopposed estrogen or estrogen plus progestin. Future studies with more recently collected data could evaluate this issue further.
In conclusion, the results from this large, pooled analysis of data from four large prospective studies suggest that nulliparity does not modify the risks associated with established hormone-related endometrial cancer risk factors. Continued innovative investigations of potential risk-factor differences across at-risk groups of interest may help to advance the understanding of how parity reduces the risk of endometrial cancer.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the NIH and the National Cancer Institute. Dr. Greenlee was supported in part by N01-CN-25518
NIH-AARP: Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University. Cancer incidence data from California were collected by the California Department of Health Services, Cancer Surveillance Section. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, State of Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System under contract to the Department of Health (DOH). The views expressed herein are solely those of the authors and do not necessarily reflect those of the contractor or DOH. Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Medical Center in New Orleans. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey State Department of Health and Senior Services. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services. Cancer incidence data from Nevada were collected by the Nevada Central Cancer Registry, Center for Health Data and Research, Bureau of Health Planning and Statistics, State Health Division, State of Nevada Department of Health and Human Services.
We are indebted to the participants in the NIH-AARP Diet and Health Study for their outstanding cooperation. We also thank Sigurd Hermansen and Kerry Grace Morrissey from Westat for study outcomes ascertainment and management and Leslie Carroll at Information Management Services for data support and analysis.
BCDDP: We are grateful to the study participants. We thank Dave Campbell and Leslie Carroll at Information Management Services for data management support.
PLCO: This research was supported by contracts from the Division of Cancer Prevention, National Cancer Institute, NIH, DHHS. The authors thank Drs. Christine Berg and Philip Prorok, Division of Cancer Prevention, National Cancer Institute, the Screening Center investigators and staff of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, Mr. Tom Riley and staff, Information Management Services, Inc., Ms. Barbara O'Brien and staff, Westat, Inc. Most importantly, we acknowledge the study participants for their contributions to making this study possible.
USRT: We are grateful to the radiologic technologists who participated in the USRT Study; Jerry Reid of the American Registry of Radiologic Technologists for continued support of the study; Diane Kampa, Allison Iwan, and Richard Hoffbeck of the University of Minnesota for study coordination, data collection, and data management; and Jeremy Miller of Information Management Services for biomedical computing support.
Footnotes
The authors declare that they have no conflict of interest
The authors have no financial disclosures.
REFERENCES
- 1.Dossus L, Allen N, Kaaks R, et al. Reproductive risk factors and endometrial cancer: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2010;127(2):442–51. doi: 10.1002/ijc.25050. [DOI] [PubMed] [Google Scholar]
- 2.Karageorgi S, Hankinson SE, Kraft P, De Vivo I. Reproductive factors and postmenopausal hormone use in relation to endometrial cancer risk in the Nurses' Health Study cohort 1976–2004. Int J Cancer. 2010;126(1):208–16. doi: 10.1002/ijc.24672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfeiffer RM, Mitani A, Landgren O, et al. Timing of births and endometrial cancer risk in Swedish women. Cancer Causes Control. 2009;20(8):1441–9. doi: 10.1007/s10552-009-9370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21(3):427–33. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 5.Key TJ, Pike MC. The dose-effect relationship between `unopposed' oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer. 1988;57(2):205–12. doi: 10.1038/bjc.1988.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JJ, Chapman-Davis E. Role of progesterone in endometrial cancer. Semin Reprod Med. 2010;28(1):81–90. doi: 10.1055/s-0029-1242998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kvale G, Heuch I, Nilssen S. Reproductive factors and cancers of the breast and genital organs--are the different cancer sites similarly affected? Cancer Detect Prev. 1991;15(5):369–77. [PubMed] [Google Scholar]
- 8.Baird DD, Dunson DB. Why is parity protective for uterine fibroids? Epidemiology. 2003;14(2):247–50. doi: 10.1097/01.EDE.0000054360.61254.27. [DOI] [PubMed] [Google Scholar]
- 9.Brinton LA, Westhoff CL, Scoccia B, et al. Causes of infertility as predictors of subsequent cancer risk. Epidemiology. 2005;16(4):500–7. doi: 10.1097/01.ede.0000164812.02181.d5. [DOI] [PubMed] [Google Scholar]
- 10.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) National Cancer Institute; Bethesda, MD: Apr, 2012. http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site. [Google Scholar]
- 11.Dye JL. Current Population Reports. US Census Bureau; Washington, DC: 2010. Fertility of American Women: 2008. [Google Scholar]
- 12.Lacey JV, Jr., Brinton LA, Lubin JH, Sherman ME, Schatzkin A, Schairer C. Endometrial carcinoma risks among menopausal estrogen plus progestin and unopposed estrogen users in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1724–31. doi: 10.1158/1055-9965.EPI-05-0111. [DOI] [PubMed] [Google Scholar]
- 13.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–25. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 14.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(6 Suppl):273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 15.Sigurdson AJ, Doody MM, Rao RS, et al. Cancer incidence in the US radiologic technologists health study, 1983-1998. Cancer. 2003;97(12):3080–9. doi: 10.1002/cncr.11444. [DOI] [PubMed] [Google Scholar]
- 16.Schonfeld SJ, Pfeiffer RM, Lacey JV, Jr., et al. Hormone-related risk factors and postmenopausal breast cancer among nulliparous versus parous women: An aggregated study. Am J Epidemiol. 2011;173(5):509–17. doi: 10.1093/aje/kwq404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fritz AG. International classification of diseases for oncology : ICD-O. 3rd ed. World Health Organization; Geneva: 2000. [Google Scholar]
- 18.Beral V, Bull D, Reeves G. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005;365(9470):1543–51. doi: 10.1016/S0140-6736(05)66455-0. [DOI] [PubMed] [Google Scholar]
- 19.Chang SC, Lacey JV, Jr., Brinton LA, et al. Lifetime weight history and endometrial cancer risk by type of menopausal hormone use in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev. 2007;16(4):723–30. doi: 10.1158/1055-9965.EPI-06-0675. [DOI] [PubMed] [Google Scholar]
- 20.Lacey JV, Jr., Leitzmann MF, Chang SC, et al. Endometrial cancer and menopausal hormone therapy in the National Institutes of Health-AARP Diet and Health Study cohort. Cancer. 2007;109(7):1303–11. doi: 10.1002/cncr.22525. [DOI] [PubMed] [Google Scholar]
- 21.Crosbie EJ, Zwahlen M, Kitchener HC, Egger M, Renehan AG. Body mass index, hormone replacement therapy, and endometrial cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19(12):3119–30. doi: 10.1158/1055-9965.EPI-10-0832. [DOI] [PubMed] [Google Scholar]
- 22.Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA. 1996;275(5):370–5. doi: 10.1001/jama.1996.03530290040035. [DOI] [PubMed] [Google Scholar]
- 23.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291(1):47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 24.Britt K, Ashworth A, Smalley M. Pregnancy and the risk of breast cancer. Endocr Relat Cancer. 2007;14(4):907–33. doi: 10.1677/ERC-07-0137. [DOI] [PubMed] [Google Scholar]
- 25.Combination oral contraceptive use and the risk of endometrial cancer. The Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development. JAMA. 1987;257(6):796–800. [PubMed] [Google Scholar]
- 26.Brinton LA, Hoover RN. Estrogen replacement therapy and endometrial cancer risk: unresolved issues. The Endometrial Cancer Collaborative Group. Obstet Gynecol. 1993;81(2):265–71. [PubMed] [Google Scholar]
- 27.Levi F, La Vecchia C, Gulie C, et al. Oral contraceptives and the risk of endometrial cancer. Cancer Causes Control. 1991;2(2):99–103. doi: 10.1007/BF00053128. [DOI] [PubMed] [Google Scholar]
- 28.Rubin GL, Peterson HB, Lee NC, Maes EF, Wingo PA, Becker S. Estrogen replacement therapy and the risk of endometrial cancer: remaining controversies. Am J Obstet Gynecol. 1990;162(1):148–54. doi: 10.1016/0002-9378(90)90838-x. [DOI] [PubMed] [Google Scholar]
- 29.Schouten LJ, Goldbohm RA, van den Brandt PA. Anthropometry, physical activity, and endometrial cancer risk: results from the Netherlands Cohort Study. J Natl Cancer Inst. 2004;96(21):1635–8. doi: 10.1093/jnci/djh291. [DOI] [PubMed] [Google Scholar]
- 30.Stanford JL, Brinton LA, Berman ML, et al. Oral contraceptives and endometrial cancer: do other risk factors modify the association? Int J Cancer. 1993;54(2):243–8. doi: 10.1002/ijc.2910540214. [DOI] [PubMed] [Google Scholar]
- 31.Weiderpass E, Adami HO, Baron JA, Magnusson C, Lindgren A, Persson I. Use of oral contraceptives and endometrial cancer risk (Sweden) Cancer Causes Control. 1999;10(4):277–84. doi: 10.1023/a:1008945721786. [DOI] [PubMed] [Google Scholar]
- 32.Setiawan VW, Pike MC, Kolonel LN, Nomura AM, Goodman MT, Henderson BE. Racial/ethnic differences in endometrial cancer risk: the multiethnic cohort study. Am J Epidemiol. 2007;165(3):262–70. doi: 10.1093/aje/kwk010. [DOI] [PubMed] [Google Scholar]
- 33.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10–7. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 34.Allen NE, Tsilidis KK, Key TJ, et al. Menopausal Hormone Therapy and Risk of Endometrial Carcinoma Among Postmenopausal Women in the European Prospective Investigation into Cancer and Nutrition. Am J Epidemiol. 2010 doi: 10.1093/aje/kwq300. [DOI] [PubMed] [Google Scholar]
- 35.Razavi P, Pike MC, Horn-Ross PL, Templeman C, Bernstein L, Ursin G. Long-term postmenopausal hormone therapy and endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(2):475–83. doi: 10.1158/1055-9965.EPI-09-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaakkola S, Lyytinen HK, Dyba T, Ylikorkala O, Pukkala E. Endometrial cancer associated with various forms of postmenopausal hormone therapy: a case control study. Int J Cancer. 2011;128(7):1644–51. doi: 10.1002/ijc.25762. [DOI] [PubMed] [Google Scholar]
- 37.Phipps AI, Doherty JA, Voigt LF, et al. Long-term use of continuous-combined estrogenprogestin hormone therapy and risk of endometrial cancer. Cancer Causes Control. 2011;22(12):1639–46. doi: 10.1007/s10552-011-9840-6. [DOI] [PMC free article] [PubMed] [Google Scholar]