Abstract
Clinical factors such as tall stature, lean body mass, obstructive sleep apnea, alcohol or caffeine, smoking, endurance sports, and genetic factors are proposed as risk factors for lone atrial fibrillation (LAF). The KORAF (KORean Atrial Fibrillation) study is a retrospective multicenter registry that enrolled 3,570 consecutive atrial fibrillation (AF) patients. Data on risk factors were available for 2,133 patients, of whom 398 (18.7%) were identified as having LAF. In univariate analysis, patients with LAF were more likely to be men (82.4% vs 59.8%, P < 0.001) and current smokers (25.9% vs 15.6%, P < 0.01), alcohol drinkers (55.3% vs 31.2%, P < 0.01) and frequent consumers of caffeinated beverages (> 2 cups/day) (31.7% vs 19.3%, P < 0.01), and have a family history of AF (9.0% vs 2.6%, P < 0.001) than the non-LAF patients. Multivariate analysis showed that male gender (OR, 2.30; 95% CI, 1.61-3.27, P < 0.01), family history of AF (OR, 3.12; 95% CI, 1.91-5.12, P < 0.01), current alcohol use (OR, 2.01; 95% CI, 1.46-2.76, P < 0.01), and frequent caffeinated beverage consumption (OR, 1.66; 95% CI, 1.20-2.29, P < 0.01) were independently associated with LAF. In Korean patients, LAF is more closely associated with male gender, family history of AF, current alcohol and frequent caffeinated beverage consumption than non-LAF.
Keywords: Lone Atrial Fibrillation, Risk Factors
INTRODUCTION
Atrial fibrillation (AF) is the most common tachyarrhythmia in adults (1) and is associated with increased morbidity and mortality, especially congestive heart failure, stroke, and death. AF has been associated with advanced age, male gender, hypertension, diabetes mellitus, thyroid disease, pulmonary disease, and structural heart disease such as congestive heart failure, coronary artery disease, cardiomyopathy, and valvular heart disease (2, 3).
However, 2%-10% of AF patients are younger than 60 years old, and have no cardiac or other etiologic diseases (4). These patients are classified as having lone atrial fibrillation (LAF). Recently, emerging risk factors for AF, including height (5), obesity (6), obstructive sleep apnea (7), excessive alcohol intake (8), caffeine (9), smoking (10), participating in endurance sports (11), increased pulse pressure (12), genetic factors (13), and inflammation (14) have been investigated. However, research comparing patients with LAF and those with non-lone atrial fibrillation (non-LAF) for clinical risk factors is scarce.
The aim of this study was to determine the proportion of LAF in Korean AF patients and to identify risk factors in patients with LAF compared to those with non-LAF.
MATERIALS AND METHODS
Study population
The KORAF (KORean Atrial Fibrillation study) is a retrospective multicenter registry that from January 2006 through December 2007 enrolled 3,570 consecutive AF patients in Korea. Among these, 1,437 were excluded because of incomplete data. The remaining 2,133 were included in study analyses. All patients were adults over 17 yr of age. Height, weight, and blood pressure were measured and participants underwent 12-lead electrocardiogram, blood test, urine analysis, chest radiography, and transthoracic echocardiography. Participants answered questionnaires about family history of AF, cardiovascular disease (hypertension, hyperlipidemia, congestive heart failure, coronary artery disease, valvular heart disease, cardiomyopathy, peripheral vascular disease, and congenital heart disease), other disease (diabetes mellitus, neoplasms, hyperthyroidism, obstructive sleep apnea, chronic obstructive pulmonary disease, hypothyroidism, and chronic renal failure), risk factors (smoking status, alcohol drinking, and caffeinated beverage intake), and triggering factors for paroxysmal AF (emotional stress, use of caffeinated beverages, heavy meals, alcohol use, sleeping, exercise, and smoking).
Each patient underwent a structured interview that included a standard question regarding a family history of AF. All subjects were asked if they had a first degree family member (a biologically related parent, sibling, or child) with a known history of AF. If subjects were certain of the answer, the answer was regarded as positive.
Based on questionnaire answers, current smokers were defined as reporting current smoking for at least 6 months; exsmokers were defined as having smoked, but having quit, and never smokers had never smoked. Lifelong pack-years of exposure were assessed by determining the average packs of cigarettes smoked per day for specific age periods, beginning with the age at initiation. Alcohol intake was evaluated using semiquantitative questionnaire including detailed questions on the use of alcoholic beverages. The subjects were asked in multiple choice questions whether they drank beer, soju, hard liquors "daily", "weekly", "monthly", or "hardly ever/never". The duration of use was also recorded. A questionnaire to assess consumption of caffeinated beverages was "Do you drink caffeinated beverage?" Options for answers to that question were "more than 2 cups/day, 1 cup/day, 3-4 cups/week, 1-2 cups/week, 1-2 cups/month and never or seldom". Body mass index (BMI) was categorized as less than 18.5 kg/m2, underweight; 18.5 to < 25 kg/m2, normal; 25 to < 30 kg/m2, overweight; and ≥ 30 kg/m2, obese (15).
Types of AF
AF was classified as first diagnosed, paroxysmal, persistent and permanent according to the ESC guidelines (16). Patients who were detected as having AF for the first time were defined as having a first-diagnosed AF, irrespective of the duration, presence, or severity of AF-related symptoms. Paroxysmal AF was defined as AF that self-terminated within 48 hours or lasted within 7 days. Persistent AF was AF that required pharmacologic or electrical cardioversion or lasted more than 7 days. Permanent AF was defined when the presence of arrhythmia was accepted as irreversible by the patient and physician, and rhythm control intervention was not pursued.
Definition of LAF
LAF was defined as AF in individuals younger than 60 yr old without cardiac or other disease (2).
Statistical analysis
Categorical variables are presented as number (%) and continuous variables as mean ± standard deviation. Comparisons between groups were performed using Student's t-test for continuous variables and chi-square test for categorical data. To determine independent clinical predictors for LAF compared to non-LAF, multivariate logistic regression analysis was performed. The multivariate model included variables that were significant at a P < 0.2 in the univariate analysis. Bonferroni correction was used to adjust for multiple comparisons. Values of P < 0.05 were considered significant. Statistical analysis was performed with SPSS 18.0 (SPSS Interactive Graphics, version 18.0, SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical characteristics of study subjects
Among 2,133 patients, 398 (18.7%) were identified as having LAF and 1,735 (81.3%) were defined as non-LAF patients. Demographic baseline characteristics of the participants are in Table 1. The mean age of the study population was 62.2 ± 12.8 yr and 1,366 (64%) were men. Mean BMI was 24.3 ± 3.2 and 81 (3.8%) patients had a family history of AF. Underlying cardiovascular diseases were hypertension (42.9%), hyperlipidemia (15.4%), congestive heart failure (13.2%), coronary artery disease (12.5%), valvular heart disease (7.8%), cardiomyopathy (2.6%), peripheral vascular disease (0.6%), and congenital heart disease (0.4%). Underlying comorbid diseases were diabetes mellitus (13.7%), neoplasms (4.7%), hyperthyroidism (3.6%), obstructive sleep apnea (3.0%), chronic obstructive pulmonary disease (2.9%), hypothyroidism (1.4%), and chronic renal failure (1.3%).
Table 1.
Baseline characteristics of 2,133 Korean patients with atrial fibrillation
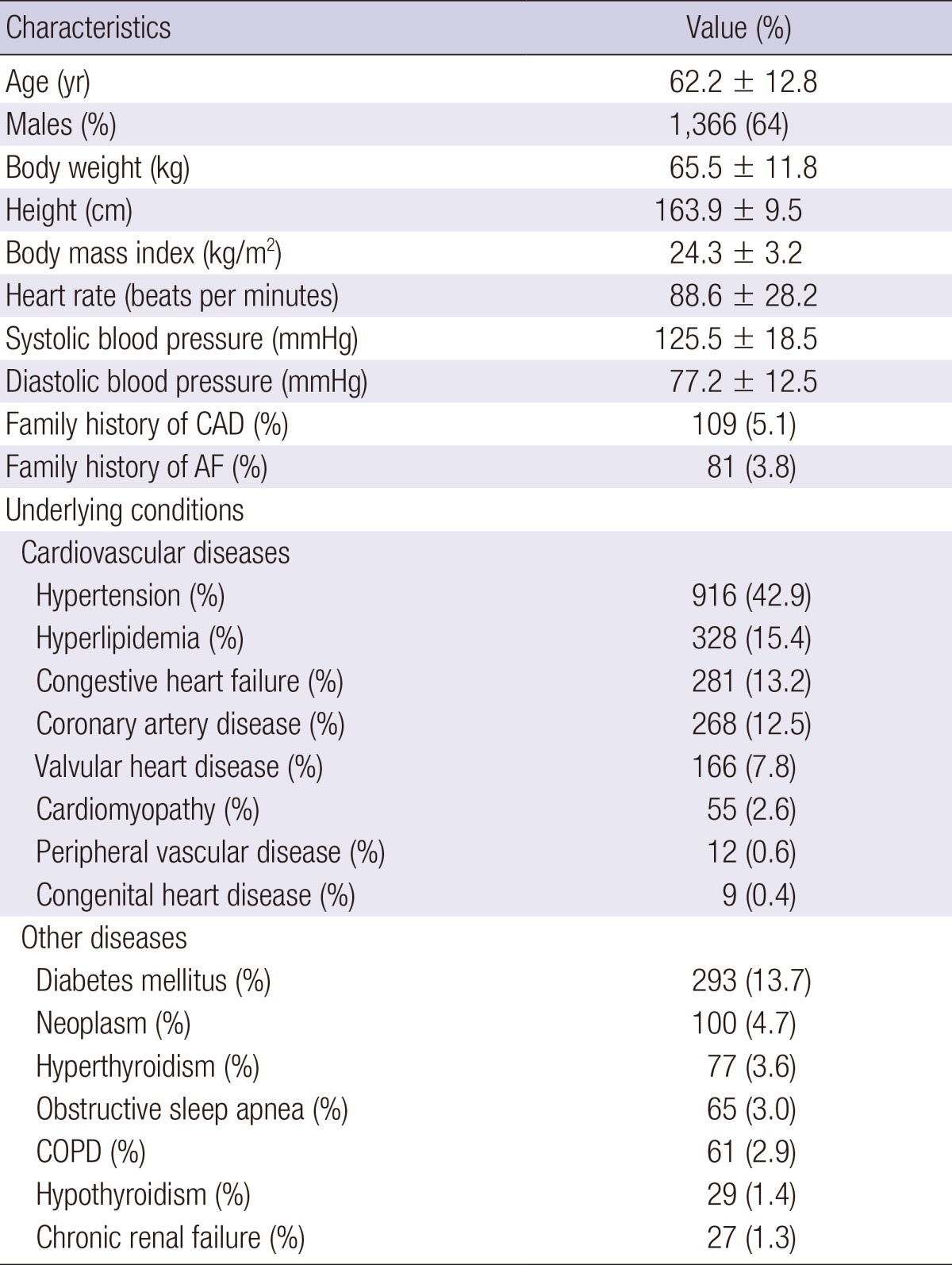
Values are mean ± SD or number (%). CAD, coronary artery disease; AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease.
Comparison of clinical characteristics
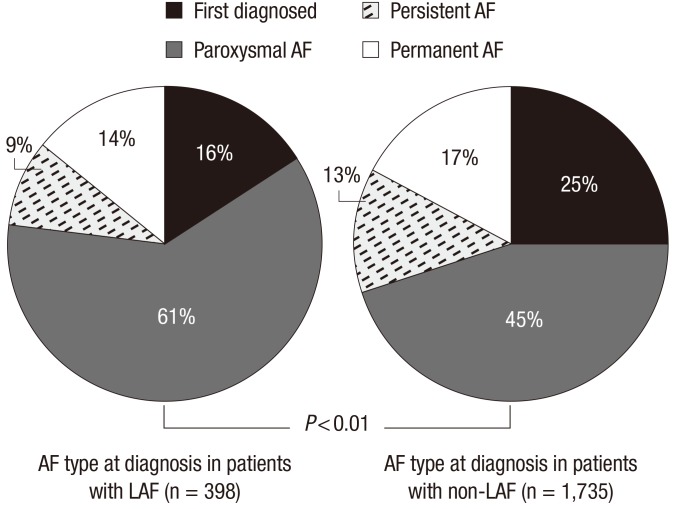
Clinical characteristics of non-LAF and LAF- > LAF and non-LAF patients are in Table 2. The mean age was 48.1 ± 8.5 yr for LAF patients and 65.5 ± 11.3 yr for non-LAF patients (P < 0.001). Men were predominant in the LAF group than in the non-LAF group (82.4% vs 59.8%, P < 0.001). Patients with LAF had a higher mean weight (71 ± 10 kg vs 64 ± 11 kg, P < 0.001) and were taller than those with non-LAF (169 ± 7 cm vs 162 ± 9 cm, P < 0.001). However, there were no significant differences in body weight (70 ± 12 kg vs 70 ± 9 kg, P = 0.99) and height (168 ± 8 cm vs 167 ± 7 cm, P = 0.08) between two groups after adjustment for age and gender matched comparison. BMI was slightly higher in patients with LAF than in those with non-LAF (24.7 ± 2.6 vs 24.2 ± 3.3, P = 0.003). Patients with LAF had lower systolic blood pressure (122 ± 13 mmHg vs 126 ± 19 mmHg, P < 0.001) and pulse pressure (44 ± 9 mmHg vs 49 ± 14 mmHg, P < 0.001) than those with non-LAF but diastolic pressure showed no significant difference between the two groups. The proportion of participants with a family history of coronary artery disease was similar in both groups (5.5% vs 5.0%, P = 0.67), but family history of AF was more frequently observed in the LAF group (9.0% vs 2.6%, P < 0.001). Hyperlipidemia was less frequently observed in patients with LAF than in those with non-LAF (10.3% vs 16.5%, P = 0.002). However, obstructive sleep apnea (3.3% vs 3.1%, P = 0.28) showed no significant difference between the LAF and non-LAF groups. A comparison of the proportions of AF type are in Fig. 1. In patients with LAF, paroxysmal AF was more frequently noted (61% vs 45%, P < 0.01) but less persistent (9% vs 13%, P < 0.01) and permanent (14% vs 17%, P < 0.01) than patients with non-LAF.
Table 2.
Clinical characteristics in LAF and non-LAF groups
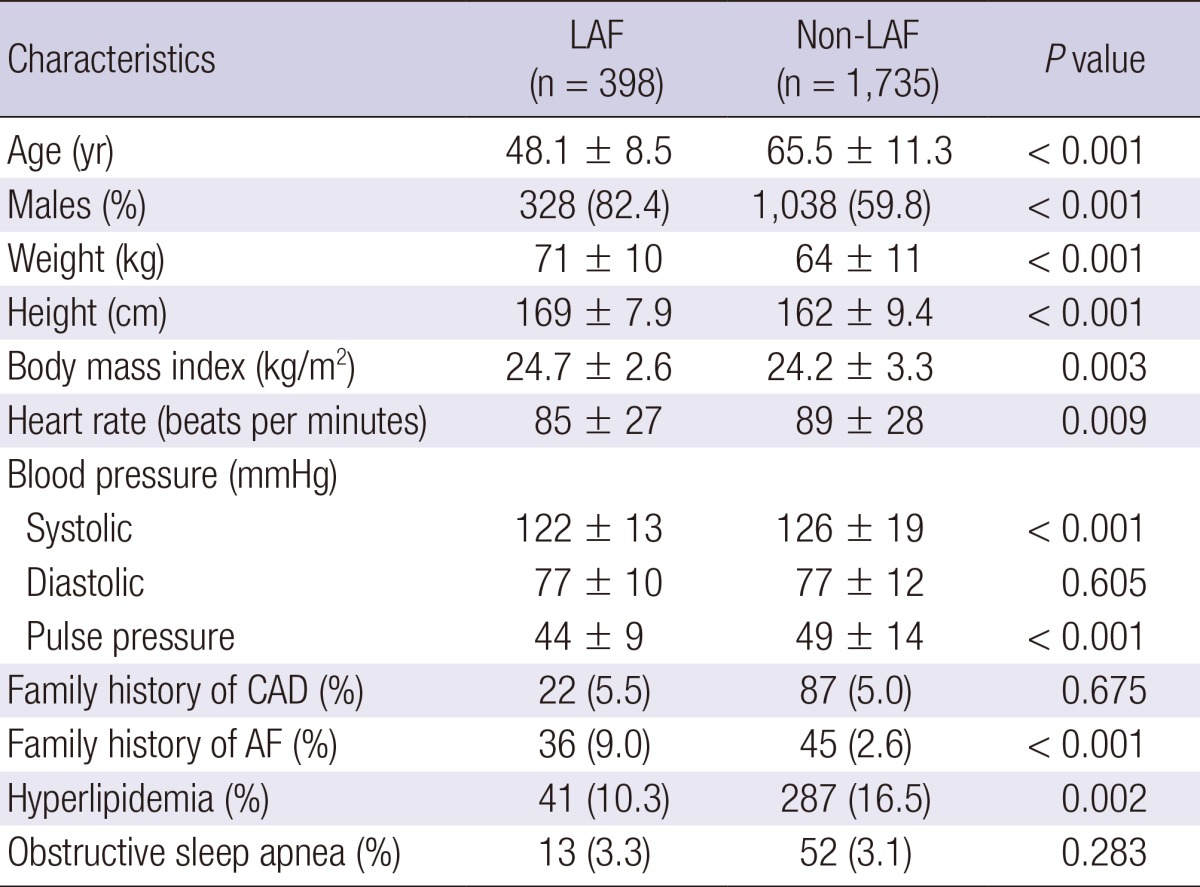
Values are mean ± SD or number (%). CAD, coronary artery disease; LAF, lone atrial fibrillation; Non-LAF, non-lone atrial fibrillation.
Fig. 1.
Comparison of AF type in LAF and non-LAF groups.
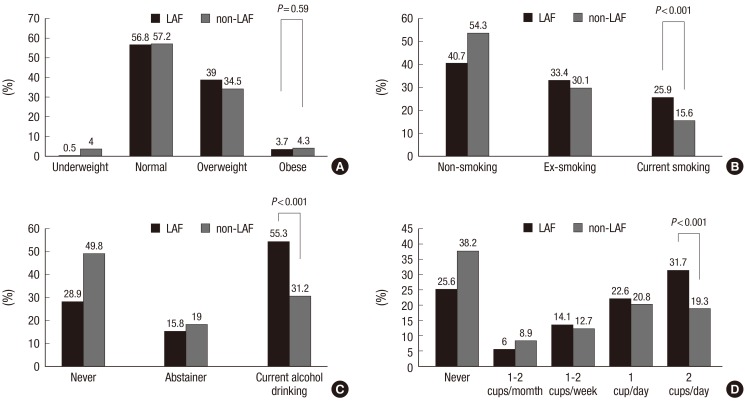
There were no significant differences in BMIs by grade between groups (Fig. 2A). Patients with LAF were more likely to be current smokers (25.9% vs 15.6%, P < 0.001) (Fig. 2B), current alcohol drinkers (55.3% vs 31.2%, P < 0.001) (Fig. 2C) or consumers of more than 2 cups of caffeinated beverages per day (31.7% vs 19.3%, P < 0.001) (Fig. 2D).
Fig. 2.
Comparison of risk factors in LAF and non-LAF groups. Body mass index (A), smoking (B), alcohol drinking (C), caffeinated beverage intake (D) in LAF and non-LAF groups.
Comparison of triggering factors
Triggering factors causing paroxysmal AF were more frequently observed in LAF patients (52.4% vs 34.7%, P < 0.01) than non-LAF patients. More frequently noted in LAF patients than non-LAF were emotional stress (32.8% vs 19.5%, P < 0.01), alcohol (18.9% vs 7.5%, P < 0.01), heavy meal (4.5% vs 1.7%, P = 0.01), and sleeping (5.7% vs 2.5%, P = 0.01) (Table 3). We did not observe significant differences in the triggering factors of exercise, caffeinated beverage intake, or smoking between two groups.
Table 3.
Triggering factors for paroxysmal AF in LAF and non-LAF groups
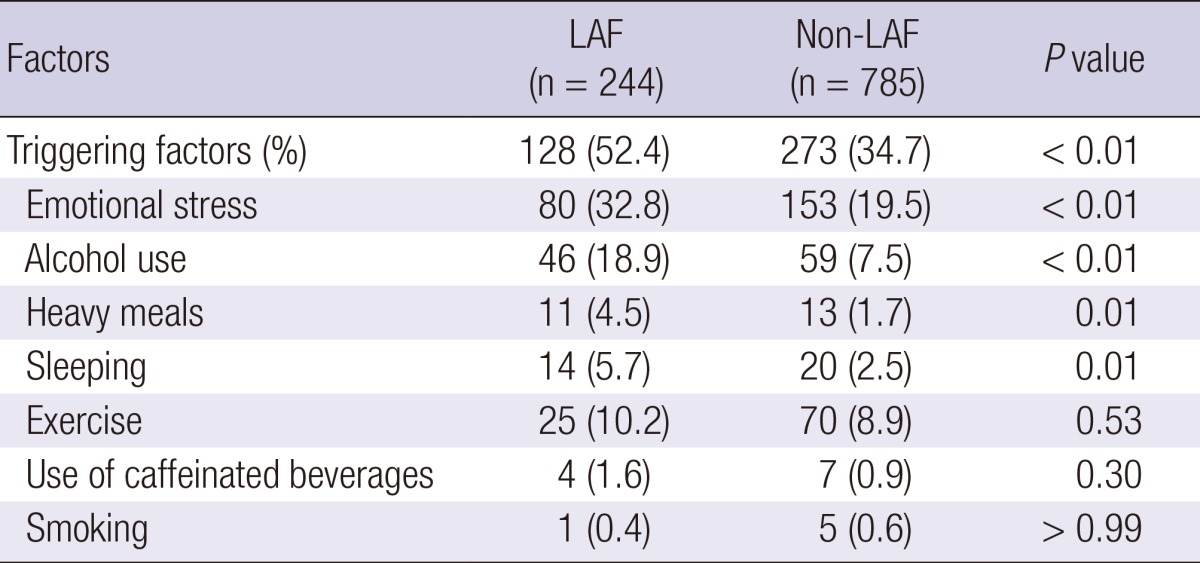
Values are mean ± SD or number (%). LAF, lone atrial fibrillation; Non-LAF, non-lone atrial fibrillation.
Risk factors associated with LAF
Male gender (OR, 3.14; 95% CI, 2.38-4.14, P < 0.001), higher BMI (OR, 1.04; 95% CI, 1.01-1.08, P = 0.007), family history of AF (OR, 3.73; 95% CI, 2.37-5.87, P < 0.001), current alcohol use (OR, 3.05; 95% CI, 2.37-3.91, P < 0.001), current smoking (OR, 2.21; 95% CI, 1.66-2.92, P < 0.001), or caffeinated beverage intake of more than 2 cups per day (OR, 2.44; 95% CI, 1.82-3.27, P < 0.01) were significantly associated with LAF in univariate analysis. Multivariate logistic regression analysis showed that male gender (OR, 2.30; 95% CI, 1.61-3.27, P < 0.01), family history of AF (OR, 3.12; 95% CI, 1.91-5.12, P < 0.01), current alcohol drinking (OR, 2.01; 95% CI, 1.46-2.76, P < 0.01), or caffeinated beverage intake of more than 2 cups per day (OR, 1.66; 95% CI, 1.20-2.29, P < 0.01) were independently associated with LAF (Table 4).
Table 4.
Univariate and multivariate logistic regression analysis of LAF risk factors
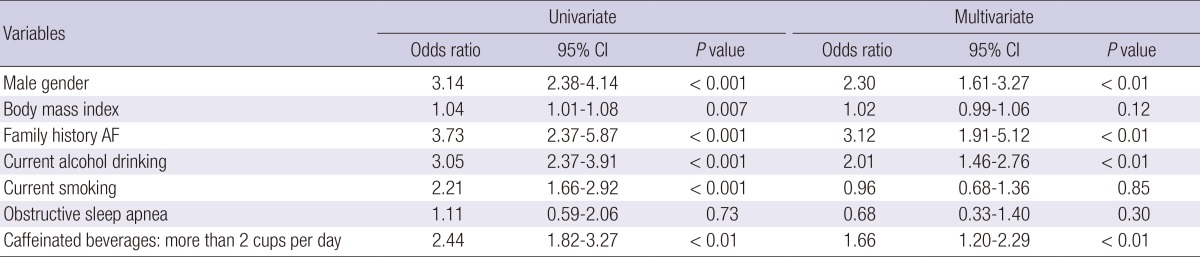
LAF, lone atrial fibrillation; AF, atrial fibrillation.
DISCUSSION
This study showed that compared to patients with non-LAF, patients with LAF were more predominantly male, with a family history of AF, currently alcohol drinkers, and frequent consumers of caffeinated beverages.
LAF was defined as AF in individuals under the age of 60 without cardiac or other etiologic disease. Therefore, the pathophysiology of LAF is not substrate related, such as dilated atria with stretch and fibrosis, which is more related to electrophysiological phenomena in structurally normal atria (17). This could be the reason why paroxysmal AF is more prevalent (18) and does not often progress to persistent or permanent AF (19). In our study population, a larger proportion of patients with LAF had paroxysmal AF and had less persistent and less permanent AF than patients with non-LAF.
Consistent with a previous study showing a male-to-female ratio of 3:1 to 4:1 for LAF (4), we found that LAF patients were more predominant male (male to female ratio; 4.6:1). Family members of patients with LAF have a substantially increased prevalence of AF compared to the general population (20). Darbar et al. (21) reported that at least 5% of AF and 15% of LAF patients had a family history of the disease. The risk of LAF strongly increases with early age at onset, multiple affected relatives, and LAF in first-degree relatives (22). Our results showed a similar pattern as a study that showed that family history of AF was more often observed in patients with LAF than patients with non-LAF. Focusing on male predominance and frequent family history, Chen et al. (23) found that male predilection in sporadic LAF and a possible familial proband could be partly due to X-linked recessive inheritance. However, male predominance decreased in confirmed familial probands as the likelihood of dominant Mendelian inheritance increased. The authors suggested that the increased frequency of sporadic LAF in men might be explained by unrecognized X-linked recessive disease. However, male gender and the genetic inheritance pattern have not been confirmed. Further investigation of the Mendelian inheritance of LAF incidence needs to be studied.
Wang et al. (24) found that obesity is a potential risk factor for AF and this mechanism appears to be mediated by left atrial dilatation. However, patients with LAF tend to be taller and leaner than average (5, 11). Individuals with these anthropometric measurements tend to be relatively parasympathetic dominant (25). Parasympathetic stimulation can cause shortening of the atrial effective refractory period and hyperpolarization of atrial fibers, leading to increased conduction velocity (26). Vagally mediated AF occurs in about two-thirds of LAF patients, with mixed or adrenergic AF in the rest (25). Vagally mediated AF occurs more frequently in men and at night or early in the morning (26). It is associated with participating in endurance sports (27) as autonomic nervous system imbalance such as marathon and cycling (11, 28). Consistent with previous studies, we found that patients with LAF had more vagally mediated stimulations (sleeping, heavy meal) as triggering factors.
Several mechanisms have been suggested by which alcohol consumption influences the development of AF. Alcohol causes AF by a direct toxic effect on cardiac myocytes, hyperadrenergic state and impaired vagal tone, and an increase in intra-atrial conduction time (17). A previous study found that LAF patients drank more in the previous week than controls (29). Our results showed that current alcohol drinking was more frequent in LAF than in non-LAF patients.
However, some reports found that the amount of alcohol was associated with risk of AF (30, 31). Djousse et al. (30) reported that consuming more than 36 g of alcohol per day increased the risk of developing AF by 34%. Mukamal et al. (31) found that an alcohol intake of more than 35 drinks a week was associated with a higher risk of AF in men. We did not estimate the amount of alcohol and association with LAF because of difficulty in determining the different kinds of alcohol intake and correcting for alcohol intake amounts. The cumulative effect of alcohol on LAF needs to be further studied.
Our finding showed current smoking was more common in the LAF group. This result might be explained by an earlier study reported that nicotine changes atrial conduction and refractoriness in isolated rat hearts. Younger rat hearts were more susceptible to AF than older rat hearts after nicotine administration (10). In our results, current smoking was associated with LAF in univariate analysis. A younger population might be more sensitive to a smoking effect on AF. However, Goette et al. (32) found that, in patients hospitalized for coronary bypass surgery, the only factor related to the amount of fibrosis in right atrial appendages was the number of pack-years. Fibrosis was related to the development of postoperative AF (32). However, our study did not estimate the cumulative effect of smoking on LAF.
Studies on the association between caffeinated beverage intake and AF risk have had conflicting results (9, 33). Our study found that frequent caffeinated beverage consumption (more than 2 cups of coffee per day) was significantly associated with LAF. These results corresponded with the results of earlier studies reported that coffee consumption resulting from acute, stress-induced changes in lifestyle is associated with an increased risk of acute LAF (33). A possible mechanism for this association might be neurohormonal stimulation and sympathetic activation (34). However, our results are different from that of Frost and Vestergaard (9), in which consumption of caffeine was not associated with risk of AF or flutter. Further studies are necessary to clarify the relation between caffeine consumption and risk of LAF.
This study had several limitations. First, we were unable to investigate the cumulative effect of risk factors such as alcohol, smoking, caffeinated beverage consumption on LAF because of incomplete data and lack of standard measurements. Several studies reported that the amount of alcohol and the number of pack-years of smoking were more closely associated with risk of AF than simply alcohol or cigarette use (30-32). Further studies on dose-response relationships are needed. Second, this study had a cross-sectional retrospective design. The association of substances such as alcohol, smoking, and caffeinated beverages with LAF might not be a cause-effect relationship. Third, a detailed family history of AF including whether first- or second-degree relatives had AF, and how many relatives were affected, and AF patients were familial probands or sporadic cases were not obtained.
In conclusion, LAF is more closely associated with male gender, family history of AF, current alcohol drinking, and frequent caffeinated beverage consumption than non-LAF. Lifestyle modification such as abstaining from drinking alcohol or reducing caffeinated beverage consumption might decrease the AF burden in patients with LAF. Additionally, further research on genetic factors that predispose to LAF might be needed.
DISCLOSURE
The authors have no conflicts of interest to disclose.
APPENDIX
The following investigators and institutions participated in the KORAF Investigators:
Sung Won Jang, Division of Cardiology, The Catholic University of Korea, St Paul's Hospital, Seoul; Yong-Seog Oh, Division of Cardiology, The Catholic University of Korea, St Mary's Hospital, Seoul; Young Soo Lee, Division of Cardiology, Daegu Catholic University College of Medicine, Daegu; Yong Gyeun Cho, Division of Cardiology, Kyungpook National University College of Medicine, Daegu; Tae Joon Cha, Division of Cardiology, Kosin University College of Medicine, Busan; Dong-Jin Oh, Division of Cardiology, Hallym University College of Medicine, Seoul; In Suck Choi, Division of Cardiology, Gachon University College of Medicine, Gil Hospital, Incheon; Myung Yong Lee, Division of Cardiology, Dankook University College of Medicine, Cheonan; Seil Oh, Division of Cardiology, Seoul National University College of Medicine, Seoul; Moon-Hyoung Lee, Division of Cardiology, Yonsei University College of Medicine, Severance Hospital, Seoul; Kee-Joon Choi, Division of Cardiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul; Nam-Ho Kim, Division of Cardiology, Wonkwang University College of Medicine, Iksan; Dae-Kyung Kim, Division of Cardiology, Inje University College of Medicine, Busan Paik Hospital, Busan; June Namgung, Division of Cardiology, Inje University College of Medicine, Ilsan Paik Hospital, Goyang; Dae-Hyuck Kim, Division of Cardiology, Inha University College of Medicine, Incheon; Kyoung-Suk Lee, Division of Cardiology, Chonbuk National University College of Medicine, Jeonju; Jun Hyung Kim, Division of Cardiology, Chungnam National University College of Medicine, Daejeon; Myung-Chan Cho; Division of Cardiology, Chungbuk National University College of Medicine, Cheongju, Korea.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort: the Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 3.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 4.Kopecky SL, Gersh BJ, McGoon MD, Whisnant JP, Holmes DR, Jr, Ilstrup DM, Frye RL. The natural history of lone atrial fibrillation: a population-based study over three decades. N Engl J Med. 1987;317:669–674. doi: 10.1056/NEJM198709103171104. [DOI] [PubMed] [Google Scholar]
- 5.Mont L, Tamborero D, Elosua R, Molina I, Coll-Vinent B, Sitges M, Vidal B, Scalise A, Tejeira A, Berruezo A, et al. Physical activity, height, and left atrial size are independent risk factors for lone atrial fibrillation in middle-aged healthy individuals. Europace. 2008;10:15–20. doi: 10.1093/europace/eum263. [DOI] [PubMed] [Google Scholar]
- 6.Dublin S, French B, Glazer NL, Wiggins KL, Lumley T, Psaty BM, Smith NL, Heckbert SR. Risk of new-onset atrial fibrillation in relation to body mass index. Arch Intern Med. 2006;166:2322–2328. doi: 10.1001/archinte.166.21.2322. [DOI] [PubMed] [Google Scholar]
- 7.Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 8.Kodama S, Saito K, Tanaka S, Horikawa C, Saito A, Heianza Y, Anasako Y, Nishigaki Y, Yachi Y, Iida KT, et al. Alcohol consumption and risk of atrial fibrillation: a meta-analysis. J Am Coll Cardiol. 2011;57:427–436. doi: 10.1016/j.jacc.2010.08.641. [DOI] [PubMed] [Google Scholar]
- 9.Frost L, Vestergaard P. Caffeine and risk of atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Clin Nutr. 2005;81:578–582. doi: 10.1093/ajcn/81.3.578. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi H, Omichi C, Miyauchi Y, Mandel WJ, Lin SF, Chen PS, Karagueuzian HS. Age-related sensitivity to nicotine for inducible atrial tachycardia and atrial fibrillation. Am J Physiol Heart Circ Physiol. 2003;285:H2091–H2098. doi: 10.1152/ajpheart.00371.2003. [DOI] [PubMed] [Google Scholar]
- 11.Molina L, Mont L, Marrugat J, Berruezo A, Brugada J, Bruguera J, Rebato C, Elosua R. Long-term endurance sport practice increases the incidence of lone atrial fibrillation in men: a follow-up study. Europace. 2008;10:618–623. doi: 10.1093/europace/eun071. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell GF, Vasan RS, Keyes MJ, Parise H, Wang TJ, Larson MG, D'Agostino RB, Sr, Kannel WB, Levy D, Benjamin EJ. Pulse pressure and risk of new-onset atrial fibrillation. JAMA. 2007;297:709–715. doi: 10.1001/jama.297.7.709. [DOI] [PubMed] [Google Scholar]
- 13.Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, Jin HW, Sun H, Su XY, Zhuang QN, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 14.Ellinor PT, Low A, Patton KK, Shea MA, MacRae CA. C-reactive protein in lone atrial fibrillation. Am J Cardiol. 2006;97:1346–1350. doi: 10.1016/j.amjcard.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 15.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report: National Institutes of Health. Obes Res. 1998;6:51S–209S. [PubMed] [Google Scholar]
- 16.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 17.Schoonderwoerd BA, Smit MD, Pen L, Van Gelder IC. New risk factors for atrial fibrillation: causes of 'not-so-lone atrial fibrillation'. Europace. 2008;10:668–673. doi: 10.1093/europace/eun124. [DOI] [PubMed] [Google Scholar]
- 18.Patton KK, Zacks ES, Chang JY, Shea MA, Ruskin JN, Macrae CA, Ellinor PT. Clinical subtypes of lone atrial fibrillation. Pacing Clin Electrophysiol. 2005;28:630–638. doi: 10.1111/j.1540-8159.2005.00161.x. [DOI] [PubMed] [Google Scholar]
- 19.Jahangir A, Lee V, Friedman PA, Trusty JM, Hodge DO, Kopecky SL, Packer DL, Hammill SC, Shen WK, Gersh BJ. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation. 2007;115:3050–3056. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- 20.Ellinor PT, Yoerger DM, Ruskin JN, MacRae CA. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118:179–184. doi: 10.1007/s00439-005-0034-8. [DOI] [PubMed] [Google Scholar]
- 21.Darbar D, Herron KJ, Ballew JD, Jahangir A, Gersh BJ, Shen WK, Hammill SC, Packer DL, Olson TM. Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol. 2003;41:2185–2192. doi: 10.1016/s0735-1097(03)00465-0. [DOI] [PubMed] [Google Scholar]
- 22.Oyen N, Ranthe MF, Carstensen L, Boyd HA, Olesen MS, Olesen SP, Wohlfahrt J, Melbye M. Familial aggregation of lone atrial fibrillation in young persons. J Am Coll Cardiol. 2012;60:917–921. doi: 10.1016/j.jacc.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 23.Chen LY, Herron KJ, Tai BC, Olson TM. Lone atrial fibrillation: influence of familial disease on gender predilection. J Cardiovasc Electrophysiol. 2008;19:802–806. doi: 10.1111/j.1540-8167.2008.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang TJ, Parise H, Levy D, D'Agostino RB, Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 25.Chambers PW. Lone atrial fibrillation: pathologic or not? Med Hypotheses. 2007;68:281–287. doi: 10.1016/j.mehy.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 26.Podrid PJ. Etiology and pathogenesis of atrial fibrillation. In: Kowey P, Naccarelli GV, editors. Atrial Fibrillation. New York: Marcel Dekker; 2005. pp. 27–60. [Google Scholar]
- 27.Mont L, Sambola A, Brugada J, Vacca M, Marrugat J, Elosua R, Paré C, Azqueta M, Sanz G. Long-lasting sport practice and lone atrial fibrillation. Eur Heart J. 2002;23:477–482. doi: 10.1053/euhj.2001.2802. [DOI] [PubMed] [Google Scholar]
- 28.Baldesberger S, Bauersfeld U, Candinas R, Seifert B, Zuber M, Ritter M, Jenni R, Oechslin E, Luthi P, Scharf C, et al. Sinus node disease and arrhythmias in the long-term follow-up of former professional cyclists. Eur Heart J. 2008;29:71–78. doi: 10.1093/eurheartj/ehm555. [DOI] [PubMed] [Google Scholar]
- 29.Koskinen P, Kupari M, Leinonen H, Luomanmäki K. Alcohol and new onset atrial fibrillation: a case-control study of a current series. Br Heart J. 1987;57:468–473. doi: 10.1136/hrt.57.5.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Djoussé L, Levy D, Benjamin EJ, Blease SJ, Russ A, Larson MG, Massaro JM, D'Agostino RB, Wolf PA, Ellison RC. Long-term alcohol consumption and the risk of atrial fibrillation in the Framingham Study. Am J Cardiol. 2004;93:710–713. doi: 10.1016/j.amjcard.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Mukamal KJ, Tolstrup JS, Friberg J, Jensen G, Grnbaek M. Alcohol consumption and risk of atrial fibrillation in men and women: the Copenhagen City Heart Study. Circulation. 2005;112:1736–1742. doi: 10.1161/CIRCULATIONAHA.105.547844. [DOI] [PubMed] [Google Scholar]
- 32.Goette A, Lendeckel U, Kuchenbecker A, Bukowska A, Peters B, Klein HU, Huth C, Röcken C. Cigarette smoking induces atrial fibrosis in humans via nicotine. Heart. 2007;93:1056–1063. doi: 10.1136/hrt.2005.087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattioli AV, Bonatti S, Zennaro M, Melotti R, Mattioli G. Effect of coffee consumption, lifestyle and acute life stress in the development of acute lone atrial fibrillation. J Cardiovasc Med (Hagerstown) 2008;9:794–798. doi: 10.2459/JCM.0b013e3282f64554. [DOI] [PubMed] [Google Scholar]
- 34.Strubelt O, Diederich KW. Experimental treatment of the acute cardiovascular toxicity of caffeine. J Toxicol Clin Toxicol. 1999;37:29–33. doi: 10.1081/clt-100102405. [DOI] [PubMed] [Google Scholar]